Abstract
Background:
Fruit extracts of Xylopia aethiopica are used traditionally in the management of pain disorders including headache and neuralgia. An animal model of vincristine-induced sensory neuropathy was developed after repeated intraperitoneal injection in rats and used in the present work to study the effects of the ethanolic extract of X. aethiopica (XAE) and its diterpene xylopic acid (XA) in vincristine-induced neuropathic pain.
Materials and Methods:
Vincristine (0.1 mg kg-1 day-1) was administered during two cycles of five consecutive days to induce chemotherapy-induced neuropathic pain. Static tactile anti-allodynic, anti-hyperalgesic, and cold anti-allodynic effects of XAE (30-300 mg kg-1) and XA (10-100 mg kg-1) were assessed using Von Frey filaments of bending forces of 4, 8, and 15 g, the Randall-Selitto paw pressure test, and cold water (4.5°C), respectively.
Results:
Administration of vincristine caused the development of allodynia and hyperalgesia with no significant motor deficit, spontaneous pain, and foot deformity. XAE (30-300 mg kg-1) and XA (10-100 mg kg-1) exhibited anti-hyperalgesic, tactile, and cold anti-allodynic properties with XA exhibiting greater potency than XAE. Pregabalin (10-100 mg kg-1) used as control produced similar effect.
Conclusion:
These findings establish the anti-allodynic and anti-hyperalgesic effects of the ethanolic fruit XAE and its major diterpene XA in vincristine-induced neuropathtic pain.
Keywords: Pregabalin, randall-sellito, vincristine, Von Frey filaments
INTRODUCTION
Fruits of Xylopia aethiopica (Dunnal) A. Rich (Family: Annonaceae), popularly called African pepper in West Africa, is used for the treatment of rheumatism, headache, neuralgia, and colic pain.[1]
The fruit of X. aethiopica contains kaurenoic and xylopic acid (XA) which are kauranes, a class of diterpenes. Biological activities of kauranes include antimicrobial, cytotoxic, antiparasitic, insect antifeedant, anti-HIV, and anti-inflammatory activities.[2,3] The anti-inflammatory activity of the kauranes has been shown to involve the impairment of inflammation signaling through inhibition of NF-κB activity.[4,5] Kaurenoic acid (ent-kaur-16-en-19-oic acid), an ent-kaurene diterpene, has several biological activities including analgesia,[6] diuretic, vasorelaxant, anti-inflammatory, and antipyretic effects in rodents.[7,8] Xylopic acid [15β-acetoxy-(-)-kaur-16-en-19-oic acid; Figure 1] and its epimer, acetylgrandifloric acid [15α-acetoxy-(-)-kaur-16-en-19-oic acid), also exhibit antibacterial activity.[9] Some studies have also shown that XA and the ethanol fruit extract of X. aethiopica (XAE) have a low toxicity profile.[7,10] A recent study from our laboratory established the analgesic properties of XAE and its major diterpene, XA, as well as its possible mechanisms of action.[11,12]
Figure 1.
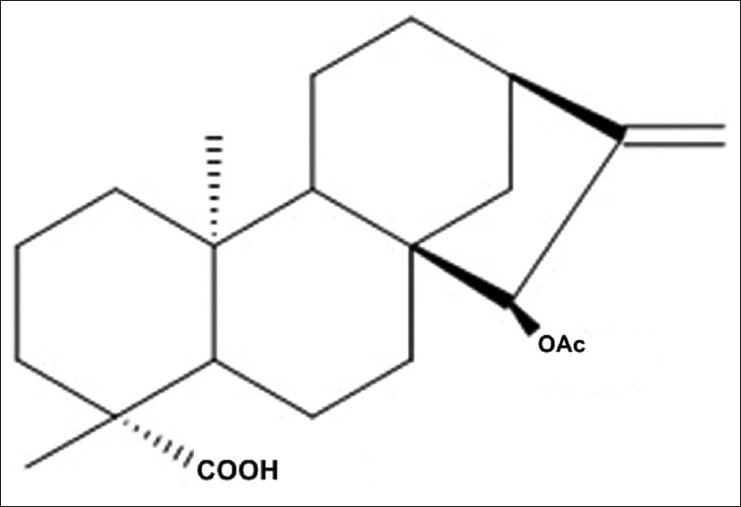
Chemical structure of 15 α-Acetoxy-(-)-kaur-16-en-19-oic acid (xylopic acid)
Neuropathic pain normally results from a number of metabolic, toxic, or traumatic insults to the central or peripheral nervous system and accounts for enormous morbidity and societal cost in both health care expenditure and lost work.[13] Neuropathic pain responds poorly to many classical analgesics and is costly to manage.[14,15,16] Moreover, with the available treatments, a small number of patients experience some pain relief with most patients being resistant to available analgesics and are persistently in pain.[16,17,18] Over the years, the survival rate of cancer patients have increased but this comes with the cost of patients developing peripheral neuropathy and subsequent neuropathic pain which is related to chemotherapeutic treatment.[19] Consequently, there is still a considerable need to explore novel treatment modalities for neuropathic pain management especially chemotherapy-induced neuropathic pain.
