Abstract
Current therapies for attention deficit hyperactivity disorder (ADHD) have varying efficacy in individuals with fetal alcohol spectrum disorders (FASD), suggesting that alternative therapeutics are needed. Developmental exposure to ethanol produces changes in dopamine (DA) systems, and DA has also been implicated in ADHD pathology. In the current study, lobeline, which interacts with proteins in dopaminergic presynaptic terminals, was evaluated for its ability to attenuate neonatal ethanol-induced locomotor hyperactivity and alterations in dopamine transporter (DAT) function in striatum and prefrontal cortex (PFC). From postnatal days (PND) 1–7, male and female rat pups were intubated twice daily with either 3 g/kg ethanol or milk, or were not intubated (non-intubated control) as a model for “third trimester” ethanol exposure. On PND 21 and 22, pups received acute lobe-line (0, 0.3, 1, or 3 mg/kg), and locomotor activity was assessed. On PND 23–25, pups again received an acute injection of lobeline (1 or 3 mg/kg), and DAT kinetic parameters (Km and Vmax) were determined. Results demonstrated that neonatal ethanol produced locomotor hyperactivity on PND 21 that was reversed by lobeline (1 and 3 mg/kg). Although striatal DAT function was not altered by neonatal ethanol or acute lobeline, neonatal ethanol exposure increased the Vmax for DAT in the PFC, suggesting an increase in DAT function in PFC. Lobeline ameliorated this effect on PFC Vmax at the same doses that decreased hyperactivity. Methylphenidate, the gold standard therapeutic for ADHD, was also evaluated for comparison with lobeline. Methylphenidate decreased DAT Vmax and Km in PFC from ethanol-treated pups. Thus, lobeline and methylphenidate differentially altered DAT function following neonatal ethanol exposure. Collectively, these findings provide support that lobeline may be a useful phar-macotherapy for some of the deficits associated with neonatal ethanol exposure.
Keywords: ethanol, dopamine transporter, prefrontal cortex, lobeline, hyperactivity, preadolescent
Exposure to ethanol (EtOH) during development is the leading preventable cause of mental retardation in the United States (Krulewitch, 2005). Studies report the prevalence of individuals meeting the full criteria for fetal alcohol syndrome (FAS) at 0.2–1.5 per 1000 births (Astley et al., 2002; Bertrand et al., 2004). Children diagnosed with FAS exhibit specific clinical symptoms, including pre and/or postnatal growth retardation (Abel, 1985; Zuckerman and Hingson, 1986), changes in craniofacial features (Sant'Anna and Tosello, 2006), and alterations in CNS structure or function (Smith and Eckardt, 1991; Welch-Carre, 2005). However, this only represents a very small proportion of children affected by ethanol with a 3–10-fold increase in prevalence when considering the number of individuals that meet the criteria for any fetal alcohol spectrum disorder (FASD) (Stratton et al., 1996). In either of these conditions, some of the more common behavioral characteristics include impulsivity, hyperactivity, and attentional problems (O'Malley and Nanson, 2002; Kodituwakku et al., 2006; Lopez, 2006) similar to attention deficit hyper-activity disorder (ADHD) (Nanson and Hiscock, 1990; O'Malley and Hagerman, 1998; Coles, 2001; O'Malley and Nanson, 2002). In fact, recent estimates suggest that up to 94% of individuals with heavy prenatal alcohol exposure have been diagnosed with ADHD (Peadon and Elliott, 2010). There is currently significant interest in determining characteristics that might differentiate individuals with ADHD from those with an FASD (Coles et al., 1997; Crocker et al., 2009, 2011); it is not known what proportion of children with a diagnosis of ADHD may actually have an FASD (see Peadon and Elliott, 2010). Preclinical literature provides further support for overlapping characteristics between prenatal ethanol exposure and ADHD, showing that prenatal and/or neonatal exposure to ethanol results in attentional deficits and hyperactivity (Slawecki et al., 2004; Gilbertson and Barron, 2005; Hausknecht et al., 2005; Dursun et al., 2006).
The suggestion has been made that the hyperactivity associated with both FASDs and ADHD is mediated, at least in part, by dysfunction of the dopaminergic neurotransmitter system (Viggiano et al., 2004). Clinical studies demonstrate alterations in extracellular dopamine (DA) concentration in brain regions including striatum and prefrontal cortex (PFC), which are correlated with hyperactivity (Mehler-Wex et al., 2006; Brennan and Arnsten, 2008; Arnsten, 2009). Preclinical evidence that extracellular DA concentrations mediates hyperactivity includes observations that mice lacking the gene for the DA transporter (DAT) have increased extracellular DA and display robust hyperactivity (Giros et al., 1996; Gainetdinov et al., 1999). Ethanol increases cell surface DAT and membrane insertion rates in human embryonic kidney (HEK-293) and neuronal SK-N-SH neuroblastoma cell expression systems, which results in increased DA uptake (Mayfield et al., 2001; Riherd et al., 2008; Methner and Mayfield, 2010). Findings from the cell expression systems may prove helpful in determining underlying mechanisms of the effect of ethanol on DAT function in brain. In adult rats, ethanol increases the number of DAT binding sites in numerous brain regions, including olfactory tubercle, striatum, hippocampus, ventral tegmental area, and substantia nigra (Jiao et al., 2006). In contrast, prenatal ethanol decreases DAT binding sites in frontal cortex, striatum, ventral tegmental area, and substantia nigra in adulthood (Druse et al., 1990; Szot et al., 1999), which may explain the hyperactivity observed following developmental ethanol exposure.
Clinical studies investigating the efficacy of stimulant medications, such as methylphenidate (MPH) and amphetamine, to treat ADHD symptoms in FAS children have reported positive results (Oesterheld et al., 1998), although other reports suggest little efficacy of these medications in this unique population (Snyder et al., 1997). Preclinical studies show that prenatal ethanol-induced hyperactivity may actually be increased by these stimulant medications (Ulug and Riley, 1983; Means et al., 1984; Hannigan and Pilati, 1991), suggesting exacerbation of the ADHD symptomology in FAS children (O'Malley and Hagerman, 1998; Oesterheld et al., 1998). Therefore, considering the uncertain efficacy of current ADHD medications in this special population and the negative impact on society resulting from the diversion of ADHD medications for recreational use (Poulin, 2007), new non-stimulant ADHD medications are needed.
Lobeline (LOB), a major alkaloid in Lobelia inflata, interacts with presynaptic proteins on dopaminergic terminals including DAT and the vesicular monoamine transporter-2 (VMAT2), which regulate intracellular and extracellular DA concentrations (Santha et al., 2000; Dwoskin and Crooks, 2002). In preclinical behavioral studies, lobeline has been shown to inhibit hyperactivity induced by psychostimulants (Miller et al., 2003; Polston et al., 2006). Importantly, lobeline does not produce hyperactivity and is not self-administered by rats, suggesting low abuse potential (Dwoskin and Crooks, 2002; Harrod et al., 2003). Further, lobeline is currently in phase II clinical trials as a novel therapeutic for ADHD (National Institute of Mental Health, 2008). Efficacy of lobeline to treat ADHD-like symptoms in FAS children has not been evaluated; preclinical evidence supporting the use of this candidate pharmacotherapy to ameliorate ADHD-like symptoms in models of FAS would augment further clinical evaluation.
