Abstract
Aim:
The aim of the present study is to evaluate the antiepileptic activity of chloroform extract of aerial parts of Acalypha fruticosa in mice.
Materials and Methods:
The antiepileptic activity of chloroform extract of A. fruticosa at the doses of 30, 100 and 300 mg/kg, p.o. was evaluated by maximum electroshock (MES), pentylenetetrazole (PTZ) and isoniazid (INH)-induced convulsions in mice. Statistical analysis was carried out by one-way analysis of variance followed by Dunnett's test.
Results:
In MES method, the chloroform extract significantly protected the mice from convulsions induced by electroshock method in a dose-dependent manner and exhibited more activity at the dose of 300 mg/kg when compared with diazepam treated animals. In PTZ method, the extract inhibited convulsions in mice potent than phenobarbitone sodium. In INH method, it delayed the latency of convulsions in mice in a dose-dependent manner but failed to protect the mice against mortality.
Conclusion:
The chloroform extract exhibited significant and dose-dependent antiepileptic activity, which may be due to the presence of antioxidant principles like flavanoids.
Keywords: Acalypha fruticosa, diazepam, epilepsy, isoniazid, pentylenetetrazole, phenobarbitone sodium
INTRODUCTION
Epilepsy is a brain disorder characterized by convulsive seizures or loss of consciousness or both. Epilepsy is a major neurological disorder and up to 5% of the world population has epilepsy in their lifetime.
Drug therapy of epilepsy with currently available antiepileptic drugs (AED) is associated with side-effects, dose-related and chronic toxicity that involves virtually every organ system. Hazards of anticonvulsant therapy in pregnancy and teratogenic effects are well-known. Moreover, all the currently available AED have potential for adverse effects on cognition and behavior. This made man to search for alternative medicine from natural source.[1,2]
Medicinal plants used for the therapy of epilepsy in traditional medicine have been shown to possess promising anticonvulsant activities. The folk or traditional medicinal uses of plants represent “leads” that could short cut the discovery of modern medicines with novel structures, which can be much cheaper and less time-consuming. Several useful medicines derived from plants have been discovered from scientific investigation of traditional and folklore claims.[3,4,5]
Acalypha fruticosa (CAF) is one such plant commonly known as “Chinnichedi” and “Birch-leaved acalypha” is a shrub belonging to the family of Euphorbiaceae. Tribal people of different parts of the world uses this plant to treat various diseases such as convulsions, cough, cold, scabies, constipation, malaria, liver problems, skin diseases. Leaf decoction of this plant is used to treat epilepsy by tribals of Tanzania. The Paliyar and Irula tribes in Western Ghats of South India use leaves and roots of A. fruticosa to treat skin diseases, wounds, stomachache and poisonous bites. The Suiei hunter-gatherers of northern Kenya drunk the root decoction to treat convulsions, fever, colds and swellings of the scrotum.[6,7] However until today, there were no reports to justify its ethnobotanical claim. Hence the present work was designed to evaluate the antiepileptic activity of chloroform extract of aerial parts of A. fruticosa against the convulsions induced by maximum electroshock (MES), pentylenetetrazole (PTZ) and isoniazid (INH) in mice.
MATERIALS AND METHODS
Drugs and chemicals
Diazepam (Calmpose inj., Ranbaxy), phenobarbitone sodium (Luminal, Bayer AG), PTZ (Sigma Aldrich Chemical Co.) and INH (S.D. Fine-Chem. LTD) were used in this study.
Plant collection
The aerial parts of A. fruticosa were collected from Tirupati, Andhra Pradesh, India. They were identified and authenticated by Prof. K. Madhava Chetty, Department of Botany, Sri Venkateswara University. The plant specimen was deposited at Sri Venkateswara University Herbarium, Tirupati with voucher number 1252.
