Abstract
Objective:
In order to discover whether the eNOS/NO (endothelial nitric oxide synthase/nitric oxide) pathway is involved in the protective mechanisms of ischemic myocardium of DGSND (Dang Gui Si Ni Decoction) in MIRI (myocardial ischemia-reperfusion injury) SD rats.
Materials and Methods:
We made I/R (ischemia-reperfusion) model by ligating the left anterior-descending branch of the coronary artery (LAD) for 30 min and releasing the ligature for 120 min. eNOS (nitric oxide synthase) mRNA (message ribonucleic acid) and iNOS (inducible nitric oxide synthase) mRNA were measured by the methods of real-time RT-PCR (Real time Polychainase Chain Reaction), peNOS (phosphorylated eNOS) and iNOS protein were measured by the means of western blot.
Results:
In PPC group, real-time RT-PCR and western-blot analysis showed that eNOS mRNA and peNOS protein increased markedly (P < 0.05); iNOS mRNA and protein decreased significantly (P < 0.05).
Conclusion:
These results indicate that ischemic preconditioning (IPC) of GFPC from DGSND plays a protective role in I/R heart through regulating the eNOS/NO signal pathway, which could increase the eNOS gene expression and decrease the expression of iNOS mRNA.
Keywords: Cinnamic acid, eNOS, ferulic acid, glycyrrhizic acid, MIRI, NO, paeoniflorin
INTRODUCTION
DGSND is a classical decoction which is used to treat blood-deficiency and cold syncope in Shanghanlun. Modern pharmacological studies shown that DGSND have objective effects of anticoagulation, expand blood vessels, analgesia and anti-inflammation,[1] which was mainly applied to treat vascular ischemic diseases, such as frostbite, thromboangiitis obliterans, Raynaud's syndrome, coronary heart disease, lower extremity arterial occlusion and venous thrombosis.[2] We have extracted four active ingredients: Glycyrrhizic acid, ferulic acid, paeoniflorin and cinnamic acid from DGSND, which all have intervention effects on myocardial ischemia-reperfusion injury (MIRI).
eNOS/NO Pathway plays an important role in the pathogenesis of MIRI.[3] Some clinical reports and studies showed that both DGSND and its ingredients have significant effects on vascular ischemic diseases, especially the MIRI.[4,5,6,7,8,9,10,11] However, is the protective mechanism of DGSND for MIRI from the eNOS/NO Pathway or not isn’t thoroughly discovered.
NO (nitric oxide) is reported to protect cells from the deleterious effects of some reactive oxygen species such as peroxide.[12] Ischemic preconditioning could promote the composition of NO induced by eNOS. NO may contribute to the ischemic preconditioning. In the previous study we selected, we have selected the best combination from DGSND against I/R injury by the means of orthogonal design: Glycyrrhizic acid 50 mg/kg ferulic acid 400 mg/kg, paeoniflorin 100 mg/kg, cinnamic acid 400 mg/kg GFPC[13] made an ischemic preconditioning in MIRI rats, and probed whether the eNOS/NO pathway is involved in the cardioprotective effects and mechanisms of DGSND in MIRI rat.
MATERIALS AND METHODS
Myocardial I/R
SD rats (250-300g, from Guangdong laboratory animal central) were maintained under conditions of standard lighting (alternating 12-h light/dark cycles), temperature (22°C ± 0.5°C) and humidity (60% ±10%) for at least 1 wk before the experiments. The rats were anesthetized with sodium pentobarbital (40 mg/kg intraperitoneally). The trachea was cannulated with a PE-90 catheter, and artificial respiration was provided by a respirator with an FiO2 (fraction of inspired oxygen) of 0.80, a frequency of 100 strokes/min and a tidal volume of 0.8 to 1.2 mL to maintain normal PO2 (partial pressure of oxygen), PCO2 (partial pressure of carbon dioxide) and pH. A left lateral thoracotomy was made in the fourth intercostals space; the skin, muscles and ribs were retracted; and the pericardial sac was removed. The left-anterior branch of the descending coronary artery (LAD) was occluded by ligation with a 4-0 silk suture. The LAD ligation was performed by using an easily opened knot set on a PE50 silicon tube lying over the LAD. After 30 min of ischemia, the ligation was loosened and reperfusion occurred. Rats were killed at 180 min of reperfusion. The sham control animals were subjected to the entire surgical procedure and the silk suture was passed beneath the coronary artery, but the LAD was not ligated.[14]
The reliability and stability of the model was observed by ECG changes and myocardial HE staining.
