Abstract
A new, simple, precise, rapid, and selective high performance thin layer chromatographic (HPTLC) method has been developed and validated for the estimation of 1, 8-cineole in volatile oil of leaves of Callistemon Citrinus obtained by hydro distillation. The method was validated as per ICH guidelines and can be utilized for routine analysis. The retention factor for 1, 8-cineole was found to be 0.52. The linearity was found to be in the range of 3 μg-12 μg. The recovery obtained for 1, 8-cineole was 98%, which is satisfactory. The result obtained in validation indicate the accuracy, reproducibility, and reliability of the developed HPTLC method for determination of 1, 8-cineole.
Keywords: 1, 8-cineole; Callistemon Citrinus; essential oil; high performance thin layer chromatographic
INTRODUCTION
Callistemon is a genus of 34 species of shrubs in the family Myrtaceae, all of which are endemic to Australia. The genus Callistemon is known in folk medicine for its anti-cough, anti-bronchitis, and insecticidal effects and its volatile oils have been used as anti-microbial and anti-fungal agents.[1] Callistemon lanceolatus D.C. (Myrtaceae) is a slow-growing ornamental shrub that grows to a height of around 10 meters. It is commonly known as crimson bottle brush tree because of its spiky inflorescence that resembles a bottle brush. The plant has been used by tribal communities of India for the treatment of gastrointestinal disorders, pain, and infectious diseases.[2]
Essential oils (EO) are plant secondary metabolites that are known for their fragrance and food flavor properties. They consist of a complex mixture of mono- and sesquiterpenes, phenyl propanoids, and oxygenated compounds. EOs can be present in different plant organs and materials, and their storage is related to specialized secretory structures.[3] Therapeutically, the essential oils exert wide spectrum of activities like antiseptic, stimulant, carminative, diuretic, anthelmintic, analgesic, anti-rheumatic, aromatic, counter irritant and many other activities. Apart from food and pharmaceutical uses, they are also used as insect repellants, insecticides, pesticides, and deodorants.[4]
Callistemon Citrinus (Curtis) Skeels (syn: Metrosideros citrina Curtis; commonly known as crimson or lemon bottlebrush) is a handy medium shrub to large tree (ca. 5-7 m tall). C. citrinus is the most widely cultivated member of the genus Callistemon. The main component in the essential oil of Callistemon Citrinus is 1, 8-cineole (61.4%) and alpha pinene (13.4%).[5] Callistemon Citrinus syn. Callistemon lanceolatus has shown to possess activities like anti-caries, antioxidant, spasmolytic, anti-inflammatory, hypoglycemic, hepatoprotective, cytotoxic, cardio-protective, anti-Helicobacter pylori, and anti-bacterial activity.[6,7,8,9,10,11,12,13,14,15]
The literature revealed that no HPTLC method has yet been reported for estimation of 1, 8-cineole in the essential oil of Callistemon Citrinus (Curtis) Skeels. The method validation has been carried out as per ICH guidelines.[16] Thus, this method is simple, accurate, precise, and cost-effective method for estimation of 1, 8-cineole in the volatile oil of Callistemon Citrinus.
MATERIALS AND METHODS
Reagents and materials
All the chemicals and solvents used were of analytical grade and obtained from E. Merck (Darmstadt, Germany). The standard 1, 8-cineole was procured from Carol Aromas, Mumbai, India (purity > 99%). The aluminum-backed pre-coated TLC plates (silica gel 60F254, 20 × 20 cm, 0.2 mm thick) were obtained from E. Merck.
Plant material
The fresh leaves of Callistemon Citrinus were collected from Karad, Maharashtra in July 2012. A voucher specimen (APS 01) for the plant material was identified at Yashwantrao Chavan College of Science (Karad). The leaves were washed and shade-dried for 24 hours. The leaves were then grounded to form coarse powder.
Extraction of essential oil for HPTLC analysis
The coarsely powdered leaves (100 g) of Callistemon Citrinus were hydro-distilled for 3 hours using Clevenger type apparatus. The resulting oil was collected, dried over anhydrous sodium sulfate, preserved in an amber-colored glass bottle, and stored in refrigerator till further analysis.