Preparation of the extract
The fresh aerial parts of A. fruticosa were collected and washed under running tap water. They were shade dried at room temperature and 1 Kg of the dried aerial parts was made in to coarse powder. The powder was passed through a No. 60 mesh sieve. Then chloroform extract was prepared by following maceration method.[8]
Preliminary phytochemical investigations
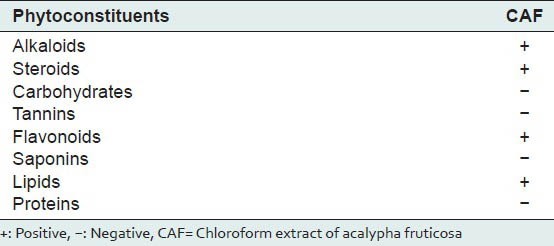
The extract was subjected to qualitative chemical tests for various phytoconstituents like alkaloids, carbohydrates, saponins, tannins, proteins, lipids, flavonoids and steroids.[9]
Pharmacological investigations
Animals
Adult Swiss albino mice (25-30 g) were used for this study. The animals were housed at 24°C ± 2°C and relative humidity 55 ± 5 with 12:12 h light and dark cycle. They were provided food and water ad libitum. The animals were acclimatized before the study. The experimental protocol was approved by the Institutional Animals Ethics Committee of Talla Padmavathi College of Pharmacy, Warangal, Andhra Pradesh (CPCSEA no. 1505/PO/a/11/CPCSEA).
Acute toxicity study
Acute toxicity study was performed for the extract to ascertain safe dose by acute oral toxic class method of Organization of Economic Co-operation and Development, as per 423 guidelines.[10]
Evaluation of antiepileptic activity
MES in mice
Five groups of six Swiss albino mice (25-30 g) of either sex were used. Group I received the vehicle, Group II, III and IV received different doses (30, 100 and 300 mg/kg, p.o.) of chloroform extract of Acalypha fruticosa (CAF) respectively. Group V received the standard drug, diazepam at the dose of 3 mg/kg, p.o. The test was started 1 h after oral treatment with the extract or the vehicle or the standard. An apparatus with corneal electrodes was used to deliver the stimuli. The intensity of the stimulus was dependent on the apparatus, e.g.: 45 mA, 50 Hz for 0.2 s has been used. Under these conditions all vehicle treated mice showed the characteristic extensor tonus. The animals were observed closely for 2 min. Disappearance of the tonic hind limb extensor was used as positive criterion. Percentage of inhibition of seizures relative to control was calculated.[11]
PTZ-induced convulsions in mice
Mice of either sex were randomly allotted to five different groups of six mice each. Group I received the vehicle, Group II, III and IV received CAF at the doses of 30, 100 and 300 mg/kg, p.o. respectively. Group V received the standard drug, phenobarbitone sodium at the dose of 40 mg/kg, i.p. Group I mice were administered with PTZ (75 mg/kg, i.p.) 1 h after vehicle. Group V mice received PTZ 15 min after phenobarbitone sodium (40 mg/kg, i.p.). Group II, III and IV mice received different doses of plant extracts, p.o. 1 h before PTZ. Onset time as well as duration of convulsions were recorded.[12]
INH-induced convulsions in mice
Five groups of six Swiss albino mice (25-30 g) of either sex were used. Group I received the vehicle, group II, III and IV received CAF at the doses of 30, 100 and 300 mg/kg, p.o. Group V received the standard drug, diazepam at the dose of 4 mg/kg, i.p. 1 h after the administration of different extracts of A. fruticosa, INH at a dose of 300 mg/kg, s.c. was administered. The mice were placed in isolated perplex chamber, during the next 120 min the latency of convulsions was recorded.[13]
Statistical analysis
The data were analyzed using one-way analysis of variance, followed by Dunnett's test. P < 0.05 was considered as statistically significant. The data are expressed as mean ± standard deviation.
RESULTS
Preliminary phytochemical investigations
Preliminary phytochemical investigations of CAF were presented in Table 1 which showed the presence of alkaloids, steroids, flavanoids and lipids.
Table 1.
Preliminary phytochemical investigations
Pharmacological investigations
Acute toxicity study
In the acute toxicity study, CAF was found to be toxic at a dose of 2000 mg/kg, p.o. body weight in mice and CAF was found to be safe up to 1000 mg/kg, p.o. So, three doses i.e., 30, 100 and 300 mg/kg body weight were selected for the evaluation of antiepileptic activity.
