Abstract
The regulatory information for phenotype, proliferation, and growth of normal and tumor cells must be maintained through genome replication in the S-phase and cell division during mitosis. Epigenetic mechanisms that include DNA methylation, posttranslational modifications of histones, selective utilization of histone variants, and inheritable RNA molecules play pivotal roles in maintaining cellular identity through mitotic divisions. Recent studies demonstrate that mitotic occupancy of genes, which are determinants of cell fate, growth and proliferation, by lineage restricted transcription factors is a key epigenetic mechanism for retention and transmission of cellular expression memory. Evidence is emerging for the presence of distinct transcriptional regulatory microenvironments in mitotic chromosomes where the genes bookmarked for reactivation post-mitotically reside. Importantly, some oncoproteins are present in mitotic microenvironments where they occupy target genes during mitosis and may contribute to perpetuating the transformed phenotype. We will discuss emerging regulatory implications of epigenetically bookmarking genes during mitosis for physiological control as well as for the onset and progression of cancer.
INTRODUCTION
Mitosis, the process of cell division, poses a challenge to maintain cellular identity. In normal diploid cells, lineage-restricted transcription must resume in an efficient and faithful manner following mitosis. In tumor and transformed cells, there is an equivalently stringent requirement to sustain the cancer phenotype through successive cell divisions. Genetic information plays a key role in maintaining cellular identity, but it is becoming increasingly evident that epigenetic mechanism(s) are pivotal to sustaining cellular memory. Studies over the past decade have provided insights into epigenetic mechanisms that maintain cellular identity in lineage-committed as well as in cancer cells. Mitotically heritable post-translational modifications and stoichiometry among variants of histone proteins, as well as methylation of CpG islands present in DNA regulatory elements of developmental, phenotypic and cancer-related genes are key in marking genes for reactivation immediately after cell division. These epigenetic mechanisms and their roles in heritable cellular memory are well established and have been reviewed [(1–4)]. Here, we will focus on mitotic bookmarking of genes by transcription factors as an emerging epigenetic mechanism that sustains cellular memory. As discussed below, much of our current understanding of the mitotic gene bookmarking stems from studies carried out in cell differentiation and proliferation models. We will present the case that similar bookmarking mechanism may be operative in the onset, progression and maintenance of cancer phenotype.
MITOTIC CHROMOSOMAL MICROENVIRONMENT
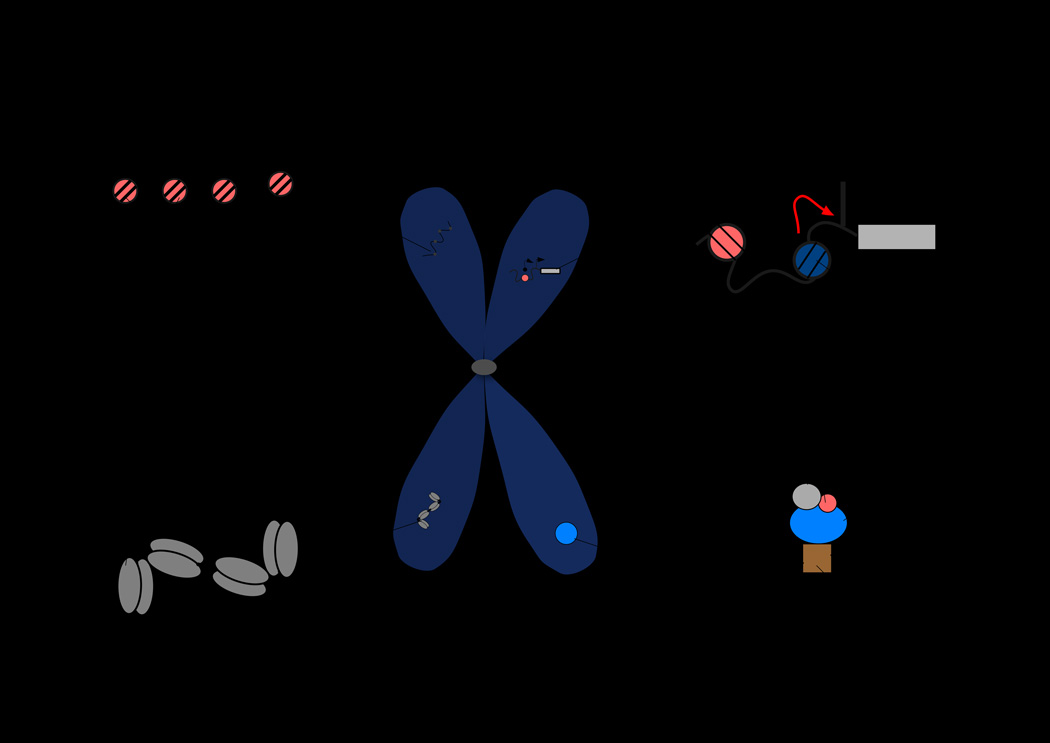
Chromatin reconfiguration during replication and mitosis poses a major regulatory challenge for cells to perpetuate identity as well as transcriptional regulatory commitment through architectural remodeling that includes chromatin unfolding, replication, condensation and segregation. Compared to replication, chromatin undergoes striking structural and physical changes during mitosis; the 10nm nucleosomal fiber condenses more than 70-fold to configure mitotic chromosomes [reviewed in (5)]. The highly compacted chromatin organization of mitotic chromosomes, combined with historically significant observations from pulse-chase radiolabelling approaches suggested that transcription is universally inhibited during mitosis, and that the condensed chromosomes are not readily accessible to nuclear proteins [(6,7)]. Subsequently, experiments carried out on pure mitotic cell populations demonstrated selective nuclease accessibility of mitotic chromosomes, suggesting that components of the mitotic chromatin may not be transcriptionally inert [(8)]. Ligation-mediated polymerase chain reaction studies of specific gene promoters during mitosis further corroborated the observation that regions within mitotic chromosomes are accessible to regulatory proteins [(9)]. Evidence is emerging for specific regulatory microenvironments present in mitotic chromosomes where genes involved in biological control and cancer are bookmarked for rapid reactivation post-mitotically (Figure 1).
