Abstract
Introduction:
Metronomic chemotherapy (MC) is a novel therapeutic variation for resistant cancers, wherein chemotherapeutic drugs are administrated in low doses with no prolonged drug-free break. It lessens the level of toxicity, is better tolerated and enhances the quality of life. This retrospective analysis was undertaken to evaluate whether anatomical (computed tomography [CT]) or functional (positron emission tomography [PET]) imaging be used for response assessment in patients on MC.
Materials and Methods:
A total of 16 males and 27 females with age range of 12-83 years on MC who underwent PET/CT were assessed by new response evaluation criteria in solid tumors (RECIST 1.1) and PET response criteria in solid tumors (PERCIST 1.0).
Results:
Concordance between RECIST 1.1 and PERCIST was seen in 32 (75%) patients. There was discordance in 11 (25%) patients. In patients with discordance, the results were confirmed by follow-up imaging. PET upstaged the disease in 81% of patients (9/11) and down-staged the disease in 19% of patients (2/11).
Conclusions:
Metabolic response accurately identified the disease status as assessed by clinical or imaging follow-up. Alteration in morphology takes time to manifest, which is demonstrated by CT or magnetic resonance; whereas in MC which brings about tumor dormancy, assessing metabolic response by PET would be more appropriate. MC is usually given in palliative setting but in few cases complete metabolic response was demonstrated in our study. In such a scenario this form of treatment has the potential to become an adjunct mode of treatment in some tumors. This needs to be evaluated with larger, homogenous patient population in a prospective mode.
Keywords: Chemotherapy, computed tomography, metronomic cancer, positron emission tomography, positron emission tomography/computed tomography, positron emission tomography response criteria in solid tumors, response evaluation criteria in solid tumors, response evaluation
INTRODUCTION
Metronomic chemotherapy (MC) is an alternate strategy to fight resistant cancers. This is in contrast to the regular cytotoxic chemotherapy drugs that either damage the deoxyribonucleic acid or inhibit the microtubules and kill or inhibit the rapidly dividing cancer cells.[1] Maximum tolerated doses (MTD) of these drugs are designed to kill as many tumor cells as possible. Such doses require prolonged periods of break between successive therapies so as to allow the normal tissues to recover and also to allow recovery from the harmful side-effects. Though the cure rate is high, relapses are commonly seen in more aggressive tumors.
In the past decade, the concept that smaller individual doses other than the MTD could be used emerged; which could be more effective with lesser toxic effects. This reduces the harmful side-effects and also improves antitumor effects.[2,3,4,5] This concept of regular chemotherapy scheduling in low doses, below the MTD with no prolonged drug-free breaks is called “MC”.[2] This type of chemotherapy inhibits tumor growth by anti-angiogenic mechanisms and also reduces the undesirable side-effects. This has been proven by in vitro and in vivo preclinical studies.[1,4,6,7]
Few studies have shown that in patients with cancer that are either refractory to treatment or which have relapsed after conventional chemotherapy, MC can induce satisfactory tumor stabilization, also the duration of clinical benefit is longer with this type of low dose scheduling than the conventional chemotherapy with high doses.[8,9]
The conventional chemotherapy targets the proliferating tumor cells, whereas in the low dosage metronomic therapy the target is the endothelial cell of the growing vasculature of a tumor.[10] Thus, MC is also called as “anti-angiogenic chemotherapy” as coined by Browder et al.[10] This low dose MC is one of the most efficient “anti-angiogenic” chemotherapy and also has beneficial immunologic effects.[11,12,13] This type of treatment leads to a long term asymptomatic control of the disease by inducing angiogenic dormancy.[14]
Many clinical trials have shown favorable results in patients who were untreated or had earlier received conventional chemotherapy in various types of cancer.[15,16,17,18,19,20,21,22] Few studies have shown that no improved patient survival was seen in patients on MC,[23,24] but MC does induce tumor stabilization[3,10] and the duration of clinical benefit can be longer than the benefit obtained with the conventional chemotherapy.[8,9]
Most of these studies have assessed clinical parameters in assessing response of this low dose anti-angiogenic chemotherapy. With this background, we undertook this retrospective analysis to evaluate whether anatomic imaging modality like computed tomography (CT) scan or a functional imaging modality like positron emission tomography (PET) should be used for assessment of treatment response in patients on MC.