Evaluation of antiepileptic activity
MES in mice
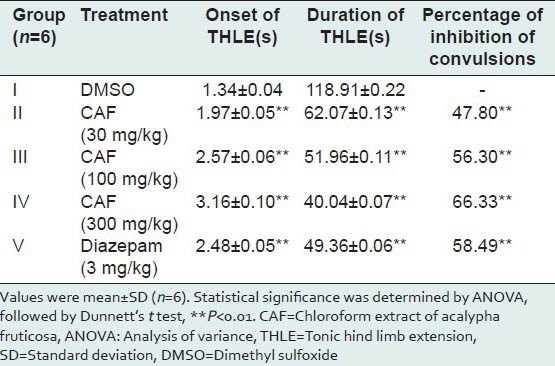
The average time of onset, duration of tonic hind limb extension (THLE) and percentages of inhibition of convulsions were presented in Table 2. Albino mice pretreated with CAF at the doses of 30, 100 and 300 mg/kg, p.o. exhibited delay in the onset time of THLE when compared with the mice belonging to control group. CAF treated mice also exhibited a decrease in duration of THLE when compared with the control group mice. Albino mice pretreated with CAF at doses 30, 100 and 300 mg/kg were provided significant protection from convulsions induced by electroshock method in a dose-dependent manner (P < 0.01). Animals pretreated with CAF exhibited significant antiepileptic activity and more percentage inhibition of convulsions at the dose of 300 mg/kg when compared to diazepam treated animals.
Table 2.
Effect of CAF on maximal electroshock-induced convulsions in mice
PTZ-induced convulsions in mice
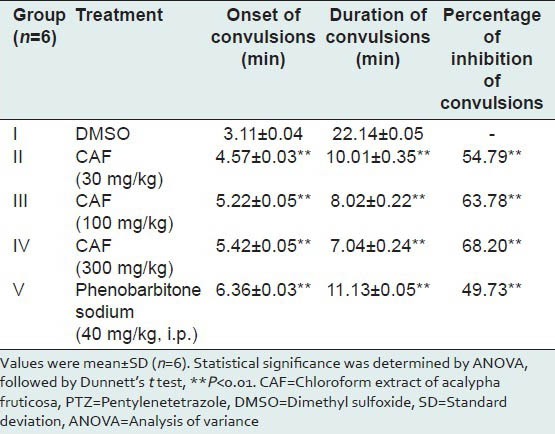
The average time of onset, duration of convulsions and percentages of inhibition of convulsions were presented in Table 3. CAF treated mice not only exhibited delay in the onset time of convulsions at the doses of 30, 100 and 300 mg/kg, p.o. but also showed reduced duration of convulsions when compared with the control group mice. All the three doses of CAF afforded significant protection in a dose-dependent manner against convulsions induced by PTZ (P < 0.01). Animals pretreated with CAF at all the three doses exhibited significant antiepileptic activity and more percentage of inhibition of convulsions when compared with Phenobarbitone sodium treated animals.
Table 3.
Effect of CAF on PTZ-induced convulsions in mice
INH-induced convulsions in mice
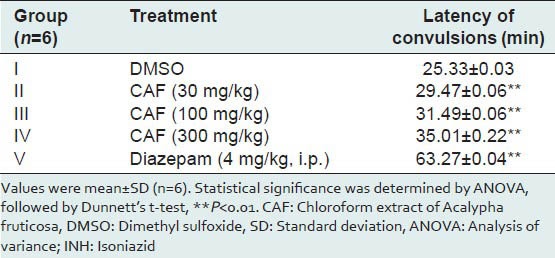
The average latency of convulsions was presented in Table 4. All the three doses of CAF showed the latency of convulsions more than that of control and less than that of standard i.e. diazepam (4 mg/kg, i.p.) and although CAF delayed the latency of convulsions in a dose-dependent manner but failed to protect the mice against mortality.
Table 4.
Effect of CAF on INH-induced convulsions in mice
DISCUSSION
Epilepsy is a group of chronic neurological disorders characterized by sporadic episodes of convulsive seizures, sensory disturbance, abnormal behavior and loss of consciousness or all of these symptoms resulting from a brain dysfunction or an abnormal discharge of cerebral neurons.[14]
Higher prevalence, lack of awareness, cultural and social stigma and non-availability of proper diagnostic and treatment facilities are among the major problems in the developing countries. Drug therapy of epilepsy with currently available AED is associated with side effects, dose-related and chronic toxicity that involves virtually every organ system.[1,2] There is a pressing need for further research especially in the field of pharmacotherapy of epilepsy to find drugs. Search for anti-epileptic agents has made man turn to alternative sources, indigenous system of medicine.