Figure 1. Distinct epigenetic regulatory microenvironments for gene bookmarking that are present in mitotic chromosomes.
Emerging evidence suggest that mitotic chromosomes exhibit distinct epigenetic regulatory microenvironment where various mechanisms of gene bookmarking are operative. These include A) incorporation of histone H3.3 variant into genes that are bookmarked for reactivation post-mitotically, B) `incorporation of histone H2A.Z into nucleosome closer to transcription start site (TSS) and its sliding on to TSS prior to mitosis for post-mitotic expression, C) maintenance of nuclease accessible regions by cohesin where transcription factors are recruited after cell division, and 4) target gene occupancy by sequence transcription factors for post-mitotic gene activation. These microenvironments are not mutually exclusive and may function in concert with other epigenetic mechanisms (e.g., DNA methylation) for maintenance of cellular potential for lineage commitment, growth and proliferation or even disease initiation, progression and perpetuation.
A. Properties of Nuclear Proteins that Bookmark Genes in Mitosis
Recent advances in high-resolution microscopy as well as genome-wide biochemical approaches (e.g., chromatin immunoprecipitation followed by high throughput sequencing) have provided an unbiased and comprehensive assessment of transcriptional dynamics in mitotic cells. Accruing evidence from studies in a variety of lineages suggest that transcription factors that play essential roles in conferring lineage identity often remain associated with target genes on mitotic chromosomes. The evidence is limited but compelling. Here we outline some of the general properties of nuclear proteins that are retained with target genes during mitosis:
I. Sequence Specificity
Although it has been shown that many sequence specific transcription factors are displaced from condensed chromosomes during mitosis [(10)], several functionally significant nuclear proteins remain associated with mitotic chromosomes. Mitotically retained nuclear proteins include components of basal transcription machinery, co-regulatory proteins that include activators, suppressors and chromatin remodeling factors, as well as lineage-restricted transcription factors that interact with cognate binding motifs in regulatory elements of target genes. The Runx (AML/Cbfα) family of transcription factors is an initial example of mitotically retained nuclear regulatory proteins [(11)]. Runx factors share a highly conserved DNA binding domain (designated Runt homology domain for its homology to the Drosophila Runt protein) and exhibit recognition of a unique DNA binding motif within the regulatory regions of target genes [(12)]. Recently, it has been shown that the liver-related transcription factor FoxA1 and the hematopoietic regulator GATA1 are among many transcription factors that bind to lineage-restricted target genes during mitosis [(13,14)]. These sequence specific interactions mediate post-mitotic reactivation of their respective differentiation programs. With few exceptions [(15), (16)], most mitotically retained proteins exhibit sequence specific recognition of regulatory DNA elements in target genes, indicating that sequence specificity is an important property of nuclear regulatory proteins that remain associated with mitotic chromosomes.