When comparing rats with humans in terms of CNS development, gestational days 1–11 represent the “first trimester equivalent,” gestational days 12–22 represent the “second trimester equivalent,” and postnatal days (PND) 1–10 comprise the “third trimester equivalent” (Dobbing and Sands, 1979; Bayer et al., 1993). The third trimester equivalent, known as the “brain growth spurt,” is a period of rapid, transient neuronal proliferation and of extreme sensitivity to teratogenic insult (Dobbing and Sands, 1971, 1979, 1981; Bayer et al., 1993; Eriksson, 1997). In the current study, ethanol was administered from PND 1–7, to assess its effect during the sensitive period of the brain growth spurt. Specifically, the effects of neonatal ethanol exposure on locomotor activity and DAT function in striatum and PFC were determined in preadolescent rat pups. Additionally, the current study determined whether acute lobeline attenuates neonatal ethanol-induced alterations in activity and DAT function.
Experimental Procedures
Subjects
Male and female Sprague–Dawley rats (60–90 days of age) obtained from Harlan Laboratories, Inc. (Indianapolis, IN, USA) were used for breeding in the facility in the Psychology Department at the University of Kentucky. Once seminal plugs were identified, females were individually housed in plastic cages in a light- and temperature-controlled nursery (12-h light/dark cycle, 70°+/−2 F) with food and water available ad libitum. On the first day following birth (PND 1), litters were culled to 10 pups, maintaining a 1:1 sex ratio whenever possible. Litters of less than 6 pups were not included in the studies. The number of animals were chosen to allow for appropriate statistical analysis and suffering of the animals was minimized. All experimental animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Materials
[3H]DA (dihydroxyphenylethylamine, 3,4-[7-3H], specific activity 28.0 Ci/mM) was purchased from PerkinElmer Life and Analytical Sciences, Inc. (Boston, MA USA). Lobeline hemisulfate was purchased from ICN Biomedicals, Inc. (Costa Mesa, CA, USA). 3-Hydroxytyramine (DA), 1-[2-[Bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride (GBR-12909), ethylenediaminetetraacetic acid (EDTA), pargyline hydrochloride, paroxetine hydrochloride, desipramine hydrochloride, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). All other chemicals were purchased from Fisher Scientific Corporation (Pittsburgh, PA, USA).
Drug administration
Pups were weighed daily on PND 1–7 (day of birth was PND 0) and intubated with either 6 g/kg/d ethanol or 0 g/kg/d ethanol as an intubated control. Ethanol was added to a milk-based diet designed to mimic rat milk (West et al., 1984) and administered twice daily at 10:00 h and 14:00 h daily. Blood ethanol concentrations were not determined in these subjects to minimize any stress associated with blood collection. However, previous studies from our laboratory have demonstrated that this ethanol administration paradigm results in blood ethanol concentrations of 220–240 mg/dl within 90 min of the second ethanol administration (Lewis et al., 2007; Rubin et al., 2009). Liquid diets were administered via oral intubation using a syringe connected to PE-50 tubing (Becton Dickinson and Corporation, Franklin Lakes, NJ, USA) connected to a PE-10 feeding tubing. Corn oil was used to ease passage down the esophagus into the stomach. The amount of time needed for intubation did not exceed 15 s/pup. A non-intubated control group was also included. On PND 21 and 22, pups from the ethanol, intubation control, and non-treated control groups were assigned randomly to lobeline treatment groups and were administered lobeline (0.3, 1, or 3 mg/kg, sc) or saline (1 ml/kg body weight; controls) 15 min before being placed in the open field.
Open-Field testing
Open-field behavior was assessed on PND 21 and 22. Immediately before behavioral testing, dams were removed from the home cage, and a portion of the home cage was placed on a heating pad until all subjects were tested. Pups were weighed, injected with lobeline or saline, and moved to the testing room for 10 min of habituation. Activity testing (and habituation) was performed between 10:00 h and 15:00 h (light cycle was from 7:00 h to 19:00 h) each day under red light conditions. Each pup was tested individually in a circular open-field apparatus (55 cm diameter), and activity was recorded for 30 min (Polytracker, San Diego Instruments, Inc., San Diego, CA, USA). The circular maze was used to preclude the natural tendency to hide in corners of the maze. Activity was expressed as total distance traveled (cm; in 10-min blocks). In addition, the number of entries into the center zone of the test chamber (the inner 25% circle of the open field) was measured in 10-min blocks. This measure is typically used as a measure of anxiety, as rodents have a preferential bias to move close to the walls of an open field (thigmotaxis). After completion of testing, dams and pups were returned to the home cage.
[3H]DA uptake assay
Kinetic parameters (Vmax and Km) of [3H]DA uptake by DAT were determined in synaptosomes prepared from striatum and PFC using minor modifications of a previously published method (Zhu et al., 2004). On PND 23–25, rats were administered lobeline (1 or 3 mg/kg, sc) or methylphenidate (20 mg/kg, sc), and after 15 or 60 min, respectively, striatum or PFC was homogenized in 20 ml ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer. PFC from two rats (same sex and treatment group from the same litter) was pooled to ensure that each sample would have sufficient DAT protein for assay. Homogenates were centrifuged at 2,000×g for 10 min at 4 °C, and resulting supernatants were centrifuged at 20,000×g for 15 min at 4 °C. Resulting pellets from striatum and PFC were resuspended in 4.8 and 2.4 ml, respectively, of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM l-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). Non-specific [3H]DA uptake was determined in the presence of 10 μM GBR 12909, a DAT inhibitor. Because DA is transported by DAT, norepinephrine transporter (NET), and serotonin transporter (SERT) in PFC, kinetic analysis of [3H]DA uptake by DAT in the PFC was assessed in the presence of desipramine (50 nM) and paroxetine (5 nM) to prevent [3H]DA uptake by NET and SERT, respectively (Zhu etal., 2004). Desipramine potently (IC50= 1.2 nM) inhibits DA uptake by NET, but has a lower potency (IC50=9.3 μM) inhibiting DA uptake by DAT (Zhu et al., 2004). Paroxetine is a potent (IC50=0.3 nM) SERT inhibitor that has low potency inhibiting DA uptake at DAT (IC50=50 μM; Nemeroff and Owens, 2003; Norrholm et al., 2007). Thus, the concentrations of desipramine (50 nM) and paroxetine (5 nM) used in the current study did not inhibit DAT function.
