Abstract
Background:
Ayurveda recommends several plants and plant preparation for conditions of urogenital disorders as per its principles. Objectives: Ayurvedic plants Tamala (Cinnamomum tamala); Daruhalad (Berberis aristata); Ativish (Aconitum heterophyllum) were studied for mechanisms of prostatic hyperplasia induced in rats.
Materials and Methods:
Prostatic enlargement was induced in castrated rats by testosterone injection s.c. for 21 days and simultaneously plants were dosed orally daily. On day 22 rats were sacrificed and prostate was removed; weight and volume of prostate was measured; histopathology performed. Inflammation was induced by injecting carrageenan in rat hind paw and inhibition was studied by measuring rat paw oedema at different time points.
Results:
Tamala showed significant effect where it reduced prostatic enlargement and improved hyperplastic changes, while Daruhalad and Ativisha did not show any significant effect. All of them showed mild to moderate anti-inflammatory activity.
Conclusion:
Study concludes that Tamala may benefit in prostate disorder by virtue of inhibition of androgen mechanisms in prostate and modulating inflammatory mediators in prostate. Daruhalad and Ativisha did not show any effect in this model of prostate enlargement while the anti-inflammatory effect may propose one of the useful properties when included in various formulations.
Keywords: Ativisha (Aconitum heterophyllum), daruhalad (Berberis aristata), prostatic hyperplasia, tamala (Cinnamomum tamala)
INTRODUCTION
Benign Prostatic Hyperplasia (BPH) and associated Lower Urinary Tract Symptoms (LUTS) and Protatitis are one of the major urogenital disorders affecting elderly mens.[1,2,3]
Ayurveda, traditional system of medicine recommends many plants and plant preparations for management of urogenital disorders as per the principles of its pathology. Present study is done to understand the effects of plants and plant preparations mentioned in Ayurveda viz. Tamala (Cinnamomum tamala), Daruhalad (Berberis aristata), Ativisha (Aconitum heterophyllum) in experimental enlargement of prostate and inflammation in rats, conditions usually found in BPH patients.
MATERIALS AND METHODS
Plants
Tamala consisting of dried mature leaves of Cinnamomum tamala (Buch. Ham.) Nees and Eberm. (Fam. Lauraceae) and Daruhalad consisting of dried stem of Berberis aristata DC. (Fam. Berberidaceae) were used for studies. Ativisha consisting of dried, tuberous roots of Aconitum heterophyllum Wall. ex. Royle (Fam. Ranunculaceae) were selected for study.
Plants were obtained from local market, authenticated and quality evaluated as per Ayurvedic Pharmacopeia of India. Plant material was powdered and passed through mesh of size 85 and used for studies.[4]
Chemicals
Chemicals were obtained as follows: Testosterone Propionate (German Remedies, India-Testoviron); Finasteride (Dr. Reddy, India-Finax); Carrageenan and Indomethacin from Sigma Aldrich.
Animals
Male rats of Sprague-Dawley strain weighing between 240-300 g were used for the experiments. All the animals were obtained from Animal House of National Toxicology Centre, Pune. Animals were fed ad libitum with standard pellet (Chakan Oil Mills, Pune, India) diet and had free access to water. All the protocols of animal experiments were approved by the Institutional Animal Ethics Committee. Animals were maintained at a temperature of 25 ± 1°C and relative humidity of 45 to 55% under 12 h light: 12-h dark cycle. They were housed individually in the polypropylene cages after surgery.