Experimental groups
32 SD rats (body weight 250-300 g) were randomly divided into a sham group (non-ischemic myocardial microvascular endothelial cell, conditions) an I/R group (heart subject to ischemia reperfusion) a PPC group (heart subjected to ischemia-reperfusion treated with GFPC) a ppc + L group (heart subjected to ischemia-reperfusion treated with GFPC and pre-treated with L-NAME, the eNOS inhibitor, Nω-Nitro-L-arginine methylester, 15 min before reperfusion, 30 mg/kg), 8 per group.
Nitrate reduction for NO
Serum NO level was tested by methods of nitrate reduction according to the instruction of the kit.
Real-time quantitative reverse transcription polymerase chain reaction for NOS
Quantitative multiplex reverse-transcribed polymerase chain reaction (RT-PCR) was used to determine mRNA levels of the constitutive eNOS and the inducible iNOS isoforms in rat ventricular tissue. Myocardial samples (n = 8/group), fixed in liquid nitrogen and stored at -80°c were homogenized in 800 mL of RNA Fast Solution (Celbio, Milan, Italy). Total RNA was isolated as recommended by the manufacturer. RNA was dissolved in DEPC-treated water and quantified spectrophotometrically at 260 nm. First-strand cDNA was generated by adding RNA (0.1 μg) to a mixture containing 1 mM deoxynucleoside-tri-phosphates (d-NTP), 1 U/μL RNase inhibitor, 2.5 U/μL Moloney murine leukemia virus reverse transcriptase, 2.5 μM random h examers, 5 mM MgCl2, 10 × PCR buffer in a final volume of 20 μL. Reverse transcription was performed at 42°c for 50 min followed by heat inactivation of reverse transcriptase at 95 for 5 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified from the same amount of RNA to correct for variation of different samples. The PCR solution contained 10 μL of first-strand cDNA, 4 μL 10 × PCR buffer, 2 mM MgCl2, 0.15 mM of both sense (5’-ACC ACA GTC CAT GCC ATC AC-3’) and antisense (5’- TCC ACC ACC CTG TTG CTG TA-3’) GAPDH primers, 0.15 mM of both sense (5’-CGA GAT ATC TTC AGT CCC AAG C-3’) and antisense (5’- GTG GAT TTG CTG CTC TCT AGG-3’) eNOS, or 0.15 mM of both sense (5’-TCT GTG CCT TTG CTG ATG AC-3’) and antisense (5’- CAT GGT GAA CAC GTT CTT GG-3’) iNOS primers, 2 U Thermophilus Aquaticus (Taq) DNA polymerase (Celbio, Milan, Italy), and water to a final volume of 50 mL. These samples were overlaid with mineral oil and subjected to 35 cycles at 95°c for 60 s, 60°c for 60 s, and to one cycle at 72°c for 7 min. PCR products were run on 2% agarose gel electrophoresis and photographed after ethidium bromide staining. Bands on the gel were scanned and quantified using a computerized densitometric system (Bio Rad Gel Doc 1000, Milan, Italy).[15]
Western blotting
Phosphorylation of eNOS and iNOS protein were measured by the means of western blot. Extracting about 200 g tissues from the left ventricular, equal amounts of protein (50 μg) were fractionated on 10% sodium dodecyl sulfate-polyacrylamide gels in running buffer (25 mmol/L Tris, 0.25mol/L glycine, 0.1% sodium dodecyl sulfate, pH 8.3) at 90 V and then electroblotted to nitrocellulose membranes. eNOS and iNOS were detected by monoclonal antibodies (Santa Cruz Biotech, Inc. Santa Cruz, CA). Membranes were blocked at room temperature with 5% nonfat milk in Tris-buffered saline containing 0.05% Tween-20 and then incubated overnight at 4°c with the following primary antibodies: GAPDH (Sigma company, USA; dilutions, 1:5000). GAPDH was used as internal control to correct for variations of different samples. Then the membranes were washed three times in Tween-20 and incubated with the corresponding secondary antibody (Santa Cruz Biochemicals; dilutions, 1:10000) conjugated to horseradish peroxidase at room temperature. Immunoreactive bands were visualized with the chemoluminescence kit (Santa Cruz Biochemicals) according to the manufacturer's instructions. Band intensities and molecular weight were quantified by using a gel image processing system (Bio-Rad, USA).
