Abstract
Background:
Non alcoholic steatohepatitis (NASH), severe form of diseases belonging to the spectrum of the Non alcoholic fatty liver disease (NAFLD). It is an asymptomatic disease which leads to fibrosis and finally to cirrhosis, an end stage liver disease.
Objective:
To study the effect of pioglitazone, quercetin and hydroxy citric acid on hepatic biomarkers and various biochemical parameters in experimentally induced non alcoholic steatohepatitis (NASH).
Materials and Methods:
Male Wister rats were divided into 8 groups. The activities of alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH) and γ-Glutamyl Transferase (GGT) were assayed in serum. The levels of various other biochemical parameters such as serum albumin, total bilirubin, creatinine, urea, uric acid and glucose were also estimated in experimental NASH.
Results:
The NASH group produced severe liver injury by significantly increasing the serum levels of ALT, AST, GGT and LDH compared with that of the control. However, the experimental NASH rats treated with pioglitazone, with quercetin and with hydroxy citric acid showed an obvious decrease in ALT, AST, GGT and LDH levels when compared with that of NASH induced group. A significant increase in the levels of albumin, creatinine, urea, uric acid, glucose and total bilirubin was noticed in experimentally induced NASH group (group 2) when compared to rats in control group (group 1).
Conclusion:
It could be inferred from this study that, pioglitazone, quercetin and hydroxy citric acid may afford protection to the liver against NASH, as evidenced by the results of this study on the levels of various biochemical parameters such as glucose, urea, uric acid, creatinine and bilirubin. Whereas from the results of hepatic marker enzymes, it is evident that optimal protection was observed after quercetin treatment against experimental NASH whereas pioglitazone and hydroxy citric acid also confers protection to some extent against NASH.
Keywords: Biochemical parameters, experimentally induced NASH, hydroxy citric acid, liver marker enzymes, non alcoholic steatohepatitis, pioglitazone, non alcoholic fatty liver disease, non alcoholic steatohepatitis, quercetin
INTRODUCTION
Noninvasive panels of serological markers have been developed to evaluate the presence of steatosis and hepatic necroinflammation to avoid liver biopsy. Avoiding liver biopsy is desirable because it has certain disadvantages as it is an invasive procedure, prone to sampling errors and suffers from inter-observer variability.[1] Till date, no study has demonstrated that a single biomarker or a panel of biomarkers can be used as an alternative method to liver biopsy to diagnose NASH. Since, the majority of NASH patients are asymptomatic, the specific investigation usually begins after detection of abnormal liver enzymes on routine evaluation.[2]
Since, the pathogenesis of NASH involved interplay of three possible mechanisms[3] such as hyper-insulinemia, lipotoxicity and oxidative stress, we have chosen three categories of drugs, pioglitazone as insulin sensitizer, quercetin as hepatoprotectant and antioxidant and hydroxy citric acid (HCA) as a lipid lowering agent and anti-obesity agent.
Pioglitazone hydrochloride is a widely used drug in the treatment of insulin resistance diabetes.[4] Quercetin is a plant-derived substance, or a phytochemical, which is known as a flavonoid.[5,6] Emerging research suggests that quercetin may reduce the risk of upper respiratory tract infection during intense physical exercise, which is likely attributable to its antioxidant, anti-inflammatory and anti-pathogenic effects.[6] The physiological and biochemical effects of (-)-HCA have been studied extensively for its unique regulatory effect on fatty acid synthesis, lipogenesis, appetiteand weight loss.[7] The derivatives of (-)-HCA have been incorporated into a wide range of pharmaceutical preparations in combination with other ingredients for the claimed purpose of enhancing weight loss, cardioprotection, correcting conditions of lipid abnormalities and endurance in exercise.[8,9]
MATERIALS AND METHODS
The experimental model of NASH in rats by feeding high fat diet for 8 weeks[10] and this model were used to conduct a comparative study of role of pioglitazone, quercetin and hydroxy citric acid on various parameters in experimental model of non alcoholic steatohepatitis. Male Wistar rats weighing approximately 150gm were housed in solid-bottomed polypropylene cages under strict veterinary supervision and maintained in control rooms with 12 h light/dark cycle. The animals received commercial rat diet, standard diet, high-fat diet and water ad libitum as per the experimental protocol. This study conformed to the guiding principles of Institutional Animal Ethical Committee (IAEC), Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Guide for the care and use of laboratory animals (IAEC Approval Number: 001/006/2010 and 01/007/2011).