Previous reports evidenced that many plants like Hibiscus rosa, Cyperus articulatus, Delphinium denudatum, Carissa edulis showed good protection against epilepsy.[15,16,17,18] A. fruticosa is one such plant whose leaf decoction is used to treat epilepsy by tribal of Tanzania. The chloroform extract was evaluated for antiepileptic activity by three animal models involving gamma-amino butyric acid (GABA) ergic neurotransmission i.e. MES in mice, PTZ and INH-induced convulsions in mice.
The data obtained in the present study demonstrated for the first time that the CAF had significantly inhibited the convulsions induced by MES, PTZ and INH.
GABA is known to be an important inhibitory neurotransmitter in the brain, whereas glutamate is the excitatory neurotransmitter. GABA acts on the GABA receptors and glutamate acts through the N-methyl-D-aspartate (NMDA) and non-NMDA receptors. Activation of these receptors modifies various voltage-gated Na+, K+, Ca++ and Cl- ion channels and excites or inhibits the neuron.[19] Abnormalities in the GABA system have been found in neurological and psychiatric diseases such as Huntingdon's chorea, anxiety, panic attacks, schizophrenia and epilepsy. One major factor in epileptogenesis seems to be a decreased function of GABAA synapses.[11]
The MES test in mice is a suitable model for grand mal epilepsy. MES test in mice is used primarily as an indication for compounds, which are effective in grand mal epilepsy. THLE are evoked by electric stimuli which are suppressed by antiepileptics. PTZ-induced convulsions in mice are a suitable model for petit mal epilepsy. PTZ is GABA antagonist. This assay has been used primarily to evaluate AED. Drugs which antagonize PTZ-induced seizures are generally useful in petit mal epilepsy. It has been indicated that PTZ-induced seizures can be prevented by drugs that reduce T-type Ca2+ currents, such as ethosuximide and also by drugs that enhance GABAA receptor-mediated inhibitory neurotransmission, such as benzodiazepines and Phenobarbital. INH can precipitate convulsions in patients with seizure disorders. INH is regarded as a GABA-synthesis inhibitor. Clonic tonic seizures are elicited in mice which are antagonized by AED.[11,20] The results of our study reveal that CAF significantly inhibited the convulsions induced by MES, PTZ and INH.
Previous phytochemical investigations revealed the presence of flavonoids, tannins, terpenoids, coumarins, quinones, anthraquinones, alkaloids, steroids, phenols and saponins.[21] It is also found that many flavonoids could act as benzodiazepine-like molecules in the central nervous system and modulate GABA-generated chloride currents in animal models of anxiety, sedation and convulsion.[22]
CONCLUSION
Based on the above investigations, it may be concluded that the chloroform extract of leaves of A. fruticosa exhibited significant antiepileptic activity. These findings justify the traditional use of this plant in the control and/or treatment of convulsions and epilepsy as the plant is unexplored yet. The presence of flavanoids may partially contribute the significant activity of chloroform extract of aerial parts of A. fruticosa by enhanced GABAergic neurotransmission. Further detailed phytochemical investigations are required to identify the phytoconstituent/s responsible for the antiepileptic effect.
