Abstract
Objective:
To investigate the aphrodisiac potential of polyherbal formulations prepared from different parts of Tribulus terrestris, Curculigo orchioides, Allium tuberosum, Cucurbita pepo, Elephant creeper, Mucuna pruriens, and Terminalia catappa in Albino rats in specified ratio as suspension.
Materials and Methods:
The different concentrations of prepared polyherbal formulations i.e. 150, 300, and 600 mg/kg and sildenafil citrate as standard (5 mg/kg) and vehicle (control) were administered orally to rats (n = 6 animals per group) for 3 weeks. Mating behavior parameters in male rats was monitored in first week and third week week of treatment pairing with receptive females. After termination of drug treatment, the mating performance, hormonal analysis, sperm count, and testes-body weight ratio were also evaluated.
Results:
The polyherbal formulation showed a significant increase in mating behavior as well as mating performance, serum hormonal levels, sperm count, and testes-body weight ratio with dose-dependent relationship as compared to vehicle control. But the dose of 600 mg/kg of polyherbal formulation assumes closer resemblance of above parameters with the standard used.
Conclusion:
The results of the study strongly suggest that the polyherbal formulations have a good aphrodisiac activity on rats in the above experimental models, which may be an alternative weapon for various sexual dysfunctions in future.
Keywords: Aphrodisiac, mating behavior, polyherbal formulations, serum hormonal levels, sexual dysfunctions, testes-body weight ratio
INTRODUCTION
Sexual dysfunction includes erectile dysfunction or impotence, ejaculation dysfunction, hypogonadism, etc.[1] It is a serious public health problem among young as well as old men worldwide, with a prevalence of more than 20%.[2,3] This dysfunction leads to risk for aging and other etiological factors, including degenerative diseases, increase in injuries, and stress associated with industrialized lifestyles. Sexual relationship is among the most important social and biological relationship of human life by boosting the mood as well as interpersonal functioning. It does not only affect life expectancy, but also have a significant negative impact on an individual's wellbeing and quality of life.[4] People have searched for ways to achieve sexual desire or sexual techniques from ancient times.[5] In ancient history, most cultures helped society to improve the sexual life as evident by writings holy texts and sculptures in Hindu temples. Successful treatment of sexual dysfunctions may improve not only sexual relationships, but also the overall superiority of life.[6] It can be treated by both medical and surgical modalities. To achieve better sexual desire has led to the development and use of different substances known as aphrodisiacs. An aphrodisiac enhances sex drive or sexual pleasure by crossing the blood brain barrier and mimicking or stimulating some area of sexual arousal in the central nervous system. These substances also act physiologically to increase blood flow to the penile area, or increase the duration of sexual activity by numbing the genital area or even mimic the burning sensation of sexual intercourse.
Management of sexual dysfunction includes counseling of patient by an experienced psychiatrist or psychologist to restore confidence and improve patient's ability to obtain adequate erection, the use of vacuum erection devices, the use of surgical penile implants, hormonal treatments mainly with testosterone, or the use of specific drugs which increases firmness, maintenance of erection, frequency of orgasm, and level of desire.[7] Side effects of these treatments can’t be neglected, which include high cost, complicacy of infections in surgical procedures, mechanical failure of devices, acceptability, side effects of drugs such as headache, flushing, dizziness, visual disturbances, nasal congestions and priapism. So there is need to develop safe or cost effective drug despite advances in modern and orthodox medicines. For several hundred years, people around the world have used locally grown plants as supplements to energize, vitalize, and eventually to improve sexual functions. However, plant-derived and herbal remedies continue to be a popular alternative to treat sexual disorders[8] and have also proven effective in improving sexual desire and sexual behavior in male animals.[9]
Many indigenous plants have been claimed to have a sex-stimulating effect in Ayurvedic system. Among the several plants, Terminalia catappa,[10] Allium tuberosum,[11] Bryonia laciniosa,[12] Elephant creeper,[13] Montana tomentosa,[14] Mucuna pruriens,[15] Cucurbita pepo,[16] Tribulus terrestris,[17] Hypericum perforatum,[18] Senecio cardiophyllus,[19] Ginkgo biloba,[20] Pausinystalia yohimbe[21] Asteracantha longifolia,[22] Curculigo orchioides,[12] Microdesmis keayana,[23] etc., Most studies published on this regard have generally targeted one plant at a time even though in the traditional medicine, most of the plants are used in formulations of groups of two or four plants or even more.[10] In the present study, a polyherbal formulation (PHF) was made, on the scientific basis of the use of dried fruits of Tribulus terrestris, rhizomes of Curculigo orchioides, seeds of Allium tuberosum, seeds of Cucurbita pepo, roots of Elephant creeper, seeds of Mucuna pruriens, and seeds of Terminalia catappa, as an aphrodisiac combination and to determine the effects of the prepared formulation as suspension on sexual behavior of male rats.
