Abstract
Objectives
Some women with isolated gestational proteinuria (IGP) later develop hypertension and are diagnosed with pre-eclampsia (PE). This study was performed to determine whether clinical features of such proteinuria preceding PE (P-PE) differ from those of other PE (O-PE).
Design
Retrospective observational study after approval of the institutional review board of ethics.
Setting
A single university hospital. Proteinuria was defined as a protein-to-creatinine ratio (mg/mg; P/Cr) of ≥0.27 in the spot urine specimen. IGP was defined as proteinuria in the absence of hypertension. P-PE was defined as PE in which proteinuria preceded hypertension by more than 2 days.
Participants
All of 10 and 18 consecutive women with P-PE and O-PE, respectively, who gave birth between January 2008 and August 2013.
Results
Proteinuria appeared earlier (at 30.2±3.0 vs 35.3±4.3 weeks, p=0.001), the P/Cr level was greater at birth (7.28±2.14 vs 3.19±2.49, p<0.001), net maternal weight gain during the last antenatal 1 week was greater (3.1±1.8 vs 1.3±1.7 kg, p=0.023) and length of pregnancy was shorter (32.5±1.9 vs 36.1±3.6 weeks, p=0.001) in women with P-PE than in O-PE. The duration of IGP was 10.0±5.9 days (range 3–20), and the time interval until delivery after diagnosis of PE was 6.1±8.2 days (range 0–23) in 10 women with P-PE. The P/Cr levels at birth were significantly inversely correlated with the antenatal lowest antithrombin activity and fibrinogen levels among the 28 women with PE.
Conclusions
Women with P-PE were likely to exhibit greater proteinuria in the urine, greater water retention in the interstitial space and more enhanced coagulation–fibrinolysis, thus suggesting that they may constitute a more severe form of PE than women with O-PE do.
Strengths and limitations of this study.
This study included all women with pre-eclampsia without known renal or autoimmune diseases who gave birth at a single centre during the period between January 2008 and August 2013.
This study focused on the timing of the onsets of hypertension and significant proteinuria defined as a protein-to-creatinine ratio (mg/mg) ≥0.27 in the spot urine specimen, classifying women into two groups: proteinuria preceding pre-eclampsia and other pre-eclampsia.
This study confirmed that a considerable number of women with pre-eclampsia (36% in this study) develop significant proteinuria prior to the development of hypertension.
This study indicated that pre-eclamptic women with isolated gestational proteinuria constitute a severe pre-eclampsia group with respect to degrees of proteinuria, oedema and enhanced coagulation–fibrinolysis.
This study demonstrated that women with proteinuria preceding pre-eclampsia were likely to give birth at an earlier stage of pregnancy compared to those with other pre-eclampsia. However, this may have been distorted by incidental bias due to the small size of the study population.
Introduction
Pre-eclampsia (PE) remains a leading cause of maternal and perinatal mortality and morbidity.1 Hypertensive disorders are responsible for approximately one quarter of all maternal deaths in Latin America and the Caribbean.2 Hypertensive disorders encoded O-10 to O-16 by ICD-10 accounted for 8.1% and 4.4% of all maternal mortalities in Japan in 2005 and 2010, respectively.3
The current criteria for diagnosis of PE are systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg with proteinuria of ≥0.3 g/day. Thus, assessment of proteinuria is an important constituent of antenatal care to detect women with PE. Although proteinuria has been considered as a late sign of the clinical course of PE,4 it was recently demonstrated that some women exhibit proteinuria first in the absence of hypertension, so-called ‘isolated gestational proteinuria (IGP)’, and develop hypertension later and are diagnosed as having PE.5 6 The IGP was proposed to be an early clinical sign of PE.7 Then, the established PE risk factors, such as higher body mass index, younger age, nulliparity and twin pregnancy, were shown to be independently associated with the occurrence of IGP in late gestation in healthy term pregnancies, suggesting that IGP represents an early manifestation of PE.8 As approximately 15–26% of women with new onset of hypertension alone in pregnancy develop proteinuria later and are diagnosed as having PE5 9; there are two types of women with PE: those who develop proteinuria first significantly earlier than hypertension, and those who develop hypertension first or hypertension and proteinuria simultaneously.
Frequent laboratory workup, at least twice in a week, is recommended in women with PE.10 However, there have been no extensive studies regarding whether clinical and laboratory features of such women with proteinuria preceding PE (P-PE) differ from those of other women with PE.