II. Scaffolding Proteins
Another shared property of transcription factors that bookmark target genes during mitosis is that these proteins reside in specific nuclear microenvironments in the interphase nucleus and assemble multi-protein regulatory complexes at strategic sites of target gene promoters that often contain chromatin remodeling factors, co-activators and co-suppressors [(17)]. To what extent the cohort of co-regulatory proteins is retained on mitotic chromosomes with the scaffolding proteins remains to be determined. Experimental evidence is provided by the Runx2 transcription factor. Runx2 coordinates cell proliferation, growth and differentiation by regulating both the RNA Pol I and RNA Pol II genes [(18,19)]. During mitosis, Runx2 selectively occupies target genes regulated by RNA Pol II, as well as the RNA Pol I ribosomal RNA genes. Biochemical and in situ studies reveal that Runx2 remains associated with TLE2, a co-suppressor of Runx target genes, but dissociates from HDAC1, another co-regulator [(20)]. Additional examples of co-regulatory proteins without known DNA binding activity include bromodomain containing BRD proteins that recognize acetylated histones and remain associated with mitotic chromosomes [(15,16,21)]. Global proteomics and multiplex in situ studies are warranted to further explore the complete cohort of co-regulators that remain associated with mitotically retained transcription factors.
III. Lineage Master Regulators
Most of the transcription factors that bookmark target genes during mitosis are also master regulators of respective lineages. For example, GATA1, which remains associated with target genes during mitosis for their reactivation after cell division, is a master regulator that controls myeloid lineage differentiation from hematopoietic stem cells [(13,22)]. Similarly, FoxA1 regulates specification of liver cell differentiation [(14,23)]. Other examples include Runx factors that regulate the hematopoietic (Runx1), osteogenic (Runx2), and gastro-intestinal/neurogenic (Runx3) lineages, the key adipogenic regulator C/EBPα, and MyoD, the master regulator of muscle differentiation [(11,12,18,24–29)]. These observations raise an interesting possibility that lineage identity is, in part, sustained by mitotic gene bookmarking. Additional studies are required to establish whether mitotic bookmarking of genes is a common property of all lineage determining transcription factors.
IV. Dynamics of Mitotic Bookmarking
It is becoming increasingly evident that mitotic bookmarking of target genes for rapid activation is a shared property of phenotypic transcription factors. However, the mechanistic underpinnings of the process remain underexplored. Insights into the process have been provided by live cell imaging experiments that combined the power of live cell microscopy and fluorescence recovery after bleach to establish kinetics of gene reactivation post-mitotically [(21,30,31)]. For example, Spector and colleagues have shown that specific gene loci that are active prior to mitosis exhibit increased kinetics of activation in post-mitotic cells. Recruitment kinetics for the RNA Pol II and chromatin modifier BRD4 are different in interphase and mitotic cells. Mechanistically, enhanced BRD4 recruitment results from increased levels of H4K5Ac on the previously activated locus. BRD4 accelerated the dynamics of messenger RNA synthesis by de-compacting chromatin and hence facilitating transcriptional re-activation. Previous studies showed that components of the transcriptional regulatory and RNA processing machineries sequentially enter the telophase nuclei after the nuclear envelope is reformed [(31)]. These studies reveal that genes activated prior to mitosis are marked for rapid reactivation following mitosis, and the machinery is assembled in situ to resume transcription immediately after cell division [(30)]. Such single cell studies elucidate kinetics of gene activation following mitosis. However, use of GFP for live cell microscopy requires the expression of artificially constructed transgenes are ectopically expressed in cells above physiological levels. It remains to be established if the endogenous proteins under physiological conditions follow similar kinetics. Additional considerations include quantitative determination of transcription factor concentration/association/dissociation analyses and mobility of proteins involved in epigenetic bookmarking.