Duplicate striatal and PFC synaptosomes (∼150 μg protein/100 μl and ∼50 μg protein/30 μl, respectively) were incubated in a metabolic shaker for 5 min at 34 °C. Then, one of eight [3H]DA concentrations (0.01–3.0 μM) was added to each synaptosomal sample (500-μl total volume), and samples were incubated for 10 min at 34 °C. Incubation was terminated by the addition of 3 ml of ice-cold assay buffer, followed by immediate filtration through Whatman GF/B (glass fiber) filters (presoaked with 1 mM pyrocatechol for 3 h) using a cell harvester (MP-43RS; Brandel Inc., Gaithersburg, MD, USA). Filters were washed three times with 4 ml of ice-cold Krebs' buffer containing catechol (1 μM). Radioactivity retained by the filters was determined by liquid scintillation spectrometry (B1600 TR scintillation counter; PerkinElmer, Inc., Waltham, MA, USA). Protein concentrations were determined by protein-dye binding (Bradford, 1976).
Lobeline-induced inhibition of [3H]DA uptake into rat striatal synaptosomes was also determined. Samples (final volume of 500 μl) were incubated at 34 °C for 10 min in absence or presence of lobeline (10–1000 nM). Then, 10 nM [3H]DA was added, and samples were incubated for 10 min at 34 °C. Non-specific [3H]DA uptake was determined in the presence of 10 μM GBR 12909. Assays were terminated by the addition of 3 ml of ice-cold Krebs' buffer and rapid filtration through Whatman GF/B filters using a cell harvester. Radioactivity retained on the filters was determined as previously described.
Data analysis
Locomotor activity was analyzed using analysis of variance (ANOVA) with SPSS statistical software. Neonatal treatment group, lobeline treatment, and sex were between-subjects factors with distance traveled and number of entries into the center zone (10-min blocks nested within day [day 1, day 2]) as within-subjects repeated measures. To determine whether lobeline selectively reduced hyperactivity following neonatal ethanol exposure, treatment with ethanol and/or lobeline was evaluated also using a 2 (ethanol vs. 0)×4 (lobeline dose: 0, 0.3, 1, or 3 mg/kg) factorial design. Data from the intubated and non-intubated control groups were pooled because the ANOVA revealed no differences between these control groups. For ease of presentation, data were collapsed across sex, unless a main effect or interaction with sex was observed.
Statistically significant three- or four-way interactions were examined by additional one- or two-way ANOVAs to determine the nature of the interaction. To further evaluate the interactions between ethanol and lobeline, t-tests with Bonferroni corrections were performed. A probability value of 0.05 was considered statistically significant.
Neurochemical data are presented as mean±SEM, and n represents the number of independent experiments for each treatment group. Specific binding and uptake were determined by subtracting the non-specific from total binding and uptake, respectively. For kinetic analyses, Vmax and Km were determined using one-site binding curves generated via an iterative curve-fitting program (Prism 5.0; GraphPad Software Inc., San Diego, CA, USA). To analyze the kinetic parameters, three-way ANOVA with neonatal treatment (ethanol, control), sex, and drug (control, lobeline, methylphenidate) as between-groups factors was performed on the arithmetic Vmax and log Km values. When appropriate, Tukey's post hoc analyses were used to make pairwise comparisons. For inhibition curves, concentrations of lobeline that produced 50% inhibition of the specific binding or uptake (IC50 values) were determined from the concentration-effect curves (Prism 5.0; GraphPad Software Inc., San Diego, CA, USA). IC50 values were used to calculate inhibition constants (Ki values) using the Cheng-Prusoff equation (Cheng and Prusoff, 1973). For clarity of presentation, data from both behavioral and neurochemical studies were collapsed across sex unless a main effect or interaction with sex was observed. Significance was declared at P<0.05.
Results
Body weights
Body weights (mean±SEM) recorded on PND 22 are presented in Table 1. Subjects exposed to neonatal ethanol weighed significantly less than non-ethanol exposed offspring (F1,296=43.4, P<0.001). There were no differences in body weights between the two control groups (non-treated and intubated controls), indicating that intubation with the milk diet did not alter somatic growth. Statistical analysis revealed no effect of sex on body weight. As expected, acute administration of lobeline on PND 22 did not alter body weight.
Table 1. Neonatal ethanol treatment, but not preadolescent lobeline treatment, significantly decreases mean body weight.
| Neonatal treatment | Lobeline (mg/kg) | Weight (g) | n |
|---|---|---|---|
| Non-treated control | 0.0 | 59±1.2 | 34 |
| 0.3 | 62±1.4 | 24 | |
| 1.0 | 59±1.5 | 21 | |
| 3.0 | 61±1.6 | 20 | |
| Intubated control | 0.0 | 59±1.3 | 29 |
| 0.3 | 60±1.5 | 21 | |
| 1.0 | 61±1.3 | 30 | |
| 3.0 | 61±1.6 | 20 | |
| Ethanol | 0.0 | 54±1.1* | 32 |
| 0.3 | 56±1.5* | 22 | |
| 1.0 | 55±1.3* | 29 | |
| 3.0 | 54±1.6* | 23 |
Indicates significant difference between rats receiving neonatal ethanol treatment when compared with control animals (non-treated control and intubated control combined). Lobeline did not impact body weights. Data are expressed as mean±SEM * P<0.05.
Lobeline attenuates neonatal ethanol-induced hyperactivity
Repeated-measures ANOVA on distance traveled revealed a lobeline×day×block interaction (F6,592=2.43, P<0.05). To further understand this interaction, activity on PND 21 and 22 was examined using separate ANOVAs. The ethanol exposed offspring were hyperactive on PND 21, but not on PND 22 as described below.
Activity (PND 21)
Further analysis of the activity data on PND 21 revealed an ethanol×lobeline interaction (F3,296=3.39, P<0.05). As shown in Fig. 1 (left), the ethanol-exposed offspring were hyperactive relative to control (F1,98=1047.15, P<0.001). The lowest dose of lobeline (0.3 mg/kg) had no effect on activity in any of the neonatal treatment groups, and thus, did not reduce the hyperactivity following neonatal ethanol exposure. Although the 1.0 mg/kg dose of lobeline had no effect on activity in the control offspring, the higher dose (3.0 mg/kg) of lobeline reduced activity in the pooled intubated and non-treated control relative to the saline-injected group (F1,100=27.0, P<0.05). Lobeline (1.0 and 3.0 mg/kg) reduced activity in the ethanol-exposed offspring compared with controls (F1,64=8.21, P<0.05; F1,58=10.56, P<0.05, respectively).
Fig. 1.