The Male wistar rats selected for the study were divided into 8 groups as follows:[11]
Group 1; Controls (n = 6): Control rats received regular standard diet for 8 weeks.
Group 2; NASH (n = 6): Rats induced with NASH by feeding high fat diet for 8 weeks.
Group 3; Pioglitazone Control (n = 6): Rats fed with standard diet for 4 weeks, then feeding them with standard diet simultaneously with intra gastric administration of Pioglitazone (4 mg/kg. b.wt) (0.5% methyl cellulose w/v) for further 4 weeks.
Group 4; Quercetin Control (n = 6): Rats fed with standard diet for 4 weeks, then feeding them with standard diet simultaneously with intra gastric administration of quercetin (20 mg/kg. b.wt) dissolved in 1% DMSO v/v for further 4 weeks.
Group 5; Hydroxy Citric Acid Control (n = 6): Rats fed with standard diet for 4 weeks, then feeding them with standard diet simultaneously with intra gastric administration of hydroxy citric acid (150 mg/kg. b.wt) for another 4 weeks.
Group 6; NASH + Pioglitazone (n = 6): Rats fed with high fat diet for 4 weeks, then feeding them high fat diet simultaneously with intra gastric administration of Pioglitazone (4 mg/kg. b.wt) (0.5% methyl cellulose w/v) for another 4 weeks.
Group 7; NASH + Quercetin (n = 6): Rats fed with high fat diet for 4 weeks, then feeding them high fat diet simultaneously with intra gastric administration of quercetin (20 mg/kg. b.wt) dissolved in 1% DMSO v/v for another 4 weeks.
Group 8; NASH + Hydroxy Citric Acid (n = 6): Rats fed with high fat diet for 4 weeks, then feeding them high fat diet simultaneously with intra gastric administration of Hydroxy Citric Acid (150 mg/kg. b.wt) for another 4 weeks.
After the experimental period, the animals were sacrificed after 12 h of fasting by cervical decapitation and blood was collected, centrifuged for 5 min at 3000 rpm/min and serum was stored at -70°C from that various biochemical analysis were carried out. Alkaline phosphatase (ALP) was assayed by the method of King.[12] The activity of aspartate transaminase (AST) was assayed by the method of Mohur.[13] The activity of alanine transaminase (ALT) was assayed by the method of Mohur.[13] The activity of lactate dehydrogenase (LDH) was measured by the method of Nieland.[14] The Max Discovery™ γ-Glutamyl Transferase (GGT) Enzymatic Assay Kit uses an enzymatic reaction to measure enzyme levels in serum according to the model of Moss and Handerson.[15] The levels of various other biochemical parameters such as serum albumin, total bilirubin, creatinine, urea, uric acid and glucose were estimated by using standard kits available commercially.
RESULTS
The effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum liver marker enzymes in experimental NASH were depicted in Table 1 and Figures 1–6. The experimental rats in NASH group (group 2) produced severe liver injury by significantly increasing the serum levels of ALT, AST, GGT and LDH compared with that of the control. Table 2 and Figures 7–12 depicts the effect of pioglitazone, quercetin and hydroxy citric acid on the levels of other biochemical parameters such as serum albumin, total bilirubin, creatinine, urea, uric acid and glucose in experimental NASH.
Table 1.
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum liver marker enzymes in experimental NASH
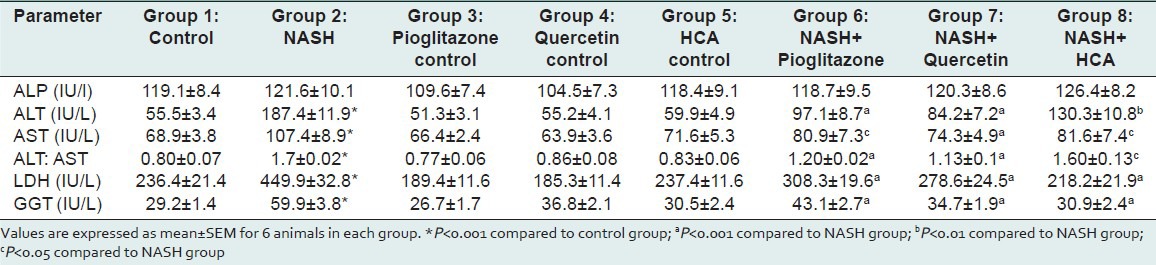
Figure 1.
