Abstract
Background:
Oxidative stress not only develops complications in diabetic (type 1 and type 2) but also contributes to beta cell destruction in type 2 diabetes in insulin resistance hyperglycemia. Glucose control plays an important role in the pro-oxidant/antioxidant balance. Some antidiabetic agents may by themselves have antioxidant properties independently of their role on glucose control.
Objective:
The present investigation draws a comparison of the protective antioxidant activity, total phenol content and the antihyperglycemic activity of the methanolic extract of Cajanus cajan root (MCC) and Tamarindus indica seeds (MTI).
Materials and Methods:
Antidiabetic potentials of the plant extracts were evaluated in alloxan-induced diabetic Swiss albino mice. The plant extracts at the doses of 200 and 400 mg/kg body weight was orally administered for glucose tolerance test during 1-hour study and hypoglycemic effect during 5-day study period in comparison with reference drug Metformin HCl (50 mg/kg). In vitro antioxidant potential of MCC and MTI was investigated by using 1, 1- diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity at 517 nm. Total phenolic content, total antioxidant capacity and reducing power activity was also assayed.
Results:
There was a significant decrease in fasting serum glucose level (P < 0.001), reduction in blood glucose level (P < 0.001) in 5-days study, observed in the alloxan-induced diabetic mice. The reduction efficacy of blood glucose level of both the extracts is proportional to their dose but MCC is more potent than MTI. Antioxidant study and quantification of phenolic compound of both the extracts revealed that they have high antioxidant capacity.
Conclusion:
These studies showed that MCC and MTI have both hypoglycemic and antioxidant potential but MCC is more potent than MTI. The present study suggests that both MCC and MTI could be used in managing oxidative stress.
Keywords: Alloxan; antioxidant activity; 1, 1- diphenyl-2-picrylhydrazyl; hyperglycemia; metformin hydrochloride; oxidative stress
INTRODUCTION
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are products of normal cellular metabolism. At low-to-moderate concentrations, they possess various physiological roles ranging from cellular signal transduction to defense against pathogens.[1] During oxidative stress, there is an overproduction of ROS and RNS on one side and a deficiency of enzymatic and nonenzymatic antioxidant defense system on the other, resulting in degradation of cellular components, DNA, carbohydrates, proteins and lipids. Oxidative stress is currently suggested as mechanism underlying diabetes and diabetic complications,[2] a devastating illness with significant morbidity and mortality, which has increased steadily worldwide.[3] During diabetes or insulin resistance, glucose concentrations in blood remain high due to failure of insulin-stimulated glucose uptake by fat and muscle. Consequently, glucose uptake by insulin-independent tissues increases resulting in enhancement of oxidant production and impairs antioxidant defenses by multiple interacting nonenzymatic, enzymatic catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) and mitochondrial pathways.[4,5] The level of these antioxidant enzymes critically influences the susceptibility of various tissues like beta-islet (insulin-releasing cell), which is among those tissues that have the lowest levels of intrinsic antioxidant defenses and is associated with the development of complications in diabetes (kidney, eye, blood Vessel and nerve damage).[6,7] Diseases linked with hyperglycemia-induced oxidative stress medicated free radicals generation can be prevented by antidiabetic and antioxidant therapy.[8] Despite progress in the management of diabetes by synthetic drug (insulin) and oxidative stress using synthetic antioxidants like butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT), rutin, tertiary butylated hydroquinon and gallic acid (GA) esters are less effective and suspected to cause health hazard.[9] So, current researches are directed towards improved, safe and natural antidiabetic and antioxidative plant products as widespread traditional medical treatment.[10] Even for populations which use herbs traditionally, encouraging the use of species with chemopreventive actions as costs are significantly low, herbs have usually little or no toxicity during long-term oral administration and are relatively available at large scale.[11]