MATERIALS AND METHODS
Plant material
The various parts of selected plants were collected from local market of Bhopal, MP, India, and authenticated at the Department of Botany, The City College, Jiwaji University, Gwalior, Madhya Pradesh, India, where the plant voucher specimens number were deposited.
Preparation of polyherbal formulation
The fruits of Tribulus terrestris, rhizomes of Curculigo orchioides, seeds of Allium tuberosum, seeds of Cucurbita pepo, roots of Elephant creeper, seeds of Mucuna pruriens, and seeds of Terminalia catappa were selected. The selected parts were allowed to get air dried and triturated to make powder form individually. The prepared powders were mixed in the ratio of 2:2:2:1:1:1:1 (ratio depends upon aphrodisiac potency) and made as suspension in 8% gum acacia aqueous solution to form a PHF. To evaluate the dose-related aphrodisiac activity, we had chosen three doses of PHF as PHF-I, II, and -III on basis of acute toxicity study.
Experimental animals
Swiss Albino rats (8-10 months) weighing around 125-150 g of either sex housed in standard conditions of temperature (22 ± 2°C), relative humidity (55 ± 5%), and light (12 hrs light/dark cycles) were used. They were fed with standard pellet diet and water ad libitum. The experimental protocol was approved by Institutional Animal Ethical Committee as per the guidance of CPCSEA, Ministry of Social Justice and Empowerment, Government of India (Protocol No. 379/01/Ab/CPCSEA).
Acute toxicity evaluation and treatment schedule
The acute toxicity study of PHF was done by up and down method.[24] The rats were fasted for 18 hours with water ad libitum. The PHF was administered in six different doses: 250, 500, 750, 1000, 1500, and 2000 mg/kg body weight, per oral. The animals were observed for clinical signs and symptoms of toxicity every 30 min up to 6 hours on the first day and thereafter, everyday up to 7 days. Acute toxicity studies showed no mortality or changes in behavior observed at the dose up to 2000 mg/kg. Dose selected for aphrodisiac activity was 150, 300, and 600 mg/kg. Volume of oral administration was 1 ml/100 g of mouse. Animals were randomly divided into five groups with six animals per group. Group I represented the control animals treated with normal water only; Groups II, III, and IV were treated with oral suspension of PHF at 18:00 h, at doses of 150, 300, and 600 mg/kg, respectively; and Group V served as a positive control treated with Sildenafil citrate of 5 mg/kg body weight. All the treatments were continued for 3 weeks only.