Effect in experimentally induced prostatic hyperplasia in rats
The rats were castrated by removing their testes and 6 days after castration animals were divided into following groups of negative control, test groups of Tamala, Daruhalad and Ativish and positive control with 6 animals in each group. Six normal non-castrated rats were taken as normal control. Test groups, were divided in dose range of 270 and 540 mg/kg for Tamala; 500 and 1000 mg/kg for Daruhalad; 180 and 360 mg/kg for Ativish and dosage were given orally. Positive control Finasteride was given at dose of 5 mg/kg orally. Negative control received water orally. One hour after oral dosing the negative control, test groups and positive control group received testosterone propionate 0.5 mg/0.1 mL by s.c. route. Dosing was done for 3 weeks. 24 hours after last dosing animals were sacrificed and prostate removed. Prostate was weight and volume was measured. The volume was measured (by the formula: 1/2(a × b2), where a and b refer to longer and shorter dimension, respectively). Prostate was fixed in 10% formalin. The formalin fixed tissues were embedded in paraffin and thin sections were taken and stained by H and E staining process.[5,6]
Anti-inflammatory activity
The rats were divided into normal vehicle control, test groups, and positive control with 6 rats in each group. Acute inflammation was produced in all rats by subplantar administration of 0.1 mL of 1% carrageenan in normal saline in the right hind paw of rats. Test groups were divided in dose range of 270 and 540 mg/kg for Tamala; 500 and 1000 mg/kg for Daruhalad; 180 and 360 mg/kg for Ativish. Positive control received Indomethacin 10 mg/kg orally. Vehicle control received only water orally. The paw volume was measured at 0, 1, 2, 3 and 4 h after carrageenan administration by plethysmometer (Ugo Basile). Dosing was done 1 h before carrageenan administration. The amount of inflammation was compared with the 0 h reading of the same rat and percentage anti-inflammatory activity calculated by comparing difference in percentage inflammation with vehicle control group.[6]
Statistical analysis
The data was expressed as mean ± SEM. The results were analyzed statistically using ANOVA and student's t test. The minimum level significance was considered as P < 0.05.
RESULT
Negative control shows that testosterone at dose of 0.5 mg/0.1 mL significantly increased prostate weight and volume of castrated animals compared to normal control animals indicating enlargement [Table 1 and Figure 1]. Tamala powder after 21 days treatment at dose of (270 and 540 mg/kg) significantly reduced the volume and weight of prostate when compared to negative control. The effect was more pronounced than positive control Finasteride (5 mg/kg). Ativish powder at dose (180 and 360 mg/kg) and Daruhalad at dose (500 and 1000 mg/kg) did not show any significant effect on volume and weight of prostate after 21 days treatment [Table 1 and Figure 1].
Table 1.
The effect of plants on testosterone- induced growth of volume and weight index of prostate gland of castrated rat
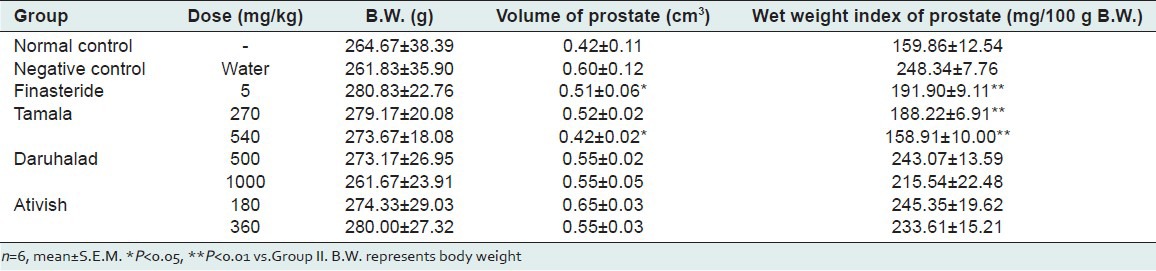
Figure 1.
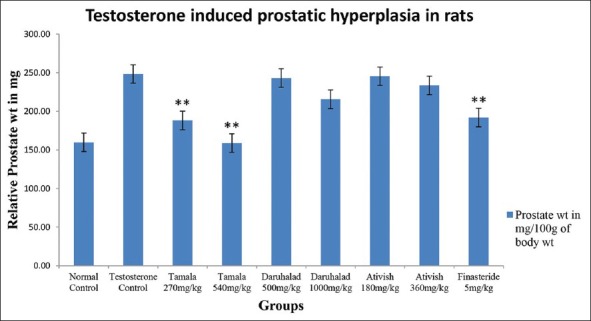
Relative prostate weight of rats
Histopathological observations show that normal control rat show regular acini, cuboidal epithelial cells with round nuclei at basal position; intact basal membrane of epithelial lining and slightly irregular interstitial stroma region [Table 2 and Figure 2]. Testosterone-treated rat show irregular acini growth with irregular stroma. Epithelial lining shows hyperplasia with low cuboidal to tall cells and basal membrane was slightly discontinuous. There was high chronic edematous interstitial and intraluminal infilteration and WBC exudates. Lesions of prostatitis and fibrosis were observed [Table 2 and Figure 3].