Being a tropical country, Bangladesh is abundant in medicinal plants used in traditional medicine. In this connection two Bangladeshi medicinal plant of known hypoglycemic effect Cajanus cajan and Tamarindus indica were subjected for study in order to assess their antidiabetic and oxidative stress inhibitory effect. C. cajan,local name Arhar (family- Fabaceae), is a perennial shrub native to Asia, most common pulse eaten all over Asian subcontinent. The extracts or components of C. cajan are commonly used all over the world for the treatment of diabetes, dysentery, hepatitis and measles, as a febrifuge to stabilize the menstrual period.[12,13,14] As a traditional Chinese medicine, the leaves of C. cajan have been widely used to arrest blood, relieve pain and kill worms.[15] Nowadays, the leaves are used for the treatment of wounds, aphtha, bedsores and malaria, as well as diet-induced hypercholesterolemia,among others.[16,17,18] Protective effects of extracts from C. cajan leaf against hypoxic-ischemic brain damage and alcohol-induced liver damage have also been reported.[19] Chemical constituent investigations have indicated that C. cajan leaves are rich in flavonoids and stilbenes, which are considered responsible for the beneficial efficacies of C. cajan leaves on human health.[20,21,22]
T. indica, local name tetul, is a perennial herb belonging to the dicotyledonous family of Fabaceae. It grows naturally in tropical and subtropical regions and now is one of the most important plant resources as food materials and is accepted as herbal medicine in parts of the world.[23] It was used as a traditional medicine for the management of diabetes mellitus inhuman and experimental animals.[24,25] The potential antioxidant activity of Tamarind seeds has already been reported.[26,27] T. indica seed coat may play an important role in chemical protection from oxidative damage by possessing endogenous antioxidants such as phenolic compounds. The potential antioxidant activity of Tamarind seeds have already been reported and the isolated antioxidant components are, 2-hydroxy-30, 40-dihydroxyacetophenone, methyl 3, 4- ihydroxybenzoate, 3, 4-dihydroxyphenylacetate and epicatechin in addition to oligomeric proanthocyanidins, phenolic compounds are procyanidin B2, epicatechin, procyanidin trimer, procyanidin tetramer, procyanidin pentamer, procyanidin hexamer, polymeric tannins, polymeric tannins.[28] It has been shown that aqueous extract of T. indica seeds have potent antidiabetic and antihyperlipidemic activities in STZ-induced diabetic male rat.[29] Here we studied the methanolic extract of C. cajan root (MCC) and methanolic extract of T. indica seeds (MTI) to compare their hypoglycemic activity. As antidiabetic activity is closely related with antioxidant activity, in this communication, we have also assessed the antioxidant activity and total phenol content of these two plants.
MATERIALS AND METHODS
Collection of plant parts
Roots of the plant C. cajan and seeds of T. indica, were collected during the month of May 2011 from the area of Konda Thana, Dhaka, Bangladesh. The plants were taxonomically identified and voucher specimen No- DACB 36424 and DACB 36425, have been maintained for C. cajan and T. indica, respectively, in the Bangladesh National Herbarium, Dhaka, Bangladesh.
Preparation of methanolic extract of C. cajan root
About 387 g of dried powdered material was refluxed with 500 ml of 95% methanol for 3 h. The extracts were then filtered through filter paper (Double Rings filter paper 102, 11.0 cm). The filtrates (methanol extract) were concentrated at 50°C under reduce pressure using rotary evaporator (STUART RF3022C, UK) to afford an oily concentrate of greenish black color of extract. Finally, we got 16 g methanolic extract. The extract transferred to a closed container for further use and protection.
Preparation of Methanolic extract of T. indica Seeds
The seeds of T. indica were dried in an oven for 2 days at 40°C, crushed in an electrical grinder and then powdered. Extraction was performed by taking 25-g powder in 250 ml of distilled water for 18 h in a soxhlet apparatus and a deep brown aqueous extract was obtained. The extract was dried at reduced pressure and transferred to a closed container for further use and protection.
Instruments
The molecular absorption spectra and absorbance at specific wavelengths were recorded with a HACH DR 4000U UV-visible spectrophotometer equipped with quartz cells of 1-cm light path.
Chemicals and reagents
All chemicals and drugs were obtained commercially and were of analytical grade. DPPH (Sigma Chemical Co., USA.), Alloxan (Fluka, Germany), ammonium molybdate (Merck, Germany), sodium phosphate (BDH, England), potassium ferricyanide K3 [Fe(CN)6 ], trichloroacetic acid (CCl3 COOH), Folin–Ciocalteu reagent, GA [C6 H2 (OH)3 COOH], ascorbic acid (AA) and metformin hydrochloride (General Pharmaceutical Bangladesh Ltd) and glucose estimation kit (Human, Germany).
In vivo study for assessment of antihyperglycemic activity
Experimental animal
Experiment was conducted on adult albino mice of mix sex with the weights of 50-55 g procured from International Centre for Diarrhoeal Disease Research Bangladesh (ICDDRB). All mice were fed normal laboratory chow food containing 16% protein, 66% carbohydrate, 8% fats and water. All mice were housed at a (12:12) h light and dark cycle at 25°C and relative humidity (60-70%).