B. Properties of Genes that are Bookmarked in Mitosis
As discussed above, for decades, it had been assumed that highly compact and condensed mitotic chromosomes are not accessible to non-histone proteins to the chromatin. It has, however, become increasingly evident that despite compaction, some regions of the mitotic chromosomes remain in an open conformation and accessible to regulatory proteins and that the mitotically bookmarked genes share several properties:
I. Nuclease Accessible
In mid 70s, pulse-chase radiolabelling experiments on mitotic chromosomes of kangaroo rat revealed that the compacted mitotic chromosomes were indeed amenable to nuclease digestion [(8)]. Subsequent studies using LM-PCR identified several genes, including Hsp70 and Myc among others that were accessible to nuclease and were occupied by regulatory proteins [reviewed in (32)]. For example, the Myc gene, which is repressed during mitosis, is rapidly re-activated after cells divide. Many sequence-specific proteins that recognize and bind to single-stranded DNA (e.g. hnRNP K and FBP) regulate Myc gene transcription. Interestingly, several genes that are rapidly reactivated after mitosis are selectively sensitive to permanganate only during mitosis, but not in other stages of the cell cycle. These observations indicate a unique and selective behavior of bookmarked genes during mitosis, where they are accessible to single-strand DNA binding proteins for rapid reactivation post-mitotically. Genome-wide studies are required to assess whether nuclease accessibility is a common feature of all mitotically bookmarked genes.
II. Enrichment of Selective Histone Modifications and/or Histone Variants H2A.Z and H3.3 in Gene Promoters
There is emerging evidence that the nucleosomes of bookmarked genes are enriched in histone variants, particularly in H2A.Z and H3.3. Gurdon and colleagues have shown that the memory for the myogenic gene MyoD expression, where histone H3.3 has been incorporated into the nucleosomes, can persist through 24 cell divisions in the absence of transcription. Mechanistically, the association of a mutated histone H3.3, which lacks the methylatable H3.3 lysine 4, with promoter DNA eliminates memory, indicating a requirement of H3.3 K4 for memory [(33)]. An alternate mechanism of gene bookmarking by histone variants in provided by genome-wide association of H2A.Z [(34)]. Jones and colleagues have shown that in active gene promoters +1 nucleosomes (immediately downstream the transcription start sites or TSSs) containing H2A.Z shift upstream to occupy TSSs during mitosis, significantly reducing nucleosome-depleted regions. This change appears to be specific to active genes that are silenced during mitosis and rapidly reactivated post-mitotically (e. g. GRP78 gene). Importantly, these authors find that trimethylation of lysine 4 at histone H3 (H3K4me3) is also maintained enriched at these promoters during mitosis, whereas other epigenetic markers of active chromatin are lost.
Histone modifications play a key role in epigenetically regulating gene expression in the interphase cells and there role in bookmarking genes during mitosis has been well-studied [reviewed in (1)]. Additional evidence for a role of histone modifications in mitotic gene bookmarking is suggested by observations at the level of a higher order chromosome architecture [(35)]. In this study, Turner and colleagues have found a striking similarity in the distribution patterns of histone H3K4me3 between interphase and metaphase cells. It is notable that the methylation of histone H3 at lysine 4, in combination with histone H2A.Z variant, provides a mark for activation-associated acetylation of genes in the interphase [(36)]. Although it remains to be investigated whether a gene that is active in G2 cells is selectively marked during mitosis via H3K4me3 modification, and is acetylated prior to reactivation in G1 cells, these observations suggest that a mitosis-specific histone code may, in part, orchestrate gene bookmarking. Together, these observations indicate that various chromatin marks that include selective incorporation of histone variants and retention of specific epigenetic post-translational modifications contribute to mitotic gene bookmarking.