MATERIALS AND METHODS
All patients on metronomic therapy who were referred to our department for a PET CT study prior to start of the treatment and for a response evaluation after 12-14 weeks, between May 08 and January 12 were included in this retrospective evaluation.
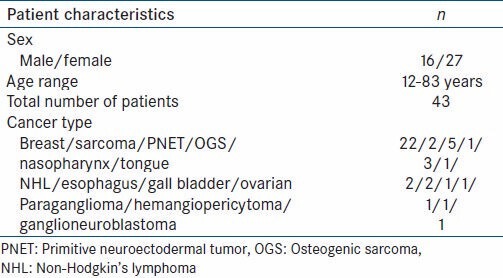
Patient characteristics
A total of 43 patients of various cancers, on metronomic therapy who underwent PET CT were assessed by CT and PET using new response evaluation criteria in solid tumors (RECIST 1.1) and PET response criteria in solid tumors (PERCIST 1.0) criteria. There were 16 males, 27 females with age range 12-83 years. There were 22 patients of breast cancer, 7 of musculoskeletal cancer (soft tissue sarcoma-2, primitive neuroectodermal tumor [PNET]-5, Osteogenic sarcoma-1), 4 of head and neck cancer (nasopharyngeal-3, tongue-1), 2 of non Hodgkins lymphoma, 2 of esophageal and 1 each of gall bladder, ovarian cancer, hemangiopericytoma, ganglioneuroblastoma and 1 a paraganglioma [Table 1].
Table 1.
Patient characteristics
Patient preparation and PET-CT imaging protocol
All patients were asked to fast for 4-6 h prior to the study and blood glucose levels were checked and confirmed to be <150 mg/dl. The studies were performed 60-90 min following intravenous administration of 5 MBq/kg of 18F-fluorodeoxyglucose (18F-FDG). Imaging was performed on a discovery ST PET-CT system (general electric medical systems). It combines a 16 slice CT scanner with a dedicated PET scanner (bismuth germanium oxide crystal).
CT was performed over 5-8 bed positions from the skull base to the mid-thigh; using multislice (16 slice) CT component of the system. CT parameters included 140 kV, 110-210 mA, 0.8 s/rotation, pitch of 1.75:1, field of view (FOV) 50 cm, length of scan 1.0-1.6 m, 0.625 spatial resolution and slice thickness of 3.75 mm. Intravenous and oral contrast was not routinely administered in all patients unless there was a specific indication and request to do so. This was followed immediately by acquisition of PET data in the same anatomic locations with 15.4 cm axial FOV acquired in 3D mode with 3 min/bed position.
Image reconstruction and interpretation
The images were reconstructed using a standard vendor provided reconstruction algorithm which incorporated ordered subset expectation maximization. Image fusion was performed using co-ordinate based fusion software and subsequently reviewed at a workstation that provided multiplanar reformatted images and displayed PET images, CT images and PET-CT fusion images.
The images were evaluated by experienced radiologist and Nuclear medicine physician blinded to each other's results. The CT findings were analyzed using the RECIST 1.1 and the PET findings by PERCIST 1.0 criteria respectively.
Any area with intensity greater than background that could not be identified as physiological activity or which on CT correlation did not fit into benign (infective/inflammatory/degenerative) was considered to be suggestive of tumor on the PET study.