Table 2.
Results of histopathology of prostate glands
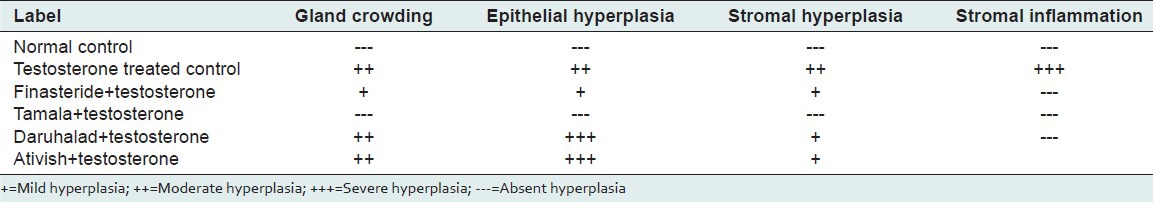
Figure 2.
Normal control
Figure 3.
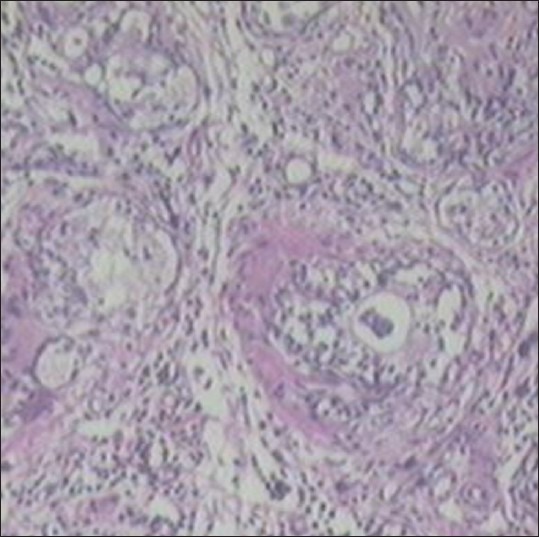
Testosterone treated control
Finasteride-treated group show regular acini, cuboidal to tall epithelial cells with some hyperplasia, intact basal membrane with slight irregular stroma [Table 2 and Figure 4]. Tamala-treated group show regular acini, cuboidal epithelial cells with round nuclei at basal position, intact basal membrane and slightly irregular interstitial stroma [Table 2 and Figure 5]. Daruhalad-treated group show irregular acini growth with irregular stroma. Epithelial lining shows hyperplasia with cuboidal to tall cells and intact basal membrane [Table 2, Figure 6]. Ativish showed irregular acini growth with slight irregular stroma and mild focal epithelial hyperplasia, cuboidal to tall epithelial cells with round nuclei at basal position and intact basal membrane [Table 2, Figure 7].
Figure 4.
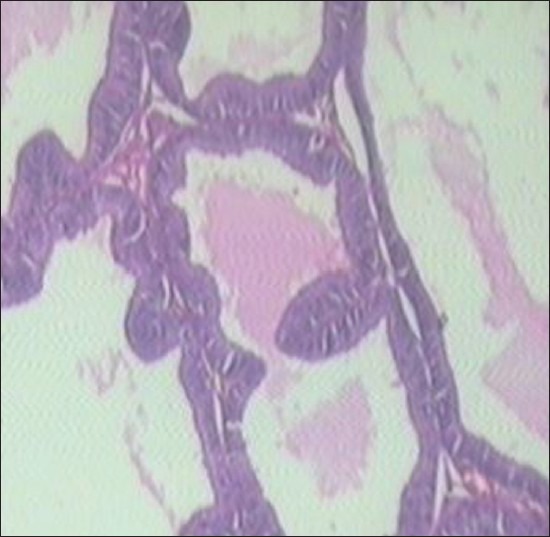
Standard Finasteride
Figure 5.