Instrumentation
The method was developed on CAMAG HPTLC system consisting of Linomat V applicator (Camag, Muttenz, Switzerland), CAMAG twin trough chamber (10 × 10 cm and 20 × 20 cm), CAMAG TLC Scanner equipped with WinCATS software (version 1.4.6), CAMAG syringe of 100 μl capacity, CAMAG derivatization chamber, and CAMAG TLC plate heater. Separation, derivatization, and identification of 1, 8-cineole were done on aluminum-backed pre-coated TLC plates.
Preparation of working standard solution of 1, 8- cineole
The standard stock solution of standard 1, 8-cineole was prepared by dissolving 15 mg of 1, 8-cineole up to 10 ml with methanol. The concentration of resulting stock solution was 1500 μg/ml of 1, 8-cineole.
Preparation of sample solution of oil of Callistemon Citrinus
The stock solution of the sample was prepared by dissolving 15 mg of oil up to 10 ml with methanol, and the concentration of the sample solution was 1500 μg/ml of oil.
Chromatographic conditions
The experiment was performed on a silica gel 60F254 (0.2 mm thick) HPTLC plates (20 × 10 cm) and (10 × 10 cm) without pre-washing. Samples were applied to the plates as 8 mm bands with CAMAG Linomat V applicator. The plates were developed by ascending technique to a distance of 80 mm, at 25 ± 5° C, relative humidity 50-60%, in a CAMAG twin trough chamber with a stainless steel lid. The mobile phase composed of toluene: Ethyl acetate: Methanol: Formic acid (9: 0.5: 0.5: 0.5) for 1, 8-cineole. The chamber saturation time was 10 mins. After development, the plates were dried and then viewed in a CAMAG visualizer at 254 nm, 366 nm, and white light. The plate was then derivatized using anisaldehyde sulfuric acid reagent in CAMAG derivatization chamber and then heated at 100°C using CAMAG TLC plate heater. The plate was cooled and then scanned in CAMAG TLC scanner using WinCATS software (version 1.4.6) in absorption mode with slit dimensions 6.00 × 0.45 mm. The detection wavelength was 540 nm. The Rf value for 1, 8-cineole was found to be 0.52.
Calibration curve for standard 1, 8-cineole
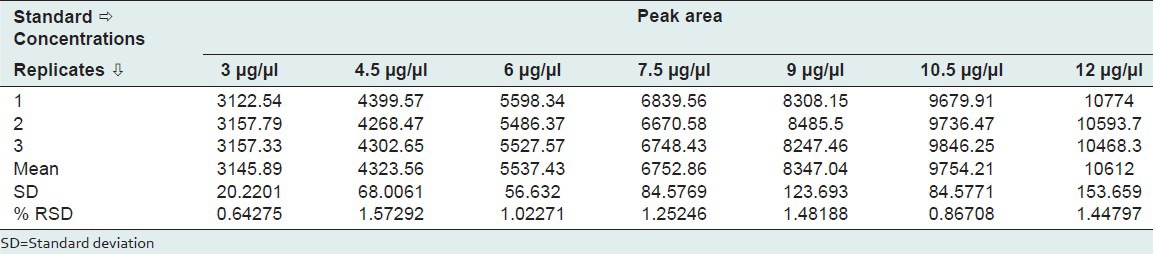
The standard solutions of 1, 8-cineole of 3 μg-12 μg were applied on TLC plate, and further it was developed and scanned as per the chromatographic conditions. The peak areas were recorded. The calibration curve of 1, 8-cineole was obtained by plotting peak area against concentration of 1, 8-cineole applied.
VALIDATION OF PROPOSED METHOD
ICH guidelines were followed for the validation of the analytical method developed (precision, accuracy, ruggedness, robustness, linearity, LOD, LOQ, and specificity).