The response criteria as per RECIST 1.1[25] was as follows-complete response (CR) was disappearance of all target lesions, partial response (PR) as 30% or more decrease in sum of diameters of target lesions, progressive disease (PD) as 20% or more increase in sum of diameters of target lesions and also an absolute increase of at least 5 mm and/or appearance of one or more new lesions, stable disease (SD) – who did not qualify for either sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
The therapeutic response as per PERCIST 1.0[26] was as follows–complete resolution of FDG uptake within the measurable target lesion that is less than the mean liver activity and indistinguishable from surrounding background, with appearance of no new lesion, was labeled as complete metabolic response (CMR). Reduction of minimum of 30% of standardized uptake value Lean (SULpeak) in the target volume in the same lesion as the baseline measurement was grouped under partial metabolic response (PMR). Progressive metabolic disease (PMD) was a 30% increase in the SULpeak of the FDG uptake or appearance of FDG avid new lesions that was morphologically typical of cancer. Stable metabolic disease (SMD) was disease, which did not qualify for CMR, PMR or PMD. The SUL was calculated by a 1.2 cm diameter volume region of interest (ROI) placed on the hottest site of the tumor. It was also determined whether the SULpeak of the tumor was higher than 1.5 times the liver SUL mean + 2 SDs (in a 3 cm-diameter spheric ROI in the normal right lobe of liver).
Statistical analysis
The responses were graded as follows: PD-1, SD-2, PR-3, CR-4 and non-classifiable – 0 for RECIST and PMD-1, SMD-2, PMR-3, CMR-4 and non-classifiable-0 for PERCIST. These values were statistically compared using Wilcoxon signed-rank test.
RESULTS
The response was concordant in 32 patients and discordant in 11 patients. There was concordance in 75% of patients and discordance in 25% of patients [Table 2].
Table 2.
Results
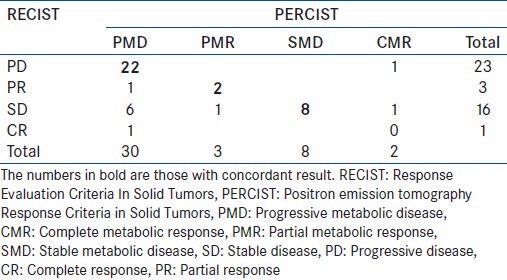
In patients with concordant result, 2 patients showed PR/PMR, 8 patients showed SD/SMD and 22 patients showed PD/PMD, as assessed by RECIST 1.1 for morphologic criteria and PERCIST for metabolic criteria, respectively.
PR/PMR was seen in two[2] patients of breast cancer as there was decrease in size and metabolic activity of the lesions by >30% [Figure 1a–c].
Figure 1.
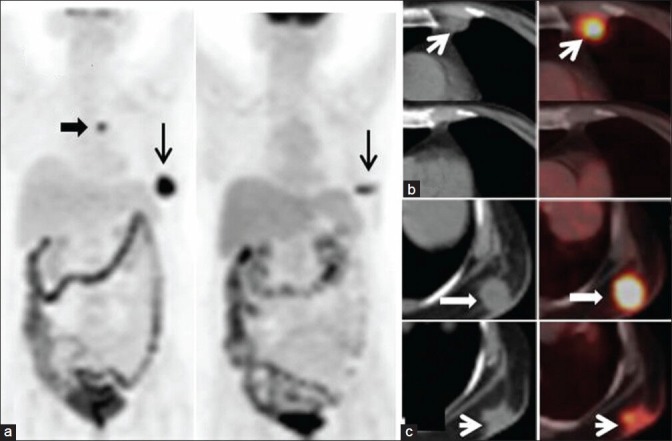
A case of carcinoma breast with partial response (concordant result). (a) Maximum Intensity Projection image shows left internal mammary node (block arrow) and left chest wall deposit (arrow). Subsequent scan shows complete resolution of the internal mammary node and partial metabolic response in the chest wall deposit (arrow). (b) Transaxial images of hypermetabolic internal mammary node (arrows) with complete metabolic and morphologic regression. (c) Transaxial images of hypermetabolic left chest wall deposit (block arrow) with residual disease (arrow)
SD/SMD was seen in 8 patients as there was no significant change in size and metabolic activity of the lesions to classify for either PR/PMR or PD/PMD.