Mating behavior study
Mating behavior studies were carried out in a separate room under dim red illumination according to the standard procedure. Healthy male albino rats showing brisk sexual activity and female animals showing regular oestrus cycle were selected for the study. The male rats were placed in a rectangular plexiglass chamber, 10 minutes before the introduction of a primed female and get acclimatized to the chamber conditions. The primed female was then introduced into the chamber with one female to one male ratio and the mating behaviors observed for first week and third week after commencement of the PHF treatment. The following mating behavior parameters were recorded: (a) Mount frequency (MF): The number of mounts without intromission from the time of introduction of the female until ejaculation; (b) Intromission frequency (IF): The number of intromissions from the time of introduction of the female until ejaculation; (c) Mount latency (ML): The time interval between the introduction of the female and the first mount by the male; (d) Intromission latency (IL): The interval from the time of introduction of the female to the first intromission by the male (characterized by pelvic thrusting and springing dismount); (e) Ejaculation latency (EL): The time interval between the first intromission and ejaculation (characterized by longer, deeper pelvic thrusting, and slow dismount followed by a period of inactivity), (f) Post-ejaculatory interval (PEI): The time interval between ejaculation and the first intromission of the following series. The experiment was terminated when the male rat begins to mount the female followed by intromission after a brief period of inactivity (which normally results following ejaculation). The values of the observed parameters were measured at first week and third week of drug administration and compared with control.[25,26]
Mating performance test
After 3 week treatment, the male mouse of each group was placed in a separate cage with oestrus female animals for 1 day (male: female = 1:5). The next day morning, the vaginal smear of each female mouse was examined under a microscope for the presence of sperm. The number of sperm-positive females was recorded in each experimental group and compared with control.[26]
Hormonal analysis
The blood was collected from retro orbital venous plexus of all animals after termination of experiment. Blood samples were spun at 2500 rpm for 10 min in a table top centrifuge. The serum samples were separated to measure the concentration of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone. Serum FSH was measured by a radioimmunoassay kit (Board of Radiation and Isotope Technology, Mumbai, India); FSH concentration was estimated by a microplate chemiluminescence immunoassay (CLIA) kit; and total testosterone was measured by a double antibody ELISA kit (Eiagen Testosterone kit, Italy), according to the protocol provided with each kit.[27]
Reproductive organ and spermal analysis
At the end of study, the animals were killed by an overdose of anesthesia. Immediately after the respiration ceases, the animals were fixed by transcardial perfusion with normal saline after flushing the blood. Before perfusion, right-hand side of the epididymis was removed and used for sperm analysis and left-hand side was used for a morphological study. Main and accessory reproductive organs were dissected and weighed.[15]
Statistical analysis
The results of various studies were expressed as Mean ± SEM and analyzed using one-way ANOVA followed by Student's t-test using software SYSTAT 7.0, to find out the level of significance. Data were considered statistically significant at minimum level of P < 0.01.
RESULTS
Effect of PHF on mating behavior of rats
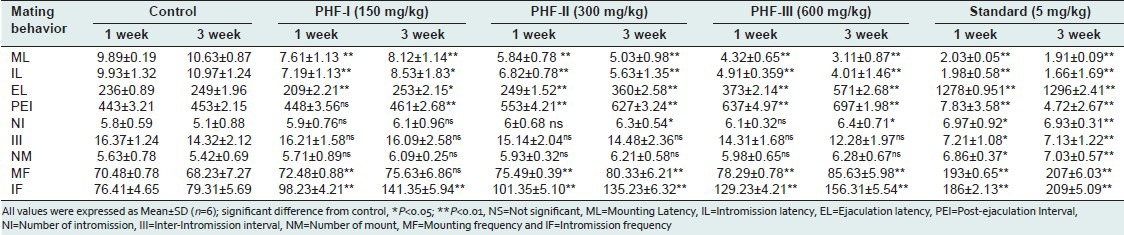
A mating behavior study revealed that continuous administration for 3 weeks, all the doses of PHF were able to significantly decrease mount and intromission latencies, when compared to vehicle control and standard drug-treated rats. It also significantly decreases the ejaculatory latency and post ejaculatory interval. Ultimately it resulted in an increased percentage of mounting frequency and intromission frequency in comparison to control and standard drug [Table 1]. However, the PHF at 600 mg/kg significantly increased the frequency of mounting and intromission and other reflexes of sexual behavior, as nearly equal to standard drug. Also, the pre-coital sexual behaviors, such as chasing, nosing, and anogenital sniffing, were prominently observed in this group. All the doses of PHF were followed by dose-dependent progression at first and third week interval consecutively on the mating behavior of male rats as shown in Table 1. There was an overall increase in the sexual behavior parameters in PHF-treated groups of rats as reflected in MF, IF and EF, and reduction in ML, IL, EL, and PEI. These results were also statistically significant.
Table 1.