The analgesic properties of XAE and its major diterpene, XA as well as its possible mechanisms has been recently reported.[11,12] In the present study, an animal model of vincristine-induced neuropathic pain was used to evaluate the effect of XAE and XA in neuropathic pain.[20,21]
MATERIALS AND METHODS
Collection of plant material, preparation of XAE and isolation and purification of XA (15β-Acetoxy-(-) - kaur-16-en-19-oic Acid).
Dried fruits of X. aethiopica were collected from the Botanical Gardens (06°41’6.39”N; 01°33’45.35”W) of Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana, between the months of August and December 2008. The fruits were authenticated by Dr. Kofi Annan of the Department of Herbal Medicine, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health Sciences, KNUST. A voucher specimen (No. FP/09/77) has been kept at the herbarium of the Faculty.
Preparation of XAE and isolation and purification of XA (15β-Acetoxy-(-) - kaur-16-en-19-oic Acid) were as previously described.[11,12,22]
Animals
Sprague-Dawley rats (150-200 g) of both sexes were purchased from Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, Ghana, and housed in the animal facility of the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST). The animals were housed in groups of six in stainless steel cages (34×47×18 cm) with soft wood shavings as bedding, fed with normal commercial pellet diet (GAFCO, Tema, Ghana), given water ad libitum, and maintained under laboratory conditions (temperature 24-25°C, relative humidity 60-70%, and 12 h light-dark cycle). All procedures and techniques used in these studies were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85 -23, 1985, revised 1996). All protocols used were approved by the Departmental Ethics Committee.
Drugs and chemicals
Pregabalin (Lyrica®) was purchased from Pfizer Pharmaceuticals, New York, USA and vincristine sulfate from Health Biotech Limited, Chandigarh, India.
Vincristine-induced neuropathic pain
Vincristine sulfate was dissolved in saline and stored as a stock concentration of 1 mg l-1 at 4°C. The animals received intraperitoneal (i.p.) injection of vincristine at a final concentration of 0.1 mg kg-1 day-1 during two cycles of five consecutive working days (i.e. days 1-5 and days 8-12 with 2 days off). This dose was chosen because it produces hyperalgesia with no significant motor deficit.[21] On day 15, baseline response was measured in the Randall-Sellito test, Von Frey test (4 g, 8 g and 15 g) and cold allodynia (cold water at 4.5°C). The animals were later treated with XAE (30-300 mg kg-1 p.o.), XA (10-100 mg kg-1 p.o.), pregabalin (10-100 mg kg-1 p.o.), or saline. Three sets of experiments were then performed in order to evaluate the effects of XAE, XA, and pregabalin in vincristine-induced neuropathic pain.
Experimental design
Assessment of tactile allodynia
To evaluate the effect of XAE, XA, and pregabalin on static tactile allodynia, animals were placed in a restrainer. Tactile allodynia was assessed using von Frey filaments (IITC Life Science Inc. Model 2888, Woodland Hills, CA, USA) with bending forces of 4 g. Chemotherapy-induced responses to 4 g are best described as tactile allodynia (pain induced by a normally innocuous stimulus) because normal rats never withdraw from this stimulus.[23,24] In ascending order of force, each filament was applied to the mid-plantar area (avoiding the base of the tori) of each hind paw five times, with each application being held for 5 s. Withdrawal responses to the von Frey filaments from both hind paws were counted and then expressed as an overall percentage response, i.e. if a rat withdrew 6 times out of a total 10 von Frey applications, this was recorded as 60% overall response to that von Frey filament.[23]
Assessment of intermediate and mechanical hyperalgesia with Von Frey
Intermediate and mechanical hyperalgesia were assessed with Von Frey filaments of 8 and 15 g, respectively. Responses to 15 g are best described as hyperalgesia (heightened pain response from a normally painful stimulus) because normal rats withdraw from this stimulus 5-10% of the time. The responses to 8 g are intermediate.[23,24] The percentage overall responses to Frey filaments of 8 and 15 g were measured as described earlier.