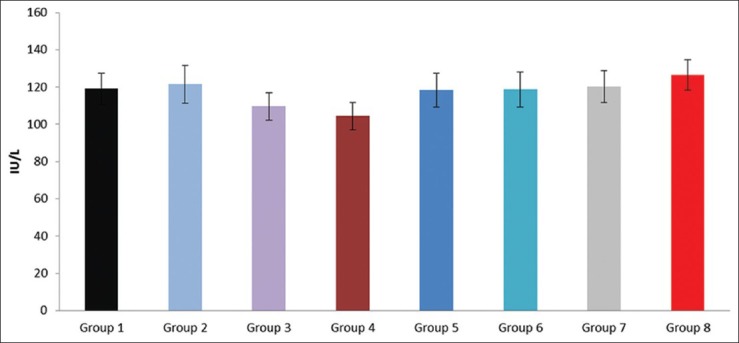
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum alkaline phosphatase (ALP) in experimental NASH
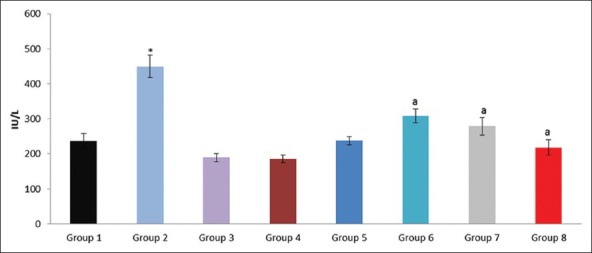
Figure 6.
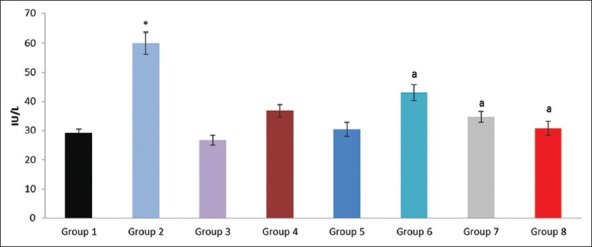
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum gamma glutamyl transferase (GGT) in experimental NASH. *P<0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Table 2.
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of various biochemical parameters in experimental NASH
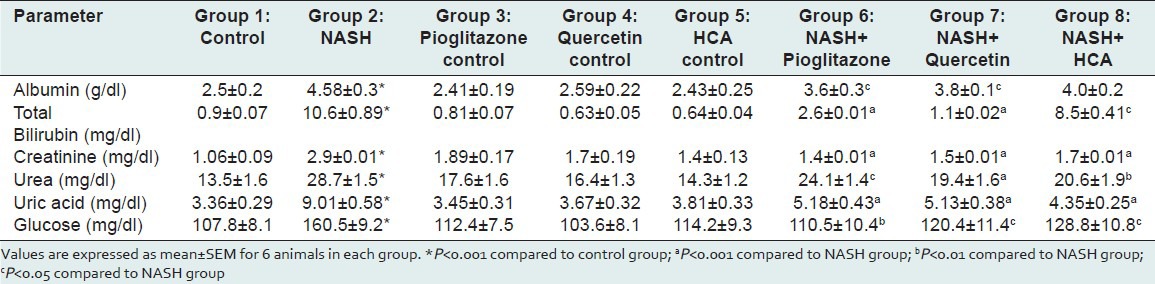
Figure 7.
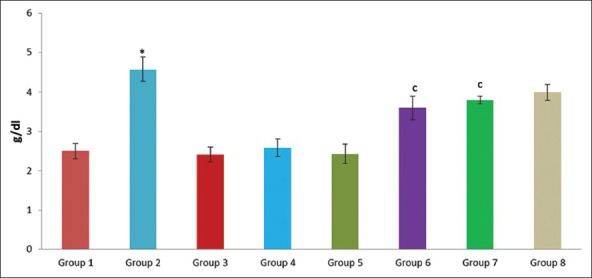
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum albumin in experimental NASH. *P<0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 12.