Statistics analysis
All results are reported as mean ± S.D. Statistical analysis was performed using analysis of variance of orthogonal design, 1-way and 2-way analysis of variance (ANOVA) for multiple-group comparisons, Chi-square test for data count. The probability of null hypothesis < 0.05 (P < 0.05) was considered statistically significant.
RESULTS
Effect of GFPC on eNOS and iNOS
To test the hypothesis that eNOS/NO pathway acts to protect ischemic myocardium in ischemic preconditioning of GFPC, we examined the gene and protein expressions of eNOS and iNOS by real-time PCR and western blot analysis. As shown in Figure 1a and b, compared with the sham group, eNOS mRNA and protein of I/R group significantly decreased (P < 0.05); compared with the I/R group, eNOS mRNA and eNOS protein of PPC group significantly increased (P < 0.05); compared with the PPC group, eNOS mRNA and peNOS protein of PPC + L group significantly reduced (P < 0.05). Compared with the sham group, iNOS mRNA and protein of the I/R group significantly increased (P < 0.05); Compared with the I/R group, iNOS mRNA and protein of PPC group decreased significantly (P < 0.05); but compared with the PPC group, iNOS mRNA and protein of the PPC + L group changes were not statistically significant (P > 0.05)
Figure 1.
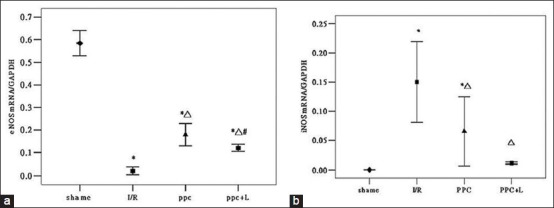
(a) eNOS mRNA statistical analysis (b) iNOS mRNA statistical analysis
Figure 1a shows real-time RT-PCR analysis of eNOSmRNA in rat hearts of different groups (sham, I/R, PPC, PPC + L). The expression of eNOS mRNA attenuated in the I/R group decreased (*P < 0.05 versus shame group) After administration of GFPC, the amount of eNOSmRNA significantly increased (ΔP < 0.05 versus the I/R group); and in the PPC + L group, the amount of eNOS mRNA was significantly reduced (# P < 0.05 versus the PPC group).
Figure 1b shows the gene expression of iNOS in rat hearts of different groups (shame, I/R, PPC, PPC + L) evaluated by real-time quantitative RT-PCR. The expression of iNOS mRNA was markedly increased in the I/R group (*P < 0.05 versus sham group)After administration of GFPC, the amount of iNOSmRNA significantly decreased (ΔP < 0.05 versus the I/R group); The amount of iNOS mRNA has no obvious difference (P > 0.05) between ppc group and ppc + L group.
Figures 2b and 3b show western blot analysis of peNOS and iNOS protein of different groups in rat hearts. A. significant reduction in amount of peNOS protein was observed in the I/R group (P < 0.05 versus the sham group); After administration of GFPC, the amount of peNOS protein was increased in PPC (P < 0.05 versus the I/R group); and in the PPC + L group, the amount of peNOS protein was reduced significantly (P < 0.05 versus the PPC group).
Figure 2.
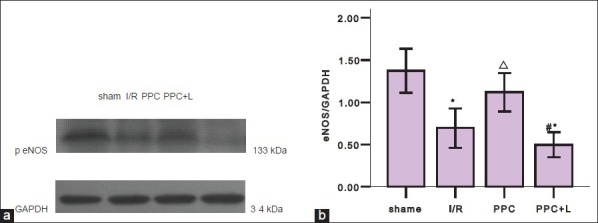
(a) Western blot analysis of peNOS of different groups in rat hearts (b) statistics of western blot
Figure 3.