Ethical Approval: Animal experiment was done by following guidelines followed by ICDDRB which were approved by the institutional animal ethical committee.[30]
Acute toxicity test
Acute toxicity study was performed for the extract according to the acute toxic classic method as per the method of Lorke.[31] The animals were divided into six groups containing 10 animals each. The MCC and MTI suspension were administrated orally in increasing dose up to 1500 mg/kg, b.w as we were investigating in low doses. These animals were observed for mortality and toxicity for 14 days.
Preparation of the drug solution
MCC and MTI were dissolved separately in normal saline to prepare dose level of 200 and 400 mg/kg/day, body weight for administration into mice.
Oral glucose tolerance test
A glucose tolerance test is the administration of glucose to determine how quickly it is cleared from the blood. This study includes measurement of the blood sugar levels of fasted mice and after 30 min, 1 and 2 h after taking glucose drink in same mice. For this, the normal nondiabetic mice, who had fasted overnight, were randomly divided into six experimental groups. Each group contained six mice.
Group 1: Treated with distilled water (Negative control normal group).
Group 2: Treated with Metformin HCl 50 mg/kg body weight (positive control normal group).
Groups 3 and 4: Treated with 200 mg/kg body weight of MCC and MTI separately (test groups).
Groups 5 and 6: Treated with 400 mg/kg body weight of MCC and MTI separately (test groups).
The non-diabetic mice in all groups were orally feed glucose (3 g/kg body weight) after 30 min of the above pretreatment.
Experimental design
Animals were divided into seven groups and for each group four animals were taken. The investigation was carried out for 4 weeks. The first 2 weeks were for the induction of diabetic condition in mice and the following 2 weeks were experimental period with crude MCC and MTI on fasted mice.
Group-1: Normal saline-treated mice.
Group-2: Normal saline-treated alloxan-induced diabetic mice.
Group-3 and 4: Each MCC and MTI of 200 mg/kg body weight treated alloxan-induced diabetic mice, respectively.
Group-5 and 6: Each MCC and MTI of 400 mg/kg body weight treated alloxan-induced diabetic mice, respectively.
Group-7: Metformin hydrochloride 50 mg/kg body weight treated alloxan-induced diabetic mice.
Induction of experimental diabetes
Diabetes was induced in Swiss albino mice of mix sex by intravenous injection of aqueous alloxan monohydrate (55 mg/kg, i.v).[32] Two week after alloxan injection, mice with plasma glucose level (blood collected from tail vein) of greater than 10 mmol/L were included in the study.
Statistical analysis
The data obtained in the animal experiments was subjected to statistical analysis. All values are expressed as Mean ± S.E.M (Standard Error of Mean). The data were assessed by the analysis of variance (ANOVA) and the group means were evaluated by the post-hoc Dunnet test using SPSS program (SPSS 15.0, USA). Mean values were considered significantly different if P < 0.05, 0.001.
In vitro study for antioxidative activity evaluation DPPH radical scavenging effect
DPPH radical scavenging effects of MCC and MTI were performed according to the method of Braca.[33] The 0.1 mmol/L solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 mL of extract's solution at different concentrations (5, 10, 25 and 50 μg/mL). After 30 min, absorbance was measured at 517 nm. Vitamin C (AA) was used as a reference drug. The percentage inhibition was evaluated by comparing the absorbance values for the control and experimental samples[34] following the equation:
Percentage of inhibition = [(Ao - A1)/Ao] × 100
Where Ao is the Absorbance of the control, A1 is the Absorbance of the plant extract/standard.
IC50 value was calculated from the equation of line obtained by plotting a graph of concentration (μg/mL) versus % inhibition.
Total antioxidant activity capacity test
The antioxidant activity of MCC and MTI were evaluated by the phosphomolybdenum method according to the procedure describe by Prieto.[35] The assay is based on the reduction of Mo (VI)–Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acid pH. The antioxidant activity is expressed as the number of equivalents of AA using the following equation:
C = (c x V)/m
Where, C= Total antioxidant activity of extract, in AA (mg/mL), c =The concentration of AA established from the calibration curve (mg/ml), V= The volume of extract (mL), m = the weight of pure plant methanolic extract (g).
Determination of total phenolic content
The concentration of total phenolic was based on the method described by Velioglu[36] with some modification. Phenols of extracts react with phosphomolybdic acid in Folin-Ciocalteu reagent in alkaline media and produce a blue-colored complex (molybdenum blue). That can be anticipated colorimetrically at 760 nm after 2 h against a blank. GA was used to construct a standard curve (0-50 mg/L). The amount of total phenols were expressed as milligram gallic acid equivalents (GAE)/dried weight, calculated from the calibration curve.
