Abstract
Enormous progress in understanding the role of four populations of benzodiazepine-sensitive GABAA receptors was paralleled by the puzzling findings suggesting that substantial separation of behavioral effects may be accomplished by apparently non-selective modulators. We report on SH-I-048A, a newly-synthesized chiral positive modulator of GABAA receptors characterized by exceptional subnanomolar affinity, high efficacy and non-selectivity. Its influence on behavior was assessed in Wistar rats and contrasted to that obtained with 2 mg/kg diazepam. SH-I-048A reached micromolar concentrations in brain tissue, while the unbound fraction in brain homogenate was around 1.5%. The approximated electrophysiological responses, which estimated free concentrations of SH-I-048A or diazepam are able to elicit, suggested a similarity between the 10 mg/kg dose of the novel ligand and 2 mg/kg diazepam; however, SH-I-048A was relatively more active at α1- and α5-containing GABAA receptors. Behaviorally, SH-I-048A induced sedative, muscle relaxant and ataxic effects, reversed mechanical hyperalgesia 24 hours after injury, while it was devoid of clear anxiolytic actions and did not affect water-maze performance. While lack of clear anxiolytic actions may be connected with an enhanced potentiation at α1-containing GABAA receptors, the observed behavior in the rotarod, water maze and peripheral nerve injury tests was possibly affected by its prominent action at receptors containing the α5 subunit. The current results encourage further innovative approaches aimed at linking in vitro and in vivo data in order to help define fine-tuning mechanisms at four sensitive receptor populations that underlie subtle differences in behavioral profiles of benzodiazepine site ligands.
Keywords: equilibrium dialysis, free brain concentration, diazepam, rat
1. INTRODUCTION
Ionotropic receptors for the inhibitory neurotransmitter γ-aminobutyric acid (GABA) are the most widely distributed receptors in the central nervous system (Watanabe et al., 2002) and at the same time successfully exploited drug targets (Sieghart, 2006). Among drugs acting through GABAA receptors, benzodiazepines (BZs) have been established as especially versatile GABA-modulatory drugs. However, a plethora of regulatory roles of GABA itself is reflected in a wide range of pharmacological actions of BZs and an increased burden of side effects. The recognition that the molecular substrate of BZ action comprises four populations of GABAA receptors which contain the α1, α2, α3 or α5 subunit, in addition to the γ2 subunit, has given an impetus for synthesis and development of newer ligands. It was hypothesized that four populations of BZ-sensitive GABAA receptors mediate distinct sets of behavioral responses, enabling separation of desirable effects mediated by one, from undesirable effects supposedly mediated by the other subtype of receptors (Olsen and Sieghart 2008; Rudolph and Möhler, 2006).
A number of subtype selective ligands acting at the BZ site has been reported on in recent years, with the final aim of obtaining the optimal balance between efficacy and safety and possibly broadening the current spectrum of indications (Rudolph and Knoflach, 2011). This concept pursued the results of genetic studies, which have attributed the sedative and ataxic properties of BZs to the predominantly expressed α1 subunit-containing GABAA receptors, the anxiolytic, muscle relaxant and antihyperalgesic actions to the α2/α3 subtype-containing receptors, and anticonvulsant activity to all, the α1/α2/α3 GABAA receptors (reviewed in Rudolph and Möhler, 2006). Despite promising preclinical data, no clear clinical breakthroughs have been made to date (Atack et al. 2011) and, indeed, the whole concept has been questioned to a certain degree (Skolnick, 2012). Especially puzzling were the results suggesting that substantial improvement in the behavioral profile may be accomplished by non-selective ligands active at all four populations of BZ-sensitive GABAA receptors (Auta et al., 2010; Rabe et al., 2007), or even predominantly active at GABAA receptors containing the α1 subunit (Lippa et al., 2005; Popik et al., 2006). Such findings were clearly at odds with results from genetic studies showing that sedation and motor impairments exerted by BZs depend to a high degree on positive modulation of α1 GABAA receptors (Atack, 2010). The predominantly, though not exclusively hippocampus-based α5 GABAA receptors are thought to contribute to amnesic effects of BZs (Rudolph and Möhler, 2006), but their biological role is also the subject of debate, because a number of results suggest that activation of these receptors may facilitate memory processing (Koh et al., 2013; McEown and Treit, 2013), as well as reduce psychotic symptoms (Gill et al., 2011).
To tackle the problem in a stepwise way, we hereby report on a newly-synthesized chiral positive modulator of GABAA receptors characterized by high affinity, high efficacy and non-selectivity, which was examined for its brain exposure and pharmacodynamic profile in rats. In order to estimate pharmacologically active drug concentrations, we calculated the unbound concentrations in brain (Hammarlund-Udenaes, 2010; Read and Braggio, 2010). In vitro and in vivo properties, as well as brain exposure of SH-I-048A were directly compared with data generated, under identical experimental conditions, for the standard non-selective BZ site positive modulator, diazepam. The chosen battery of behavioral tests included those assessing motor status of the animal (spontaneous locomotor activity, rotarod, grip strength), its anxiety level (open field, elevated plus maze), learning and memory capability (Morris water maze) and pain susceptibility (model of peripheral nerve injury). We hypothesized that a ligand with an in vitro/ex vivo profile demonstrating superiority or, at least, non-inferiority to diazepam, in terms of availability at the receptor site and its subsequent modulation, should exert a set of in vivo actions comparable to those repeatedly reported for diazepam (Rudolph and Knoflach, 2011). Potential failures to support the hypothesis would prove that assigning various behavioral effects to any of specific GABAA receptor populations demands further refining and obtaining more comprehensive experimental data than usually presented (cf. Skolnick, 2012).
2. RESULTS
2.1. In vitro affinity
The in vitro binding data for SH-I-048A, in parallel with those for diazepam, are presented in Table 1. The novel ligand showed very high (subnanomolar) affinity for BZ-sensitive recombinant human GABAA receptors, considerably higher than that of diazepam. Furthermore, no major selectivity in binding at one over the other subtype was noticed (quite similar to diazepam).
Table 1.
Binding affinity (Ki, nM) of SH-I-048A and diazepam at human recombinant GABAA receptors containing β3, γ2 and named α subunit, stably expressed in mouse fibroblast L(tk−) cells; ND – not detected. [3H] flunitrazepam was used as the radiolabelled ligand in the competition binding assay. Data for diazepam were published in Savić et al. 2010.
| Ligand | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| SH-I-048A | 0.77 | 0.17 | 0.38 | ND | 0.11 | ND |
| diazepam | 14.0 | 7.8 | 13.9 | ND | 13.4 | ND |
2.2. Electrophysiological experiments
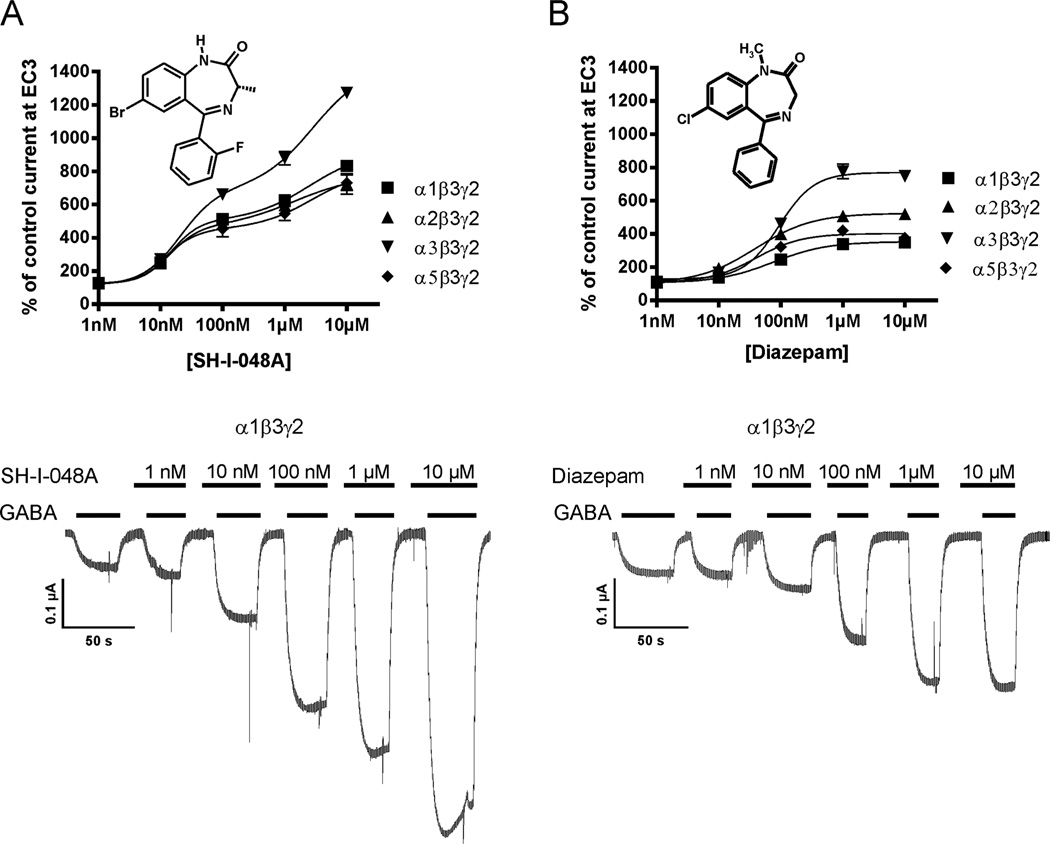
Electrophysiological behavior of SH-I-048A and the reference benzodiazepine diazepam in a wide range of concentrations (1 nM – 10 µM) is presented in Fig. 1. The novel ligand exerted higher positive modulation than diazepam at all four rat recombinant GABAA receptor subtypes. In addition, it seemed to interact with a second binding site at concentrations >1 µM, as indicated by the mathematical fit of the data points by a biphasic dose-response simulation using GraphPad Prism. EC50 values used by this program for fitting the SH-I-048A data were 14 nM and 2560 nM; 14 nM and 1410 nM; 20 nM and 2830 nM; or 12 nM and 2670 nM, for α1β3γ2, α2β3γ2, α3β3γ2, or α5β3γ2 containing receptors, respectively. Although an interesting finding, we did not further investigate this low potency interaction of SH-I-048A because the high free concentrations of the ligand required to generate relevant effects via this second site (probably well above 100 nM) were not reached under the conditions used (see in 2.3.1) and thus could not have contributed to the results of the present study.