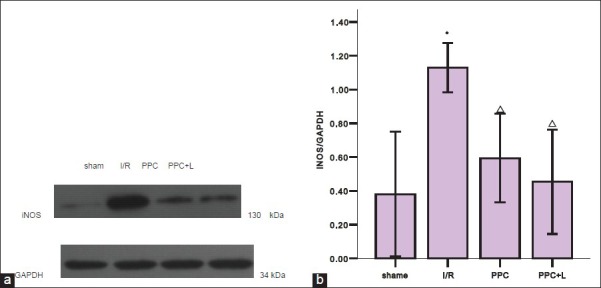
(a) Western blot analysis of iNOS of different groups in rat hearts (b) statistics of western blot
Figure 1 shows the amount of iNOS protein increased significantly in the I/R group (P < 0.05 versus the shame group); After administration of GFPC, the amount of iNOS protein was decreased in PPC (P < 0.05 versus the I/R group); and between the PPC and PPC + L, the amount of iNOS protein changes was not significant (P > 0.05).
DISCUSSION
In previous studies of L-arginine-NO pathway in I/R injury, NO was found as an endothelium-derived relaxing factor by Palmer in 1987.[16] L-arginine-NO pathway contributed to free radical generation, which lead to ischemia-reperfusion injury. NO synthase inhibitors decreased coronary sinus free radical concentration and tissue peroxynitrite formation in an ischemic-reperfusion canine model.[17] Various competitive inhibitors of the NOS enzyme have been shown to reduce I/R injury in various settings by reducing myocardial infarct size and improving myocardial contractile function.[18,19] L-arginine aggravated myocardial staining through production of peroxynitrite.[20] In contrast to these above studies, some studies have shown that an important protective role of NO in the ischemic preconditioning.[3,21] NO precursor arginine ameliorated the endothelial dysfunction resulting from global ischemia-reperfusion sequences in an isolated working rat heart model.[22] In the basic state, NO released from vascular endothelial cells plays an important role in maintaining the cardiovascular system at a relaxation state, regulating blood pressure, ameliorating coronary artery vascular tone and I/R injury. The generation of nitric oxide contributes to the marked antiarrhythmic effects of preconditioning in the canine myocardium, probably through elevation of cyclic GMP.[23]
The results of our study provided the experimental evidence that iNOS expression elevate, while eNOS expression reduce in ischemia-reperfusion myocardium. During reperfusion, the resulting formation of NO decreases. GFPC could increase the expression level of eNOSmRNA and phosphorylation of eNOS protein, and then promote the L-arginine, NO precursor synthesize nitric oxide. At the same time, GFPC decreases the expression level of iNOS and protein. A large number of iNOS will produce the toxicity NO and inflammatory factors, which will aggravate ischemia reperfusion. Preconditioning of GFPC plays a protective role on ischemic myocardium by increasing eNOS and inhibiting iNOS. But after administration of L-NAME, eNOS inhibitor, phosphorylation of eNOS protein was inhibited, hence the generation of NO reduced. It is concluded from these results that the generation of nitric oxide is based on the changes of NOS isozyme. Preconditioning of GFPC plays a major protective role on myocardial ischemia in MIRI through the eNOS/NO pathway.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Miao M, Wang S. 11th ed. Beijing: Tsinghua University Press; 2004. Modern dosimetry; p. 885. [Google Scholar]
- 2.Yang J. Advance in the clinical applacation of danguisini decoction in recent five years. Clin J Tradit Chin Med. 2008;20:538–40. [Google Scholar]
- 3.Hotta Y, Otsuka-Murakami H, Fujita M, Nakagawa J, Yajima M, Liu W, et al. Protective role of nitric oxide synthase against ischemia-reperfusion injury in guinea pig myocardial mitochondria. Eur J Pharmacol. 1999;380:37–48. doi: 10.1016/s0014-2999(99)00531-2. [DOI] [PubMed] [Google Scholar]