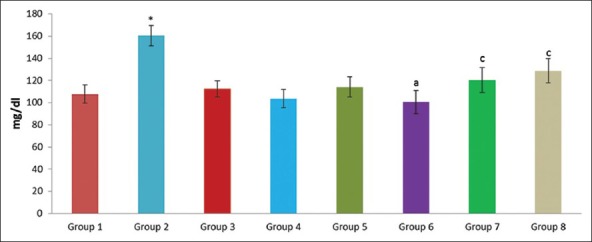
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of blood glucose in experimental NASH. *P <0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 2.
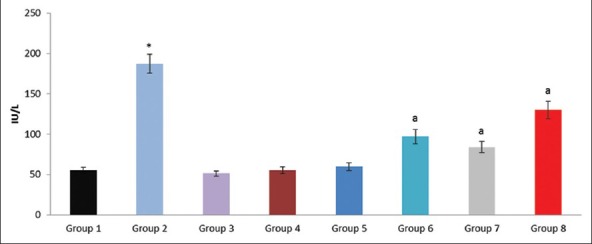
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum alanine transaminase (ALT) in experimental NASH. *P <0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 3.
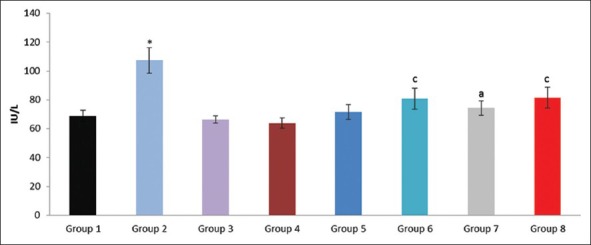
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum aspartate transaminase (AST) in experimental NASH. *P <0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 4.
Effect of pioglitazone, quercetin and hydroxy citric acid on the ratio of serum alanine transaminase (ALT) and aspartate transaminase (AST) in experimental NASH. *P<0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
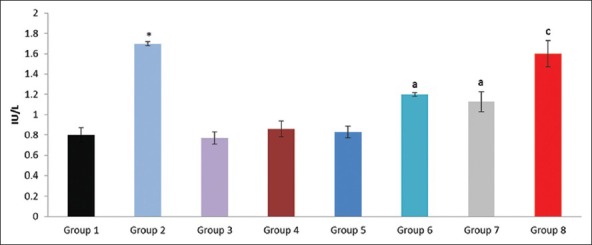
Figure 5.
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum lactate dehydrogenase (LDH) in experimental NASH. *P<0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 8.
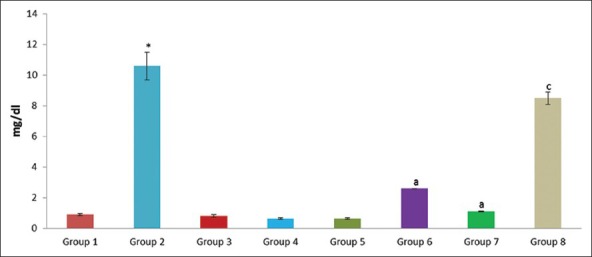
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum total bilirubin in experimental NASH. *P <0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 9.
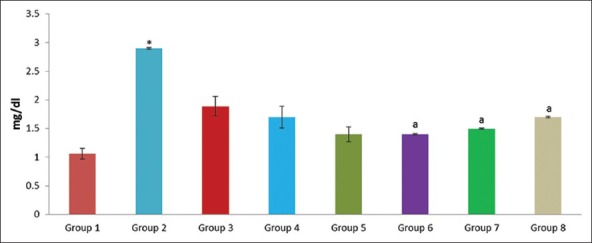
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum creatinine in experimental NASH. *P <0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 10.
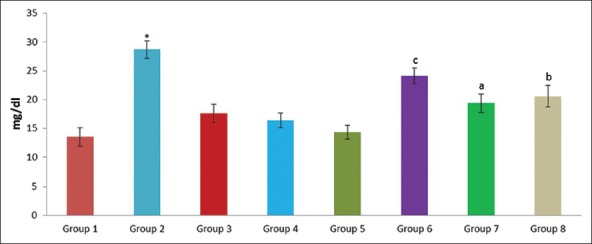
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum urea in experimental NASH. *P<0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
Figure 11.