Fig. 1.
Effects of SH-I-048A and diazepam at different GABAA receptor subtypes. A) Concentration-response curves of SH-I-048A at α1β3γ2, α2β3γ2, α3β3γ2 and α5β3γ2 receptors (n=3–5). Below: Individual current traces of GABA EC3 in the absence or presence of increasing SH-I-048A concentrations at α1β3γ2 receptors. B) Concentration-response curves of diazepam at α1β3γ2, α2β3γ2, α3β3γ2 and α5β3γ2 receptors (n=5–9). Below: Individual current traces of GABA EC3 in the absence or presence of increasing diazepam concentrations at α1β3γ2 receptors. Data are mean values ±SEM.
2.3. Estimation of free plasma and brain concentrations
2.3.1. Quantification in rat plasma and brain tissue
The concentrations of SH-I-048A in rat plasma (nmol/L) and brain tissue (nmol/kg), both total and free (estimated), are shown in Table 2. Free concentrations of SH-I-048A were calculated by multiplying the measured total plasma and brain concentrations with the appropriate estimated free fractions.
Table 2.
Total and estimated free concentrations of SH-I-048A (dosed at 0.5, 2 and 10 mg/kg) in plasma and brain samples after 20 and 60 min.
| dose (mg/kg) | 0.5 | 2 | 10 | |||
|---|---|---|---|---|---|---|
| time (min) | 20 | 20 | 60 | 20 | 60 | |
|
plasma (nmol/L) |
total | 187.59±26.66 | 818.82±57.06 | 617.87±149.35 | 1591.41±5.15 | 1396.19±492.70 |
| free | 8.43 | 36.79 | 27.76 | 71.50 | 62.73 | |
|
brain (nmol/kg) |
total | 231.12±52.27 | 750.59±307.14 | 739.37±182.94 | 2096.33±858.32 | 1035.23±421.19 |
| free | 3.26 | 10.60 | 10.45 | 29.62 | 14.63 | |
2.3.2. Plasma protein and brain tissue binding studies
Free fractions of SH-I-048A in rat plasma and brain tissue determined by rapid equilibrium dialysis were 4.49% and 1.41%, respectively.
2.3.3. Assessment of in vivo efficacy
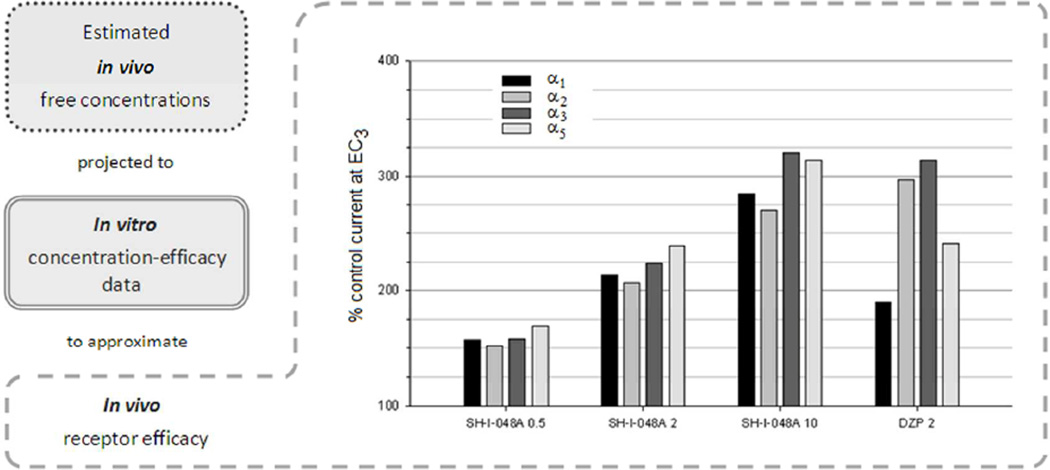
The exact in vitro efficacy values for SH-I-048A and diazepam at each of four recombinant GABAA receptor subtypes, used for the mathematical fit presented in Figure 2, were applied to calculate linear equations between two concentrations adjacent to the estimated in vivo free brain concentrations, which are presented in Table 2 for SH-I-048A, while the estimated free concentration of 2 mg/kg diazepam equals 58.46 nmol/kg (Savić et al., unpublished data). For example, in vitro efficacies of SH-I-048A at α1β3γ2 receptors are 124% and 256%, for concentrations of 1 nM and 10 nM, respectively, giving rise to potentiation of 157% when in vivo free concentration of 3.26 nM was applied to the corresponding linear equation; the values calculated by this approach are presented in Figure 7.
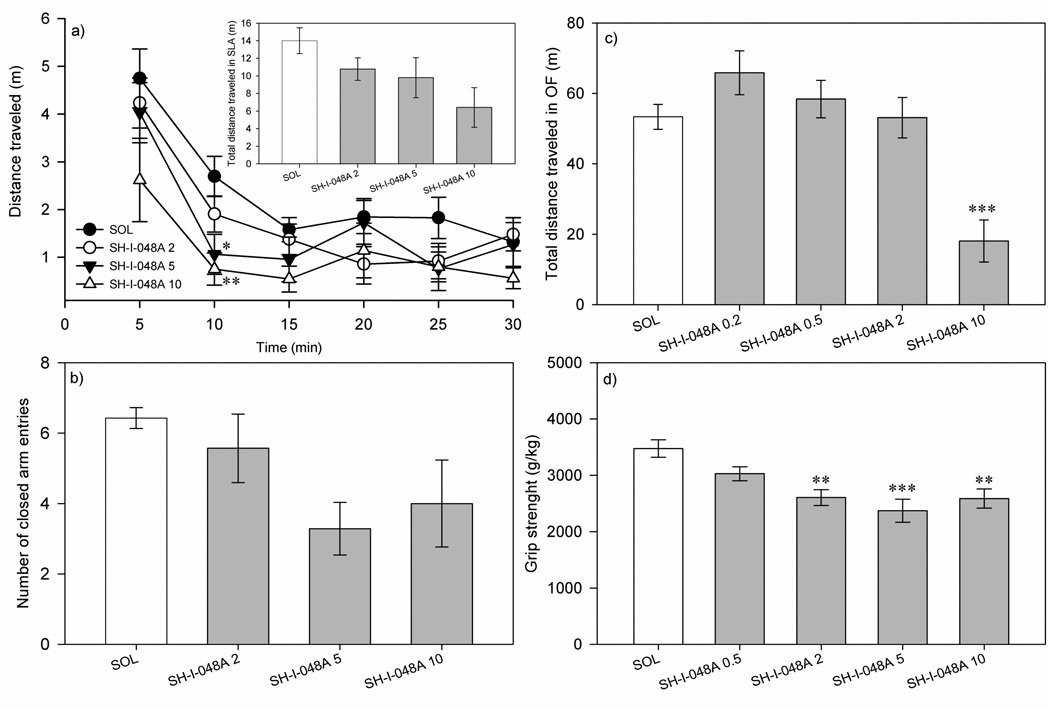
Fig. 2.