Effect of PHF on mating behavior after 1 week and 3 weeks treatment in male rats
Effect of PHF on mating performance of rats
In Figure 1, daily administration of prepared PHF for 3 weeks to male rats resulted in a dose-dependent increase in the mating performance as compared to the control group. The prepared PHF as the dose of 150, 300, and 600 mg/kg showed 52.66%, 63.83%, 71% mating performance, respectively, against 38.33% of the control group, whereas the standard showed 79.66% mating performance. The prepared PHF of 600 mg/kg showed closely resemblance with standard treatment and plays a significant role in mating performance of rats as compared to control.
Figure 1.

Effect of PHF on mating performance in male rats. All values were expressed as Mean ± SD (n=6); **P < 0.01 considered significant as compared to control
Effect of PHF on serum testosterone, LH and FSH in male rats
The PHF had significant (**P < 0.01) effect on testosterone, LH, and FSH concentration in the serum in comparison to the control group as shown in Figure 2. The level of testosterone, LH, and FSH increased gradually with dose dependency in all the experimental groups. The dose of PHF 600 mg/kg showed an increase of serum hormonal level as nearly as standard.
Figure 2.

Effects of PHF on serum testosterone, FSH and LH level in male rats. All values were expressed as Mean ± SD (n=6); **P < 0.01 considered significant as compared to control
Effect of PHF on testes-body weight ratio and sperm count after termination
The effect of the PHF on sexual organ and body weight is summarized in Table 2. After 3 week of treatment, the PHF showed increasing ratio of testes-body weight in a dose-dependent manner, and also found significance with control. The epididymal sperm parameters revealed an increase in the number of sperms in all tested groups as compared to control, i.e. 190, 220, 235, 264, and 287 million/ml in groups I, II, III, IV, and V, respectively.
Table 2.
Effect of PHF on testes-body weight ratio in male rats

DISCUSSION
This study examined the effect of PHF on male sexual competence in rats, with sildenafil citrate as positive reference drug. To the best of our knowledge, this is the first study to report the prepared PHF enhanced the sexual behaviors of male rats compared with control. The present study provides special evidence that the PHF is a potent stimulator of sexual behavior, particularly on sexual arousal in male rats.
The mating behavior study revealed that the doses of prepared PHF significantly increased MF and IF, compared with the control group, though the effect was less than that of standard. All the doses of PHF also caused significant reductions in ML and IL, compared with control animals, while highly significant decreases in ML and IL were observed in animals treated with standard. MF and IF are considered to be indices of libido and potency, while ML and IL are also indicators of sexual arousal.[10,28,29,30] The significant increases in MF and IF and the decreases in ML and IL indicate that libido and potency were enhanced by prepared PHF. Furthermore, the prolongation of EL is an indicator of prolonged duration of coitus. PEI is considered to be an index of potency, libido, and the rate of recovery from exhaustion after the first series of mating. This indicates that the treatment of different doses of PHF remarkably delayed EL, with no negative effect on the other parameters of sexual behavior, and with no locomotor alterations throughout the observation period. The delayed EL and increased penile erection in treated male rats indicated the involvement of NO in the intervention.[31] These observations support the role of PHF in improvising sexual function.
The continued administration of various doses of PHF for 3 weeks increased testosterone and LH levels. An increase in testosterone level has been associated with increase of sexual desire, penile tumescence, and rigidity, as well as the accessory muscles which help to provide additional sexual activity.[31,32,33] Research with various animal and human models indicates there is a strong correlation between sexual behavior and brain neurotransmitters like dopamine, 5-HT etc.[34] The motor control of ejaculation in animals is modulated by serotonin and its receptors.[35] Testosterone may also facilitate male sexual behavior by increasing dopamine release in the medial preoptic area and potentiating nitrergic neurotransmission in brain, which resulted in stimulation of hypothalamic-pituitary-gonadal axis.[36,37] Also, increase in the testicular weight indicates the number as well as motility of sperms.[38] Increased serum testosterone levels after administration of PHF could thus be considered as one of the contributing factors responsible for the overall increased sexual performance in the treated groups, especially for the lengthening of EL and increased copulatory ability in rats. Overall, these results suggest that PHF at dose 600 mg/kg might represent an interesting alternative for the treatment of pretreatment ejaculation.