Out of the 22 patients who showed PD/PMD by both the morphologic and metabolic criteria, 17 showed PD by virtue of new lesions, which were hypermetabolic [Figure 2a and b]. In 5 patients, there was increase in sum total of the diameter and SULpeak of the target lesions by >20% and >30% respectively.
Figure 2.
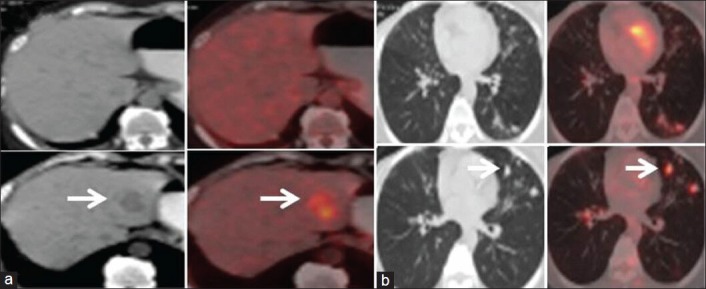
A case of carcinoma breast with new hypermetabolic lesions (concordant result). (a) Transaxial images show new hypermetabolic liver lesion in lower panel (arrows) suggesting progressive disease. (b) New hypermetabolic nodules in the left lung in the lower panel (arrow), suggesting progressive disease by new response evaluation criteria in solid tumors and PET response criteria in solid tumors
In 11 patients (25%) there was discordance in the result assessed by morphologic (RECIST 1.1) and metabolic criteria (PERCIST) [Table 3]. Totally 5 patients had SD by RECIST 1.1, of which 4 showed PMD by PERCIST. In all these 4 patients, there was an increase in the metabolism (change in SULpeak between 32 and >100%) with no significant change morphologically. On follow-up, 2 of them had PD both morphologically and metabolically on subsequent follow-up and 1 patient expired due to disease progression. In the fourth patient, the increased metabolism persisted. In 1 patient there was PMR–decrease in metabolism of liver lesions by 61.7% with no change morphologically [Figure 3]. The patient was clinically stable on 1 year follow up.
Table 3.
Results of patients with discordant result
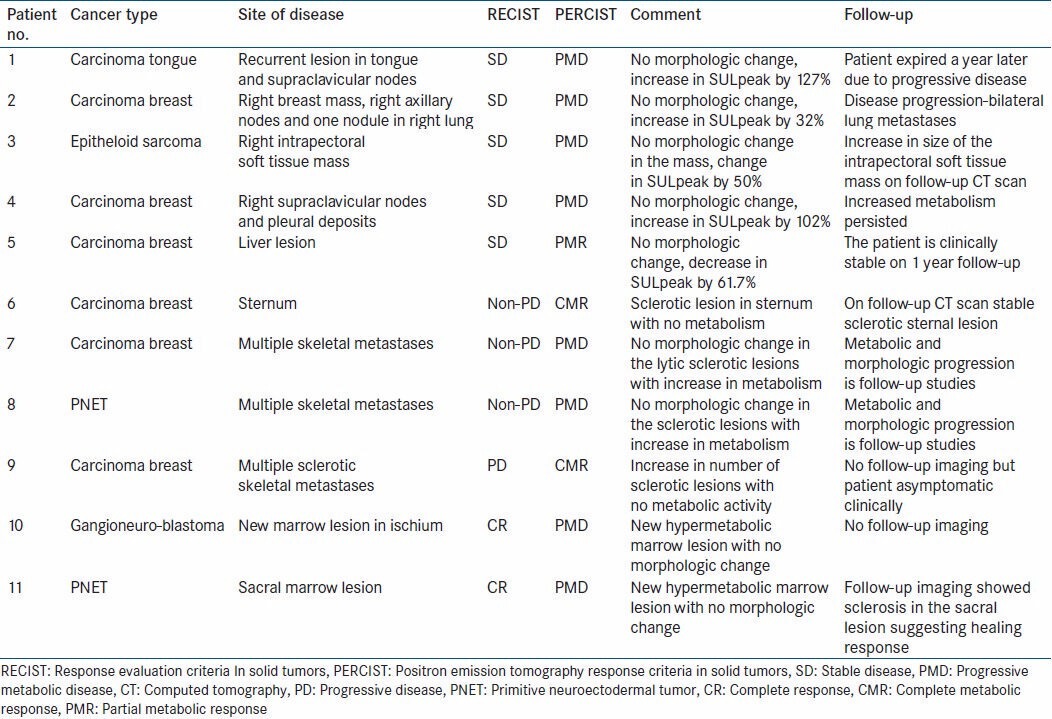
Figure 3.