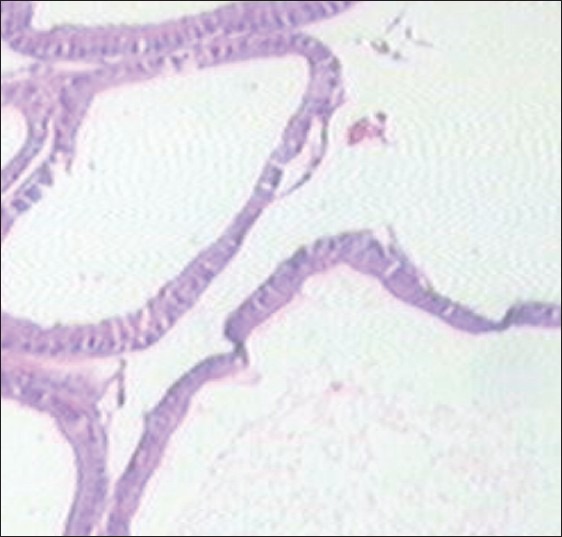
Tamala
Figure 6.
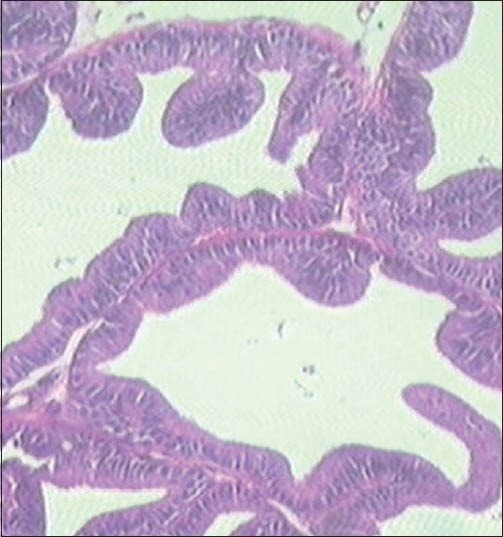
Daruhalad
Figure 7.
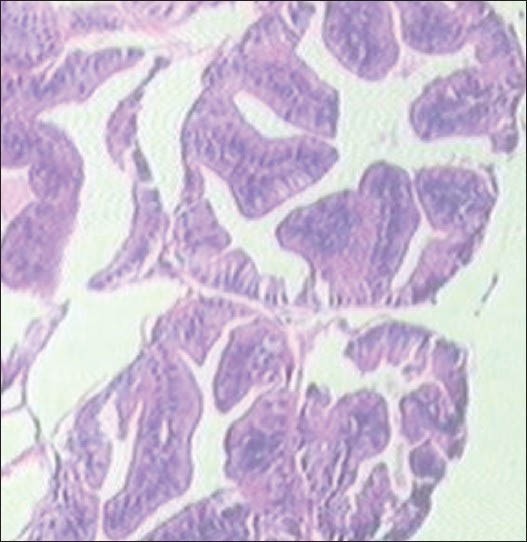
Ativish
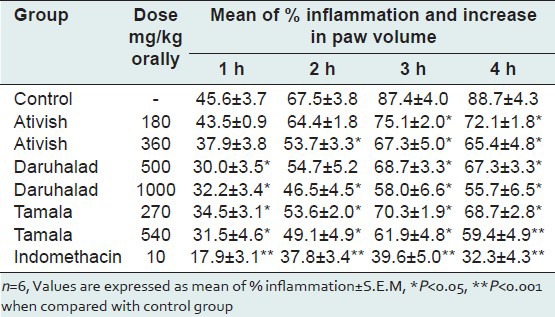
Ativish at dose of 360 mg/kg, Daruhalad at 1000 mg/kg and Tamala at 540 mg/kg reduced carrageenan-induced paw edema by 23.04%, 33.65% and 29.21%, respectively, indicating mild to moderate anti-inflammatory activity [Table 3].
Table 3.