Cold allodynia
Potential cold anti-allodynic effect of XAE, XA, and pregabalin was assessed by immersion of the rat's hind paw into a water bath containing cold water (4.5°C). The latency to paw withdrawal was measured using a digital timer as described.[19] Only one hind paw was assessed during each immersion at a time with a cut-off time of 20 s. For each animal, two recordings were made for each hind paw, and the withdrawal responses were reported as the mean of both hind paw values.
Mechanical hyperalgesia using Randall-Selitto
Mechanical hyperalgesia was measured using the Randall-Selitto paw pressure test (IITC Life Science Model 2888 Woodland Hills, CA, USA) as described.[25] Briefly, the rat's hind paw was placed into the pressure applicator, and a steadily increasing pressure stimulus (maximum cut-off of 250 g) was applied to the dorsal surface of the paw until withdrawal or vocalization. This was recorded as the nociceptive threshold value. For each animal, two recordings were made for each hind paw, and the data were reported as the mean of both hind paw values.
Statistical analysis
In all experiments, a sample size of seven-eight animals (n = 7-8) were used. All data are presented as mean ± S.E.M. The time-course curves were subjected to two-way (dose × time) repeated measures analysis of variance (ANOVA) with Holm-Sidak's post hoc test. Total nociceptive score for each treatment was calculated in arbitrary unit as the area under the curve (AUC). To determine the percentage inhibition for each treatment, the following equation was used.

Differences in AUCs were analyzed using one-way ANOVA with drug treatment as a between subject factor. Further comparisons between vehicle- and drug-treated groups were performed using the Holm-Sidak's post hoc test.
Doses for 50% of the maximal effect (ED50) for each drug were determined by using an iterative computer least squares method, with the following nonlinear regression (three parameter logistic) using the equation
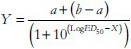
Where X is the logarithm of dose and Y is the response. Y starts at a (the bottom) and goes to b (the top) with a sigmoid shape.
The fitted midpoints (ED50 s) of the curves were compared statistically using F test.[26,27] GraphPad Prism for Windows version 6 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses and ED50 determinations. P < 0.05 was considered statistically significant.
RESULTS
Tactile allodynia
Intraperitoneal injection of vincristine for 2 weeks produced a marked, prolonged dynamic tactile allodynia in rats. One hour after the various drug treatments, tactile allodynia was measured using Von Frey filament of 4 g and the control animals showed increase response to tactile allodynia compared to the treated animals. XAE and XA increased the latency to paw response (F3,28 =6.03, P = 0.0060; F3,28 =4.19, P = 0.0228, respectively) with the highest doses used achieving a tactile anti-allodynia of 61.3 ± 11.4% and 65.8 ± 23.8%, respectively [Figure 2a and b]. Pregabalin significantly and dose-dependently inhibited tactile allodynia (F3,28 =7.14, P = 0.0029): With the highest dose of pregabalin (100 mg kg-1) producing an anti-allodynic effect of 62.4 ± 8.93% [Figure 2c]. XA was more potent and efficacious than pregabalin and XAE. Pregabalin was, however, more potent and efficacious than XAE [Table 1 and Figure 6].
Figure 2.
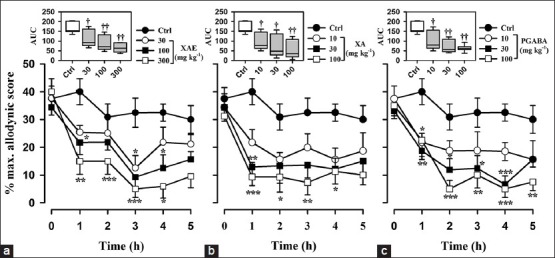
Effect of (a) XAE (30-300 mg kg-1 p.o.), (b) XA (10-100 mg kg-1 p.o.), and (c) pregabalin (10-100 mg kg-1 p.o.) on the time course of vincristine-induced neuropathic pain (tactile allodynia, von Frey 4 g) in rats. Each point represents mean ± S.E.M (n = 8). *P von Frey P von Frey 4P von Frey 4 g) in rats. Each point represent-way repeated measures ANOVA followed by Holm-Sidak's post hoc). The box-and-whisker plots (insets) depict AUCs derived from the respective time course curves. The plots show the 25th and 75th percentiles, the median (horizontal line within the box), and the 10th and 90th percentiles (whiskers). †P (whisker ††P† whiskers).-way ANOVA followed by Holm-Sidak post hoc)
Table 1.