There are some possible bioactive agents responsible for increasing endogenous testosterone levels and enhancing male sexual behavior. The mechanism of these agents includes steroids by rising androgen production;[39,40] flavonoids by enhancing testosterone synthesis or by preventing its metabolic degradation;[41,42] alkaloids by dilating the blood vessels in the sexual organs;[24] and saponins by activating gonadal tissues and CNS via NO-dependent mechanism.[43] Thus, the improvements in sexual function demonstrated in the current study might be due to the presence of such compounds in prepared PHF.
Organ-body weight ratio is an index of inflammation or cellular constriction. The increase in the testes-body weight ratio observed [Table 2] may be attributed to increase the secretory activity of the testes, i.e. increase in the concentrations of testosterone, LH, FSH, cholesterol, protein, sialic acid, etc., Increased testicular weight and high protein concentration of the testes indicate enhancement of testicular growth as FSH. Testosterone, LH, and FSH are hormonal markers of androgenicity.[44] Increase of testicular weight and hormonal concentration indicates the presence of androgenic potential in prepared PHF. FSH is responsible for the initiation, maintenance, and production of normal sperms in pubertal rats. The significant increase in the serum FSH suggests an enhancement of sperm cell in sertoli cells. Increase of sperm count was also observed of 3 week treatment of PHF.
The study concluded that the cumulative dose of PHF could enhance overall sexual function and performance in male rats by increasing the levels of FSH, LH, testosterone, spermatozoa concentration. The results suggest that the prepared PHF may be a new promising aphrodisiac combination, which can be used to improve the sex life of many troubled men. This aphrodisiac property may be due to possible synergistic action of selected plants used in the prepared PHF. However, further studies are warranted to establish molecular mechanism for aphrodisiac activity.
ACKNOWLEDGEMENT
The authors thank to Dr M. L. Kori (Director cum Principal) of Vedica College of Pharmacy, RKDF University, Bhopal, MP, India, for providing the lab facilities and financial support for the present work.
Footnotes
Source of Support: Vedica college of Pharmacy, Bhopal
Conflict of Interest: None declared.
REFERENCES
- 1.Ho CC, Singam P, Hong GE, Zainuddin ZM. Male sexual dysfunction in Asia. Asian J Androl. 2011;13:537–42. doi: 10.1038/aja.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laumann EO, Nicolosi A, Glasser DB. Sexual problems among women and men aged 40-80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 3.Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J, et al. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: Prevalence, comorbidities, and professional help-seeking. Eur Urol. 2007;51:816–24. doi: 10.1016/j.eururo.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Fugl-Meyer KS, Fugl-Meyer AR. Impact of erectile dysfunction on quality of life patient and partner perspectives. Int J Impot Res. 2000;12:144–6. doi: 10.1038/sj.ijir.3900594. [DOI] [PubMed] [Google Scholar]
- 5.Jain N, Goyal S, Ramawat KG. Desert Plants. Vol. 4. Berlin, Heidelberg: Springer-Verlag; 2010. Biotechnological approaches to aphrodisiac plants of Rajasthan, India; pp. 479–95. [Google Scholar]
- 6.Shin BC, Lee MS, Yang EJ, Lim HS, Ernst E. Maca (L. meyenii) for improving sexual function: A systematic review. BMC Complement Altern Med. 2010;10:44–6. doi: 10.1186/1472-6882-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Taher TS, Matalka Z, Taha HA, Badwan AA. Ferula harmonis`zallouh’ and enhancing erectile function in rats: Efficacy and toxicity study. Int J Impot Res. 2001;13:247–51. doi: 10.1038/sj.ijir.3900706. [DOI] [PubMed] [Google Scholar]