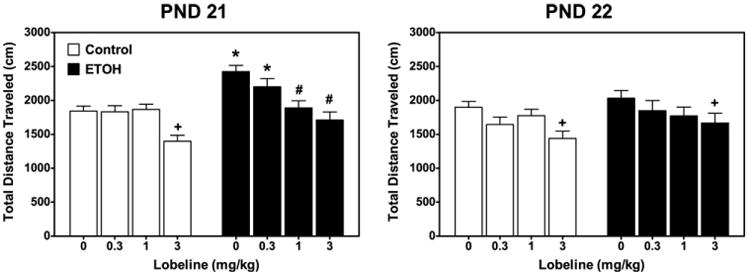
Neonatal ethanol-mediated hyperactivity in preadolescent rats is reversed by acute administration of lobeline. Preadolescent rats were injected with lobeline (0.3, 1, or 3 mg/kg, sc) or saline (control) 15 min before being placed in an open-field chamber on PND 21 and 22. Activity was measured as total distance traveled (cm) measured during a 30-min test session. Data represent mean±SEM total distance traveled (cm). + Indicates lower than control/saline group, P<0.05. * Indicates higher than control/saline, P<0.05. n=10–19 for each treatment group. # Indicates lower than ethanol (EtOH)/saline group. The control group is composed of the pooled data from subjects from non-treated and intubated control groups. Data are collapsed across sex.
Activity (PND 22)
Neonatal ethanol had no effect on locomotor activity on PND 22 (Fig. 1, right). The only significant effect observed on day 2 was a main effect of lobeline (F3,296=24.5, P<0.005), with post hoc analyses revealing a decrease in activity with the high dose of lobeline (3 mg/kg) compared with the pooled intubated and non-intubated controls that received saline injection before activity testing.
Neonatal ethanol increases the frequency of center zone entries
The repeated-measures ANOVA on the number of entries into the center zone revealed a main effect of ethanol (F1,296=13.18, P<0.01) that did not interact with either day or block within-subject measures. Regardless of the day tested, offspring exposed neonatally to ethanol entered the center zone more frequently than non-ethanol exposed pups (see Fig. 2). Treatment with lobeline had no effect on this behavioral measure.
Fig. 2.
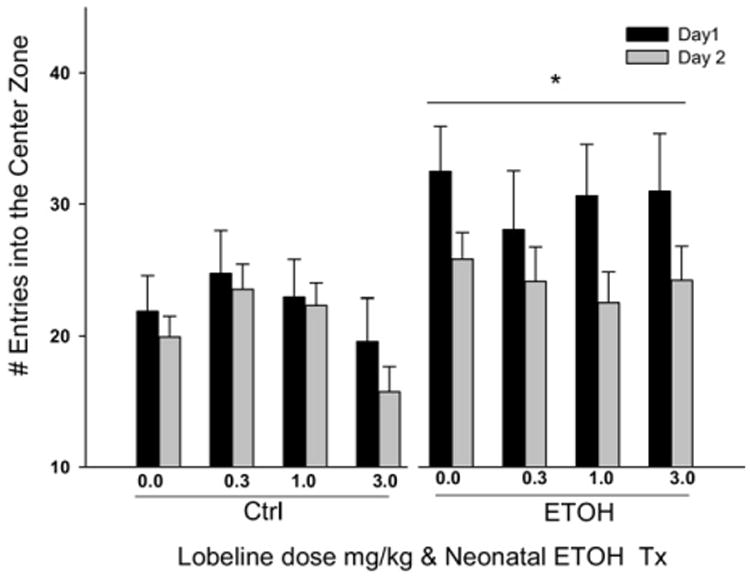
Neonatal ethanol exposure increased the number of entries into the center. Preadolescent rats were injected with lobeline (0.3, 1, or 3 mg/kg, sc) or saline (control) 15 min before being placed in an open-field chamber on PND 21 and PND 22. The center was defined as the inner 25% of the round activity chamber. Data are presented collapsed across the 30-min test session. Data represent the frequency±SEM of the number of entries. Ethanol-exposed offspring entered the center more frequently than controls. Lobeline had no effect on this dependent variable. The control group is composed of the pooled data from subjects from non-treated and intubated control groups. Data are collapsed across sex. * Indicates a main effect of EtOH, P<0.05.
Effects of neonatal ethanol and acute lobeline on DAT function in striatum
The effect of neonatal ethanol on DAT function in striatum of preadolescent (PND 23–25) rats was evaluated using kinetic analysis of [3H]DA uptake. Also, the acute effect of the high dose of lobeline (3 mg/kg) on DAT function was evaluated. Separate three-way ANOVAs revealed no main effects or interaction of neonatal ethanol, lobeline, or sex for either Vmax or Km values (Fig. 3). Thus, neonatal ethanol and/or acute lobeline did not alter maximal velocity of DA uptake or affinity of DA for DAT in striatum of preadolescent rats.
Fig. 3.
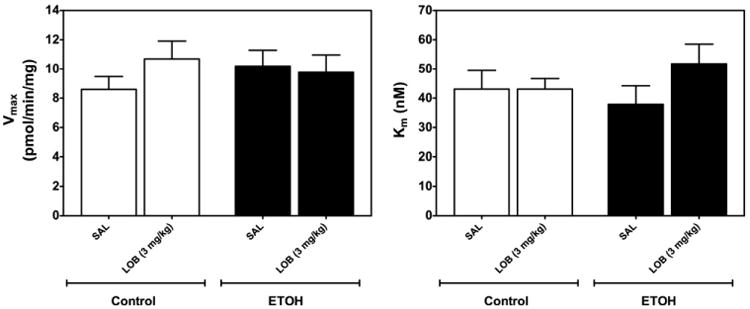
Neonatal ethanol treatment does not alter DAT function in striatum of preadolescent rats. Rat pups were injected (sc) with lobeline (3 mg/kg) or saline (controls), and striatum was obtained 20 min later. Kinetic analysis of synaptosomal [3H]DA uptake was determined in the presence of GBR 12909 (10 μM). Vmax (picomoles per milligram per minute), and Km (nM) are expressed as mean±SEM n=10–14 for each treatment group. The control group is composed of the pooled data from subjects from non-treated and intubated control groups. Data are collapsed across sex.
Neonatal ethanol did not alter the affinity (Ki) of lobeline for DAT in striatum (Fig. 4). Using non-linear regression, the lobeline concentration-response curve fit a single-site model (R2=0.97 and 0.95 for control and ethanol-treated rats, respectively, P<0.05). Lobeline-induced inhibition of [3H]DA uptake was not different between neonatal ethanol (Ki=21.7±1.37 μM) and control (Ki=22.2±1.42 μM), indicating that ethanol did not alter the ability of lobeline to inhibit DAT in striatum.
Fig. 4.