ED50s and Emax values for X. aethiopica extract, xylopic acid, and pregabalin in the various models
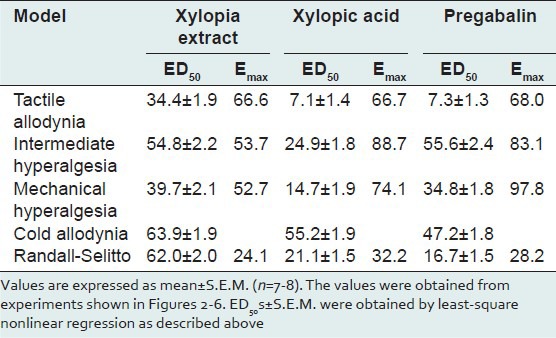
Figure 6.
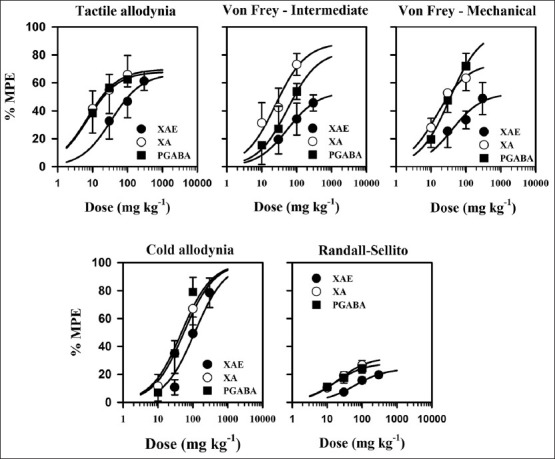
Dose-response curves of the effects of XAE (30-300 mg kg-1 p.o.), XA (10-100 mg kg-1 p.o.), and pregabalin (10-100 mg kg-1 p.o.) in the vincristine-induced neuropathic pain models in rats. Data was derived from the AUCs and curve obtained by non-linear regression as described under the Materials and Methods section
Intermediate hyperalgesia with Von Frey 8 g
Von Frey filaments of 8 g was used to assess the effect of XAE, XA, and pregabalin on mechanical hypernociception, intermediate to tactile allodynia, and mechanical hyperalgesia. One hour after the various drug treatments, pain was measured using Von Frey filament of 8 g. The control animals showed increased response to pain compared to the treated animals. XAE, XA, and pregabalin produced anti-hyperalgesia (F3,28 =3.20, P = 0.0515; F3,28 =4.05, P = 0.0272; F3,28 =2.59, P = 0.0889; figure 3 upper panel) in this test. The highest doses of XAE, XA, and pregabalin produced possible anti-hyperalgesic effect of 454.6 ± 11.5%, 66.46 ± 10.3%, and 53.8 ± 11.4% (Figure 3 upper panel), respectively. XA was more potent and efficacious than pregabalin and XAE. Pregabalin was also more potent and efficacious than XAE [Table 1 and Figure 6].
Figure 3.
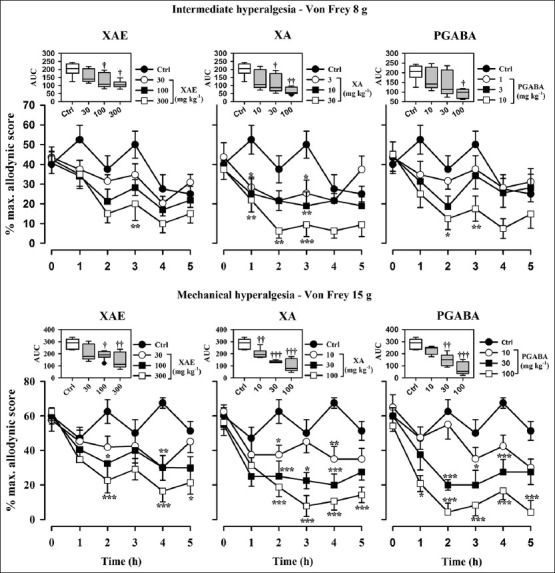
Effect of XAE (30-300 mg kg-1 p.o.), XA (10-100 mg kg-1 p.o.), and pregabalin (10-100 mg kg-1 p.o.) on the time course of vincristine-induced neuropathic pain (intermediate hyperalgesia, von Frey 8 g) in rats (upper panels). The lower panels show the effect of XAE (30-300 mg kg-1 p.o.), XA (10-100 mg kg-1 p.o.), and pregabalin (10-100 mg kg-1 p.o.) on the time course of vincristine-induced neuropathic pain (mechanical hyperalgesia, von Frey 15 g) in rats. Values represent mean ± S.E.M (n = 8). *P an ± S.E.MP an ± S.E.M P an ± S.E.M (n = 8). *ts. Values represent s -way repeated measures ANOVA followed by Holm-Sidak's post hoc). The box-and-whisker plots (insets) depict AUCs derived from the respective time course curves. The plots show the 25th and 75th percentiles, the median (horizontal line within the box), and the 10th and 90th percentiles (whiskers). Outlying points are shown individually as symbols. †P s symbo ††P †s symbo ††P †s symbolsin-way ANOVA followed by Holm-Sidak's post hoc test)
Mechanical hyperalgesia using Von Frey 15 g