Methods
This study was conducted with the approval of the Institutional Review Board of Hokkaido University Hospital, a tertiary teaching hospital managing mainly high-risk pregnant women. Laboratory tests, including complete blood count, biochemistry and parameters of coagulation–fibrinolysis, such as antithrombin activity, are performed routinely in pregnant women visiting our clinic even for minor symptoms as well as those admitted to the hospital for management of various obstetric and incidental complications.
Definition of terms used in this study and study protocol
Pregnant Japanese women receive 14–15 antenatal care visits before delivery and undergo determination of blood pressure, proteinuria by dipstick testing and body weight at each visit.11 A spot urine specimen with ≥1+ on dipstick test at antenatal visits was used for determination of protein and creatinine concentrations in this study. Proteinuria was defined as a protein-to-creatinine ratio (mg/mg; P/Cr) ≥0.27 in the spot urine specimen, and IGP was defined as proteinuria in the absence of hypertension. Hypertension was defined as the occurrence of SBP ≥140 mm Hg and/or DBP ≥90 mm Hg on at least two occasions recorded more than 12 h apart. Gestational hypertension (GH) was defined as hypertension occurring on and after gestational week (GW) 20 in the absence of proteinuria. PE was defined as the presence of hypertension and proteinuria. The GW at new onset of hypertension and proteinuria was specified in each patient, and each woman with PE was classified into either type of PE according to timing of the onsets of hypertension and proteinuria: P-PE in which the duration of IGP was 3 days or more and other PE (O-PE) in which the duration of IGP was 2 days or less. Subanalysis was performed in women with O-PE classified into two groups: those with a duration of IGP or GH of 2 days or less (simultaneous PE, S-PE) and those with a duration of GH of 3 days or more (hypertension preceding PE, H-PE). Women with IGP or GH were followed up once or more per week on an outpatient basis in principle, but underwent laboratory workup at each visit, including complete blood count, coagulation–fibrinolysis and blood chemistry. Women with a diagnosis of PE were admitted to our hospital and underwent determination of blood pressure at least three times a day, body weight daily and laboratory workup, including blood tests and P/Cr test, at least twice a week.
Patient selection
A total of 1665 women gave birth on or after GW 22 at Hokkaido University Hospital during the study period between January 2008 and August 2013. A total of 28 PE women without known renal or autoimmune diseases were abstracted from the database of discharge records. We were provided data from the institutional central laboratory regarding blood and urine test results for these 28 women. The data included complete blood count, biochemistry and coagulation–fibrinolysis parameters, and protein and creatinine concentrations in the urine. The clinical data, including patient age, parity and clinical outcomes, were obtained from medical charts.
Indication for an early delivery in women with PE
Induced labour or caesarean section were indicated in women with PE with one or more of the following: uncontrollable hypertension exceeding SBP ≥180 mm Hg and/or DBP ≥110 mm Hg even with antihypertensive agents; gradual increase in proteinuria exceeding P/Cr (mg/mg) ≥5.0 in the spot urine; liver dysfunction evidenced by an elevated aspartate aminotransferase (>45 IU/L) level concomitant with a reduced platelet count (<120×109/L) and/or a reduced antithrombin activity level (<65% of normal activity level); and a net weight gain ≥5 kg/week.
Statistical analyses
Data are presented as means±SD. Statistical analyses were performed using the JMP10 statistical software package (SAS, Cary, North Carolina, USA). Differences between the means were tested by analysis of variance and Tukey-Kramer HSD (honestly significant difference) test between each group, and categorical variables were compared using the χ2 test or Fisher's exact probability test. In all analyses, p<0.05 was taken to indicate statistical significance.
Results
Of the 28 women with PE, 10 (36%) had a duration of IGP ≥3 days, and therefore had P-PE, and the remaining 18 women (64%) exhibited no IGP or had a duration of IGP <3 days, and therefore had O-PE (table 1). Of the 18 women with O-PE, the duration of GH was 3 days or more in 8 women (H-PE group). There were no significant differences in clinical background between women with H-PE and S-PE (in whom the duration of IGP or GH was ≤2 days; table 1). Women with P-PE were significantly more likely to develop proteinuria and hypertension earlier and gave birth earlier compared to those with O-PE.
Table 1.