Ferric reducing/antioxidant power assay
The reducing power of MCC and MTI was determined according to the method described by Oyaizu.[37] For the measurement of the reductive ability, transformation of Ferric ion (Fe3+) to Ferrus ion (Fe2+) was investigated in the presence of extracts. Increased absorbance of the reaction mixture indicated increased reducing power. AA was used as the standard. Phosphate buffer (pH 6.6) was used as blank solution. The absorbance of the final reaction mixture of two parallel experiments was taken and is expressed as mean ± standard deviation.
RESULTS
Acute toxicity
During the acute toxicity study of the extracts, either mortality or any considerable symptoms of toxicity was not found after the oral administration of MCC and MTI upto a dose of 1 g/kg body weight in mice. Even no significant changes in general behavior was observed in mice up to 14-days study.
Antidiabetic investigations
Oral glucose tolerance test
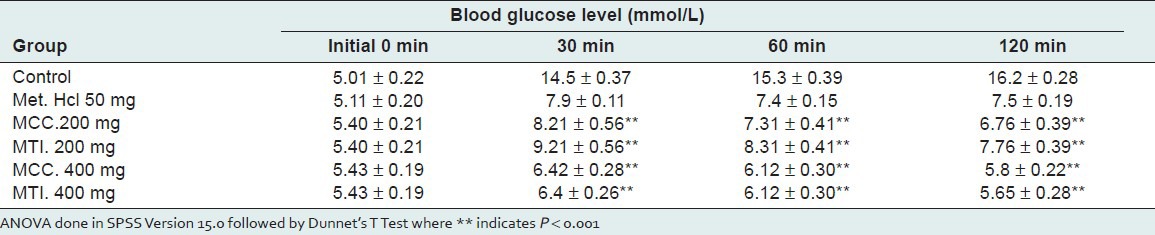
The result of glucose tolerance test are summarized in Table 1. The results of OGTT showed that after a single dose load of 200 mg/kg and 400 mg/kg of both the extracts in mice, there was a significant reduction (P < 0.001) of fasting blood glucose level during the 2-h study period. Within 30 min of administration of glucose load, there was a progressive increase in the postprandial blood glucose level (BGL) of all the rats which peaked at 30 min. At 30 min, the MCC treated groups (200 and 400 mg/kg) had 52.04% and 18.23% increase in BGL compared to control (189.42%). Hence treatment with MCC suppressed the rise in BGL at 180 min by 17.66% (200 mg/kg) and 59.67% (400 mg/kg). The MCC evoked a progressive, significant and nondose-related decrease in BGL up to 180 min, at which the BGL were close to basal levels [Table 1]. At 30 min, the MTI-treated groups (200 and 400 mg/kg) had 70.55% and 17.86% increase in BGL compared to control (189.42%). Hence treatment with MTI suppressed the rise in BGL at 180 min by 15.74% (200 mg/kg) and 11.72% (400 mg/kg). Thus MTI also evoked a progressive, significant and nondose-related decrease in BGL up to 180 min, at which the BGL were close to basal levels.
Table 1.
Glucose tolerance test after administration of 200 and 400 mg/kg body weight of methanolic extract of Cajanus cajan root (MCC) and methanolic extract of Tamarindus indica seeds (MTI) in mice
Result of long-term antidiabetic study
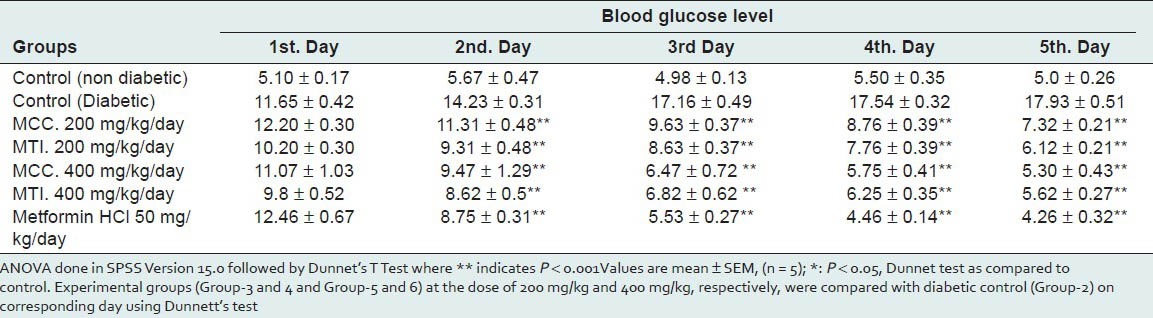
The results after chronic administration of MCC and MTI summarized in Table 2, showed significant difference (P < 0.001) which was observed between experimental and diabetic control mice in lowering fasting blood glucose level. At a dose of 200 mg/kg body weight, MCC significantly lowered blood glucose level and showed reduction of 37.17% and MTI lowered 47.47% blood glucose level of on day 5. The extracts at 400 mg/kg body weight dose, produced maximum reduction of 54.51% and 51.76% of blood glucose level for MCC and MTI, respectively, on day 5 whereas inhibition of 63.43% was found for metformin HCl on day 5 as a peak. So both the extracts have potent hypoglycemic effect.