The effects of SH-I-048A on the: a) total distance traveled in spontaneous locomotor activity test, b) number of closed arm entries of elevated plus maze, c) total distance traveled in open field test, and d) grip strength. ** p<0.01 and *** p<0.001, compared to the solvent (SOL) group. Data represent mean ± SEM. Number of animals per each treatment group in a) and d) was 8, while in b) and c) was 7.
Fig. 7.
Approximated electrophysiological behavior of the estimated free brain concentrations, based on total brain concentrations measured 20 min after i.p. administration of SH-I-048A (0.5, 2 and 10 mg/kg) and diazepam (2 mg/kg; DZP 2). The estimated free concentrations of SH-I-048A are given in Table 2, while the estimated free concentration of 2 mg/kg diazepam equals 58.46 nmol/kg (Savić et al., unpublished data). The bars indicate the stimulation of the individual αxβ3γ2 GABAA receptors at these concentrations, as extrapolated from data presented in Fig. 1.
2.4. Behavioral experiments
2.4.1. Spontaneous locomotor activity
The influence of SH-I-048A (dosed at 2, 5 and 10 mg/kg) on the total distance traveled during 30 min of monitoring did not reach statistical significance, as assessed by a one-way ANOVA (F(3,28)=2.76, p=0.061) (the inset of Figure 2a). When the analysis of distance traveled was developed into 5-min bins (Figure 2a), it turned out that rats treated with SH-I-048A, dosed at 5 and 10 mg/kg, traveled shorter distances than the solvent-treated animals during the second time interval (5–10 min) (p=0.017 and p=0.008, respectively).
2.4.2. Elevated plus maze
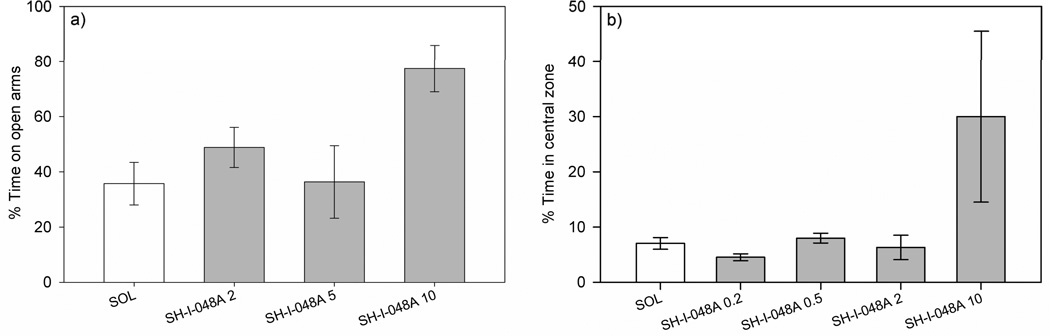
A one-way ANOVA showed no significant effect of treatment on the number of closed arm entries (F(3,24)=2.64, p=0.072; Figure 2b). Regarding the anxiety-related parameters, the same statistical analysis revealed that the effect of treatment on the percentage of the time spent on open arms was not significant, but again a statistical trend was noted (F(3,24)=2.37, p=0.095; Figure 3a). However, ANCOVA revealed that the trend of SH-I-048A to increase this parameter, related to anxiolytic-like activity, disappeared when the concomitant influence on closed arm entries was taken into account (F(3,23)=2.22, p=0.113). The doses of 0.5 and 2 mg/kg tested in a preliminary study were devoid of any influences on the EPM parameters (data not shown).
Fig. 3.
The effects of SH-I-048A (2, 5 and 10 mg/kg) on the: a) percentage of time spent on open arms and b) percentage of spent in the central zone of the open field arena. Data represent mean ± SEM.
2.4.3. Open field test
A one-way ANOVA revealed a significant effect of treatment on the total distance traveled in the open field arena (F(4,30)=11.48, p<0.001; Figure 2c). Dunnett’s post hoc test showed that only SH-I-048A (10 mg/kg) significantly decreased the total distance traveled, when compared to the solvent group (p<0.001). Anxiety-related parameter (the percent of time spent in the central zone) was not significantly affected, as assessed by a one-way ANOVA (F(4,3=0.60, p=0.669; Figure 3b).
2.4.4. Grip strength test
A one-way ANOVA revealed a significant effect of treatment on grip strength (F(4,35)=7.48, p<0.001; Fig. 2d). Post hoc Dunnett’s test showed that rats treated with SH-I-048A (2, 5 and 10 mg/kg) experienced significant muscle relaxation compared to the control (solvent) group (p=0.002, p<0.001 and p=0.002, respectively).
2.4.5. Rotarod test
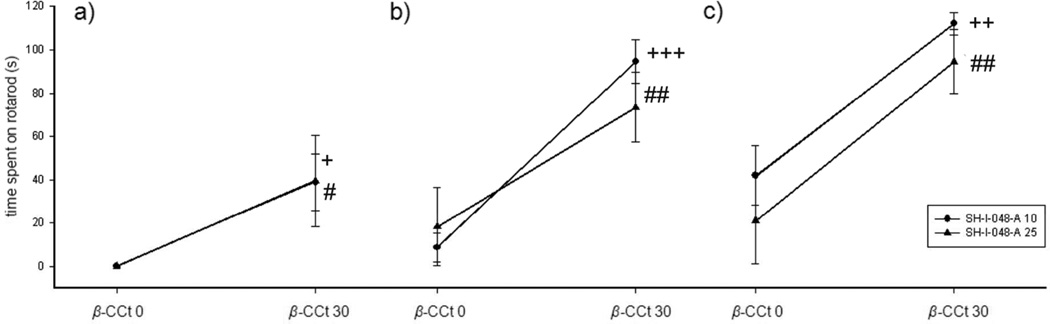
When three separate two-way ANOVAs (SH-I-048A and β-CCt, as factors) were applied at each time point, the only significant factor was β-CCt (after 0.5 h: F(1,24)=9.91, p=0.004; after 1 h: F(1,24)=30.02, p<0.001, and after 1.5 h: F(1,24)=26.38, p<0.001). Post hoc Tukey test showed that the addition of β-CCt to both 10 and 25 mg/kg SH-I-048A increased latency to fall from the revolving rod at 0.5 h (p=0.031 and p=0.040, respectively; Fig. 4a), after 1 h (p<0.001 and p=0.008, respectively; Fig. 4b), and finally after 1.5 h (p=0.001 and p=0.002, respectively; Fig. 4c).
Fig. 4.
The effects of SH-I-048A (10 and 25 mg/kg) in the absence (β-CCt 0) and the presence of 30 mg/kg β-CCt (β-CCt 30) on ataxia in the rotarod test: a) 30 min, b) 60 min, and c) 90 min after treatment. + p<0.05, ++ p<0.01 and +++ p<0.001 compared to SH-I-048A 10 + β-CCt 0 group; # p<0.05 and ## p<0.01 compared to SH-I-048A 25 + β-CCt 0 group. Data represent mean ± SEM. Number of animals per treatment varied from 6 to 8.
2.4.6. Morris water maze
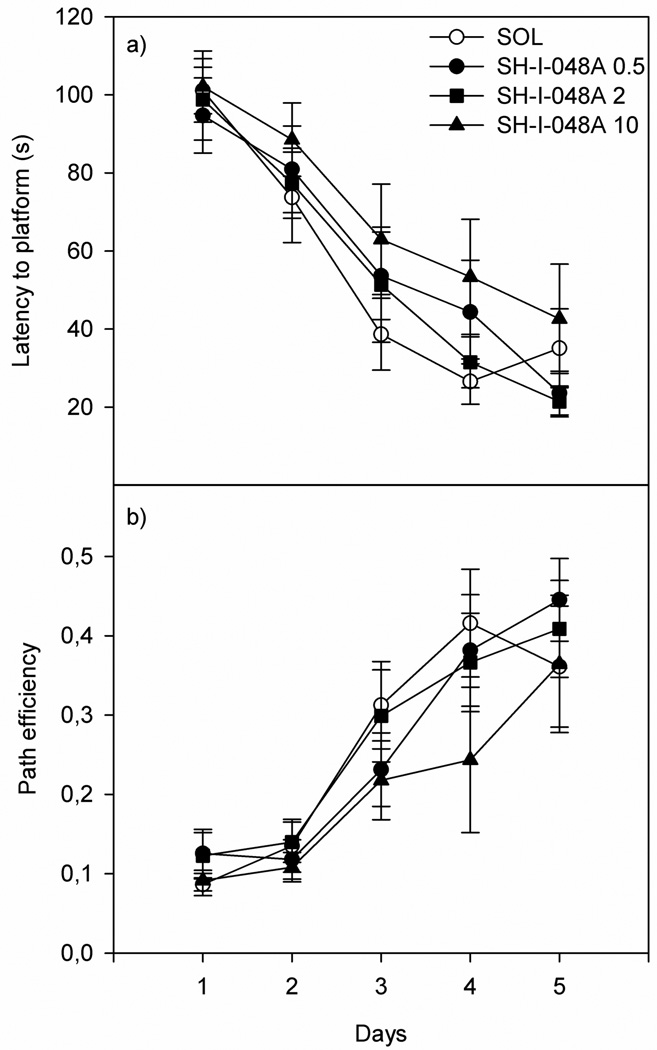
Regarding the dose response study of SH-I-048A in the water maze, a two-way ANOVA with repeated measures revealed that only factor Days was significant, for both the latency to find the platform (Treatment: F(3,24)=0.88, p=0.467; Day: F(4,96)=46.77, p<0.001; and Treatment×Day: F(12,96)=0.59, p=0.843; Fig. 5a) and the path efficiency (Treatment: F(3,24)=0.89, p=0.463; Day: F(4,96)=28.40, p<0.001; and Treatment×Day: F(12,96)=0.61, p=0.825; Fig. 5b). A one-way ANOVA applied on the data from the probe test revealed no significant effect of factor Treatment on both parameters measured: the latency to the first entry to the target zone (F(3,24)=0.28, p=0.841) and the path efficiency to the first entry to the target zone (F(3,24)=0.73, p=0.542).