- 4.He X, Xing D, Ding Y, Li Y, Xu L, Du L. Effects of cerebral ischemia-reperfusion on pharmacokinetic fate of paeoniflorin after intravenous administration of Paeoniae Radix extract in rats. J Ethnopharmacol. 2004;94:339–44. doi: 10.1016/j.jep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Chen DM, Xiao L, Cai X, Zeng R, Zhu XZ. Involvement of multitargets in paeoniflorin-induced preconditioning. J Pharmacol Exp Ther. 2006;319:165–80. doi: 10.1124/jpet.106.104380. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–50. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 7.Yogeeta SK, Gnanapragasam A, Senthilkumar S, Subhashini R, Devaki T. Synergistic Salubrious effect of ferulic acid and ascorbic acid on membrane-bound phosphatases and lysosomal hydrolases during experimental myocardial infarction in rats. Life Sci. 2006;80:258–63. doi: 10.1016/j.lfs.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Yan Y, Zhu D, Yu B, Wang Q. Protective effects of FBD-an experimental Chinese traditional medicinal formula on memory dysfunction in mice induced by cerebral ischemia- reperfusion. J Ethnopharmacol. 2005;97:477–83. doi: 10.1016/j.jep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Qin F, Zhang HM, Xiao HB, Wang LX, Zhang XY, et al. Cardioprotection by Guanxin II in rats with acute myocardial infarction is related to its three compounds. J Ethnopharmacol. 2009;121:268–73. doi: 10.1016/j.jep.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H, Nassiri Asl M, Parvardeh S. The effects of carbenoxolone, a semisynthetic derivative of glycyrrhizinic acid, on peripheral and central ischemia-reperfusion injuries in the skeletal muscle and hippocampus of rats. Phytomedicine. 2005;12:632–7. doi: 10.1016/j.phymed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Gu Y, Song X, Zuo J. HPLC for measuring the contents of four active ingredients in Dangguisini decoction. J Shen Yang Pharm Univ. 2008;25:200–3. [Google Scholar]
- 12.Okayama N, Grisham MB, Kevil CG, Eppihimer LA, Wink DA, Alexander JS. Effect of reactive oxygen metabolites on endothelial permeability: Role of Nitric oxide and iron. Microcirculation. 1999;6:107–16. [PubMed] [Google Scholar]
- 13.Qian G, Zhao G. Best combination of four active imgredients of anti-MIRI of DGSND. J Chin Med Mater. 2011;34:81–5. [Google Scholar]
- 14.Zhang J, Bian HJ, Li XX, Liu XB, Sun JP, Li N, et al. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16:307–15. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Napoli P, Antonio Taccardi A, Grilli A, Spina R, Felaco M, Barsotti A, et al. Simvastatin reduces reperfusion injury by modulating nitric oxide synthase expression: An ex vivo study in isolated working rat hearts. Cardiovasc Res. 2001;51:283–93. doi: 10.1016/s0008-6363(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 16.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Bissing JW, Xu L, Ryan AJ, Martin SM, Miller FJ, Jr, et al. Nitric oxide synthase inhibitors decrease coronary sinus free radical concentration and ameliorate myocardial stunning in an ischemia-reperfusion model. J Am Cell Cardiol. 2001;38:546–54. doi: 10.1016/s0735-1097(01)01400-0. [DOI] [PubMed] [Google Scholar]
- 18.Patel VC, Yellon DM, Singh KJ, Neild GH, Woolfson RG. Inhibition of nitric oxide limits infarct size in the insitu rabbit heart. Biochem Biophys Res Commun. 1993;194:234–8. doi: 10.1006/bbrc.1993.1809. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Zweier J. Measurement of nitric oxide and peroxynitrite generation in the post-ischemic heart. J Biol Chem. 1996;271:23–30. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 20.Mori E, Haramaki N, Ikeda H, Imaizumi T. Intracoronary administration of L-arginine aggravates myocaridal stunning through production of peroxynitrite in dogs. Cardiovasc Res. 1998;40:13–23. doi: 10.1016/s0008-6363(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 21.Jones KW, Flaherty MP, Tang XL, Qiu Y, Banerjee S, Bolli R. Ischemic preconditioning regulates expression of NOS in RNA in conscious rabbits via an NO-dependent mechanism (abstr) Circulation. 1998;98:I–620. [Google Scholar]
- 22.Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE, 3rd, Deaton DW, et al. Constitutive nitric oxide release is impaired after ischemia and reperfusion. J Thorac Cardiovasc Surg. 1995;110:1047–53. doi: 10.1016/s0022-5223(05)80173-4. [DOI] [PubMed] [Google Scholar]
- 23.Vegh A, Szekeres L, Parrat J. Preconditioning of the ischemic myocardium; involvement of the L-arginine nitric oxide pathway. Br J Pharmacol. 1992;107:648–52. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