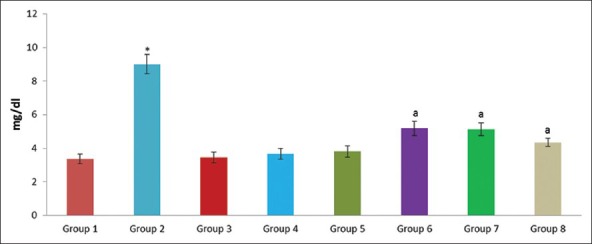
Effect of pioglitazone, quercetin and hydroxy citric acid on the levels of serum uric acid in experimental NASH. *P <0.001 compared to control group; aP <0.001 compared to NASH group; bP <0.01 compared to NASH group; cP <0.05 compared to NASH group
The experimental rats in NASH group (group 2) produced severe liver injury as evidenced by the significantly increased levels of ALT, AST, GGT and LDH in serum, compared with that of the control. However, the experimental NASH rats treated with pioglitazone (group 6; NASH + pioglitazone), with quercetin (group 7; NASH + quercetin) and with hydroxy citric acid (group 8; NASH + HCA) showed an obvious decrease in ALT, AST, GGT and LDH levels when compared with that of NASH induced group (group 2). Rats fed with standard diet simultaneously with pioglitazone (group 3; pioglitazone control), with quercetin (group 4; quercetin control) and with hydroxy citric acid (group 5; HCA control) does not show any significant effect on the liver marker enzymes compared to control group (group 1).
A significant increase in the levels of albumin, creatinine, urea, uric acid, glucose and total bilirubin was noticed in experimentally induced NASH group (group 2) when compared to rats in control group (group 1). The experimental NASH rats treated with pioglitazone (group 6; NASH + pioglitazone), with quercetin (group 7; NASH + quercetin) and with hydroxy citric acid (group 8; NASH + HCA) showed significant reduction in serum albumin, total bilirubin, creatinine, urea, uric acid and glucose levels when compared with that of NASH induced group (group 2). Rats fed with standard diet simultaneously with pioglitazone (group 3; pioglitazone control), with quercetin (group 4; quercetin control) and with hydroxy citric acid (group 5; HCA control) does not show any significant effect on serum albumin, total bilirubin, creatinine, urea, uric acid and glucose compared to control group (group 1).
DISCUSSION
Laboratory tests that are routinely included in the evaluation of patients with suspected NASH include a serum panel of liver tests (alanine aminotransferase, ALT, aspartate aminotransferase AST, alkaline phosphatase, ALP, gamma-glutamyl-transpeptidase, GGT) albumin, prothrombin time and complete blood counts. Serum aspartate aminotransferase (AST) and more commonly, alanine aminotransferase (ALT) show mild to moderate elevation in NASH patients. Therefore, the most common laboratory abnormalities of patients with NAFLD are mild to moderate (twofold to threefold) elevations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), or both.[16] However, as fibrosis advances, this ratio can reverse and lose its diagnostic value in assessing steatohepatitis. Other liver enzymes (gamma-glutamyl transferase) may be elevated two to three times above the normal range.[17]
Drugs like troglitazone (now withdrawn because of hepatotoxicity), rosiglitazone and pioglitazone proved to improve the serum aminotransferase levels.[18] Thus, considering the markers of liver damage, GGT, ALT and AST were correlated to the severity of the hepatic damage and also stated that amino transferase abnormalities probably occur at an earlier stage, different from GGT that would require a greater hepatic damage to be altered. The role of GGT, as a molecular marker for disease severity and diagnostics is still obscure in NASH. The findings of our present study were supported by various other studies report which showed a significant difference between GGT level and the severity of liver fibrosis.[19]
NASH is typically associated with an ALT level greater than the AST level[16,20] in the absence of cirrhosis. Elevation of aminotransferase levels were widely used to diagnose NASH but the estimation of amino transferase levels lacks adequate sensitivity to detect patients with or without NASH and are entirely nonspecific in predicting liver injury.[21] Age >45 years, the presence of obesity or type 2 diabetes mellitus and ALT/AST ratio >1 have been identified as independent predictors of the liver fibrosis.[17] The ratio between alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was predictive for the severity of the liver disease, with an ALT/AST ratio >1 suggesting cirrhosis or advanced fibrosis.[17,22]