Fig. 5.
The effects of SH-I-048A (0.5, 2 and 10 mg/kg) on the water maze parameters: a) the latency to reach the platform and b) the path efficiency. Data represent mean ± SEM. Number of animals per each treatment was 7.
2.4.7. Peripheral nerve injury model
A two-way ANOVA with repeated measures showed that both factors were significant (Treatment: F(3,20)=7.83, p=0.001; Time: F(2,40)=124.50, p<0.001; Fig. 6). Since a significant interaction between these two factors was also determined (F(6,40)=3.07, p=0.015), we decided to use one-way ANOVAs (for each of three levels of factor Time) to analyze simple main effects. A one-way ANOVA followed by Tukey’s post hoc analysis showed no significant differences between all groups at the baseline time (0 h). After 24 h, the same statistical procedure showed that rats treated with three doses of SH-I-048A (10 mg/kg) exhibited significantly higher paw withdrawal threshold compared to the solvent group (p=0.015), those treated with only one dose (p<0.001), as well as to rats treated with two doses of SH-I-048A (p=0.019). A one-way ANOVA applied on the data measured 48 h after surgery revealed no significant effect of factor Treatment on paw withdrawal threshold (F(3,2)=1.93, p=0.157). Regarding the analysis of data for left (uninjured) hind paws, a two-way ANOVA with repeated measures showed that both factors, as well as their interaction, were without significant effect (Treatment: F(3,20)=1.99, p=0.148; Time: F(2,40)=1.073, p=0.352; and Treatment×Time F(6,40)=1.98, p=0.091).
Fig. 6.
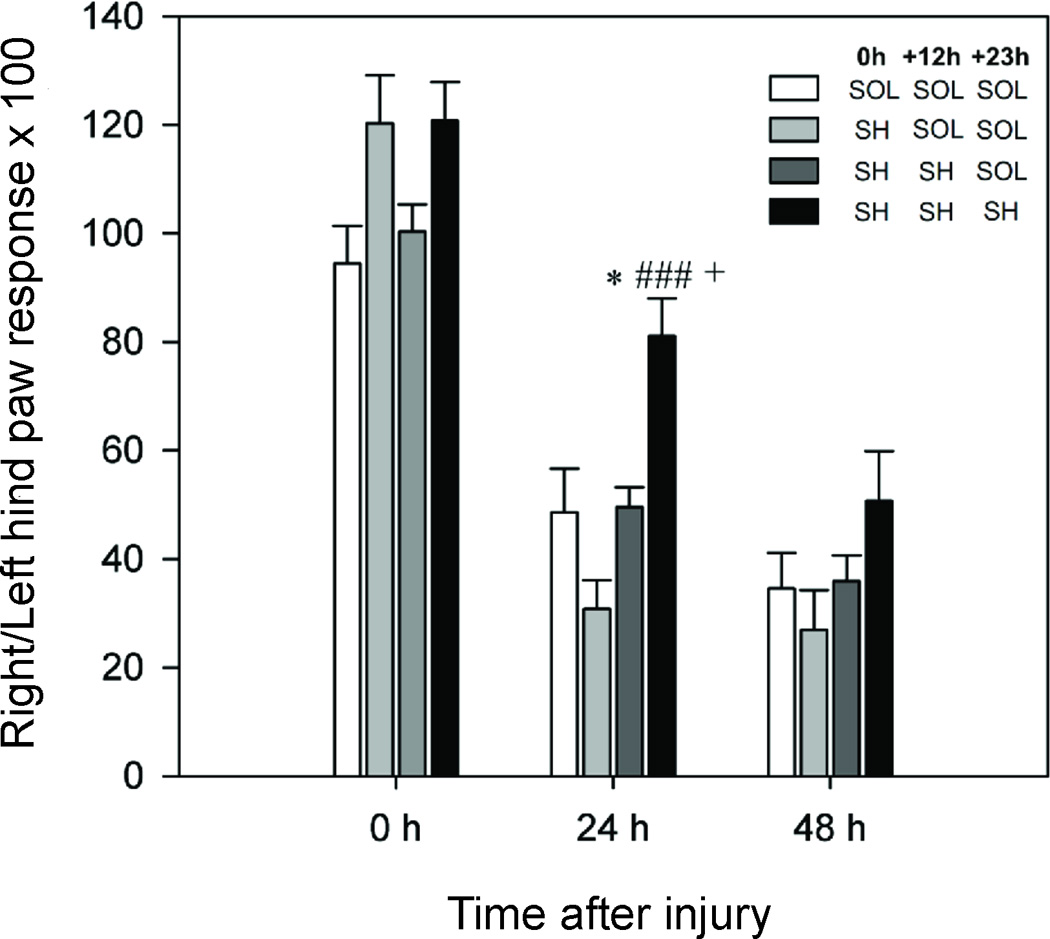
The effects of three treatment combinations comprising solvent (SOL) and/or 10 mg/kg SH-I-048A (SH), administered immediately after surgery (0 h), 12 h later (+ 12 h) and after 23 h (+ 23 h), on the ratios between the right (injured) hind paw and left hind paw responses, obtained by dividing the two values and then multiplying the result by 100, in the peripheral nerve injury model, measured at the baseline (0 h), 24 and 48 hours after injury. * p<0.05 compared to 3×SOL group, ### p<0.001 compared to 1×SH-I-048A + 2×SOL group, and + p<0.05 compared to 2×SH-I-048A + 1×SOL group. Data represent mean + SEM. Number of animals per each treatment group was 6.
2.4.8. Studies with diazepam as positive control
Table 3 demonstrates the results of behavioral studies with 2 mg/kg diazepam in comparison to the appropriate control group and, in parallel, the above-presented data for 10 mg/kg SH-I-048A and its control. Although the effects of ligands were generally similar, the observed behavioral inactivity of SH-I-048A in MWM was distinctly different from the incapacitating effect of diazepam during five days of acquisition. This finding was not accompanied by significant differences in the overall latencies during five acquisition days for solvent-treated animals from experiments with diazepam and SH-I-048A, respectively (F(1,69)= 3.169, p=0.100). The only significant difference between controls in two experiments was for the percent time spent in the central zone in OF (data not shown); this discrepancy was probably related to subtle differences in illumination used and also to the well-known variability of results obtained in this test (Prut and Belzung, 2003).
Table 3.
Numeric values for diazepam (DZP, 2 mg/kg), SH-I-048A (10 mg/kg) and their appropriate solvent controls (SOL) in behavioral experiments. Each value is the mean ± SEM of 6–8 rats. Data for SH-I-048A and its solvent control are also graphically presented in Figures 2–5.