Transaxial computed tomography (CT) and fused positron emission tomography CT images of a patient with carcinoma breast [patient no. 5, Table 3]. The upper panel shows a hypermetabolic liver lesion. The lower panel shows mild decrease in the uptake visually, with no significant morphologic change. Stable disease (SD) and partial metabolic response (PMR)-discordant result
In 3 patients, the disease was Non-PD (all three had sclerotic bone lesions). Out of these, 1 showed CMR as the sclerotic sternal lesion was non-metabolic. This patient on follow-up showed stable sternal sclerotic lesion. In 2 patients, there was an increase in metabolism with no morphologic change and hence showed a PMD. On follow-up imaging, both these patients had metabolic and morphologic progression of disease.
In 1 patient of breast cancer [Table 3, patient no. 9], there was increase in number of sclerotic lesions in the vertebrae and hence showed PD as per RECIST 1.1 whereas there was no metabolic activity in these lesions and showed CMR as per PERCIST. In this particular case, it is likely that PET may have missed the lesions as it is known that sclerotic lesions in patients with breast cancer may not be hypermetabolic.[27] However there is no follow-up imaging that is available, but the patient remains asymptomatic.
In a 12-year-old girl, a case of Ganglioneuroblastoma [Table 3, patient no. 10], a new hypermetabolic marrow lesion in the ischium was detected on PET (PMD) and not seen on CT with other lesions showing no morphologic change (PR).
In another patient of PNET [Table 3, patient no. 11], a sacral marrow lesion was not detected on CT but was picked up by PET- thus the disease was CR by RECIST and PMD by PERCIST. On subsequent follow up imaging when the patient was on metronomic treatment, there was sclerosis (healing response) in the sacral marrow lesion and was then evident on CT [Figure 4].
Figure 4.
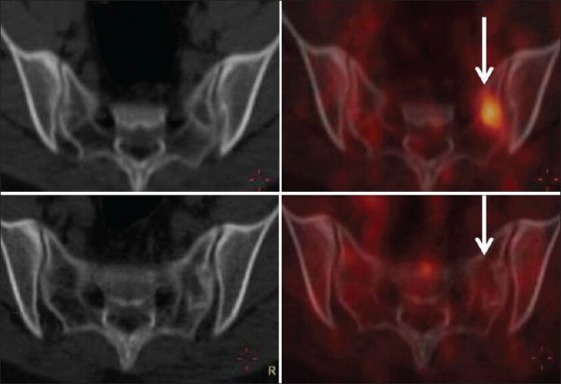
Transaxial computed tomography (CT) and fused positron emission tomography (PET) CT images of a patient with primitive neuroectodermal tumor [patient no. 11, Table 3]. The upper panel shows no lesion in the sacrum on the CT image with a hypermetabolic marrow lesion in the left sacral ala in the fused PET CT image (arrow) (complete response and progressive metabolic disease-discordant result). The lower panel is follow up study showing minimal sclerosis in the left sacral ala in the CT image with no hypermetabolism in the fused PET CT image (arrow) suggesting response
PET upstaged the disease in 81% of patients (9/11) and down staged the disease in 19% of patients (2/11).