Anti-inflammatory activity of Ativish
DISCUSSION
Benign prostatic hyperplasia is a complex disease where various mechanisms are leading to enlargement of tissue and retention of urine. Age-related hormonal imbalance of testosterone/estrogen and activity of testosterone and dihydrotestosterone after binding to cellular androgen receptors set complex secondary reactions that signal the cell to produce growth factors resulting in hyperplastic growth of prostate. High amounts of 5-alpha reductase enzyme activity that convert testosterone to dihydrotestosterone (active form of androgen in prostate) in prostate as well as abundance of estrogen receptor are responsible for BPH prostates.[7,8,9,10,11,12,13,14]
Exogenous testosterone accelerate the growth of an immature prostate, castration prior to puberty prevents prostatic development and testosterone administration to castrated adult male rat causes the gland to grow to normal size.[15] Castration of adult male rats causes extensive atrophy of prostate with induction of apoptosis, majorly of the ventral prostate epithelial cells.[16] Further administration of testosterone exogenously to castrated rats caused suppression of apoptosis and prevents epithelial cell atrophy. Finasteride, an inhibitor of 5α-reductase enzyme, reduced the testosterone-induced prostatic hyperplasia in rats. Tamala at doses of 270 mg and 540 mg/kg showed good dose response in reducing prostate size and improving hyperplastic changes induced by testosterone. Daruhalad and Ativish did not show any significant effect in reducing prostate enlargement and hyperplasia induced by testosterone. The results are indicative of anti-androgenic activity and inhibition of proliferative processes in prostate.
Prostatic inflammation represents an important factor in influencing prostatic growth and progression of symptoms. Inflammatory mediators secreted by prostatic stromal cells is mediating low level, cumulative increase in proliferation of both epithelial and stromal cells that characterizes age-related development of BPH. Activation of CD4+ lymphocytes, prostate-specific antigen (PSA) and initiation of pro-inflammatory processes by presence of growth factors and cytokines, autoimmune mechanisms in prostate, oxidative stress by Nitric Oxide (NO) and other oxygen species, presence of COX (Cycloxygenase) activity leads to activation of hyperproliferative pathways in prostate. COX-2 inhibition is reported to increase apoptotic activity in prostatic cell in human BPH tissue.[17,18,19,20,21,22]
The use of carrageenan as an irritant agent to induce rat paw inflammation introduced by Winter et. al.[23] and is one of the most popular methods for drug testing and evaluation of anti-inflammatory therapies.[23,24] Carrageenan is believed to result from the action of locally released several mediators sequentially like histamine, serotonin and bradykinin in the initial phase (0-1 h), and an increase in the production of prostaglandins through the activation of cyclooxygenase-2 and release of nitric oxide in the later phase (1-6 h).[25] Mild anti-inflammatory activity shown by powder of Tamala, Daruhalad and Ativish in carrageenan-induced paw edema in rat is indicative of inhibition of Cox and Prostaglandin mechanisms, which are one of the factors in the growth of prostate.
CONCLUSION
The study concludes that Tamala may benefit in prostate disorder by virtue of inhibition of androgen mechanisms in prostate as well as modulation of inflammatory mediators in prostate. Although Daruhalad and Ativish are important Ayurvedic drugs included in many formulations or used individually in many related disorders, they are found to be ineffective in this model while their mild anti-inflammatory property may benefit when used as component of formulation.
ACKNOWLEDGEMENT
We authors are thankful to Dr. Kishori Apte and Mr. Yogesh Talekar, APT Research Foundation, Pune for Technical guidance for pharmacological work. Dr. Avinash Pradhan, MD Pathology for carrying out histopathological study
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Navre KR, Panshikar LV, Soman KV, editors. Part 2. Delhi: Published by Chaukhamba Sanskrit Prathistan; 2011. Nighunturatnakar; pp. 516–86. (631-37). [Google Scholar]
- 2.Tripathi B, editor. Vol. 2. Varanasi: Published by Chaukhamba Surbharati Prakashan; 2011. Charaka Samhita; pp. 541–86. [Google Scholar]
- 3.Ghanekar BG, editor. Pune: Meharchand Laxmandas; 1998. Sushrut Samhita, Nidhasthanam, Chapter 1-11; pp. 1–71. [Google Scholar]