| Test/parameter | SOL | DZP (2 mg/kg) |
p values (SOL vs. DZP) |
SOL | SH-I-048A (10 mg/kg) |
p values (SOL vs. SH-I-048A) |
|
|---|---|---|---|---|---|---|---|
| SLA | |||||||
| total distance (m) | 16.6 ± 1.51 | 7.17 ± 1.13*** | p<0.001 | 14.01 ± 1.47 | 6.42 ± 2.26* | p=0.014 | |
| EPM | |||||||
| number of closed arm entries | 5.36 ± 0.89 | 4.09 ± 0.77 | p=0.306 | 6.43 ± 0.30 | 4.00 ± 1.23 | p=0.080 | |
| % time on open arms | 40.00 ± 9.17 | 76.67 ± 6.67** | p=0.009 | 35.71 ± 7.68 | 67.43 ± 12.34* | p=0.050 | |
| OF | |||||||
| total distance (m) | 53.91 ± 4.68 | 24.50 ± 5.34*** | p=0.001 | 53.36 ± 3.56 | 15.39 ± 5.35*** | p<0.001 | |
| % time in central zone | 2.11 ± 0.42 | 3.09 ± 1.15 | p=0.437 | 7.04 ± 1.06 | 30.01 ± 15.49 | p=0.165 | |
| GS | |||||||
| grip strength (g/kg) | 2698.33 ± 211.36 | 1722.50 ± 197.65** | p=0.007 | 3477.30 ± 154.85 | 2588.83 ± 169.52** | p=0.002 | |
| RR | |||||||
| time on rotarod (s) | 120.00 ± 0.00 | 41.70 ± 9.79*** | p<0.001 | 120.00 ± 0.00 | 0.25 ± 0.16*** | p<0.001 | |
| MWM | |||||||
| latency (s) | |||||||
| Day 1: | 73.92 ± 9.14 | 103.45 ± 10.64 | p=0.057 | 101.10 ± 5.97 | 102.11 ± 9.11 | p=0.927 | |
| Day 2: | 64.84 ± 13.09 | 92.82 ± 6.40 | p=0.079 | 73.75 ± 11.58 | 88.52 ± 9.40 | p=0.342 | |
| Day 3: | 31.57 ± 6.37 | 77.83 ± 15.52* | p=0.017 | 38.68 ± 9.21 | 62.98 ± 14.13 | p=0.175 | |
| Day 4: | 37.66 ± 8.79 | 59.02 ± 9.78 | p=0.130 | 26.56 ± 5.82 | 53.39 ± 14.74 | p=0.116 | |
| Day 5: | 17.61 ± 3.10 | 56.40 ± 10.20** | p=0.003 | 35.11 ± 10.09 | 42.65 ± 13.99 | p=0.670 | |
p<0.05;
p<0.01;
p<0.001 compared to the respective SOL.
3. DISCUSSION
In recent years, several research groups, including ours, have reported on a number of ligands for the BZ site of GABAA receptors which possessed in vitro selectivity for one or two populations of BZ-sensitive receptors, and as a rule low activity at GABAA receptors containing the α1 subunit (e.g. Atack et al. 2011; Griebel et al., 2001; Mirza et al. 2008; Savić et al. 2010). Their in vivo profiles were mainly in line with the results of genetic studies, which, as a finding of special clinical relevance, associated motor impairing effects with activation of receptors containing the α1 subunit (reviewed in Rudolph and Möhler, 2006). At the same time, there have been some promising developments also of ligands reportedly non-selective in affinity and efficacy at GABAA receptors (Auta et al., 2010; Rabe et al., 2007), or even predominantly active at receptors containing the α1 subunit (Lippa et al., 2005; Popik et al., 2006), but still showing a low potential for motor deficits in animals. In order to shed some additional light on these apparently contradictory results, we discovered and pharmacologically characterized SH-I-048A, a non-selective ligand that exhibited an exceptional, subnanomolar capacity to bind to all four BZ-sensitive GABAA receptors and also strongly potentiated the inhibitory effect of GABA, mediated through these receptors. Its overall ability to change the rat’s behavior was similar, but far from identical, to that characterizing diazepam at the most commonly used 2 mg/kg dose.
The ligand SH-I-048A is structurally closely related to typical benzodiazepines such as diazepam and flurazepam, except that it contains a chiral methyl function at C-3 with the S-configuration. Previously, in another series of ligands, it was demonstrated that the binding, efficacy and in vivo activity of ligands which contain a chiral S-CH3 group at C-4 (similar to the C-3 S-CH3 function in SH-I-048A) is different than the R-CH3 isomer (Fischer et al., 2010; Savić et al., 2010). Importantly, the R-enantiomer of SH-I-048A was almost completely inactive in the same oocyte assay (not shown), consequently stimulating interest in the study of chiral S-enantiomer described in this manuscript. Hence this chiral function would be expected to alter the profile of SH-I-048A vs. diazepam. The question was to what extent and how.
It is generally accepted that the receptor effects, beside affinity and efficacy, depend on the available unbound ligand (Hammarlund-Udenaes, 2010; Read and Braggio, 2010). Here, we present an innovative approach aimed to compare brain exposure and electrophysiological data obtained for SH-I-048A to the results for diazepam, observed in the same experimental settings. The approximated electrophysiological responses, which the estimated free concentrations of two ligands are able to elicit (Figure 7), suggest that the 2 mg/kg dose of diazepam corresponds to a five times higher dose of the novel ligand, at least after intraperitoneal administration in Wistar rats. Such a relation could not have been predicted solely from knowledge of the in vitro properties. Moreover, any discrepancies in in vivo profiles could be governed by relative differences in the approximated receptor activity of diazepam and SH-I-048A. Most remarkably, a substantial difference related to α1- and α5-containing GABAA receptors, at which SH-I-048A is relatively more active than diazepam, could be noted in Figure 7.
SH-I-048A dose-dependently affected parameters of general motor activity and dexterity, in a manner similar to diazepam (Milić et al., 2012; Savić et al., 2009; Siemiatkowski et al., 2000). Among parameters of locomotor activity, the statistical significance of the sedative effect was reached for distance traveled during the recording period 5–10 min in SLA, at doses of 5 and 10 mg/kg, and the total distance traveled in OF, at the dose of 10 mg/kg. Grip strength, measuring muscle relaxant effect, was moderately, but significantly reduced at doses of 2 to 10 mg/kg. Having in mind non-selectivity of SH-I-048A, these two sets of results conform with the postulate that sedation is mediated by α1 GABAA receptors, and muscle relaxation by α2 and α3 GABAA receptors (Rudolph and Knoflach, 2011).
While our recent results showed that the administration of β-CCt fully antagonized the impairment of motor coordination in rats induced by diazepam (Milić et al. 2012), the antagonist effect of this α1-selective ligand on the prolonged rotarod incapacitation induced by SH-I-048A was significant, but still not sufficient enough to enable the rats to regain the control performance, especially at the 30 min time point, when they spent less than 40 s on the revolving rod, far different from the basal value of 120 s. Since our analysis (Fig. 7) suggested a similarity of the effects of SH-I-048A and diazepam elicited at α2 and α3 GABAA receptors, the difference in the behavioral effect has probably been contributed to by the population of α5-containing GABAA receptors, which are more activated by SH-I-048A than by diazepam.
Diazepam in a range of moderate doses commonly exerted anxiolytic-like actions in the EPM (Savić et al., 2010) and OF (Prut and Belzung, 2003), whereas SH-I-048A, applied in a wide range of doses (0.5–10 mg/kg in EPM and 0.2–10 mg/kg in OF), did not significantly affect the parameters of anxiety-related behavior in either of these two anxiety tests. Moreover, the observed tendency of SH-I-048A to induce anxiolytic-like changes at the dose of 10 mg/kg appeared to be confounded by locomotor influences, as indicated by the results of statistical analysis which involved the number of closed arm entries as a covariate. We hypothesize that the motor effects mediated by an enhanced potentiation at α1 and/or α5 GABAA receptors, relative do diazepam, probably hampered the anxiolytic actions of SH-I-048A expected to be effected by α2 and/or α3 GABAA receptors (Rudolph and Knoflach, 2011). The results from OF and tests of motor activity suggest that even diazepam at the dose of 2 mg/kg was prone to elicit suppressing motor effects which may compromise the assessment of anxiety-like behaviors.
The results obtained in MWM demonstrated a lack of BZ-characteristic impairment of learning and memory parameters during acquisition sessions and probe trial. This finding is clearly different from the well-established incapacitating actions of diazepam (Arolfo and Brioni, 1991) and generally unexpected, especially bearing in mind the noticeable activity of SH-I-048A at α1 and α5 GABAA receptors, which were shown to contribute to the water-maze incapacitation exerted by diazepam (Savić et al., 2009). Noteworthy, the rats dosed with 10 mg/kg SH-I-048A, apparently heavily sedated immediately before being placed into the water-maze, appeared vigilant when confronted with a challenging task. While seemingly unexplainable in the scope of the results of genetic studies (Rudolph and Knoflach, 2011), the observed ‘behavioral silence’ of SH-I-048A in MWM may be connected with the recent finding that potentiation of α5 GABAA receptors increases firing rates of cortical neurons during episodes of ongoing neuronal activity (up states) (Drexler et al., 2013). In this vein, the observed higher activity at α5 GABAA receptors of SH-I-048A compared to diazepam may have contributed to a kind of compensatory effect on MWM performance, noted in the current investigation.