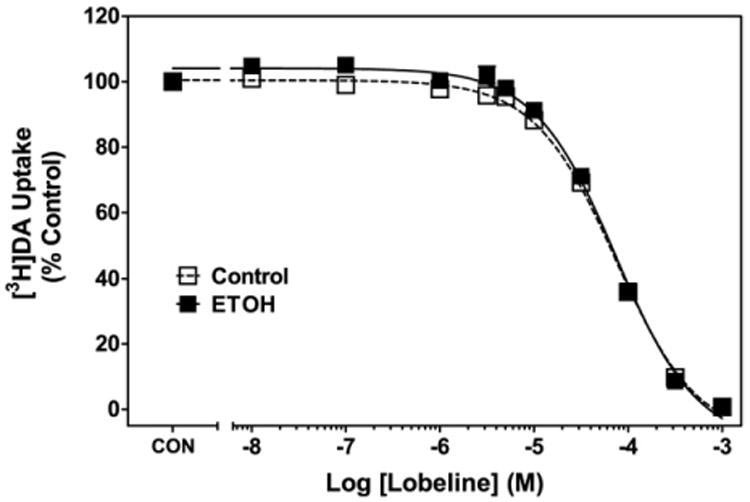
Lobeline inhibits specific [3H]DA uptake into rat striatal synaptosomes. Control represents [3H]DA uptake in the absence of lobeline. Data are mean (±SEM) specific [3H]DA uptake as a percentage of control (13.7±0.82 pmol/min/mg). n=13–14 for each group. Control group is composed of an equal number of subjects from non-treated control and intubation control groups. Data are collapsed across sex.
Lobeline attenuates the neonatal ethanol-induced increase in DAT function in PFC
To determine the effect of neonatal ethanol and/or acute lobeline (1 and 3 mg/kg) on DAT function in PFC of preadolescent (PND 23–25) rats, kinetic analyses of [3H]DA uptake were performed in the presence of desipramine and paroxetine, specific inhibitors of NET and SERT, respectively. For comparison, effects of acute methylphenidate (20 mg/kg) were determined as a positive control. Three-way ANOVA revealed only a main effect of ethanol on Vmax (F1,49=6.50, P<0.05; Fig. 5) and a two-way ethanol×drug interaction (F1,49=11.67, P<0.0001). Post hoc analysis revealed that each treatment (neonatal ethanol, 3 mg/kg lobeline and 20 mg/kg methylphenidate) increased Vmax compared with control. Lobeline (1 and 3 mg/kg) and methylphenidate each attenuated the increase in Vmax induced by neonatal ethanol, however, not back to control levels.
Fig. 5.
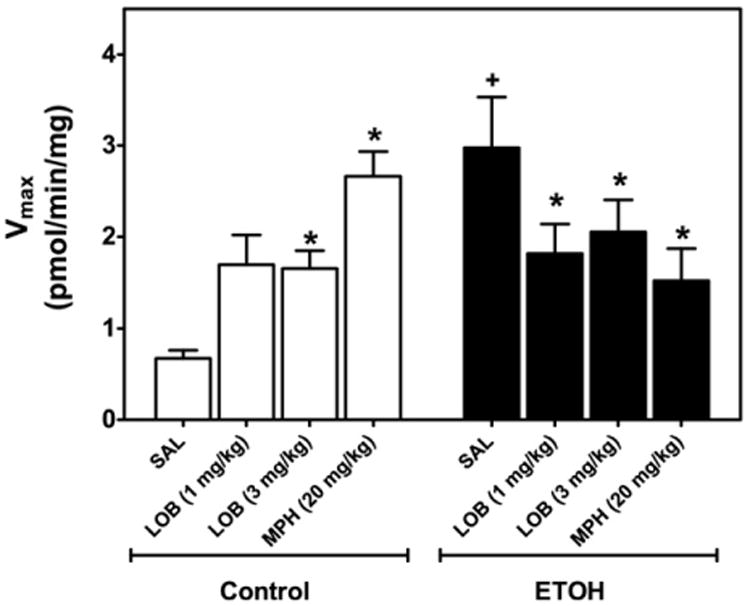
Neonatal ethanol treatment increases Vmax of [3H]DA uptake in PFC compared with control (right), which is reversed by acute lobeline or methylphenidate in preadolescent rats. Rat pups were injected (sc) with saline, lobeline (1 or 3 mg/kg) or methylphenidate (MPH, 20 mg/kg). PFC was obtained 20, 20, and 60 min after injection, respectively. Control represents an equal number of subjects from non-treated control and intubated control groups. Kinetic analysis of synaptosomal [3H]DA uptake was determined in the presence of desipramine (1 μM) and paroxetine (5 nM). Vmax (picomoles per milligram per minute) is expressed as mean±SEM n=5–7 for each group. + Indicates significant difference from control/saline injection, P<0.05. * Indicates significant difference from respective saline group, P<0.05. Data are collapsed across sex.
The effect of neonatal ethanol treatment and acute drug (i.e. lobeline or methylphenidate) administration on Km values for DA uptake by DAT in PFC (Fig. 6) was analyzed using a three-way ANOVA with neonatal treatment (ethanol, control), sex, and drug (control, lobeline, methylphenidate) as between-groups factors. The three-way ANOVA revealed a main effect of drug treatment (F3,51=5.18, P<0.01), a drug×sex interaction (F3,51 = 3.75, P<0.01), and an ethanol×drug×sex interaction (F3,51=3.06, P<0.01). Post hoc analyses revealed that methylphenidate (20 mg/kg) increased the Km value, when compared with all other treatment groups. Simple effect analyses using two-way ANOVAs were performed to identify the loci of the neonatal ethanol treatment×drug×sex interaction. In rats treated with lobeline (1 mg/kg), a neonatal ethanol treatment×sex interaction (F1,9=15.1, P<0.01) was found, which was the result of control males having higher Km values than females, whereas ethanol-treated males had lower Km values than females. In rats treated with lobeline (3 mg/kg), there was a main effect of sex (F1,10=8.96, P<0.05), with females having higher Km values than males. No other main effects or interactions were identified. Although statistically significant, it is unlikely that these statistical effects are biologically relevant given that the Km values were <2-fold different between males and females.
Fig. 6.
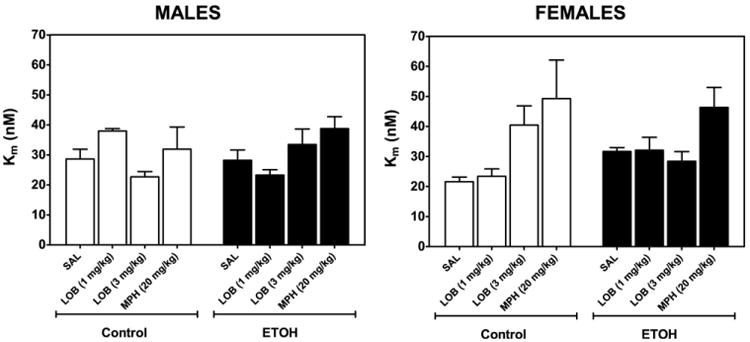
Neonatal ethanol treatment does not alter Km of [3H]DA uptake in PFC in male (left) and female rats (right), effect of acute lobeline or methylphenidate treatment. Rat pups were injected (sc) with saline or lobeline (1 or 3 mg/kg), methylphenidate (MPH, 20 mg/kg). PFC was obtained 20, 20, and 60 min after injection, respectively. Control represents an equal number of subjects from NTC and IC groups. Kinetic analysis of synaptosomal [3H]DA uptake was determined in the presence of desipramine (1 μM) and paroxetine (5 nM). Km (nM) is expressed as mean±SEM n=3–5 males or females for each group. Results indicate that overall, rats treated with methylphenidate (20 mg/kg) had higher Km values than rats injected with saline or lobeline.