III. Demarking by Cohesin
Is bookmarking of genes during mitosis a universal phenomenon? It is unclear, and unlikely, that all genes that are rapidly reactivated post-mitotically are occupied by transcription factors during mitosis. In fact, it has been shown that many sequence-specific transcription factors are selectively displaced from mitotic chromosomes [(10)]. Displacement of transcription factors from mitotic chromosomes indicates the presence of additional epigenetic mechanisms that do not involve occupancy of target genes by transcription factors. Recently, genome-wide chromatin immunoprecipitation studies followed by high throughput sequencing have revealed binding patterns for hundreds of transcription factors [(37)]. These studies show that bound transcription factors are highly clustered and that these clusters are enriched in binding motifs for several major transcription factor classes. Strikingly, most clusters are formed around cohesin, a protein required for chromosome condensation during mitosis [(38)]. Mechanistically, cohesin plays two key roles: during the S-phase of the cell cycle, it holds the replicating strands together at the TF cluster sites, and during mitosis, it remains bound to the clusters even when the transcription factors have been displaced from target genes. Functionally, loss of cohesin decreases both DNA accessibility and binding of TFs to clusters. These results provide a mechanistic explanation of decades-long observation that mitotic chromosomes are nuclease accessible [(8)]. Furthermore, these observations suggest that cohesin-binding promotes re-establishment of TF clusters and reactivation of target genes after DNA replication as well as after mitosis, thus providing a secondary mechanism for maintaining cellular memory through cell divisions.
IMPLICATIONS OF MITOTIC GENE BOOKMARKING
A. Sustained Lineage Identity
In committed cells, lineage identity must be sustained as cells replicate and equally distribute genetic information to progeny. Mitotic gene bookmarking by transcription factors, in combination with DNA methylation and histone modifications as well as selective incorporation of histone variants in the chromatin of bookmarked genes, provides a mechanistic basis for sustained lineage identity through cell divisions. Studies over the past decade have demonstrated that several phenotypic transcription factors remain associated with target genes during mitosis for rapid transcriptional control post-mitotically. So far, more than 15 transcription factors including many lineage determining factors have been reported to be associated with mitotic chromosomes [Reviewed in (39)]. Examples include the basic helix-loop-helix myogenic regulatory factors in muscle cell differentiation, CCAAT/enhancer-binding protein α in the adipocyte differentiation program, FoxA1 in liver cells, GATA1 and Runx1 in hematopoietic lineage differentiation, and Runx2 in osteoblast differentiation [(11,13,14,24,25,27)]. Frequently, these phenotypic transcription factors associate with selected target genes involved in cell growth, proliferation and differentiation, thus coordinating maintenance of not only lineage identity, but also cellular potential for a physiological balance between growth and proliferation. Despite the growing list of mitotically retained transcription factors, our current understanding for functional role(s) of mitotic gene bookmarking remains minimal. For example, it is not clear if mitotic occupancy of a target gene by the transcription factor is a prerequisite for reactivation post-mitotically. Furthermore, are all mitotically retained transcription factors functional immediately after mitosis or the mitotic retention is merely a mechanism to equally distribute regulatory proteins to progeny cells? Genome-wide experimental approaches involving endogenous transcription factors in biologically relevant systems will provide mechanistic insights into functional relevance of mitotic gene bookmarking in maintaining epigenetic cell memory in progeny cells.