The decrease of GABA neurotransmission in the spinal cord contributes to the enhanced pain sensitivity observed in pain states, especially those of neuropathic nature (Munro et al., 2009). At the behavioral level, it was shown that GABAA receptor positive allosteric modulators, such as diazepam, attenuate nociceptive transmission in animal models of neuropathic pain (Knabl et al., 2009; Malan et al., 2002). The present study has focused on sciatic crush injury (Bester et al., 2000), which mimics a common and refractory disability. SH-I-048A, administered in three 10 mg/kg doses, has reversed mechanical hyperalgesia 24 hours after injury, as measured 1 h after administration of the last dose. Motor incapacitation elicited by the novel ligand, demonstrated in tests of motor function, cannot be seen as substantially confounding these results, having in mind normal responses of uninjured hind paws. Although the antihyperalgesic influence of treatment was statistically not significant 48 h after injury, there was still a certain level of the residual effect in the group injected thrice with SH-I-048A. In the more common chronic constriction injury model, which could be regarded as similar to the crush injury model used in the present study, it was shown that diazepam was able to attenuate hind paw hypersensitivity at the highest dose tested (10 mg/kg) (Munro et al., 2008). On the contrary, zolpidem, a positive modulator highly effective at α1-, moderately effective at α2/3- and ineffective at α5 GABAA receptors (Savić et al., 2010), was completely inactive in the same model (Munro et al., 2008). Genetic and pharmacological studies have focused on the role of benzodiazepines in central pain transmission on the population of α2/3 GABAA receptors (Di Lio et al., 2011; Knabl et al., 2008). However, a number of studies have pointed to α5-containing receptors as possibly contributing to GABA-mediated antihyperalgesia (Nickolls et al., 2011). A substantial potential of antihyperalgesic action of SH-I-048A dosed thrice at 10 mg/kg may be seen as further adding to the possible role of α5 GABAA receptors, otherwise moderately present in the spinal cord (Paul et al., 2012), in modulation of pain transmission.
In conclusion, this paper presented SH-I-048A, a novel, chiral, highly potent and efficacious positive allosteric modulator at the BZ site of recombinant GABAA receptors. Apparently small differences in the simulated in vivo environment, approximated on the basis of electrophysiological, equilibrium dialysis and rat brain exposure studies, have been reflected in a number of differences in behavioral profiles of SH-I-048A and diazepam, which may be connected with relatively higher activity of SH-I-048A at α1 and/or α5 GABAA receptors. It appears crucial to establish firmer links between in vitro and in vivo data in order to help define the criteria for declaring selectivity or nonselectivity of BZ site ligands, and the presented approximation of activation of GABAA receptor subtypes may be a valuable approach. The current results suggest that diazepam, commonly seen as a prototype of nonselective BZ site ligands, does possess a kind of ‘semi-selectivity’ towards α2 and α3 GABAA receptors, far different from SH-I-048A, which thus proved to be a useful experimental tool.
4. EXPERIMENTAL PROCEDURE
4.1. Drugs
The novel chiral compound SH-I-048A ((S)-7-bromo-5-(2-fluorophenyl)-3-methyl-1Hbenzo[e][1,4]diazepin-2(3H)-one) and β-CCt (t-butyl-β-carboline-3-carboxylate), the preferential α1-containing GABAA receptor selective antagonist, were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin – Milwaukee, while diazepam was obtained from Galenika (Belgrade, Serbia).
4.2. Competition binding assays
Competition binding assays were performed in a total volume of 0.5 mL at 4 °C for 1 h using [3H]flunitrazepam as the radiolabelled ligand. A total of 6 µg of cloned human GABAA receptor DNA containing the desired α subtype along with β2 and γ2 subunits were used for transfecting HEK 293T cell lines using Fugene 6 (Roche Diagnostic) transfecting reagent. Cells were harvested 48 h after transfection, washed with Tris–HCl buffer (pH 7.0) and Tris Acetate buffer (pH 7.4) and the resulting pellets were stored at −80 °C until assayed. On the day of the assay, pellets containing 20–50 µg of GABAA receptor protein were resuspended in 50 mM Tris–acetate pH 7.4 at 4 °C and incubated with the radiolabel as previously described (Choudhary et al., 1992). Nonspecific binding was defined as radioactivity bound in the presence of 100 µM diazepam and represented less than 20% of total binding.
Membranes were harvested with a Brandel cell harvester followed by three ice-cold washes onto polyethyleneimine-pretreated (0.3%) Whatman GF/C filters. Filters were dried overnight and then soaked in Ecoscint A liquid scintillation cocktail (National Diagnostics; Atlanta, GA). Bound radioactivity was quantified by liquid scintillation counting. Membrane protein concentrations were determined using an assay kit from Bio-Rad (Hercules, CA) with bovine serum albumin as the standard.
4.3. Electrophysiological experiments
cDNAs of rat GABAA receptor subunits were used for generating the respective mRNA's that were then injected into Xenopus laevis oocytes (Nasco, WI) as described previously (Ramerstorfer et al., 2010). For electrophysiological recordings, oocytes were placed on a nylon-grid in a bath of Xenopus Ringer solution (XR, containing 90 mM NaCl, 5 mM HEPES-NaOH (pH 7.4), 1 mM MgCl2, 1 mM KCl and 1 mM CaCl2). The oocytes were constantly washed by a flow of 6 mL/min XR which could be switched to XR containing GABA and/or drugs. Drugs were diluted into XR from DMSO-solutions resulting in a final concentration of 0.1% DMSO perfusing the oocytes. Drugs were preapplied for 30 sec before the addition of GABA, which was coapplied with the drugs until a peak response was observed. Between two applications, oocytes were washed in XR for up to 15 min to ensure full recovery from desensitization. To test for modulation of GABA-induced currents by drugs, a concentration of GABA was used that was titrated to trigger 3% of the respective maximum GABA-elicited current of the individual oocyte (EC3). At this low GABA concentration, within-day and between-day currents are reproducible and effects of drugs (% of modulation) are much higher than that at higher GABA concentrations, again contributing to the accuracy of the results. In addition, such low GABA concentrations correspond to those acting at extrasynaptic receptors that represent the majority of GABAA receptors in the brain (Kasugai et al., 2010). In contrast, GABA concentrations acting at synaptic GABAA receptors in many cases fully activate these receptors. At such GABA concentrations benzodiazepines cannot enhance GABA currents anymore, but they prolong current decay. This effect, however, is antagonized by a simultaneously occurring rapid receptor desensitization making measurements unreliable. The GABA EC3 concentration was applied to the cell together with various concentrations of drugs. For current measurements the oocytes were impaled with two microelectrodes (2–3 mΩ) which were filled with 2 mM KCl. All recordings were performed at room temperature at a holding potential of −60 mV using a Warner OC-725C two-electrode voltage clamp (Warner Instruments, Hamden, CT). Data were digitized, recorded and measured using a Digidata 1322A data acquisition system (Axon Instruments, Union City, CA). Results of concentration response experiments were graphed using GraphPad Prism 4.00 (GraphPad Software, San Diego, CA).
Maximum GABA-evoked currents (1 mM GABA) were measured in each oocyte and were in the 4.5–7 microampere range for all receptor subtypes investigated, indicating an adequate expression of the different receptor subtypes. From these data, GABA EC3 values were calculated and the oocytes were then stimulated by the respective GABA concentration, confirming the calculated value. The GABA EC3 current was then modulated by 1 µM diazepam, which should nearly maximally modulate the receptors. Further measurements with the oocyte were only performed if the extent of modulation by diazepam was in a range corresponding to that shown in Fig 1b for the respective receptor subtype.
4.4. Estimation of free plasma and brain concentrations
4.4.1. Quantification in rat plasma and brain tissue
Eighteen Wistar male rats (180–220 g, Military Farm, Belgrade, Serbia) were divided into six treatment groups (3 animals per group) that intraperitoneally (i.p.) received 0.5 mg/kg SH-I-048A 20 min before decapitation, 2 or 10 mg/kg SH-I-048A 20 or 60 min before decapitation, or solvent. Blood samples were collected by cardiac puncture into heparinized tubes then centrifuged at 2500g for 10 min to obtain plasma. Thereafter, brains were rapidly removed, measured and homogenized at 16,000 rpm for 2 min by a rotor–stator blender (T 25 digital Ultra-Turrax, IKA, Germany) in 2 mL of methanol. The final volume was adjusted to 5 mL with methanol and after centrifugation (6000g for 20 min) supernatants were collected.
Concentrations of SH-I-048A in plasma and brain tissue were determined by ultra performance liquid chromatography-tandem mass spectrometry (UPLC/MS/MS). The sample pretreatment procedure was carried out using solid-phase extraction (SPE) on Oasiss HLB cartridges (Waters, Milford, MA, USA). The procedure was adapted from the method reported by Mercolini et al. (2009). Briefly, cartridges preconditioned with methanol and water were loaded with samples (plasma or supernatant of brain tissue homogenate) and the internal standard solution. Endogenous impurities were removed by washing the cartridges with water and methanol. Finally, analyte elution was carried out with 1 mL of methanol for 1 minute.