Baseline mechanical hyperalgesia taken on day 15 using the Von Frey hairs of 15 g revealed that both hind paws exhibited marked static mechanical hyperalgesia. XAE (30-300 mg kg-1, p.o.) produced a significant (F3,28 =4.05, P = 0.0256) and dose-dependent inhibition of static mechanical hyperalgesia (Figure 3 lower panel). The highest dose of XAE increased the latency to mechanical hyperalgesia by 48.3 ± 32.5% (Figure 3 lower panel). Significant (F3,28 =16.56, P < 0.0001) and dose-dependent inhibition of static mechanical hyperalgesia was produced after XA (10-100 mg kg-1, p.o.) administration, with the highest dose increasing the latency to static mechanical hyperalgesia by 63.4 ± 25.5% (Figure 3 lower panel). The administration of pregabalin (10-100 mg kg-1) significantly (F3,28 =14.98, P < 0.0001) and dose-dependently inhibited mechanical hyperalgesia and a maximum anti-hyperalgesia of 72 ± 25.1% (Figure 3 lower panel) being achieved at the highest dose administered. Pregabalin was more efficacious than XA and XAE. XA was also more efficacious than XAE [Table 1 and Figure 6].
Mechanical hyperalgesia using the Randall-Selitto test
Baseline mechanical hyperalgesia taken on day 15 using the Randall-Sellito test revealed that both hind paws exhibited marked mechanical hyperalgesia. A change in hyperalgesic state was calculated as a percentage of the maximum possible effect. XAE (30-300 mg kg-1, p.o.) produced a significant (F3,28 =21.43, P < 0.0001) and dose-dependent inhibition of mechanical hyperalgesia [Figure 5a]. The maximum effect was produced by the highest dose [Figure 4a]. XA (10-100 mg kg-1, p.o.) also produced a significant (F3,28 =21.43, P < 0.0001) and dose-dependent inhibition of mechanical hyperalgesia [Figure 4b] at all the three doses used. The highest dose administered produces the maximum anti-hyperalgesic effect [Figure 4c]. The administration of pregabalin (10-100 mg kg-1) significantly (F3,28 =12.88, P = 0.0002) and dose-dependently attenuated mechanical hyperalgesia; the maximum mechanical anti-hyperalgesic effect was achieved by the highest dose [Figure 4c]. XA was more efficacious than pregabalin and XAE. Pregabalin was also more efficacious than XAE [Table 1 and Figure 6].
Figure 5.
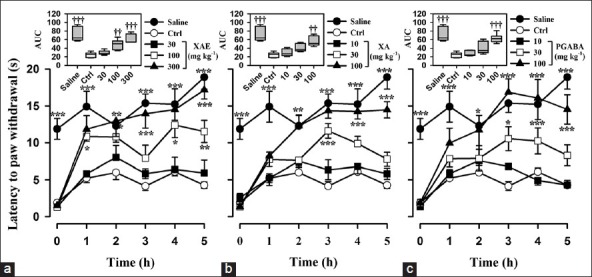
Effect of (a) XAE (30-300 mg kg-1 p.o.), (b) XA (10-100 mg kg-1 p.o.), and (c) pregabalin (10-100 mg kg-1 p.o.) on the time course curve of vincristine-induced cold allodynia. The box-and-whisker plots (insets) depict AUCs derived from the respective time course curves. The plots show the 25th and 75th percentiles, the median (horizontal line within the box), and the 10th and 90th percentiles (whiskers). Data are presented as mean ± S.E.M. (n = 8); *P < 0.05; ** P < 0.01; *P < 0.001 compared to vehicle-treated group (Two-way ANOVA followed by Holm-Sidak's post hoc test); †P < 0.05, ††P < 0.01, †††P < 0.0001 compared to vehicle-treated group (One-way ANOVA followed by Holm-Sidak's post hoc test)
Figure 4.