Statistical analysis
Wilcoxon signed-rank test was used to compare the response evaluation using RECIST and PERCIST, using the above-mentioned grades. Using a 2-tailed hypothesis, the P < 0.05 (Z-0.0445, P-0.9681) and was thus not significant.
DISCUSSION
Though RECIST[25] is a widely applied tumor response criteria being dependent on morphological changes, it has limitations. As the assessment done by PET based criteria like PERCIST[26] uses metabolic changes which are closely related to changes occurring in a malignant tissue; it would be a more accurate modality for assessing response. Furthermore PET can detect metabolic changes when there are no or minimal morphological changes as seen in our study, particularly in detection of metastatic marrow lesions. These metabolic changes can be clinically relevant in correctly assessing the response post treatment.[28]
Our results show that in patients with discordant result, PET appropriately assessed the response as was evident by follow-up imaging in most patients. PET showed morphologic progression (PMD) in 8 patients; 4 out of these on follow-up imaging had both morphologic and metabolic progression of disease (patient no. 2, 3, 7, 8). One patient (patient no 1) expired due to disease progression. In one patient (patient no 4), the increased metabolism persisted.
An important observation made in this study is that, in two patients [patient no. 10, 11] marrow lesions were detected on PET not detected by CT. On subsequent follow-up imaging one of these patients [patient no 11], showed morphologic and metabolic resolution of the disease on metronomic therapy (MC). This indicates that though MC is used in a palliative setting with effective pain palliation[14] and tumor stabilization; it may also have a curative effect in few patients. Though, this needs to be confirmed with larger studies involving large patient population. This was a retrospective observational study and a statistical difference between the response evaluation by the two methods could not be achieved. This can also be attributed to the low sample size and moreover there were 32 ties (concordance) and only 11 patients showed differences in response.
It is well-known that metabolic response depicted by FDG PET-CT well precedes the anatomic response and has been well-demonstrated in few studies.[29] The PET response is depicted by a decrease in the glycolytic metabolism of a tumor unlike anatomic response criteria which are based on size of the tumor which lags weeks and months behind the metabolic response.[30]
Many newer cancer therapies including metronomic therapy are mainly cytostatic and achieving a long standing SD is the more beneficial outcome. Such effects have been seen in patients of gastrointestinal stromal tumors treated with tyrosine kinase inhibitors where though the shrinkage in size of tumor is less the survival of patients is improved with SD.[29,31]
Major limitations of this study are the retrospective nature of the data collection and heterogeneous group of patient population (the response may vary depending on the type of cancer). A better evaluation in a prospective trial setting is warranted. Also this study does not assess clinical outcomes, but was undertaken to look at better response assessing modality.
In conclusion, as this type of metronomic treatment brings about tumor or angiogenic dormancy it is natural that the treatment response criteria based on morphologic characteristics will not be evident soon after therapy and thus using functional imaging criteria like PERCIST would be the best to monitor the treatment response.