Linearity
Standard stock solution of 3-12 μg of 1, 8-cineole was prepared and diluted to appropriate concentrations for plotting the calibration curve. At least six concentrations of the analyte solutions were analyzed in triplicate, and then the calibration curve was constructed by plotting the mean peak areas versus the concentration of each analyte.
Precision
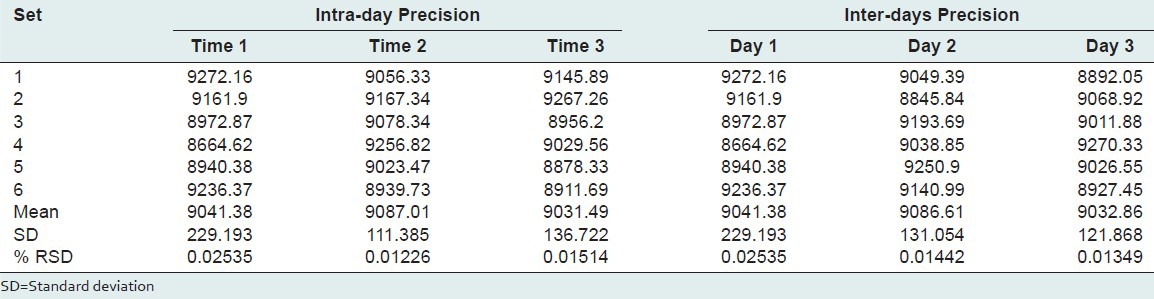
The inter-day precision (% RSD) was determined by analyzing standard solution of 1, 8-cineole over the entire calibration range for 3 different days. The intra-day precision (RSD) was determined by analyzing standard solution of 1, 8-cineole over the entire calibration range for 3 times on the same day.
Accuracy
Accuracy of the method was tested by carrying out recovery studies at different spiked level by standard addition method. Standard 1, 8-cineole solution was added at 3 different levels (80%, 100%, and 120%). At each level, 3 determinations were performed, and the results were calculated by the difference between the spiked and un-spiked sample analyzed under same conditions. The percentage recovery of 1, 8-cineole was calculated at each level.
Limit of detection
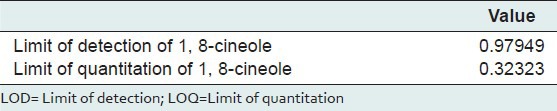
The limit of detection (LOD) was determined using following formulae:
LOD = 3.3(S. D)/S,
Where S. D = Standard deviation of response, S = average of slope of the calibration curve. The LOD was calculated for 1, 8-cineole.
Limit of quantitation
The limit of quantitation (LOQ) was determined using following formulae:
LOQ = 10(S. D)/S
Where S. D = Standard deviation of response, S = average of slope of the calibration curve. The LOQ was calculated for 1, 8-cineole.
Specificity
It was observed that the other constituents present in the oil sample did not interfere with the peak of 1, 8-cineole. Therefore, the method was specific.
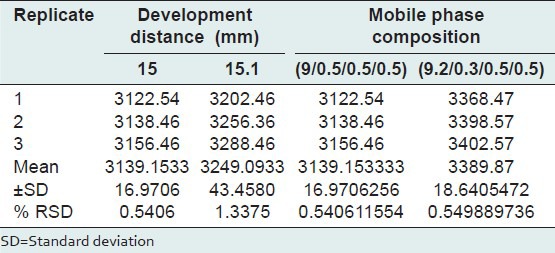
Robustness
By introducing small changes in the mobile phase composition and development distance, the effects on the results were examined. Robustness of method was done 3 times at a concentration level of 1.5 μg/spot, and the %RSD of peak area was calculated.
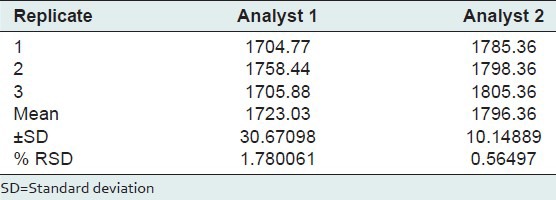
Ruggedness
Ruggedness of the method was assessed by spiking the standard 3 times with different analyst by using same equipment.