4.4.2. Plasma protein and brain tissue binding studies
A 48-well rapid equilibrium dialysis (RED) device (Thermo Scientific) was used to determine SH-I-048A free fraction in fresh rat plasma and brain tissue. Brain samples were diluted with 4 volumes of PBS and homogenized. The sample chambers of the RED device insert were loaded with 190 µL of plasma or brain tissue homogenate and 10 µL of SH-I-048A working solution in DMSO (100 µM) in order to obtain a final SH-I-048A concentration of 5 µM. A 350 µL of PBS were transferred into the buffer chamber of the insert. After incubation at 37 °C on an orbital shaker for 4 h, 50 µL of buffer, plasma and brain homogenate aliquots were transferred to microcentrifuge tubes containing equal volume of DMSO. A 50 µL volume of control buffer was added to the plasma and brain homogenate samples and 50 µL of either control brain homogenate or control plasma was added to the buffer sample to yield an identical matrix between buffer and nonbuffer samples. Sample extraction was performed by the addition of 300 µL of chilled acetonitrile containing midazolam as an internal standard. After vortexing and centrifuging, 120 µL of supernatant were transferred to vials for UPLC/MS/MS analysis. The same protocol was applied for the matrix preparation for standard curves except that 50 µL of drug-free dialyzed plasma, brain homogenate and buffer were added to microcentrifuge tubes containing 50 µL of SH-I-048A standard solutions in DMSO over the concentration range from 50 µM to 0.5 µM.
The unbound fraction in plasma was determined as the ratio of concentrations determined in buffer to that determined in plasma. The unbound fraction in undiluted brain was calculated by using the following equation: undiluted fu = {1/D*[(1/ fu (apparent) − 1) + 1/D]} (Kalvass and Maurer, 2002), where D and fu(apparent) represent dilution factor and free fraction determined as the ratio of concentrations in buffer versus diluted brain tissue, respectively.
4.5. Behavioral experiments
In vivo studies were carried out on male Wistar rats (Military Farm, Belgrade, Serbia), weighing 180–220 g at the beginning of the experiments. All procedures in the study conformed to EEC Directive 86/609 and were approved by the Ethical Committee on Animal Experimentation of the Faculty of Pharmacy in Belgrade, or, for the peripheral nerve injury model, Medical Faculty in Belgrade. The rats were group-housed four per cage (in the peripheral nerve injury model two per cage) in transparent plastic cages with tap water and food pellets available ad libitum and kept under a 12 h light/dark cycle (light on at 6:00 h). The temperature of the animal room was 22±1 °C, the relative humidity 40–70% and the illumination 120 lux. With the exception of the second treatment in the peripheral nerve injury model, all handling and testing took place during the light period of the cycle. Ligands were dissolved/suspended with the aid of sonication in solvent containing 85% distilled water, 14% propyleneglycol, and 1% Tween 80, and were administered i.p. in a volume of 1 mL/kg.
Separate groups of naïve animals were used for seven behavioral paradigms. For all behavioral studies, with the exception of the peripheral nerve injury model, the positive control experiments (2 mg/kg diazepam, with the accompanying solvent control) have been performed separately during the period when experiments with SH-I-048A were carried out. The behavior in spontaneous locomotor activity (SLA), open field (OF), Morris water maze (MWM) and elevated plus maze (EPM) was recorded by a ceiling-mounted camera and analyzed by the ANY-maze Video Tracking System software (Stoelting Co.,Wood Dale, IL, USA). The rats’ behavior in rotarod (RR) and grip strength (GS) tests, as well as in peripheral nerve injury (PNI) model, was recorded automatically by the apparatus. Where applicable, olfactory traces left in the apparatus were removed after each testing using diluted ethanol.
4.5.1. Spontaneous locomotor activity test
The rats’ spontaneous locomotor activity was monitored in a clear Plexiglas open chamber (40×25×35 cm) without any prior habituation to this apparatus. Twenty minutes after receiving the appropriate treatment, a single rat was placed in the chamber, under dimmed red light (20 lux), and activity was recorded for a total of 30 min. The total distance traveled (m) was the activity parameter evaluated in four treatment groups (solvent and SH-I-048A, dosed at 2, 5 and 10 mg/kg). Besides the total distance traveled, behavior was analyzed by breaking the locomotor activity data into 5-min bins.
4.5.2. Elevated plus maze test
The apparatus was constructed of sheet metal, with a black rubber floor. It consisted of a maze elevated to a height of 50 cm with two open (50×10 cm) and two enclosed arms (50×10×40 cm), connected by a junction area measuring 10×10 cm. A ledge of sheet metal (0.3 cm high) surrounded the open arms. The red illumination on the surface of the closed arms was 10 lux. At the beginning of the experiment, single rats were placed in the center of the maze, facing one of the enclosed arms, and their behavior was recorded for 5 min. An entry into an open or closed arm was scored when 80% of the animal crossed the virtual line separating the central square of the maze from the arm, whereas an exit occurred when more than 80% of the animal left the respective arm. In this experiment we used four treatment groups: solvent and SH-I-048A dosed at 2, 5 and 10 mg/kg. In a preliminary study, we tested also the 0.5 mg/kg dose of SH-I-048A.
4.5.3. Open field test
The OF arena consisted of a square wooden box (100×100×50 cm3), with a black rubber floor. Twenty minutes after receiving the appropriate treatment, and without any prior habituation, a single animal was placed in the center of the box. The central 16% of the floor surface (40×40 cm2) was virtually set as a central zone. Animal activity under dimmed red light (20 lux) was recorded for 15 min. The total distance traveled (m) was evaluated as an activity parameter, while the percent of time spent in the central zone, as an anxiety-related parameter, was estimated in the first 5-min period. In this experiment we used five treatment groups: solvent and SH-I-048A dosed at 0.2, 0.5, 2 and 10 mg/kg.
4.5.4. Grip strength test
Myorelaxant properties of SH-I-048A, dosed at 0.5, 2, 5 and 10 mg/kg, were examined in this study. Twenty minutes after administration of the appropriate treatment rats were allowed to grasp with their front paws a metal trapezoid wire grid attached to a grip-strength meter (Ugo Basile, Italy), while being lifted by the tail with increasing firmness until they loosened their grip. Every testing was performed by the same experimenter. The apparatus measured the pull force (expressed in grams) necessary to overcome the animal's forelimbs grip-strength. Each animal was given three consecutive trials and the median value was taken in the statistics. Dependent variable chosen was calculated by dividing the pull force with the rat’s body weight.
4.5.5. Rotarod test
Motor performance was assessed using an automated rotarod (Ugo Basile, Italy). Before testing, rats were trained for three days until they could remain on a revolving rod for 120 s with acceleration from 15 rpm to 25 rpm. During the training days, all animals were given three training sessions of 2 min each, with a 30 min inter-session interval. On the fourth day, selection was made and rats that fit the given criteria were included in the experiment. Four groups received one of the following treatments: SH-I-048A (10 and 25 mg/kg) alone or in combination with β-CCt (30 mg/kg). Latency to fall from the rotarod was recorded automatically for each animal after 30, 60 and 90 min.
4.5.6. Morris water maze test
The water maze consisted of a black cylindrical pool (diameter: 200 cm, height: 60 cm) filled to a height of 30 cm with 23 °C (±1 °C) water. The escape platform made of black plastic (15×10 cm) was submerged 2 cm below the water surface. An indirect illumination in the experimental room was provided by white neon tubes. The rats received the appropriate treatment 20 min before a swimming block, each day for 5 consecutive days of spatial acquisition. Each block consisted of 4 trials, lasting a maximum time of 120 s, the intertrial interval being 60 s. For each trial the rat was placed in the water facing the pool at one of four pseudorandomly determined starting positions. Since the platform was hidden in the middle of the NE quadrant during spatial learning, the four distal start locations were S, W, NW and SE. Once the rat found and mounted the escape platform, it was permitted to remain on it for 15 s. The rat was guided to the platform by the experimenter if it did not locate the escape within 120 s. For each training day and parameter measured in the water maze the mean value was calculated for each rat (total data/total number of trials). To assess the reference memory at the end of the learning period, a probe trial was conducted 24 h after the last acquisition day. The probe trial, starting from the novel, most distant SW location, with the platform omitted, lasted 60 s and was performed without any pre-treatment. The tracking software virtually divided the pool into four quadrants, three concentric annuli and a target region consisting of the intersection of the platform quadrant and the platform (middle) annulus, as graphically represented in Savić et al. (2009).
Dependent variables chosen for tracking during the acquisition trials were the latency to reach the platform (time from the start to the goal) and the path efficiency (the ratio of the shortest possible path length to actual path length). In addition, the latency to the first entry to the target zone and the path efficiency to the first entry to the target zone were assessed during the probe trial. In the water maze study we used four treatment groups: solvent and SH-I-048A dosed at 0.5, 2 and 10 mg/kg.