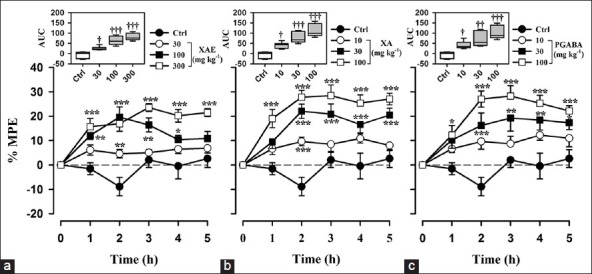
Effect of (a) XAE (30-300 mg kg-1 p.o.), (b) XA (10-100 mg kg-1 p.o.), and (c) pregabalin (10-100 mg kg-1 p.o.) on the time course curve of vincristine-induced mechanical hyperalgesia in rats in the Randall-Selitto model. The box-and-whisker plots (insets) depict AUCs derived from the respective time course curves. The plots show the 25th and 75th percentiles, the median (horizontal line within the box), and the 10th and 90th percentiles (whiskers). Data are presented as mean ± S.E.M. (n = 8); *P < 0.05; **P < 0.01; *P < 0.001 compared to vehicle-treated group (Two-way ANOVA followed by Holm-Sidak's post hoc test); †P < 0.05, ††P < 0.01, †††P < 0.0001 compared to vehicle-treated group (One-way ANOVA followed by Holm-Sidak's post hoc test)
Cold allodynia
Baseline cold allodynia was measured from both hind paws on day 15 using cold water at a temperature of 4.5°C. XAE (30-300 mg kg-1, p.o.) produced a significant (F3,28 =18.09, P < 0.0001) and dose-dependent inhibition of cold allodynia [Figure 5a] which was demonstrated as increased latency of paw withdrawal. The highest dose of XAE increased the latency of paw withdrawal to cold allodynia by 110.6 ± 5.13% [Figure 5a]. XA (10-100 mg kg-1, p.o.) also produced a significant (F3,28 =16.69, P < 0.0001) and dose-dependent inhibition of cold allodynia [Figure 5b]. The maximum time of paw withdraw after XA administration was increased by 91.8 ± 5.6% [Figure 5b] which occurred at the highest dose. The administration of pregabalin (10-100 mg kg-1) inhibited cold allodynia (F3,28 =18.04, P < 0.0001); the highest dose produced anti-allodynic effect of 111.4 ± 5.19% [Figure 5c]. XAE was more efficacious than pregabalin and XA. Pregabalin was also more efficacious than XA [Table 1 and Figure 6].
DISCUSSION
The results of the current study show clearly that XAE, XA, and pregabalin ameliorated vincristine-induced tactile and cold allodynia, as well as mechanical hyperalgesia.
Systemically administered vincristine is known to destroy Schwann cells and neurons of the dorsal root ganglion. This causes recruitment of macrophages which in turn cause the release of inflammatory cytokine IL-6, which elicits neuroinflammation and activates Janus kinase (Jak) - transcription-3 (STAT3) pathway (Jak-STAT3 pathway), leading to neuropathic pain. The p38 MAP kinase pathway also contributes to the development and maintenance of neuropathic pain in the CNS when it is activated by proinflammatory cytokines such as TNF-α.[28,29,30]
Castrillo et al. (2001) proposed that kaurene diterpenes, of which XA is an example, inhibit p38 and/or ERK1 and ERK2 pathways leading to inhibition of NF-κB activation.[4] This mechanism may contribute, at least in part, to the antinociception of XAE and XA in this experiment.