Discussion
Dysfunction of the dopaminergic neurotransmitter system likely plays a role in mediating hyperactivity associated with both FASD and ADHD (Nanson and Hiscock, 1990; Viggiano et al., 2004). This study determined if lobeline reduced hyperactivity and neurochemical deficits in a rodent model of third trimester ethanol exposure. Also, this study examined whether neonatal ethanol exposure altered DAT function in striatum and/or PFC, two brain regions involved in ADHD symptomology (Prince, 2008; Arnsten, 2009). The results from this study showed that ethanol treatment from PND 1–7 resulted in increased activity as measured by the distance traveled on PND 21. Acute lobeline administered 15 min before testing eliminated this hyperactivity. Of particular note, the 1 mg/kg dose reduced activity in the ethanol exposed offspring while having no effect on activity of the control offspring. The neurochemical studies showed that neonatal ethanol exposure also increased Vmax for DAT in the PFC, and lobeline ameliorated this effect at the same doses that decreased hyperactivity.
Previous preclinical research has shown that ethanol exposure during the developmental “brain growth spurt” produces numerous behavioral deficits, including hyperactivity (West, 1986; Melcer et al., 1994; Thomas et al., 1998; Slawecki et al., 2004; Allen et al., 2005; Gilbertson and Barron, 2005), which is consistent with our current findings. It should be noted that hyperactivity was only observed on the first day of behavioral testing (PND 21), although not on the second day (PND 22). The observation that hyperactivity was not observed on the second test day was somewhat surprising because previous studies by our laboratory and others have demonstrated neonatal ethanol-induced hyperactivity in preadolescent rats tested across multiple days (Melcer et al., 1994; Gilbertson and Barron, 2005), including PND 23–24 (unpublished observations). These results suggest that the hyperactivity observed here may be more related to the novelty of the first day in the test chamber and that habituation to the test chamber on the second day was unaffected by neonatal ethanol exposure. It should be noted that although activity levels in the alcohol-exposed offspring were similar to controls on PND 22, alcohol-related changes in DAT Vmax in the PFC were observed on PND 23–25. Thus, the potential role of PFC DAT activity following neonatal ethanol exposure as an underlying mechanism for this behavioral deficit clearly warrants further study.
Entries into the center of the open field also were increased as a function of neonatal ethanol exposure. This increase was observed on both test days and was not reduced by lobeline. As stated earlier, entries into the center are often used as a measure of anxiety, and prenatal/neonatal alcohol exposure can alter response to stress (see Hellemans et al., 2010; Kelly et al., 1991). Interpretation of entries into the center is more complicated if the organism is hyperactive, as this behavior can also be explained by hyperactivity and/or inhibitory control deficits, which have been associated with prenatal/neonatal alcohol exposure (Burden et al., 2011; see Schneider et al., 2011). Factor analyses on rat activity in locomotor chambers have shown that the majority of the variability in the open field (at least in normal rats) can be best understood by three components; exploration, fear, and shifted activity (Markel et al., 1989), and almost certainly, these components are mediated by different (although overlapping) CNS circuitry. Thus, lobeline may be more effective at reducing deficits of specific behavioral components that contribute to the complexity of hyperactivity in the open field.
Neonatal ethanol exposure did reduce body weights measured on PND 22. Reductions in body weight following developmental alcohol exposure is not surprising, and it should be noted that nutritional deficits can result in reduced or increased activity in the open field (for example, see Reyes-Castro et al., 2012). Although reduced body weight could play a role in the hyperactivity, it is unlikely that this alone explains the hyperactivity reported in the current study, as rats that received neonatal ethanol exposure plus acute lobeline also had reduced body weights, yet they were not different from controls either behaviorally or neurochemically.
Previous studies have shown that following prenatal ethanol administration, DAT expression was decreased in both terminal and cell body regions of the nigrostriatal pathway (Druse et al., 1990; Szot et al., 1999). In contrast, chronic ethanol exposure in adult rats or adult primates was reported to increase both the expression and function of striatal DAT (Budygin et al., 2003; Carroll et al., 2006; Jiao et al., 2006). The current study reports that neonatal ethanol exposure (PND 1–7, corresponding to the third trimester in humans and including the brain growth spurt) did not alter striatal DAT function. Thus, ethanol-induced changes in striatal DAT function appear to be dependent upon the developmental stage during which ethanol is administered, suggesting a temporal window of vulnerability to ethanol.
In contrast to striatum, few studies have evaluated the effects of developmental ethanol exposure on DAT function in PFC, a region linked to the pathology of ADHD (Prince, 2008; Arnsten, 2009). In the current study, ethanol treatment from PND 1–7 resulted in an increase in the maximal velocity of DA uptake in PFC, indicating enhanced DAT function in this brain region. Developmental ethanol-induced increases in DAT function in PFC, such as those observed in the current study, would be expected to increase DA clearance from the extracellular compartment and decrease extracellular DA concentrations. Consistent with this expectation, PFC DA concentrations in adult rats (PND 80–110) were reduced following prenatal ethanol treatment (6.7% ethanol in liquid diet v/v) from gestational days 6–20 (Zimmerberg and Brown, 1998); however, DAT function was not evaluated. Decreased extracellular DA is expected to result in reduced activation of D1 and D4 receptors in PFC, and consequently, inattention and diminished memory capabilities (Arnsten, 2009; Stahl, 2009).
The lowest lobeline dose (0.3 mg/kg) did not alter activity in either the ethanol-treated or control rats. A higher dose (1 mg/kg) specifically attenuated neonatal ethanol-induced hyperactivity, without producing an effect on activity when administered to control animals. In contrast, the 3 mg/kg dose decreased activity in both control and ethanol-treated rats. The decrease in activity observed following lobeline (3 mg/kg) in control rats is consistent with previous reports (Damaj et al., 1997; Miller et al., 2003; Harrod and Van Horn, 2009). Tolerance developed to the hypoactivity upon repeated lobeline administration (Miller et al., 2003; Harrod and Van Horn, 2009). Therefore, while lobeline specifically attenuates neonatal ethanol-induced hyperactivity, specificity occurred only across a narrow dose range.