B. Maintenance of Stem Cell Niches
It is hypothesized that, in contrast to embryonic stem cells, adult stem cells undergo at least one round of asymmetric cell division to generate lineage-committed cells and maintain the stem cell pool. We have discussed recent observations that lineage determining transcription factors associate with target genes during symmetrical cell division to maintain cellular identity. We suggest a requirement for asymmetric bookmarking of target genes in lineage committed stem cells by phenotypic transcription factors in adult stem cells or by oncogenes in cancer stem cells to generate committed or transformed cells. Despite broad based relevance, asymmetric cell division has been predominantly studied in yeast or Drosophila. For example, in budding yeast, the Ace2 chromatin-remodeling factor is asymmetrically accumulated in the nucleus of the daughter cell, perhaps regulating gene expression that is restricted to the progeny cell [(40)]. During Drosophila neurogenesis, Numb, an attenuator of Notch signaling, and the Prospero transcription factor are selectively distributed to progeny cells as a mechanism to maintain the stem cell population and to give rise to committed cells [(41)]. Similar mechanisms may be operative in mammalian cells. In fact, there is compelling evidence for asymmetric orientation of mitotic spindle in gut epithelium as well as in muscle stem cells that dictates lineage commitment as well as maintenance of stem cell pool [reviewed in (42–44)]. However, experimental evidence and a model system to establish the existence of asymmetrical mitotic gene bookmarking in mammals is lacking. To which extent cancer stem cells undergo asymmetric and/or symmetric cell division remains open ended and clinically and biologically relevant. This may have important implications for developing therapeutic strategies that target the persistent tumor progenitor pool or actively dividing tumor cells that have escaped from the cancer stem cell niche. Despite current experimental limitations, epigenetic bookmarking provides a flexible, yet cell type specific and context dependent mechanistic dimension to gene regulation.
C. Propagation of Disease Phenotype
Evidence is accruing that mitotic gene bookmarking may have an important role in the onset, progression, and perpetuation of disease. This is illustrated by the leukemic fusion protein AML1-ETO that blocks myeloid differentiation and enhances proliferative potential [(27)]. Interestingly, mitotic association of the leukemic AML1-ETO with the ribosomal RNA (rRNA) genes, as well as with genes controlling cell proliferation and myeloid cell differentiation upregulates rRNA and cell proliferation-related genes, but downregulates gene mediating myeloid cell differentiation, promoting and/or supporting transformed phenotype. Another recent example of cancer-related mitotic gene bookmarking is the mixed lineage leukemia protein (MLL). MLL is a chromatin remodeling factor that is associated with leukemia and regulates transcription by recruiting chromatin modifying machinery to target genes. This mitotic retention favors rapid reactivation of target genes required for the onset and progression of MLL post-mitotically [(45)]. It will be informative to establish whether mitotic bookmarking of cancer-related genes is a shared trait of all sequence-specific oncogenic proteins.
CONCLUDING REMARKS
Mitotic bookmarking of target genes by sequence specific transcription factors is emerging as a frequently invoked epigenetic mechanism to sustain lineage identity in normal cells and to perpetuate the transformed phenotype. Further insights into mechanistically understanding bookmarking requires addressing questions that include: 1) Is mitotic retention of regulatory proteins a transcriptional regulatory mechanism to reactivate gene expression post-mitotically, or some mitotically retained proteins merely use dividing chromosomes as vehicles for equal distribution into progeny cells? 2) Do signaling pathways operative prior to mitosis dictate which genes will be bookmarked? 3) What are the dynamics of co-regulatory protein complex organization and activity during mitosis? 4) Does the cohort of co-regulatory factors that remain associated with transcription factors define the post-mitotic transcriptional status of bookmarked genes? 5) Does colocalization of genes in chromatin microenvironment contribute to mitotic gene bookmarking? 6) Can the accumulation of transcription factors on mitotic chromosomes be therapeutically targeted in dividing cells? Additional studies will be necessary to further elucidate a functional link between mitotic gene bookmarking and maintenance of cellular memory within the context of biological control and pathology of cancer.
Acknowledgements
Studies that were conducted in authors’ laboratories and discussed here were in part supported by the grants from the National Institutes of Health (P01 CA082834 and P01 AR48818 to GSS.; R01 AR039588 to GSS and JBL.; R03 CA167726 to SKZ) and FONDAP (15090007 to MM).