Changes in the PNS and CNS caused by systemic administration of vincristine leads to spontaneous activity of C- and Aβ-fibers resulting in spontaneous pain and abnormal sensations both peripherally and centrally.[31] It has also been demonstrated that small diameter C- and Aδ-fibers are mainly involved in the response to cold and intense mechanical stimuli whereas large Aβ-fibers (low threshold fibers) response to tactile stimuli.[32,33] With XAE and XA inhibiting vincristine-induced tactile and cold allodynia as well as mechanical hyperalgesia as demonstrated in this study, it is likely that XAE and XA may be inhibiting pain stimuli propagation in the degenerated unmyelinated and myelinated C-, Aδ-, and Aβ-fibers
Neuropathic pain states, especially the cancer pain type, are known to be opioid resistant due to downregulation of mu-opioid receptors in dorsal spinal cord. This has been identified to be mediated through the activation of NMDA receptors and protein kinase A.[34]
Pregabalin is effective both experimentally as shown in this study and clinically in the management of neuropathic pain. This is because it is an antagonist on α2 -δ1 Ca2+ channel subunit of N-type voltage-dependent calcium channels. Inhibition of calcium channels prevent neuronal excitability and other cellular enzymatic cascade reactions that lead to pain sensation.[35,36] Kaurenoic acid and XA have been reported to exert calcium channel-blocking effects.[7] It is therefore possible that XAE (containing kaurenoic acid and XA) and XA, among other mechanisms, may have blocked pain in this model by inhibiting calcium channels similar to pregabalin leading to inhibition of pain stimuli propagation. It has also been recently reported that the antinociceptive effect of XAE and XA involved inhibition of NMDA, adrenergic (β and α), and protein kinase A/C pathways.[11] This may therefore also account for the observed anti-hyperalgesia and anti-allodynia of XAE and XA in this neuropathic pain model since NMDA receptor antagonists or protein kinase C inhibitors have been shown to suppress the development of the hyperalgesia and allodynia in neuropathic pain states.[37,38]
CONCLUSION
The ethanolic fruit extract of X. aethiopica and its major diterpene xylopic acid have anti-allodynic and anti-hyperalgesic properties in vincristine-induced neuropathic pain.
ACKNOWLEDGEMENT
The authors are grateful for the technical assistance offered by Messrs Thomas Ansah, Gordon Darku and Prosper Akortia of the Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi
Footnotes
Source of Support: Departmental Resources
Conflict of Interest: None declared.
REFERENCES
- 1.Igwe SA, Afonne JC, Ghasi SI. Ocular dynamics of systemic aqueous extracts of Xylopia aethiopica (African guinea pepper) seeds on visually active volunteers. J Ethnopharmacol. 2003;86:139–42. doi: 10.1016/s0378-8741(02)00371-9. [DOI] [PubMed] [Google Scholar]
- 2.Ghisalberti EL. The biological activity of naturally occurring kaurane diterpenes. Fitoterapia. 1997;68:303. [Google Scholar]
- 3.Garcia PA, de Oliveira AB, Batista R. Occurrence, biological activities and synthesis of kaurane diterpenes and their glycosides. Molecules. 2007;12:455–83. doi: 10.3390/12030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castrillo A, de Las Heras B, Hortelano S, Rodriguez B, Villar A, Bosca L. Inhibition of the nuclear factor kappa B (NF-kappa B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-kappa B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J Biol Chem. 2001;276:15854–60. doi: 10.1074/jbc.M100010200. [DOI] [PubMed] [Google Scholar]
- 5.Ekong DE, Ogan AU. Chemistry of the constituents of Xylopia aethiopica. The structure of xylopic acid, a new diterpene acid. J Chem Soc C: Organic. 1968:311–2. [Google Scholar]
- 6.Block LC, Santos AR, de Souza MM, Scheidt C, Yunes RA, Santos MA, et al. Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J Ethnopharmacol. 1998;61:85–9. doi: 10.1016/s0378-8741(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 7.Somova LI, Shode FO, Moodley K, Govender Y. Cardiovascular and diuretic activity of kaurene derivatives of Xylopia aethiopica and Alepidea amatymbica. J Ethnopharmacol. 2001;77:165–74. doi: 10.1016/s0378-8741(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 8.Sosa-Sequera MC, Suarez O, Dalo NL. Kaurenic acid: An in vivo experimental study of its anti-inflammatory and antipyretic effects. Indian J Pharmacol. 2010;42:293–6. doi: 10.4103/0253-7613.70205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davino SC, Giesbrecht AM, Roque NF. Antimicrobial activity of kaurenoic acid derivatives substituted on carbon-15. Braz J Med Biol Res. 1989;22:1127–9. [PubMed] [Google Scholar]
- 10.Abaidoo CS, Woode E, Alhassan A. An evaluation of the effect of ethanolic fruit extracts of Xylopia aethiopica on haematological and biochemical parameters in male rats. Der Pharm Sin. 2011;2:39–45. [Google Scholar]
- 11.Woode E, Ameyaw EO, Ainooson GK, Abotsi WK, Boakye-Gyasi E, Kyekyeku JO. Analgesic Effects of an Ethanol Extract of the Fruits of Xylopia aethiopica and Xylopic Acid in Murine Models of Pain: Possible Mechanism(s) Pharmacologia. 2013;4:285–300. [Google Scholar]