RESULT AND DISCUSSION
HPTLC procedure was optimized with a view to quantify the essential oil. Toluene: Ethyl acetate: Methanol: Formic acid (9: 0.5: 0.5: 0.5) gave good resolution with Rf value for 1, 8-cineole being 0.52. Well-defined band was obtained after chamber saturation for 10 min at room temperature. TLC plate was visualized after derivatization with anisaldehyde sulfuric acid reagent at 540 nm. The identity of 1, 8-cineole was confirmed by comparing chromatogram of standard 1, 8-cineole with that of oil extracted from Callistemon Citrinus and by comparing the retention factor of the standard with that of sample.
Linearity
Calibration plot shown in Figure 1 indicates that the response was linear function of concentration in the range of 3-12 μg for 1, 8-cineole. The correlation coefficient, intercept, and the slope for 1, 8-cineole were 0.999, 491.577, and 862.495, respectively. The linearity study is shown in Table 1.
Figure 1.
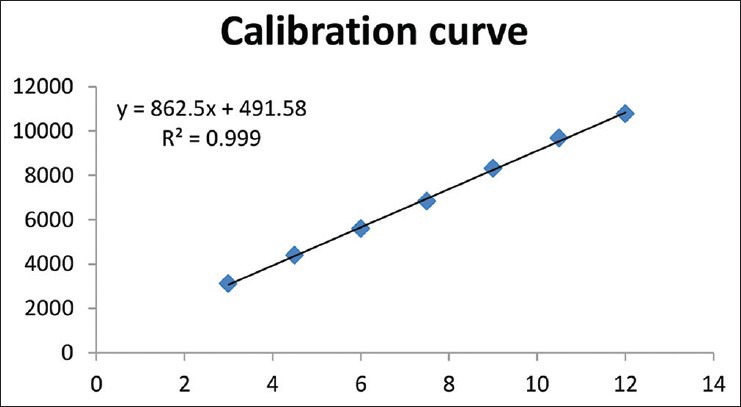
Calibration plot
Table 1.
Linearity study
Precision
The measurement of peak area three times inter day and intra-day showed %RSD (<2%), which indicated precision of method [Table 2].
Table 2.
Precision of HPTLC method
Limit of detection and limit of quantitation
The LOD and LOQ of 1, 8-cineole obtained indicate that the method is adequately sensitive [Table 3].
Table 3.
LOD and LOQ for HPTLC quantification of 1, 8-cineole
Recovery
The results from recovery study were within acceptable limit indicating accuracy of method [Table 4].
Table 4.
Recovery study of 1, 8-cineole
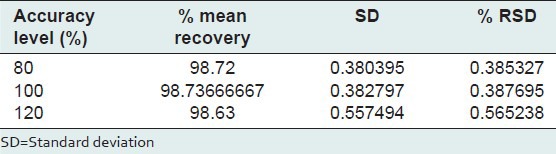
Robustness
The low value of S. D and %RSD obtained after introducing small deliberate changes in the developed HPTLC method indicates the robustness of method [Table 5].
Table 5.
Robustness of HPTLC method (n=3)
Ruggedness
Low %RSD values between the values of peak areas prove the ruggedness of method, which indicates that 1, 8-cineole gives reproducible results for the proposed method [Table 6].
Table 6.
Ruggedness of HPTLC method (n=3)
Specificity
The peak purity of 1, 8-cineole was assessed by comparing the spectra at peak start, peak apex, and peak end position of the spot. Good correlation was obtained between standard and sample of 1, 8-cineole.