References
- 1.Wang F, Higgins JMG. Histone modifications and mitosis: countermarks, landmarks, and bookmarks. Trends in Cell Biology. 2012 doi: 10.1016/j.tcb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 4.Sarkies P, Sale JE. Cellular epigenetic stability and cancer. Trends in Genetics. Elsevier Ltd. 2012;28:1–10. doi: 10.1016/j.tig.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.New Insight into the Mitotic Chromosome Structure: Irregular Folding of Nucleosome Fibers Without 30-nm Chromatin Structure. Cold Spring Harbor Symposia on Quantitative Biology. 2011;75:439–444. doi: 10.1101/sqb.2010.75.034. (null), (null), (null) [DOI] [PubMed] [Google Scholar]
- 6.Taylor JH. NUCLEIC ACID SYNTHESIS IN RELATION TO THE CELL DIVISION CYCLE*. Ann. N. Y. Acad. Sci. Wiley Online Library. 1960;90:409–421. doi: 10.1111/j.1749-6632.1960.tb23259.x. [DOI] [PubMed] [Google Scholar]
- 7.Prescott DM, Bender MA. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp. Cell Res. 1962;26:260–268. doi: 10.1016/0014-4827(62)90176-3. [DOI] [PubMed] [Google Scholar]
- 8.Bostock CJ, Christie S, Hatch FT. Accessibility of DNA in condensed chromatin to nuclease digestion. Nature. 1976;262:516–519. doi: 10.1038/262516a0. [DOI] [PubMed] [Google Scholar]
- 9.Xing H. Mechanism of hsp70i Gene Bookmarking. Science. 2005;307:421–423. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Balbás MA, Dey A, Rabindran SK, Ozato K, Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83:29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi SK, Young DW, Pockwinse SM, Javed A, Lian JB, Stein JL, et al. Mitotic partitioning and selective reorganization of tissue-specific transcription factors in progeny cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14852–14857. doi: 10.1073/pnas.2533076100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto F, Lübbert M, Stock M. Upstream and downstream targets of RUNX proteins. J. Cell. Biochem. 2003;89:9–18. doi: 10.1002/jcb.10491. [DOI] [PubMed] [Google Scholar]
- 13.Blobel SKMUJPJADJYCRHG, Udugama MI, Pawlicki JM, Achtman JC, Jain DP, Cheng Y, et al. Tissue-Specific Mitotic Bookmarking by Hematopoietic Transcription Factor GATA1. Cell. Elsevier Inc; 2012;150:725–737. doi: 10.1016/j.cell.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caravaca JM, Donahue G, Becker JS, He X, Vinson C, Zaret KS. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes & Development. 2013;27:251–260. doi: 10.1101/gad.206458.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey A, Ellenberg J, Farina A, Coleman AE, Maruyama T, Sciortino S, et al. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. Mol. Cell. Biol. 2000;20:6537–6549. doi: 10.1128/mcb.20.17.6537-6549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8758–8763. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidi SK, Young DW, Javed A, Pratap J, Montecino MA, van Wijnen AJ, et al. Nuclear microenvironments in biological control and cancer. Nat Rev Cancer. 2007;7:454–463. doi: 10.1038/nrc2149. [DOI] [PubMed] [Google Scholar]
- 18.Young DW, Hassan MQ, Pratap J, Galindo M, Zaidi SK, Lee S-H, et al. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007;445:442–446. doi: 10.1038/nature05473. [DOI] [PubMed] [Google Scholar]
- 19.Young DW, Hassan MQ, Yang X, Galindo M, Javed A, Zaidi SK, et al. Mitotic retention of gene expression patterns by the cell fate-determining transcription factor Runx2. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3189–3194. doi: 10.1073/pnas.0611419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali SA, Dobson JR, Lian JB, Stein JL, van Wijnen AJ, Zaidi SK, et al. A Runx2-HDAC1 co-repressor complex regulates rRNA gene expression by modulating UBF acetylation. Journal of Cell Science. 2012 doi: 10.1242/jcs.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell. 2009;20:4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliaccio AR, Rana RA, Vannucchi AM, Manzoli FA. Role of GATA-1 in normal and neoplastic hemopoiesis. Ann. N. Y. Acad. Sci. 2005;1044:142–158. doi: 10.1196/annals.1349.019. [DOI] [PubMed] [Google Scholar]