The degree of elevation of aminotransferases does not correlate with the severity of steatosis or fibrosis if the elevation is not higher than four times of the upper limit of normal.[23] In the majority of cases, ALT/AST ratio is >1. Higher AST and ALT levels and ALT/AST ratio were significantly associated with NASH. The findings of this study were in accordance with the previous findings. Increased lactate dehydrogenase values were observed in patients with biopsy proven NASH. Also, increased levels of GGT and LDH were associated with necro-inflammatory activity.[24] The activity of GGT was an indicator of hepatic damage and used as sensitive marker in the diagnosis of hepatic diseases and as a marker of NAFLD in patients with metabolic syndrome.[24,25] Serum levels of GGT were significantly enhanced by ethanol treated rats, along with higher concentrations of ethanol in blood and certain injury in rat liver.[26]
To analyze the effect of pioglitazone, quercetin and hydroxy citric acid alone on the liver and on normal metabolic activities, we have chosen to have three drug control groups. Rats fed with standard diet simultaneously with pioglitazone (group 3; pioglitazone control), with quercetin (group 4; quercetin control) and with hydroxy citric acid (group 5; HCA control) does not show any significant effect on the liver marker enzymes compared to control group (group 1). No metabolic alterations were observed in rats that were present in these three drug controls. All the three drugs did not produce any damage to the liver as evidenced by the levels of liver marker enzymes as shown in Table 1 and Figures 1–6.
However, the experimental NASH rats treated with pioglitazone (group 6; NASH + pioglitazone), with quercetin (group 7; NASH + quercetin) and with hydroxy citric acid (group 8; NASH + HCA) showed an obvious decrease in ALT, AST, GGT and LDH levels when compared with that of NASH induced group (group 2) as shown in Table 1 and Figures 1–6.
Pioglitazone has been established as a widely used drug in the treatment of insulin resistance diabetes. Insulin resistance was believed to be a central mechanism involved in the development of hepatic steatosis. Pioglitazone was proved to improve sensitivity of insulin significantly, transaminases and liver histology.[22,27] Rosiglitazone showed decrease in the activity of liver enzymes but was associated with many side effects.[28] In our present study the experimental NASH treated with pioglitazone and hydroxy citric acid showed significant improvement and the present investigations are also in agreement with the above reports.
In quercetin-treated rats, the serum levels of liver marker enzymes were decreased significantly when compared with NASH group (group 2) reverting back to normal levels. These results may support the hepatoprotective effect of quercetin towards NASH.[26] In support of our present findings, several studies proved the protective effect of quercetin on diabetes mellitus and improving insulin resistance.[29,30,31] The hepatoprotective effect of quercetin on liver injury is well evident, which significantly inhibits the elevation of these enzymes levels in experimental NASH treated with quercetin by keeping the structural integrity of the liver.[32]
There are several reports showing that the quantification of liver enzymes was useful for diagnosis of NASH. ALT, AST, GGT and LDH levels are fluctuated, in experimental NASH in the present study. The ratio between AST and ALT has also been found to have predictive value. Elevation of ALT activity in serum is the result of leakage from damaged cells and therefore reflects hepatocyte damage. ALT, AST, GGT and LDH levels are fluctuated in experimentally induced NASH group when compared to rats in control group. The ratio between ALT and AST has also been found to have predictive value. The protective effect of quercetin on experimental NASH showed more significant reduction of these liver enzymes in comparison with pioglitazone and hydroxy citric acid therapy.
The presence of hypoalbuminemia, prolonged prothrombin time and hyperbilirubinemia are common biochemical alterations suggest advanced NASH.[33] These patients share a common clinical feature, obesity and potentially other features of metabolic syndrome: Hyperglycemia, dyslipidemia and hypertension.[34] Several reports demonstrated the profibrinogenic nature of hyperglycemia and hyperinsulinemia.[35,36] The significant association between serum uric acid level and development of NASH suggest that high uric acid levels may play a causal role in the development of NASH.[37,38] No metabolic alterations were observed in rats that were present in these three drug controls. All the three drugs did not produce any significant alterations in the levels of albumin, total bilirubin, creatinine, urea, uric acid and glucose as evidenced by the Table 2 and Figures 7–12.