Table 2.
The effect of 5-day treatment of MCC and MTI on blood sugar of alloxan-induced diabetic mice
In vitro antioxidative potentiality
DPPH radical scavenging effect
DPPH radical scavenging activity is based on the reduction of alcoholic DPPH solution in the presence of hydrogen donating antioxidant compound due to the formation of a nonradical form (DPPH-H). In this study, DPPH was effectively scavenged by MCC and MTI and the percentage (%) of scavenging were found to be concentration dependant, i.e., scavenging capacity increases with the increase of concentration of both the extracts. The IC50 value was found to be 17.44 μg/mL and 30 μg/mL for MCC and MTI, respectively. The standard antioxidant, AA exhibited 50% inhibition at a concentration of 12 μg/mL.
Total antioxidant capacity
Total antioxidant capacities of the extracts evaluated by Prieto procedure and are expressed as the number of equivalents of AA. It was found to be 826.87 ± 10.27 mg/g eqivalent of AA for MCC and 298.38 ± 9.86 mg/g eqivalent of AA for MTI.
Total phenolic content
The total phenolic content in the extracts of MCC and MTI were determined according to the colorimetric Folin–Ciocalteu method with GA as a standard compound (data not shown). A wide range of total phenolic content was found in the plant materials to be 297.42 ± 8.4 mg/g plant extract (in GAE) for MCC and 248.97 ± 7.51 mg/g plant extract (in GAE) for MTI.
Ferric reducing/Antioxidant power (FRAP) Assay
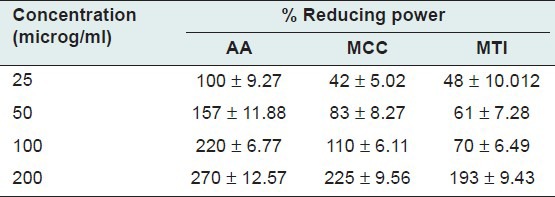
The FRAP assay measures the antioxidant effect of any substance in the reaction medium in term of its reducing ability and it reflects total antioxidant power involving the single electron transfer reaction. Table 3 shows the reductive capabilities of the plant extracts compared to AA at different concentration. The reducing power of the extracts, MCC and MTI founded remarkable and the reducing power of the extract observed to rise as the concentration of the extract gradually increased.
Table 3.
Reducing power of MCC, MTI and standard AA
DISCUSSION
The results of entire experiment indicated that both MCC and MTI established their potency against diabetes and oxidative stress by lowering postprandial hyperglycemia, serum blood glucose level in diabetic mice and greatest antioxidant capacity with huge amount of phenolic compounds. In comparative evaluation of C. cajan root (MCC) and T. indica seeds (MTI), first one was found to be more efficacious to later one.
In vivo antidiabetic effect
Insulin-dependent diabetes mellitus study model has been developed here to explore the antihyperglycemic activity of MCC and MTI. Diabetes was induced using alloxan, a beta-cytotoxin, which causes selective destruction of insulin-secreting pancreatic β-cells through reactive oxygen species-dependent oxidative damage[38] supported here by diminished serum insulin level.[39] The insulin-dependent alteration in the carbohydrate metabolism results elevation in levels of fasting blood glucose,[40] and leading to various metabolic aberrations the animals namely decreased protein content.[41] In this study the experimental groups were treated with standard (Metformin HCl,50 mg/kg/day) and test samples MCC and MTI to evaluate and compare hypoglycemic activity.