The behaviorally active dose of lobeline (3 mg/kg) was evaluated for alterations of DAT function in striatum and PFC, and results revealed no effect in striatum and an increase in maximal velocity of DA uptake in PFC. This was surprising considering previous findings that in vitro superfusion of striatal slices with lobeline decreased DAT function (Teng et al., 1997). However, the decrease in striatal DAT function in vitro was observed following high concentrations (30 μM –1 mM) of lobeline, which likely were not attained following administration of the low doses used in the current in vivo experiments. More surprising was the current observation that the 3 mg/kg dose of lobeline increased DAT maximal velocity in PFC and that the 1 mg/kg dose also tended to increase DAT function in PFC from control rats. The opposite effects on both DAT function and activity between control and neonatal ethanol exposed preadolescent rats are reminiscent of the antithetical effects of psychostimulants, such as methylphenidate, in reducing hyperactivity associated with ADHD and increasing activity in control individuals. In this regard, the current results show that methylphenidate increased PFC DAT function in control rats and conversely decreased PFC DAT function in neonatal ethanol-treated rats.
Conclusions
In conclusion, the current study provides evidence that acute lobeline treatment can reduce both behavioral and neurochemical deficits associated with third trimester ethanol exposure. Lobeline reduced hyperactivity in an open-field and reduced DAT function in the PFC at doses that had no effect in control offspring. Clearly further work is needed with this non-stimulant treatment to determine its efficacy at reducing other behavioral deficits following neonatal ethanol exposure. These findings also provide evidence for an underlying mechanism for the action of lobeline and suggest that inhibition of DAT function in PFC may represent a novel target for the development of therapeutics for the treatment of hyperactivity in children with an FASD and/or ADHD.
Acknowledgments
The research reported was supported in part by the Kentucky Tobacco Research and Development Center and NIH grants R21 AA14032 and T32 DA16176. We thank A. Gabriela Deaciuc and Ben Lewis for their assistance. For purposes of full disclosure, the University of Kentucky holds patents on lobeline, which have been licensed by Yaupon Therapeutics, Inc. (Lexington, KY). A potential royalty stream to L.P.D. may occur consistent with University of Kentucky policy, and L.P.D. is a founder of, and has financial interest in, Yaupon Therapeutics, Inc.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ANOVA
analysis of variance
- DA
dopamine
- DAT
dopamine transporter
- EDTA
ethylenediaminetetraacetic acid
- EtOH
ethanol
- FAS
fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorder
- GBR-12909
1-[2-[Bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- LOB
lobeline
- MPH
methylphenidate
- NET
norepinephrine transporter
- PFC
prefrontal cortex
- PND
postnatal day
- SERT
serotonin transporter
- VMAT2
vesicle monoamine transporter-2
- [3H]DA
dihydroxyphenylethylamine, 3,4-[7-3H]
References
- Abel EL. Prenatal effects of alcohol on growth: a brief overview. Fed Proc. 1985;44:2318–2322. [PubMed] [Google Scholar]
- Allen GC, West JR, Chen WJ, Earnest DJ. Neonatal alcohol exposure permanently disrupts the circadian properties and photic entrainment of the activity rhythm in adult rats. Alcohol Clin Exp Res. 2005;29:1845–1852. doi: 10.1097/01.alc.0000183014.12359.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. J Pediatr. 2002;141:712–717. doi: 10.1067/mpd.2002.129030. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O'Connor M, Riley EP, Johnson KA, Cohen DE National Task Force on FAS/FAE. Fetal alcohol syndrome: guidelines for referral and diagnosis. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Westerlund A, Muckle G, Dodge N, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. The effects of maternal binge drinking during pregnancy on neural correlates of response inhibition and memory in childhood. Alcohol Clin Exp Res. 2011;35:69–82. doi: 10.1111/j.1530-0277.2010.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Coles CD. Fetal alcohol exposure and attention: moving beyond ADHD. Alcohol Res Health. 2001;25:199–203. [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2009;33:2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2011;35:1114–1121. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Patrick GS, Creasy KR, Martin BR. Pharmacology of lobeline, a nicotinic receptor ligand. J Pharmacol Exp Ther. 1997;282:410–419. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Vulnerability of developing brain. IX. The effect of nutritional growth retardation on the timing of the brain growth-spurt. Biol Neonate. 1971;19:363–378. doi: 10.1159/000240430. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Vulnerability of developing brain not explained by cell number/cell size hypothesis. Early Hum Dev. 1981;5:227–231. doi: 10.1016/0378-3782(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin N, Kuo A, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. J Neurosci Res. 1990;27:233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-DoĞru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol Biochem Behav. 2006;85:345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–726. [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Barron S. Neonatal ethanol and nicotine exposure causes locomotor activity changes in preweanling animals. Pharmacol Biochem Behav. 2005;81:54–64. doi: 10.1016/j.pbb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Pilati ML. The effects of chronic postweaning amphetamine on rats exposed to alcohol in utero: weight gain and behavior. Neurotoxicol Teratol. 1991;13:649–656. doi: 10.1016/0892-0362(91)90049-3. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Lobeline does not serve as a reinforcer in rats. Psychopharmacology (Berl) 2003;165:397–404. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Van Horn ML. Sex differences in tolerance to the locomotor depressant effects of lobeline in periadolescent rats. Pharmacol Biochem Behav. 2009;94:296–304. doi: 10.1016/j.pbb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausknecht KA, Acheson A, Farrar AM, Kieres AK, Shen RY, Richards JB, Sabol KE. Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci. 2005;119:302–310. doi: 10.1037/0735-7044.119.1.302. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Paré WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006:1073–1074. 175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Mahoney JC, Randich A, West JR. Indices of stress in rats: effects of sex, perinatal alcohol and artificial rearing. Physiol Behav. 1991;49:751–756. doi: 10.1016/0031-9384(91)90314-e. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragón AS, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, May PA. Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: preliminary results. Alcohol Clin Exp Res. 2006;30:1551–1561. doi: 10.1111/j.1530-0277.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Krulewitch CJ. Alcohol consumption during pregnancy. Annu Rev Nurs Res. 2005;23:101–134. [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav. 2007;88:114–121. doi: 10.1016/j.pbb.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez FA. ADHD: new pharmacological treatments on the horizon. J Dev Behav Pediatr. 2006;27:410–416. doi: 10.1097/00004703-200610000-00008. [DOI] [PubMed] [Google Scholar]