However, The experimental NASH rats treated with pioglitazone (group 6; NASH + pioglitazone), with quercetin (group 7; NASH + quercetin) and with hydroxy citric acid (group 8; NASH + HCA) showed significant reduction in serum albumin, total bilirubin, creatinine, urea, uric acid and glucose levels when compared with that of NASH induced group (group 2) as shown in Table 2 and Figures 7–12. Pioglitazone improves insulin sensitivity and also reported the declined levels of serum liver enzymes.[33] Treatment with quercetin also resulted in the reduction of the various biochemical parameters such as albumin, total bilirubin, creatinine, urea, uric acid and glucose and offers protection to the liver against NASH.[39]
Hydroxy citric acid delays intestinal glucose absorption in rats, desirably decreasing the sugar levels and insulin responses[40] and also HCA decreases the formation of glycation products[41] suggesting the hepato-protective action. Oral treatment of HCA showed significant reduction against liver toxicity. These findings could be considered as a functional improvement of hepatocyte-induced elevated levels of urea, uric acid and creatinine. The significant reduction in the elevated levels of biochemical markers is an indication of stabilization of plasma membrane as well as repair of damage tissues.[42] It can be concluded that the HCA possess good hepatoprotective activity against NASH by virtue of its antioxidant properties.
CONCLUSION
In the present study, by the observations on the levels of various biochemical parameters, it is evident that, the pioglitazone, quercetin and hydroxy citric acid may afford protection to the liver against NASH. Whereas from the results of hepatic marker enzymes, it is evident that optimal protection was observed after quercetin treatment against experimental NASH whereas pioglitazone and hydroxy citric acid also confers protection to some extent against NASH.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 3.Cusi K. Non-alcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141–9. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- 4.Kakadiya J, Shah N. Effect of some synthetic and herbel drugs on tumer necrosis factor alpha in renal reperfusion induced renal damage in type 2 diabetic rats. Int J Preclinical Pharm Res. 2011;2:30–7. [Google Scholar]
- 5.Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26:1398–402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- 6.Alexandra B, Bentz A. Review of Quercetin: Chemistry, Antioxidant Properties and Bioavailability. J Young Investig. 2009. [Retrieved 6 October 2012]. p. 19. Available from: http://www.jyi.org/research/re.php?id=3416 .
- 7.Shara M, Ohia SE, Schmidt RE, Yasmin T, Zardetto-Smith A, Kincaid A, et al. Physico-chemical properties of a novel (–)-hydroxycitric acid extract and its effect on body weight, selected organ weights, hepatic lipid peroxidation and DNA fragmentation, hematology and clinical chemistry and histopathological changes over a period of 90 days. Mol Cell Biochem. 2004;260:171–86. doi: 10.1023/b:mcbi.0000026069.53960.75. [DOI] [PubMed] [Google Scholar]
- 8.Bennet GJ, Lee HH. Xanthones from Guttiferae. Phytochemistry. 1989;28:967–98. [Google Scholar]
- 9.Minami H, Kinoshita M, Fukuyama Y, Kodama M, Yoshizawa T, Sugiura M, et al. Antioxidant xanthones from Garcinia subliptica. Phytochemistry. 1994;36:501–6. [Google Scholar]
- 10.Surapaneni KM, Saraswathi P, Jainu M. Non alcoholic steatohepatitis (NASH) experimental model induction in rats. Int J Pharm Bio Sci. 2012;3:1085–90. [Google Scholar]
- 11.Surapaneni KM, Sarswathi P, Jainu M. Role of pioglitazone, quercetin and hydroxy citric acid against non alcoholic steatohepatitis (NASH)-histological and scanning electron microscopy (SEM) studies in an experimental model of NASH. Asian J Pharm Clin Res. 2012;5:244–7. [Google Scholar]
- 12.King J. “Practical Clinical Enzymology”. London: D Von Nostrand Co Ltd; 1965. The hydrolases-acid and alkaline phosphatses; pp. 191–208. [Google Scholar]
- 13.Mohur AF, Cooke IJ. Simple method of measuring serum level of glutamate oxaloacetic acid and glutamate pyruvate transaminase in routine laboratories. J Clin Pathol. 1975;10:394–9. doi: 10.1136/jcp.10.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieland AA. Methods in Enzymology-I. New York and London: Academic Press; 1955. Lactic acid dehydrogenase of heart muscle; pp. 449–54. [Google Scholar]
- 15.Moss DW, Henderson AR. Clinical enzymology. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: WB Saunders Company; 1999. pp. 617–721. [Google Scholar]