ACKNOWLEDGMENTS
The authors are grateful to the Management of Vaagdevi College of Pharmacy, Hanamkonda, Talla Padmavathi College of Pharmacy, Warangal and Jangaon Institute of Pharmaceutical Sciences, Jangaon, for providing necessary laboratory facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Samrén EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: A joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–90. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 2.Mattson RH. Efficacy and adverse effects of established and new antiepileptic drugs. Epilepsia. 1995;36:13–26. doi: 10.1111/j.1528-1157.1995.tb05995.x. [DOI] [PubMed] [Google Scholar]
- 3.Soejarto DD. Biodiversity prospecting and benefit-sharing: Perspectives from the field. J Ethnopharmacol. 1996;51:1–15. doi: 10.1016/0378-8741(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin MJ. Conservation, ethnobotany, and the search for new jungle medicines: Pharmacognosy comes of age. again. Pharmacotherapy. 1988;8:257–62. doi: 10.1002/j.1875-9114.1988.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 5.Holland BK. Prospecting for drugs in ancient texts. Nature. 1994;369:702. doi: 10.1038/369702a0. [DOI] [PubMed] [Google Scholar]
- 6.Schmelzer GH. Medicinal plants. Netherlands: PROTA; 2007. Acalypha fruticosa Forssk; p. 11. [Google Scholar]
- 7.Muthukumarasamy S, Mohan VR, Kumaresan S, Chelladurai V. Herbal remedies of paliyar tribe of grizzled giant squirrel wildlife sanctuary, western ghats, srivilliputhur, tamil nadu for poisonous bites. J Econ Tax Bot. 2003;27:761–4. [Google Scholar]
- 8.Hamza OJ, van den Bout-van den Beukel CJ, Matee MI, Moshi MJ, Mikx FH, Selemani HO, et al. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol. 2006;108:124–32. doi: 10.1016/j.jep.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Kokate CK. 4th ed. New Delhi: Vallabh Prakashan; 1994. Practical Pharmacognosy; p. 107. [Google Scholar]
- 10.Veeraraghavan, Prema Expert Consultant, CPCSEA, OECD Guideline No. 420. 2000 [Google Scholar]
- 11.Vogel GH. Pharmacological Assays. Germany: Springer; 1997. Drug Discovery and Evaluation; p. 487. [Google Scholar]
- 12.Dhanasekaran S, Palayan M. CNS depressant and antiepileptic activities of the methanol extract of the leaves of Ipomoea aquatica Forsk. E-J Chem. 2010;7:1555–61. [Google Scholar]
- 13.Madhu A, Keerthi PH, Jaideep S, Shivalinge GK. Antiepileptic activity of aqueous root extract of Hemidesmus indicus in rats. Arch Pharm Sci Res. 2009;1:43–7. [Google Scholar]
- 14.Jerome E, Timothy AP. A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 1997. What is Epilepsy? pp. 1–7. [Google Scholar]
- 15.Kasture VS, Chopde CT, Deshmukh VK. Anticonvulsive activity of Albizzia lebbeck, Hibiscus rosa sinesis and Butea monosperma in experimental animals. J Ethnopharmacol. 2000;71:65–75. doi: 10.1016/s0378-8741(99)00192-0. [DOI] [PubMed] [Google Scholar]
- 16.Bum EN, Schmutz M, Meyer C, Rakotonirina A, Bopelet M, Portet C, et al. Anticonvulsant properties of the methanolic extract of Cyperus articulatus (Cyperaceae) J Ethnopharmacol. 2001;76:145–50. doi: 10.1016/s0378-8741(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 17.Raza M, Shaheen F, Choudhary MI, Sombati S, Rafiq A, Suria A, et al. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from Delphinium denudatum. J Ethnopharmacol. 2001;78:73–8. doi: 10.1016/s0378-8741(01)00327-0. [DOI] [PubMed] [Google Scholar]
- 18.Ya›u J, Yaro AH, Abubakar MS, Anuka JA, Hussaini IM. Anticonvulsant activity of Carissa edulis (Vahl) (Apocynaceae) root bark extract. J Ethnopharmacol. 2008;120:255–8. doi: 10.1016/j.jep.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Satoskar RS, Bhandarkar SD. 12th ed. Mumbai: Popular Prakashan; 1991. Pharmacology and Pharmacotherapeutics; p. 10. [Google Scholar]
- 20.Costa E, Guidotti A, Mao CC. Evidence for involvement of GABA in the action of benzodiazepines: Studies on rat cerebellum. In: Costa E, Greengard P, editors. Mechanisms of Action of Benzodiazepines. Adv Biochem Psychopharmacol. Vol. 14. New York: Raven Press; 1975. pp. 113–51. [PubMed] [Google Scholar]
- 21.Gopalakrishnan S, Saroja K, Elizabeth JD. Chemical investigations of aerial parts of Acalypha fruticosa Forssk. Scholars Research Library. Der Pharma Chem. 2010;2:383–9. [Google Scholar]
- 22.Aslan M, Orhan DD, Orhan N. Effect of Gentiana olivieri on experimental epilepsy models. Pharmacogn Mag. 2011;7:344–9. doi: 10.4103/0973-1296.90419. Harborne JB, Williams CA Phytochemistry 2000;55:481. [DOI] [PMC free article] [PubMed] [Google Scholar]