The glucose tolerance test was done to assess the ability of MCC and MTI to reduce and control postprandial hyperglycemia. Postprandial elevation of blood glucose constantly fuels chronic hyperglycemia, which is a risk factor for the development of life threatening and other complications of DM.[42] The results of glucose tolerance test indicated that both the extracts suppressed the rise in blood glucose level after a heavy glucose meal with maximum suppression at 60 min, the time of peak postprandial hyperglycemia, so were able to reduce and control postprandial hyperglycemia. The underlying mechanism may be due to enhancement of gluconeogenesis, which is characteristically activated at fasting state in diabetic animals or enhanced disposal of glucose by increased insulin sensitivity.[43] Similarly antihyperglycemic effect just after a glucose load may be due to delayed absorption of glucose by inhibitory activities on α-glucosidase and α-amylase in the gut responsible for digestion of carbohydrates. Inhibitory activities on α-glucosidase and α-amylase of C. cajan linn root constituents genistein[44] and T. indica seeds were evaluated.[45]
Chronic administration of MCC and MTI in diabetic mice resulted into efficient lowering of fasting blood glucose level suggesting that both the extracts might possess insulin-like effect on peripheral tissues either by promoting glucose uptake and metabolism or inhibiting hepatic gluconeogenesis since alloxan treatment causes permanent destruction of β-cells. So in addition to hypoglycemic effect, the extracts also suppress postprandial rise in blood glucose level both of which are indices of effective glycemic control.
In respect to maximum nonfatal doses studied revealed the nontoxic nature of MCC and MTI. There was no lethality or any toxic reactions found at any of these doses selected until the end of the study period. According to toxicity classification[41] both the extracts are nontoxic.
In vitro antioxidant activity
In vitro findings indicated that both MCC and MTI attributed their antioxidative abilities in terms of total phenol content, total antioxidant capacity, DPPH radical scavenging activity and reducing power.
Total phenol content
Phenolic compounds are commonly found in plants and have been reported to have several biological activities including antioxidant properties.[46] Founded high yield of total free phenolics in both the extracts, might be due to the reflux of MCC and MTI using methanol. Because recovery of phenolic compounds from food samples is mainly depend upon the type of solvent used and the duration of extraction. In addition, the quantity of phenolic compounds in roots and seed samples is influenced by soil, environmental conditions, genotype (cultivar/variety), agronomic practices (irrigation, fertilization and pest management), maturity level at harvest and postharvest storage conditions. For instance, low temperature during the onset and duration of seed fill were shown to increase the isoflavone content by several folds in soybean.[47] Since, both the plant grows wildly in adverse environmental conditions such as drought, poor soil. A high phenolic content in the seed materials may contribute to the resistant function.
Total antioxidant activity
The observed total antioxidant activity of MCC and MTI might be the contribution of investigated high phenolic compounds in both the extracts, which may be responsible for its anti-inflammatory and chemoprotective mechanism as well as justify the basis of using this plant's extract as folkloric remedies.
DPPH radical scavenging activity
Potential DPPH radical scavenging activity revealed by MCC and MTI might confirm its hydrogen donating capacity and also its proposed ability to protect the consumers’ health from various free radical-related diseases.
Reducing power assay
Dose-dependent high yield of reducing ability of both the extracts exert antioxidant action by breaking the free radical chain by donating hydrogen atom.[48] So these antioxidant potentiality of MCC- and MTI are an important approach for the management of oxidative stress ailment.
In the entire experiment, both MCC and MTI established their potency against diabetes and oxidative stress by lowering postprandial hyperglycemia, serum blood glucose level in diabetic mice and greatest antioxidant capacity with huge amount of phenolic compounds. These is may be due to its phytoingredients viz., GA compounds present in it as they have major antioxidative activity with redox properties, adsorption and neutralization capacity to free radicals, potency to extinguish singlet and triplet oxygen and scavenging of peroxides.[49] From previous study higher positive antioxidative efficacy of this phytochemical has been established.[50]
CONCLUSIONS