- 16.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P. Management of NASH: Current and future perspectives on treatment. In: Farrell GC, George J, de laMHall P, McCullough AJ, editors. Fatty Liver Disease: NASH and Related Disorders. Malden, MA: Blackwell Publishing; 2005. pp. 194–207. [Google Scholar]
- 19.Sakugawa H, Nakasone H, Nakayoshi T, Kawakami Y, Yamashiro T, Maeshiro T, et al. Clinical characteristics of patients with cryptogenic liver cirrhosis in Okinawa, Japan. Hepatogastroenterology. 2003;50:2005–8. [PubMed] [Google Scholar]
- 20.Lee RG. Non-alcoholic steatohepatitis: A study on 49 patients. Hum Pathol. 1989;20:594–8. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 21.Adler M, Schffner F. Fatty liver hepatitis and cirrhosis in obese patients. Am J Med. 1979;67:811–6. doi: 10.1016/0002-9343(79)90740-x. [DOI] [PubMed] [Google Scholar]
- 22.Oh MK, Winn J, Poordad F. Review article: Diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:503–22. doi: 10.1111/j.1365-2036.2008.03752.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–25. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz LE, Cordero P, Torres L, Sauceda AY, Flores JP, Segura JJ. Adipokines in a group of Mexican patients with nonalcoholic steatohepatitis. Ann Hepatol. 2009;8:123–8. [PubMed] [Google Scholar]
- 25.Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramírez JC, Castro MG. γ-Glutamyl transferase: A marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24:805–10. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Yoon TJ, Kang KR, Lee KH, Kim WH, Suh YH, et al. Glycoprotein isolated from Acanthopanax senticosus protects against hepatotoxicity induced by acute and chronic alcohol treatment. Biol Pharm Bull. 2006;29:306–14. doi: 10.1248/bpb.29.306. [DOI] [PubMed] [Google Scholar]
- 27.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Sponseller CA, Hampton K, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-c ligand rosiglitazone. Hepatology. 2003;38:434–40. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 28.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: Results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–3. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 29.Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat páncreas. Pharmacol Res. 2005;51:117–23. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese zucker rats. Obesity. 2008;16:2081–7. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 31.Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res. 2009;53:859–68. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- 32.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6:135–41. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collantes R, Ong JP, Younoss ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004;71:657–64. doi: 10.3949/ccjm.71.8.657. [DOI] [PubMed] [Google Scholar]
- 34.Fierbinteanu-Braticevici C, Dina I, Petrisor A, Tribus L, Negreanu L, Carstoiu C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol. 2010;16:4784–91. doi: 10.3748/wjg.v16.i38.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: A potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–44. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 36.Munoz LE, Cordero P, Torres L, Sauceda AY, Flores JP, Segura JJ. Adipokines in group of mexican patients with non alcoholic steatohepatitis. Ann Hepatol. 2009;8:123–8. [PubMed] [Google Scholar]
- 37.Hayden MR, Tyagi SC. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome and type 2 diabetes mellitus: The urate redox shuttle. Nutr Metab (Lond) 2004;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361–2. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 39.Czerny B, Put A, Myśliwiec Z, Juzyszyn Z. The Influence of Quercetin on Some Biochemical Parameters in Rats Exposed to Environmental Contamination with Fluorine Compounds. Polish J Environ Stud. 2000;9:157–61. [Google Scholar]
- 40.Sullivan AC, Triscari J, Miller ON. The influence of (-)- hydroxycitrate on in ViVo rates of hepatic glycogenesis and cholesterogenesis. Fed Proc. 1974;33:656. [Google Scholar]
- 41.Bousova I, Bacikova E, Dobrijevic S, Drsata J. Glycation of aspartate aminotransferase by methylglyoxal, effect of hydroxycitric and uric acid. Mol Cell Biol. 2009;331:215–23. doi: 10.1007/s11010-009-0161-y. [DOI] [PubMed] [Google Scholar]
- 42.Chattopadhyay RR, Sarkar SK, Ganguly S, Banerjee RN, Basu TK, Mukherjee A. Hepatoprotective activity of Azadirachta indica leaves on paracetamol induced hepatic damage in rats. Indian J Exp Biol. 1992;30:738–40. [PubMed] [Google Scholar]