- 12.Woode E, Ameyaw EO, Boakye-Gyasi E, Abotsi WK. Analgesic effects of an ethanol extract of the fruits of Xylopia aethiopica (Dunal) A. Rich (Annonaceae) and the major constituent, xylopic acid in murine models. J Pharm Bioallied Sci. 2012;4:291–301. doi: 10.4103/0975-7406.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes-Gibby CC, Morrow PK, Buzda RA, Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain. 2009;10:1146–50. doi: 10.1016/j.jpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin RH. An overview of neuropathic pain: Syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–9. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Harden N, Cohen M. Unmet needs in the management of neuropathic pain. J Pain Symptom Manage. 2003;25(Suppl 5):S12–7. doi: 10.1016/s0885-3924(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ, Mannion RJ. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 17.Desmeules J, Constantin C, Allaz AF, Piguet V, Steiner N, Dayer P. [Predictive factors of resistance to treatment of neuropathic pain] Schweiz Med Wochenschr. 1994;124:2057–9. [PubMed] [Google Scholar]
- 18.Laird MA, Gidal BE. Use of gabapentin in the treatment of neuropathic pain. Ann Pharmacother. 2000;34:802–7. doi: 10.1345/aph.19303. [DOI] [PubMed] [Google Scholar]
- 19.Lynch JJ, Wade CL, Mikusa JP, Decker MW, Honore P. ABT-594 (a nicotinic acetylcholine agonist): Anti-allodynia in a rat chemotherapy-induced pain model. Eur J Pharmacol. 2005;509:43–8. doi: 10.1016/j.ejphar.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Thibault K, Elisabeth B, Sophie D, Claude FZ, Bernard R, Bernard C. Antinociceptive and anti-allodynic effects of oral PL37, a complete inhibitor of enkephalin-catabolizing enzymes, in a rat model of peripheral neuropathic pain induced by vincristine. Eur J Pharmacol. 2008;600:71–7. doi: 10.1016/j.ejphar.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Aley KO, Reichling DB, Levine JD. Vincristine hyperalgesia in the rat: A model of painful vincristine neuropathy in humans. Neuroscience. 1996;73:259–65. doi: 10.1016/0306-4522(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 22.Adosraku RK, Oppong Kyekyeku J. Characterisation and HPLC quantification of xylopic acid in the dried fruits of xylopia aethiopica. Int J Pure Appl Chem. 2011;6:209–13. [Google Scholar]
- 23.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–61. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006;201:507–14. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stohr T, Krause E, Selve N. Lacosamide displays potent antinociceptive effects in animal models for inflammatory pain. Eur J Pain. 2006;10:241–9. doi: 10.1016/j.ejpain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Miller JR. San Diego, CA: GraphPad Software Inc; 2003. GraphPad Version 4.0. Step-by-Step Examples. [Google Scholar]
- 27.Motulsky HJ, Christopoulos A. A practical guide to curve fitting. San Diego, CA: GraphPad Software Inc; 2003. Fitting model to biological data using linear and nonlinear regression. [Google Scholar]
- 28.Chiang AS, Wenhua X, Gary JB. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: Loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006;201:507–14. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. A new animal model of vincristine-induced nociceptive peripheral neuropathy. Neurotoxicology. 2003;24:797–805. doi: 10.1016/S0161-813X(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 30.Kiguchi N, Maeda T, Kobayashi Y, Saika F, Kishioka S. Involvement of inflammatory mediators in neuropathic pain caused by vincristine. Int Rev Neurobiol. 2009;85:179–90. doi: 10.1016/S0074-7742(09)85014-9. [DOI] [PubMed] [Google Scholar]
- 31.Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: A review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–45. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–8. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- 33.Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, et al. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;22:7746–53. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizoguchi H, Watanabe C, Yonezawa A, Sakurada S. New therapy for neuropathic pain. Int Rev Neurobiol. 2009;85:249–60. doi: 10.1016/S0074-7742(09)85019-8. [DOI] [PubMed] [Google Scholar]
- 35.Schim JD. 22nd US Psychiatric and Mental Health Congress; 2009. Neuropathic Pain: Diagnosis and New Treatment Options. [Google Scholar]
- 36.Kumar N, Laferriere A, Yu JS, Poon T, Coderre TJ. Metabotropic glutamate receptors (mGluRs) regulate noxious stimulus-induced glutamate release in the spinal cord dorsal horn of rats with neuropathic and inflammatory pain. J Neurochem. 2010;114:281–90. doi: 10.1111/j.1471-4159.2010.06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: Implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–64. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 38.Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, et al. Direct evidence for the involvement of brain derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–94. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]