- 8.Rowland DL, Tai W. A review of plant-derived and herbal approaches to the treatment of sexual dysfunctions. J Sex Marital Ther. 2003;29:185–205. doi: 10.1080/00926230390155096. [DOI] [PubMed] [Google Scholar]
- 9.Suresh Kumar PK, Subramoniam A, Pushpangadan P. Aphrodisiac activity of Vanda tessellata (Roxb) hook. Ex don extract in male mice. Indian J Pharmacol. 2000;32:300–4. [Google Scholar]
- 10.Ratnasooriya WD, Dharmasiri MG. Effects of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J Androl. 2000;2:213–9. [PubMed] [Google Scholar]
- 11.Hu G, Lu Y, Mao R, Wei D, Ma Z, Zhang H. Aphrodisiac properties of Allium tuberosum seeds extract. J Ethnopharmacol. 2009;122:579–82. doi: 10.1016/j.jep.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan NS, Rao ChV, Dixit VK. Effect of Curculigo orchioides rhizomes on sexual behaviour of male rats. Fitoterapia. 2007;78:530–4. doi: 10.1016/j.fitote.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Subramoniam A, Madhavachandran V, Ravi K, Anuja VS. Aphrodisiac property of the elephant creeper Argyreia nervosa. J Endocrinol Reprod. 2007;11:82–5. [Google Scholar]
- 14.Carro-Juarez M, Cervantes E, Cervantes-Mendez M, Rodriguez-Manzo G. Aphrodisiac properties of Montanoa tomentosa aqueous crude extract in male rats. Pharmacol Biochem Behav. 2004;78:129–34. doi: 10.1016/j.pbb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Sekar S, Elumalai P, Seppan P. Dose and time dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmacol. 2009;122:497–501. doi: 10.1016/j.jep.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Gundidza GM, Mmbengwa VM, Magwa ML, Ramalivhana NJ, Mukwevho NT, Ndaradzi W, et al. Aphrodisiac properties of some Zimbabwean medicinal plants formulations. Afr J Biotechnol. 2009;8:6402–7. [Google Scholar]
- 17.Surender S, Gupta YK. Aphrodisiac activity of Tribulus terrestris Linn. in experimental models in rats. J Men's Health. 2011;8(Suppl 1):S75–7. [Google Scholar]
- 18.Kaufman JH, Cannon-Smith T. Improved ejacultory control and sexual satisfaction in pilot study of men taking Hypericum perforatum extract. Internet J Nutr Wellness. 2007;3:2. [Google Scholar]
- 19.Carro-Juárez M, Alcazar C, Ballesteros-Polvo E, Villalobos-Peñalosa P. Increase of ejaculatory capacity by systemic administration of the oquichpatli (Senecio cardiophyllus) aqueous crude in male rats. J Ethnopharmacol. 2009;126:506–11. doi: 10.1016/j.jep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Yeh KY, Pu HF, Kaphle K, Lin SF, Wu LS, Lin JH, et al. Ginkgo biloba extract enhances male copulatory behavior and reduces serum prolactin levels in rats. Horm Behav. 2008;53:225–31. doi: 10.1016/j.yhbeh.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Carro-Juárez M, Rodríguez-Manzo G. Yohimbine reverses the exhaustion of the coital reflex in spinal male rats. Behav Brain Res. 2003;141:43–50. doi: 10.1016/s0166-4328(02)00324-8. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan NS, Sharma V, Dixit VK. Effect of Asteracantha longifolia on sexual behaviour of male rats. Nat Prod Res. 2011;25:1423–31. doi: 10.1080/14786410802588493. [DOI] [PubMed] [Google Scholar]
- 23.Zamble A, Sahpaz S, Brunet C, Bailleul F. Effects of Microdesmis keayana roots on sexual behavior of male rats. Phytomedicine. 2008;8:625–9. doi: 10.1016/j.phymed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.OECD Guideline for Testing of Chemicals, Acute Oral Toxicity- Acute Toxicity Class Method, 423. Adopted 17th December. 2001 [Google Scholar]
- 25.Gauthaman K, Adaikan PG, Prasad RN. Aphrodisiac properties of Tribulus Terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002;71:1385–96. doi: 10.1016/s0024-3205(02)01858-1. [DOI] [PubMed] [Google Scholar]
- 26.Subramoniam VM, Rajasekharan S, Pushpangadan P. Aphrodisiac property of Trichopus zeylanicus extract in male mice. J Ethnopharmacol. 1997;57:21–7. doi: 10.1016/s0378-8741(97)00040-8. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan NS, Saraf DK, Dixit VK. Effect of vajikaran rasayana herbs on pituitary-gonadal axis. Eur J Integr Med. 2010;2:89–91. [Google Scholar]