Methanolic extract of MCC and MTI were found to contain appreciable levels total free phenolics with promising antioxidant and antidiabetic properties. This scientific information can serve as an important platform for the development of further safe and effective natural medicine. Elucidation of the molecular mechanisms involved and isolation of the bioactive molecules implicated may help the development of plant-derived potent drugs which can replace the clinically toxic. Therefore, further investigations need to be undertaken for full identification and characterization of the molecules responsible for antioxidant and antidiabetic activities of MCC and MTI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Valko M, Leibfritz D, Moncol J, Cronin MT. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, John MC Gutteridge. 4th ed. London, UK: Oxford University press; 1989. Free Radicals in Biology and Medicine. [Google Scholar]
- 3.Luo JZ, Luo L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured b Cells. Evid Based Complement Alternat Med. 2006;3:365–372. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complication. Histochem Cell Biol. 2004;122:333–8. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 5.Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: A mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int J Biochem Cell Biol. 2006;38:794–803. doi: 10.1016/j.biocel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Grodsky GM, Anderson CE, Coleman DL, Craighead JE, Gerritsen GC, Hansen CT, et al. Metabolism and underlying causes of diabetes mellitus. Diabetes. 1982;31(Suppl 1 Pt 2):45–53. doi: 10.2337/diab.31.1.s45. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–4. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 8.Sannigrahi S, Mazumder UK, Pal DK, Parida S. In-Vitro anti oxidant activity of methanol extract of Clerodendrum infortunatum Linn. Oriental Pharm Expt Med. 2009;9:128–34. [Google Scholar]
- 9.El-Hela A, Abdullah A. Antioxidant and antimicrobial activities of methanol extracts of some verbena species: In vitro evaluation of antioxidant and antimicrobial activity in relation to polyphenolic content. J Appl Sci Res. 2010;6:683–9. [Google Scholar]
- 10.Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Saavedra G, Murcia MA, et al. Investigation of bovilian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci. 2003;73:1667–81. doi: 10.1016/s0024-3205(03)00488-0. [DOI] [PubMed] [Google Scholar]
- 11.Ramos A, Visozo A, Piloto J, Garcia A, Rodriguez CA, Rivero R. Screening of antimutagenicity via antioxidant activity in Cuban medicinal plants. J. Ethnopharmacol. 2003;87:241–6. doi: 10.1016/s0378-8741(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 12.Amalraj T, Ignacimuthu S. Evaluation of the hypoglycaemic effect of Cajanus cajan (seeds) in mice. Indian J Exp Biol. 1998;36:1032–3. [PubMed] [Google Scholar]
- 13.Abbiw DK. Royal Botanic Gardens, Kew. London, UK: Richmond Intermediate Technology Publications; 1990. Useful Plants of Ghana. [Google Scholar]
- 14.Tang Y, Wang B, Zhou XJ. Effect of external application of herbal cajani preparation on the fibronection content during healing process of open wound. J Guangzhou U Tradit Chin Med. 1999;16:302–4. [Google Scholar]
- 15.Aiyeloja AA, Bello OA. Ethnobotanical potentials of common herbs in Nigeria: A case study of Enugu state. Educ Res Rev. 2006;1:16–22. [Google Scholar]
- 16.Chen DH, Li HY, Lin H. Studies on chemical constituents in pigeonpea leaves. Chin Tradit Herb Drugs. 1985;16:134–6. [Google Scholar]
- 17.Luo QF, Sun L, Si JY, Chen DH. Hypocholesterolemic effect of stilbenes containing extract-fraction from Cajanus cajan L. on diet-induced hypercholesterolemia in mice. Phytomedicine. 2008;15:932–9. doi: 10.1016/j.phymed.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Huang GY, Liao XZ, Liao HF, Deng SJ, Tan YH, Zhou JY. Studies on water-soluble extracts from Cajanus cajan leaf against hypoxic-ischemic brain damage. Tradit Chin Drug Res Clin Pharmacol. 2006;17:172–4. [Google Scholar]
- 19.Kundu R, Dasgupta S, Biswas A, Bhattacharya A, Pal BC, Bandyopadhyay D, et al. Cajanus cajan Linn. (Leguminosae) prevents alcohol-induced rat liver damage and augments cytoprotective function. J. Ethnopharmacol. 2008;118:440–7. doi: 10.1016/j.jep.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Duker-Eshun G, Jaroszewski JW, Asomaning WA, Oppong-Boachie F, Christensen SB. Antiplasmodial constituents of Cajanus cajan. Phytother Res. 2004;18:128–30. doi: 10.1002/ptr.1375. [DOI] [PubMed] [Google Scholar]
- 21.Zu YG, Fu YJ, Liu W, Hou CL, Kong Y. Simultaneous determination of four flavonoids in pigeonpea [Cajanus cajan (L.) Millsp. ] leaves using RP-LC-DAD. Chromatographia. 2006;63:499–505. [Google Scholar]