CONCLUSION
A rapid, simple, accurate, and specific HPTLC method for quantitative estimation of 1, 8-cineole present in oil of Callistemon Citrinus has been developed and validated. The data could be used as a quality control standard. The method used resulted in good peak shape and enabled good resolution of 1, 8-cineole from other constituents of the essential oil since there was no interference with peak of 1, 8-cineole from other constituents of oil.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Goyal PK, Jain R, Jain S, Sharma A. A Review on biological and phytochemical investigation of plant genus Callistimon. Asian Pac J Trop Biomed. 2012;2:S1906–9. [Google Scholar]
- 2.Paluri V, Ravichandran S, Kumar G, Karthik L, Rao KV. Phytochemical Composition and In Vitro Antimicrobial Activity of Methanolic Extract of Callistemon Lanceolatus D.C. Int J Pharm Pharma Sci. 2012;4:699–702. [Google Scholar]
- 3.Rohloff J. Doctorate of Philosopy Thesis. Trondheim, Norway: Faculty of Natural Sciences and Technology; 2003. Cultivation of Herbs and Medicinal Plants in Norway- Essential Oil Production and Quality Control. [Google Scholar]
- 4.Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 36th ed. Pune: Nirali Prakashan; 2006. Terpenoids; pp. 311–3. [Google Scholar]
- 5.Oyedeji OO, Lawal OA, Shode FO, Oyedeji OA. Chemical Composition and antibacterial activity of the essential oils of callistemon citrinus and callistemon viminalis from South Africa. Molecules. 2009;14:1990–8. doi: 10.3390/molecules14061990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park Y, Lee WI, Park SY, Ahn JK, Han M. Anticariogenic activity of Callistemon citrinus extract against Streptococcus mutans. Mokchae Konghak. 2005;33:72–7. [Google Scholar]
- 7.Haque ME, Sultana A, Shibib BA, Islam MM. Antimicrobial, Antioxidant and Cytotoxic activities of Callistemon citrinus (Curtis) skeels. Dhaka Univ J Pharm Sci. 2012;11:51–4. [Google Scholar]
- 8.Niaz A, Shah SW, Ahmad B. Calcium Channel Blocking Activity of Fruits of Callistemon Citrinus. J Chem Soc Pak. 2011;33:245. [Google Scholar]
- 9.Kumar S, Kumar V, Prakash O. Pharmacognostic study and anti-inflammatory activity of Callistemon lanceolatus leaf. Asian Pac J Trop Biomed. 2011;1:177–81. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazreen S, Kaur G, Alam MM, Haider S, Shafi S, Hamid H, et al. Hypoglycemic ACTIVITY of callistemon lanceolatus leaf ethanolic extract in streptozotocin induced diabetic rats. Pharmacologyonline. 2011;1:799–808. [Google Scholar]
- 11.Jain AK, Dubey SK, Sikarwar MS, Jain SK. Hepatoprotective activity of methanolic extract of leaves of Callistemon lanceolatus. Int J Plant Sci. 2007;2:185–6. [Google Scholar]
- 12.Ali N, Ahmed G, Shah SW, Shah I, Ghias M, Khan I. Acute toxicity, brine shrimp cytotoxicity and relaxant activity of fruits of Callistemon citrinus Curtis. BMC Complement Altern Med. 2011;11:99. doi: 10.1186/1472-6882-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momin F, Kalai B, Patole N, Godse V, Shikalgar T, Naikwade N. Cardio protective activity of ethanolic extract of Callistemon lanceolatus leaves on doxorubicin-induced cardiomyopathy in rats. Bangladesh J Pharmacol. 2011;6:38–45. [Google Scholar]
- 14.Lee HK, Lee HB, Kim CS, Ahn YJ. Anti-Helicobacter pylori activity of methanol extracts from Korean native plant species in Jeju Island. Agric Chem Biotechnol. 2004;47:91–6. [Google Scholar]
- 15.Seyydnejad SM, Niknejad M, Darabpoor I, Motamedi H. Antibacterial activity of Hydroalcoholic Extract of Callistemon citrinus and Albizia lebbeck. Am J Appl Sci. 2010;7:13–6. [Google Scholar]
- 16.ICH Q2b, ICH Harmonised Tripartite Guideline, “Validation of Analytical Procedures: Methodology”, Recommended for Adoption at Step 4 of the ICH Process on 6 Nov 1996, by the ICH Steering Committee. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html .