- 28.Tajuddin AS, Latif A, Qasmi IA, Amin KM. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg) BMC Complement Altern Med. 2005;5:16. doi: 10.1186/1472-6882-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakubu MT, Akanji MA, Oladiji AT, Adesokan AA. Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyl. Stem in male Wistar rats. J Ethnopharmacol. 2008;118:508–13. doi: 10.1016/j.jep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Mbongue FG, Kamtchouing P, Essame OJ, Yewah PM, Dimo T, Lontsi D, et al. Effect of the aqueous extract of dry fruits of Piper guineense on the reproductive function of adult male rats. Indian J Pharmacol. 2005;37:30–2. [Google Scholar]
- 31.Cao JF, Zhang PY, Xu CW, Huang TT, Bai YG, Chen KS, et al. Effect of aqueous extract of Arctium lappa L. (burdock) roots on the sexual behavior of male rats. BMC Complement Altern Med. 2012;12:1–8. doi: 10.1186/1472-6882-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aversa A, Fabbri A. New oral agents for erectile dysfunction: What is changing in our practice? Asian J Androl. 2001;3:175–9. [PubMed] [Google Scholar]
- 33.Gauthaman K, Adaikan PG, Prasad RN. Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002;71:1385–96. doi: 10.1016/s0024-3205(02)01858-1. [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran S, Sridhar Y, Kishore GS, Saravanan M, Thomas LJ, Anbalagan N, et al. Aphrodisiac activity of Butea frondosa Koen. ex Roxb. extract in male rats. Phytomedicine. 2004;11:165–8. doi: 10.1078/0944-7113-00343. [DOI] [PubMed] [Google Scholar]
- 35.Giuliano F, Clement P. Physiology of ejaculation: Emphasis on serotoninergic control. Eur Urol. 2005;48:408–17. doi: 10.1016/j.eururo.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Chauhan NS, Dixit VK. Effects of Bryonia laciniosa seeds on sexual behaviour of male rats International. J Impot Res. 2010;22:190–5. doi: 10.1038/ijir.2009.62. [DOI] [PubMed] [Google Scholar]
- 37.Putnam SK, Du J, Sato S, Hull EM. Testosterone restoration of copulatory behaviour correlates with medial preoptic dopamine release in castrated male rats. Horm Behav. 2001;39:216–24. doi: 10.1006/hbeh.2001.1648. [DOI] [PubMed] [Google Scholar]
- 38.Gonzales GF, Ruiz A, Gonzales C, Villegas L, Cordova A. Effect of Lepidium meyenii (maca) roots on spermatogenesis of male rats. Asian J Androl. 2001;3:231–3. [PubMed] [Google Scholar]
- 39.Drewes SE, George J, Khan F. Recent findings on natural products with erectile-dysfunction activity. Phytochemistry. 2003;62:1019–25. doi: 10.1016/s0031-9422(02)00621-0. [DOI] [PubMed] [Google Scholar]
- 40.Gauthaman K, Adaikan PG. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction-an evaluation using primates, rabbit and rat. Phytomedicine. 2008;15:44–54. doi: 10.1016/j.phymed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Ratnasooriya WD, Fernando TS. Effect of black tea brew of Camellia sinensis on sexual competence of male rats. J Ethnopharmacol. 2008;118:373–7. doi: 10.1016/j.jep.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Yang NJ, Kaphle K, Wang P, Jang D, Wa L, Lin J, et al. Effect of aqueous extracts of “Betel Quid” and its constituents on testosterone production by dispersed mouse intestinal cell. Am J Chin Med. 2004;32:705–15. doi: 10.1142/S0192415X04002430. [DOI] [PubMed] [Google Scholar]
- 43.Murphy LL, Lee TJ. Ginseng, sex behavior and nitric oxide. Ann NY Acad Sci. 2002;962:372–7. doi: 10.1111/j.1749-6632.2002.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 44.Musa TY, Musbau AA, Adenike TO, Ayoade AA. Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyl. stem in male Wistar rats. J Ethnopharmacol. 2008;118:508–13. doi: 10.1016/j.jep.2008.05.020. [DOI] [PubMed] [Google Scholar]