- Markel AL, Galaktionov YK, Efimov VM. Factor analysis of rat behavior in an open field test. Neurosci Behav Physiol. 1989;19:279–286. doi: 10.1007/BF01236015. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Maiya R, Keller D, Zahniser NR. Ethanol potentiates the function of the human dopamine transporter expressed in Xenopus oocytes. J Neurochem. 2001;79:1070–1079. doi: 10.1046/j.1471-4159.2001.00656.x. [DOI] [PubMed] [Google Scholar]
- Means LW, Medlin CW, Hughes VD, Gray SL. Hyperresponsiveness to methylphenidate in rats following prenatal ethanol exposure. Neurobehav Toxicol Teratol. 1984;6:187–192. [PubMed] [Google Scholar]
- Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson's disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. 2006;10:167–179. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- Melcer T, Gonzalez D, Barron S, Riley EP. Hyperactivity in preweanling rats following postnatal alcohol exposure. Alcohol. 1994;11:41–45. doi: 10.1016/0741-8329(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Methner DN, Mayfield RD. Ethanol alters endosomal recycling of human dopamine transporters. J Biol Chem. 2010;285:10310–10317. doi: 10.1074/jbc.M109.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Harrod SB, Green TA, Wong MY, Bardo MT, Dwoskin LP. Lobeline attenuates locomotor stimulation induced by repeated nicotine administration in rats. Pharmacol Biochem Behav. 2003;74:279–286. doi: 10.1016/s0091-3057(02)00996-6. [DOI] [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1990;14:656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. Clinical Trials Identifier: NCT00664703. Principle Investigator: Martin, C., MD.: General Clinical Research Center, University of Kentucky; Lexington, KY, USA: 2008. Effectiveness of the non-stimulant medication lobeline in improving symptoms of attention deficit hyperactivity disorder in adults. [Google Scholar]
- Nemeroff CB, Owens MJ. Neuropharmacology of paroxetine. Psychopharmacol Bull. 2003;37(Suppl 1):8–18. [PubMed] [Google Scholar]
- Norrholm SD, Horton DB, Dwoskin LP. The promiscuity of the dopamine transporter: implications for the kinetic analysis of [3H]serotonin uptake in rat hippocampal and striatal synaptosomes. Neuropharmacology. 2007;53:982–989. doi: 10.1016/j.neuropharm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley KD, Hagerman RJ. Developing clinical practice guidelines for pharmacological interventions with alcohol-affected children. Chevy Chase, MD: National Institute on Alcohol and Alcoholism; 1998. [Google Scholar]
- O'Malley KD, Nanson J. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Can J Psychiatry. 2002;47:349–354. doi: 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- Oesterheld JR, Kofoed L, Tervo R, Fogas B, Wilson A, Fiechtner H. Effectiveness of methylphenidate in Native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: a controlled pilot study. J Child Adolesc Psychopharmacol. 1998;8:39–48. doi: 10.1089/cap.1998.8.39. [DOI] [PubMed] [Google Scholar]
- Peadon E, Elliott EJ. Distinguishing between attention-deficit hyperactivity and fetal alcohol spectrum disorders in children: clinical guidelines. Neuropsychiatr Dis Treat. 2010;6:509–515. doi: 10.2147/ndt.s7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE, Cunningham CS, Rodvelt KR, Miller DK. Lobeline augments and inhibits cocaine-induced hyperactivity in rats. Life Sci. 2006;79:981–990. doi: 10.1016/j.lfs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Poulin C. From attention-deficit/hyperactivity disorder to medical stimulant use to the diversion of prescribed stimulants to non-medical stimulant use: connecting the dots. Addiction. 2007;102:740–751. doi: 10.1111/j.1360-0443.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol. 2008;28:S39–S45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- Reyes-Castro LA, Rodriguez JS, Charco R, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E. Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int J Dev Neurosci. 2012;30(1):39–45. doi: 10.1016/j.ijdevneu.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Riherd DN, Galindo DG, Krause LR, Mayfield RD. Ethanol potentiates dopamine uptake and increases cell surface distribution of dopamine transporters expressed in SK-N-SH and HEK-293 cells. Alcohol. 2008;42:499–508. doi: 10.1016/j.alcohol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MA, Wellmann KA, Lewis B, Overgaauw BJ, Littleton JM, Barron S. Difluoromethylornithine (DFMO) reduces deficits in isolation-induced ultrasonic vocalizations and balance following neonatal ethanol exposure in rats. Pharmacol Biochem Behav. 2009;92:44–50. doi: 10.1016/j.pbb.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Anna LB, Tosello DO. Fetal alcohol syndrome and developing craniofacial and dental structures—a review. Orthod Craniofac Res. 2006;9:172–185. doi: 10.1111/j.1601-6343.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- Santha E, Sperlágh B, Zelles T, Zsilla G, Tóth PT, Lendvai B, Baranyi M, Vizi ES. Multiple cellular mechanisms mediate the effect of lobeline on the release of norepinephrine. J Pharmacol Exp Ther. 2000;294:302–307. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley EP, Ehlers CL. Neurophysiologic consequences of neonatal ethanol exposure in the rat. Alcohol. 2004;34:187–196. doi: 10.1016/j.alcohol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Eckardt MJ. The effects of prenatal alcohol on the central nervous system. Recent Dev Alcohol. 1991;9:151–164. [PubMed] [Google Scholar]
- Snyder J, Nanson J, Snyder R, Block G. Stimulant efficacy in children with FAS. Seattle: University of Washington Press; 1997. [Google Scholar]
- Stahl SM. The prefrontal cortex is out of tune in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2009;70:950–951. doi: 10.4088/jcp.09bs05416. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington, DC.: National Academies Press; 1996. [Google Scholar]
- Szot P, White SS, Veith RC, Rasmussen DD. Reduced gene expression for dopamine biosynthesis and transport in midbrain neurons of adult male rats exposed prenatally to ethanol. Alcohol Clin Exp Res. 1999;23:1643–1649. [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther. 1997;280:1432–1444. [PubMed] [Google Scholar]
- Thomas JD, Melcer T, Weinert S, Riley EP. Neonatal alcohol exposure produces hyperactivity in high-alcohol-sensitive but not in low-alcohol-sensitive rats. Alcohol. 1998;16:237–242. doi: 10.1016/s0741-8329(98)00008-1. [DOI] [PubMed] [Google Scholar]
- Ulug S, Riley EP. The effect of methylphenidate on overactivity in rats prenatally exposed to alcohol. Neurobehav Toxicol Teratol. 1983;5:35–39. [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 2004;11:97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch-Carre E. The neurodevelopmental consequences of prenatal alcohol exposure. Adv Neonatal Care. 2005;5:217–229. doi: 10.1016/j.adnc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- West JR. Long-term effects of developmental exposure to alcohol. Neurotoxicology. 1986;7:245–256. [PubMed] [Google Scholar]
- West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Brown RC. Prenatal experience and postnatal stress modulate the adult neurosteroid and catecholaminergic stress responses. Int J Dev Neurosci. 1998;16:217–228. doi: 10.1016/s0736-5748(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Zuckerman BS, Hingson R. Alcohol consumption during pregnancy: a critical review. Dev Med Child Neurol. 1986;28:649–654. doi: 10.1111/j.1469-8749.1986.tb03910.x. [DOI] [PubMed] [Google Scholar]