4.5.7. Peripheral nerve injury model
The animals were habituated in individual Plexiglas chambers for 30 min before testing. Electronic von Frey apparatus (Model 2390, IITC Life Science – USA) was used to determine paw withdrawal threshold expressed in grams. An increasing pressure of maximum 80 g was intermittently applied to the right and left rat hind paw for three times each, until the animal showed a typical paw withdrawal reaction. Baseline withdrawal scores were obtained before injury (0 h). To induce PNI model, we performed a crush injury to the right sciatic nerve. Rats were anesthetized with ketamine solution (10% Ketamidor, Richter Pharma Ag, Wels, Austria). After the right external thigh and buttock area had been shaved, the skin was properly prepared with antiseptic solution and alcohol, and small incision was made. The m. vastus lateralis and m. biceps femoralis were bluntly separated at the mid-thigh level. The nerve was kept in situ. At approximately 7 mm from the point of its emergence, the common sciatic nerve was mobilized and traumatized by a single 30-s crush with a serrated hemostat (Bridge et al., 1994). After surgery animals were separated in four groups. Treatments were administered three times: immediately after surgery, after 12 hours and finally after 23 h. The treatment groups were: solvent three times; one dose of SH-I-048A (10 mg/kg) and solvent two times; two doses of SH-I-048A (10 mg/kg) and solvent once and three doses of SH-I-048A (10 mg/kg). After 24 and 48 hours, mechanical hyperalgesia was determined in both hind paws. The graph shows the ratios between the right hind paw and left hind paw responses, obtained by dividing the two values and then multiplying the result by 100 for a better graph representation.
4.6. Statistical analysis
For electrophysiological data, as well as for behavioral data presented in Table 3, Student's t-test was used. Other behavioral data sets were checked for homogeneity of variance and normality prior to analysis by a one-way ANOVA (SLA, OF, EPM and GS tests), or a two-way ANOVA (RR test). In order to fully investigate the rats’ behavior in the EPM, an analysis of covariance (ANCOVA) was performed for the percentage of time spent on open arms, as an anxiety-related parameter, using the number of enclosed arm entries as covariate. Data from MWM and PNI model were assessed with a two-way ANOVA with repeated measures (Treatment as between-subject factor and Day or Time, respectively, as within-subject factor). In the case of significant interaction, separate one-way ANOVAs were conducted to assess the influence of one factor within individual levels of other factor. For post hoc analysis, Dunnett’s or Tukey’s test were used. Statistical analyses were performed with ANY-maze Video Tracking System software (Stoelting Co., Wood Dale, IL, USA) and Sigma Plot 11 (Systat Software Inc., Richmond, CA, USA). Differences were considered to be statistically significant when p was less than 0.05, while 0.1>p>0.05 was considered as a trend toward significance.
Acknowledgement
This work was supported in part by The Ministry of Science, R. Serbia - Grant No. 175066 (MMS) and by NIMH 46851 (JMC)
REFERENCES
- Arolfo MP, Brioni JD. Diazepam impairs place learning in the Morris water maze. Behav. Neural Biol. 1991;55:131–136. doi: 10.1016/0163-1047(91)80133-y. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABAA receptor alpha2/alpha3 subtype-selective modulators as potential nonsedating anxiolytics. Curr. Top. Behav. Neurosci. 2010;2:331–360. doi: 10.1007/7854_2009_30. [DOI] [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Street LJ, Dawson GR, Tye S, Van Laere K, et al. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J. Psychopharmacol. 2011;25:314–328. doi: 10.1177/0269881109354927. [DOI] [PubMed] [Google Scholar]
- Auta J, Kadriu B, Giusti P, Costa E, Guidotti A. Anticonvulsant, anxiolytic, and non-sedating actions of imidazenil and other imidazo-benzodiazepine carboxamide derivatives. Pharmacol. Biochem. Behav. 2010;95:383–389. doi: 10.1016/j.pbb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J. Comp. Neurol. 2000;428:45–61. doi: 10.1002/1096-9861(20001204)428:1<45::aid-cne5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, et al. Nerve crush injuries--a model for axonotmesis. Exp. Neurol. 1994;127:284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- Choudhary MS, Craigo S, Roth BL. Identification of receptor domains that modify ligand binding to 5-hydroxytryptamine2 and 5-hydroxytryptamine1c serotonin receptors. Mol. Pharmacol. 1992;42:627–633. [PubMed] [Google Scholar]
- Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler B, Zinser S, Huang S, Poe MM, Rudolph U, Cook JM, et al. Enhancing the function of alpha5-subunit-containing GABAA receptors promotes action potential firing of neocortical neurons during up-states. Eur. J. Pharmacol. 2013;703:18–24. doi: 10.1016/j.ejphar.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, et al. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Decobert M, et al. SL651498: an anxioselective compound with functional selectivity for alpha2- and alpha3-containing gamma-aminobutyric acid(A) (GABA(A)) receptors. J. Pharmacol. Exp. Ther. 2001;298:753–768. [PubMed] [Google Scholar]
- Hammarlund-Udenaes M. Active-site concentrations of chemicals - are they a better predictor of effect than plasma/organ/tissue concentrations? Basic. Clin. Pharmacol. Toxicol. 2010;106:215–220. doi: 10.1111/j.1742-7843.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Maurer TS. Influence of nonspecific brain and plasma binding on CNS exposure: implications for rational drug discovery. Biopharm. Drug. Dispos. 2002;23:327–338. doi: 10.1002/bdd.325. [DOI] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur. J. Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–152. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa A, Czobor P, Stark J, Beer B, Kostakis E, Gravielle M, et al. Selective anxiolysis produced by ocinaplon, a GABA(A) receptor modulator. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7380–7385. doi: 10.1073/pnas.0502579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- McEown K, Treit D. A2 GABAA receptor sub-units in the ventral hippocampus and α5 GABAA receptor sub-units in the dorsal hippocampus mediate anxiety and fear memory. Neuroscience. 2013;252:169–177. doi: 10.1016/j.neuroscience.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Mercolini L, Mandrioli R, Iannello C, Matrisciano F, Nicoletti F, Raggi MA. Simultaneous analysis of diazepam and its metabolites in rat plasma and brain tissue by HPLC-UV and SPE. Talanta. 2009;80:279–285. doi: 10.1016/j.talanta.2009.06.074. [DOI] [PubMed] [Google Scholar]
- Milić M, Divljaković J, Rallapalli S, van Linn ML, Timić T, Cook JM, et al. The role of α1 and α5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav. Pharmacol. 2012;23:191–197. doi: 10.1097/FBP.0b013e3283512c85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, et al. NS11394 [3'-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J. Pharmacol. Exp. Ther. 2008;327:954–968. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EØ, Larsen JS, et al. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3'-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- Munro G, Ahring PK, Mirza NR. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends. Pharmacol. Sci. 2009;30:453–459. doi: 10.1016/j.tips.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Nickolls S, Mace H, Fish R, Edye M, Gurrell R, Ivarsson M, et al. A comparison of the α2/3/5 selective positive allosteric modulators L-838,417 and TPA023 in preclinical models of inflammatory and neuropathic pain. Adv. Pharmacol. Sci. 2011;2011:608912. doi: 10.1155/2011/608912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J, Zeilhofer HU, Fritschy JM. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J. Comp. Neurol. 2012;520:3895–3911. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, et al. The anxioselective agent 7-(2-chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha1 subunit-containing GABAA receptors. J. Pharmacol. Exp. Ther. 2006;319:1244–1252. doi: 10.1124/jpet.106.107201. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rabe H, Kronbach C, Rundfeldt C, Lüddens H. The novel anxiolytic ELB139 displays selectivity to recombinant GABA(A) receptors different from diazepam. Neuropharmacology. 2007;52:796–801. doi: 10.1016/j.neuropharm.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Ramerstorfer J, Furtmüller R, Vogel E, Huck S, Sieghart W. The point mutation gamma 2F77I changes the potency and efficacy of benzodiazepine site ligands in different GABAA receptor subtypes. Eur. J. Pharmacol. 2010;636:18–27. doi: 10.1016/j.ejphar.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read KD, Braggio S. Assessing brain free fraction in early drug discovery. Expert. Opin. Drug. Metab. Toxicol. 2010;6:337–344. doi: 10.1517/17425250903559873. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug. Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Milinković MM, Rallapalli S, Clayton T, Sr, Joksimović S, Van Linn M, et al. The differential role of alpha1- and alpha5-containing GABA(A) receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. Int. J. Neuropsychopharmacol. 2009;12:1179–1193. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Majumder S, Huang S, Edwankar RV, Furtmüller R, Joksimović S, et al. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Siemiatkowski M, Sienkiewicz-Jarosz H, Członkowska AI, Bidziński A, Płaźnik A. Effects of buspirone, diazepam, and zolpidem on open field behavior, and brain [3H]muscimol binding after buspirone pretreatment. Pharmacol. Biochem. Behav. 2000;66:645–651. doi: 10.1016/s0091-3057(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends. Pharmacol. Sci. 2012;33:611–620. doi: 10.1016/j.tips.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]