CONCLUSION
Our study shows that metabolic response is a more appropriate way of assessing the response to metronomic therapy. Tumorostatic treatment regimens like metronomic therapy where alteration in morphological features would take time to manifest, assessment of metabolic response using FDG PET would be more appropriate. We thus recommend that PET should the imaging modality of choice in response assessment of patients of metronomic therapy. Though metronomic therapy is used as a palliative therapy, if response evaluation by PET shows CR, this form of treatment has the potential to become a mode of treatment rather than palliation in some tumors and this has to be evaluated with larger, homogenous patient population in a prospective mode.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–7. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparini G. Metronomic scheduling: The future of chemotherapy? Lancet Oncol. 2001;2:733–40. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 5.Kamen BA, Rubin E, Aisner J, Glatstein E. High-time chemotherapy or high time for low dose. J Clin Oncol. 2000;18:2935–7. doi: 10.1200/JCO.2000.18.16.2935. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/metronomic chemotherapy: From the research laboratory into the oncology clinic. Ann Oncol. 2002;13:12–5. doi: 10.1093/annonc/mdf093. [DOI] [PubMed] [Google Scholar]
- 7.Sarmiento R, Gasparini G. Antiangiogenic metronomic chemotherapy. Onkologie. 2008;31:161–2. doi: 10.1159/000119925. [DOI] [PubMed] [Google Scholar]
- 8.Sterba J, Valik D, Mudry P, Kepak T, Pavelka Z, Bajciova V, et al. Combined biodifferentiating and antiangiogenic oral metronomic therapy is feasible and effective in relapsed solid tumors in children: Single-center pilot study. Onkologie. 2006;29:308–13. doi: 10.1159/000093474. [DOI] [PubMed] [Google Scholar]
- 9.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–81. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 10.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 11.Pasquier E, André N, Braguer D. Targeting microtubules to inhibit angiogenesis and disrupt tumour vasculature: Implications for cancer treatment. Curr Cancer Drug Targets. 2007;7:566–81. doi: 10.2174/156800907781662266. [DOI] [PubMed] [Google Scholar]
- 12.Mabeta P, Pepper MS. A comparative study on the anti-angiogenic effects of DNA-damaging and cytoskeletal-disrupting agents. Angiogenesis. 2009;12:81–90. doi: 10.1007/s10456-009-9134-8. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69:6987–94. doi: 10.1158/0008-5472.CAN-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil V, Noronha V, D'cruz AK, Banavali SD, Prabhash K. Metronomic chemotherapy in advanced oral cancers. J Cancer Res Ther. 2012;8(Suppl 1):S106–10. doi: 10.4103/0973-1482.92223. [DOI] [PubMed] [Google Scholar]
- 15.Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, Sathornsumetee S, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br J Cancer. 2009;101:1986–94. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brizzi MP, Berruti A, Ferrero A, Milanesi E, Volante M, Castiglione F, et al. Continuous 5-fluorouracil infusion plus long acting octreotide in advanced well-differentiated neuroendocrine carcinomas. A phase II trial of the Piemonte oncology network. BMC Cancer. 2009;9:388. doi: 10.1186/1471-2407-9-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res. 2006;12:3092–8. doi: 10.1158/1078-0432.CCR-05-2255. [DOI] [PubMed] [Google Scholar]
- 18.Steinbild S, Arends J, Medinger M, Häring B, Frost A, Drevs J, et al. Metronomic antiangiogenic therapy with capecitabine and celecoxib in advanced tumor patients - Results of a phase II study. Onkologie. 2007;30:629–35. doi: 10.1159/000110580. [DOI] [PubMed] [Google Scholar]
- 19.Fontana A, Galli L, Fioravanti A, Orlandi P, Galli C, Landi L, et al. Clinical and pharmacodynamic evaluation of metronomic cyclophosphamide, celecoxib, and dexamethasone in advanced hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:4954–62. doi: 10.1158/1078-0432.CCR-08-3317. [DOI] [PubMed] [Google Scholar]
- 20.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: A trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 21.Wong NS, Buckman RA, Clemons M, Verma S, Dent S, Trudeau ME, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28:723–30. doi: 10.1200/JCO.2009.24.0143. [DOI] [PubMed] [Google Scholar]
- 22.Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, et al. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: Antitumor activity and biological effects. Ann Oncol. 2006;17:232–8. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 23.Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9:354–63. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krzyzanowska MK, Tannock IF, Lockwood G, Knox J, Moore M, Bjarnason GA. A phase II trial of continuous low-dose oral cyclophosphamide and celecoxib in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2007;60:135–41. doi: 10.1007/s00280-006-0347-x. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35:135–42. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 29.Van den Abbeele AD. The lessons of GIST - PET and PET/CT: A new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13. doi: 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]
- 30.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–4. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]