- 22.Zheng YY, Yang J, Chen DH, Sun L. Effects of the stilbene extracts from Cajanus cajan L. on ovariectomy-induced bone loss in rats. Yao Xue Xue Bao. 2007;42:562–5. [PubMed] [Google Scholar]
- 23.Siddhuraju P. Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT Food Science and Technology. 2007;40:982–90. [Google Scholar]
- 24.Maiti R, Das UK, Ghosh D. Attenuation of Hyperglycemia and Hyperlipidemia in Streptozotocin-Induced Diabetic Rats by Aqueous Extract of Seed of Tamarindus indica. Biol Pharm Bull. 2005;28:1172–6. doi: 10.1248/bpb.28.1172. [DOI] [PubMed] [Google Scholar]
- 25.Martinello F, Soaresh SM, Franco JJ, Santos AC, Sugohara A, Garcia SB, et al. Hypolipemic and antioxidant activities from Tamarindus indica pulp fruit extract in hypercholesterolemic hamsters. Food Chem Toxicol. 2006;44:810–8. doi: 10.1016/j.fct.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Dighe NS, Pattan SR, Nirmal SA, Kalkotwar RS, Gaware VM, Hole MB. Analgesic activity of Tamarindus indica. Res J Pharmacogn Phytochem. 2009;1:69–71. [Google Scholar]
- 27.Luengthanaphol S, Mongkholkhajornsilp D, Douglas S, Douglas PL, Pengsopa L, Pongamphai S. Extraction of antioxidants from sweet Thai tamarind seed coat: Preliminary experiments. J Food Eng. 2004;63:247–52. [Google Scholar]
- 28.Sudjaroen Y, Haubner R, Wu¨rtele G, Hull WE, Erben G, Spiegelhalder B, et al. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem Toxicol. 2005;43:1673–82. doi: 10.1016/j.fct.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Maiti R, Jana D, Das UK, Ghosh D. Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabeticrats. J Ethnopharmacol. 2004;92:85–91. doi: 10.1016/j.jep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 31.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;53:275–89. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 32.Badole S, Patel N, Bodhankari S, Jain B, Bhardwaj S. Antihyperglycemic activity of aqueous extract of leaves of Cocculus hirsutus (L.) Diels in alloxan-induced diabetic mice. Indian J Pharmacol. 2006;38:49–53. [Google Scholar]
- 33.Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Natural anti-oxidants from plant material in phenolic compounds in food and their effects on health. J Nat Prod. 2001;64:892–5. [Google Scholar]
- 34.Suksomtrip M, Ukrisdawithid S, Bhusawang P, Pongsamart S. Phenolic compound content, antioxidant and radical-scavenging properties of methanolic extracts from the seed coat of certain Thai tamarind cultivars. J Food Biochem. 2010;34:916–31. [Google Scholar]
- 35.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 36.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–7. [Google Scholar]
- 37.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 38.Kamalakkanan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin induced diabetic wister rats. Basic Clin Pharmacol Toxicol. 2006;98:97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and alloxan induces DNA strand breaks and poly (ADP ribose) synthatase in pancreatic islet. Nature. 1981;294:284–6. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 40.Chude MA, Orisakawe OE, Afonne OJ, Gamanial KS, Vongtau OH, Obi E, et al. Effect of Vinca rosea extracts in treatment of alloxan diabetic male albino rats. Indian J Exp Biol. 2001;39:748–59. [PubMed] [Google Scholar]
- 41.Ghosh MN. Toxicity studies. In: Ghosh MN, editor. Fundamentals of Experimental Pharmacology. Kolkata, India: Hilton & Company; 2008. pp. 176–83. [Google Scholar]
- 42.Ceriello A. Postprandial hyperglycemia and diabetes complications; is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Jahan S, Saeed N, Ijlal F, Khan MA, Ahmad M, Zafar M, Abbasi AM. Histomorphological study to evaluate anti-fertility effect of Abrus precatorius L. in adult male mice. J Med Plants Res. 2009;3:1029–33. [Google Scholar]
- 44.Kenjiro T, Yuji M, Kouta T, Tomoko M. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006;52:149–53. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 45.Mahesh BN. Investigation of invitro α-amylase & α-glucosides inhibitory activities of polyherbal extract. IJPRD. 2011;3:97–103. [Google Scholar]
- 46.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 47.Kim JA, Jung WS, Chun SC, Yu CY, Ma KH, Gwag JG. A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties. Eur J Food Res Technol. 2006;224:259–70. [Google Scholar]
- 48.Duh PD, Tu YY, Yen GC. Antioxidant activity of the aqueous extract of harn jyur (Chrysanthemum morifolium Ramat) Lebensm Wiss Technol. 2006;32:269–77. [Google Scholar]
- 49.Scalzo RL. Measurement of free radical scavenging activity of gallic acid and unusual antioxidants as sugars and hydroxy acids. EJEAF Che. 2010;9:1360–71. [Google Scholar]
- 50.Veluri R, Singh RP, Zhengjie L, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–53. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]