- 4.I. New Delhi: Published by Department of Ayush; Ministry of Health and family welfare, Govt of India. The Ayurvedic Pharmacopeia of India. 1st Ed, Part I; 2007. pp. 27–8. (153-4). vol II. p. 34-6. [Google Scholar]
- 5.Guo QL, Ding QL, Wu QZ. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol Pharm Bull. 2004;27:333–7. doi: 10.1248/bpb.27.333. [DOI] [PubMed] [Google Scholar]
- 6.Chapter H. Analgesic, anti-inflammatory and anti-pyretic activity. In: Vogel HG, editor. Drug Discovery and Evaluation Pharmacological Assays. 2nd ed. New York: Springer-Verlag Berlin Heidelberg; 2002. pp. 759–60. [Google Scholar]
- 7.Marengo SR, Chung LW. An orthotopic model for the study of growth factors in the ventral prostate of the rat: Effects of epidermal growth factor and basic fibroblast growth factor. J Androl. 1994;15:277–86. [PubMed] [Google Scholar]
- 8.Levine AC, Ren M, Huber GK, Kirschenbaum A. The effects of androgen, estrogen, and growth factors on the proliferation of cultured fibroblast derived from human fetal and adult prostates. Endocrinology. 1992;130:2413–9. doi: 10.1210/endo.130.4.1372243. [DOI] [PubMed] [Google Scholar]
- 9.Russell PJ, Bennett S, Stricker P. Growth factor involvement in progression of prostate cancer. ClinChem. 1998;44:705–23. [PubMed] [Google Scholar]
- 10.Story MT, Hopp KA, Meier DA, Begun FP, Lawson RK. Influence of transforming growth factor beta 1 and other growth factors on basic fibroblast growth factor level and proliferation of cultured human prostate-derived fibroblasts. Prostate. 1993;22:183–97. doi: 10.1002/pros.2990220302. [DOI] [PubMed] [Google Scholar]
- 11.Culig Z, Hobisch A, Cronauer MV. Regulation of prostatic growth and function by peptide growth factor. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin: Anatomy and physiology of a new regulatory system. J Steroid Biochem Mol Biol. 1991;40:813–20. doi: 10.1016/0960-0760(91)90307-q. [DOI] [PubMed] [Google Scholar]
- 13.Nakhla AM, Romas NA, Rosner W. Estradiol activates the prostate androgen receptor and prostate-specific antigen secretion through the intermediacy of sex hormone-binding globulin. J Biol Chem. 1997;272:6838–41. doi: 10.1074/jbc.272.11.6838. [DOI] [PubMed] [Google Scholar]
- 14.Farnsworth WE. Roles of estrogen and SHBG in prostate physiology. Prostate. 1996;28:17–23. doi: 10.1002/(SICI)1097-0045(199601)28:1<17::AID-PROS3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths K. Molecular control of prostate growth. In: Kirby R, McConnell JD, Fitzpatrick JM, Roehrborn CG, Boyle P, editors. Textbook of Benign prostatic hyperplasia. London: Oxford; 1996. pp. 23–55. [Google Scholar]
- 16.Colombel M, Buttyan R. Hormonal control of apoptosis: The rat ventral prostate gland as a model system. Methods Cell Biol. 1995;46:369–85. doi: 10.1016/s0091-679x(08)61936-6. [DOI] [PubMed] [Google Scholar]
- 17.Hamid AR, Umbas R, Mochtar CA. Recent role of inflammation in prostate diseases: Chemoprevention development opportunity. Acta Med Indones. 2011;43:59–65. [PubMed] [Google Scholar]
- 18.Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benignProstatic hyperplasia. RevUrol. 2011;13:147–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Fibbi B, Penna G, Morelli A, Adorini L, Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33:475–88. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 20.Sciarra A, Mariotti G, Salciccia S, Autran Gomez A, Monti S, Toscano V, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:54–60. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic Hyperplasia? Curr Opin Urol. 2006;16:25–9. [PubMed] [Google Scholar]
- 22.Kramer G, Mitteregger D, Marberger M. Is Benign Prostatic Hyperplasia (BPH) an immune inflammatory disease? Eur urol. 2007;51:1202–16. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edemas in hind paw of rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;3:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 24.Colville-Nash PR, Gilroy DW. COX-2 and the cyclopentenone prostaglandins - A new chapter in the book of inflammation? Prostaglandins Other Lipid Mediat. 2000;62:33–43. doi: 10.1016/s0090-6980(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 25.Raghavendra V, Kulkarni SK. AT1 receptor antagonism enhances angiotensin-II facilitated carrageenan-induced paw oedema. Clin Pharmacol. 2000;22:633–8. doi: 10.1358/mf.2000.22.8.802275. [DOI] [PubMed] [Google Scholar]