- 23.Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. CMLS, Cell. Mol. Life Sci. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali SA, Zaidi SK, Dacwag CS, Salma N, Young DW, Shakoori AR, et al. Phenotypic transcription factors epigenetically mediate cell growth control. Proceedings of the National Academy of Sciences. 2008;105:6632–6637. doi: 10.1073/pnas.0800970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes & Development. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Seminars in Cell & Developmental Biology. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Bakshi R, Zaidi SK, Pande S, Hassan MQ, Young DW, Montecino M, et al. The leukemogenic t(8;21) fusion protein AML1-ETO controls rRNA genes and associates with nucleolar-organizing regions at mitotic chromosomes. Journal of Cell Science. 2008;121:3981–3990. doi: 10.1242/jcs.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pande S, Ali SA, Dowdy CR, Zaidi SK, Ito K, Ito Y, et al. Subnuclear targeting of the Runx3 tumor suppressor and its epigenetic association with mitotic chromosomes. J. Cell. Physiol. 2009;218:473–479. doi: 10.1002/jcp.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes & Development. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 30.Zhao R, Nakamura T, Fu Y, Lazar Z, Spector DL. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nature Publishing Group. 2011;13:1295–1304. doi: 10.1038/ncb2341. Nature Publishing Group; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasanth KV, Sacco-Bubulya PA, Prasanth SG, Spector DL. Sequential entry of components of the gene expression machinery into daughter nuclei. Mol. Biol. Cell. 2003;14:1043–1057. doi: 10.1091/mbc.E02-10-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John S, Workman JL. Bookmarking genes for activation in condensed mitotic chromosomes. Bioessays. 1998;20:275–279. doi: 10.1002/(SICI)1521-1878(199804)20:4<275::AID-BIES1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 33.Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol. 2007;10:102–109. doi: 10.1038/ncb1674. [DOI] [PubMed] [Google Scholar]
- 34.Kelly TK, Miranda TB, Liang G, Berman BP, Lin JC, Tanay A, et al. H2A.Z Maintenance during Mitosis Reveals Nucleosome Shifting on Mitotically Silenced Genes. Molecular Cell. 2010;39:901–911. doi: 10.1016/j.molcel.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terrenoire E, McRonald F, Halsall JA, Page P. Immunostaining of modified histones defines high-level features of the human metaphase epigenome. Genome. 2010 doi: 10.1186/gb-2010-11-11-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Enge M, Whitington T, Dave K, Liu J, Sur I, et al. Transcription Factor Binding in Human Cells Occurs in Dense Clusters Formed around Cohesin Anchor Sites. Cell. Elsevier Inc; 2013;154:801–813. doi: 10.1016/j.cell.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Rappsilber J, Earnshaw WC. Building mitotic chromosomes. Current Opinion in Cell Biology. Elsevier Ltd; 2011;23:114–121. doi: 10.1016/j.ceb.2010.09.009. (null), (null), (null) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenetics & Chromatin. 2013;6:1–1. doi: 10.1186/1756-8935-6-6. Epigenetics & Chromatin; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Talia S, Wang H, Skotheim JM, Rosebrock AP, Futcher B, Cross FR. Daughter-Specific Transcription Factors Regulate Cell Size Control in Budding Yeast. PLoS Biol. Public Library of Science; 2009;7:e1000221. doi: 10.1371/journal.pbio.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirata J, Nakagoshi H, Nabeshima Y, Matsuzaki F. Asymmetric segregation of the homeodomain protein Prospero during Drosophila development. Nature. 1995;377:627–630. doi: 10.1038/377627a0. [DOI] [PubMed] [Google Scholar]
- 42.Aakre CD, Laub MT. Asymmetric cell division: a persistent issue? Developmental Cell. 2012;22:235–236. doi: 10.1016/j.devcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roegiers F, Jan YN. Asymmetric cell division. Current Opinion in Cell Biology. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Blobel GA, Kadauke S, Wang E, Lau AW, Zuber J, Chou MM, et al. A reconfigured pattern of MLL occupancy within mitotic chromatin promotes rapid transcriptional reactivation following mitotic exit. Molecular Cell. 2009;36:970–983. doi: 10.1016/j.molcel.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]