Demographic characteristics of two (three) groups
| Other types of PE |
||||
|---|---|---|---|---|
| P-PE | O-PE | H-PE | S-PE | |
| Number of women | 10 | 18 | 8 | 10 |
| Age (year) | 35.5±3.1 | 34.2±5.2 | 34.8±4.7 | 33.7±5.8 |
| Nulliparous | 5 (50%) | 11 (61%) | 4 (50%) | 7 (70%) |
| Twin pregnancy | 0 (0.0%) | 5 (28%) | 3 (38%) | 2 (20%) |
| Pre-pregnancy BMI | 19.5±2.9 | 21.2±3.5 | 21.3±3.0 | 21.1±4.0 |
| Body weight at delivery (kg) | 61.6±8.9 | 66.1±7.1 | 67.1±8.2 | 65.4±6.6 |
| Net weight gain in pregnancy (kg) | 12.2±2.9 | 13.6±5.5 | 14.5±5.1 | 12.9±6.0 |
| Caesarean delivery | 10 (100%) | 14 (78%) | 6 (75%) | 8 (80%) |
| Systolic blood pressure (mm Hg) | 164±10 | 173±14 | 173±18 | 172±12 |
| Diastolic blood pressure (mm Hg) | 96±7.2 | 97±13 | 95±13 | 98±13 |
| Gestational week at | ||||
| Proteinuria† | 30.2±3.0 | 35.3±4.3* | 34.4±4.1* | 36.0±4.5* |
| Hypertension | 31.6±2.7 | 34.6±4.4* | 32.9±3.8 | 35.9±4.5* |
| Delivery | 32.5±1.9 | 36.1±3.6* | 35.5±3.6 | 36.7±3.6* |
| <37 | 10 (100%) | 10 (56%)* | 6 (75%) | 4 (40%)* |
| Birth weight (g) | 1445±597 | 2219±692* | 2150±546* | 2281±823* |
| <2500 | 10 (100%) | 14 (78%) | 7 (88%) | 7 (70%) |
Data are presented as means±SD.
*p < 0.05 vs P-PE.
†Defined as a protein-to-creatinine ratio ≥0.27 in the spot urine.
BMI, body mass index; H-PE, hypertension preceding pre-eclampsia; O-PE, other pre-eclampsia including H-PE and S-PE; P-PE, proteinuria preceding pre-eclampsia; S-PE, simultaneous pre-eclampsia.
The P/Cr levels increased with advancing gestation in P-PE and O-PE groups (figure 1). The P/Cr level at birth was significantly higher in women with P-PE than in O-PE. The antenatal duration of proteinuria in women with P-PE, 16.1±10.3 days, did not differ from the antenatal duration of hypertension of 10.9±9.8 days in women with O-PE. The time interval until delivery after diagnosis of PE did not differ between the two groups (6.1±8.2 days for P-PE vs 5.9±6.8 days for O-PE; figure 1). Proteinuria disappeared in 4 (40%) of the 10 women with P-PE and 13 (72%) of the 18 women with O-PE at 1-month postpartum and in 7 (70%) and 18 (100%), respectively, at 2 months postpartum. Three women with P-PE and proteinuria at 2 months postpartum exhibited no proteinuria at 3 months postpartum.
Figure 1.

Changes in degree of proteinuria in relation to the timing of hypertension onset in the two groups. Open and closed circles indicate women with P-PE (n=10) and O-PE (n=18), respectively. *p<0.001 for differences between two levels. In women with P-PE , the P/Cr levels were 1.45±1.16, 5.63±2.27 and 7.28±2.14 when diagnosed with IGP, PE and on giving birth, respectively. In women with O-PE, the P/Cr levels were 1.70±1.81 and 3.19±2.49 when diagnosed with PE and on giving birth, respectively. The duration of IGP was 10.0±5.9 days (range 3–20) and the time interval until delivery after diagnosis of PE was 6.1±8.2 days (range 0–23) in women with P-PE. The duration of GH was 4.9±6.5 days (range 0–20) and the time interval until delivery after diagnosis of PE was 5.9±6.8 days (range 0–22) in women with O-PE. GH, gestational hypertension; IGP, isolated gestational proteinuria; O-PE, other pre-eclampsia; P-PE, proteinuria preceding pre-eclampsia; P/Cr, protein-to-creatinine ratio.
Net maternal weight gain in pregnancy did not differ between the P-PE and O-PE groups, although the gestation period was significantly shorter in women with P-PE than in O-PE (table 1). This may have reflected a significantly greater net maternal weight gain during the last antenatal 1 week in women with P-PE compared with O-PE (figure 2). Net maternal weight gains for the last 1 week (3.1±1.8 vs 1.3±1.7 kg, p=0.023) and for the last 3 days (2.2±1.4 vs 0.5±1.4 kg, p=0.005) were significantly greater in women with P-PE than in those with O-PE.
Figure 2.
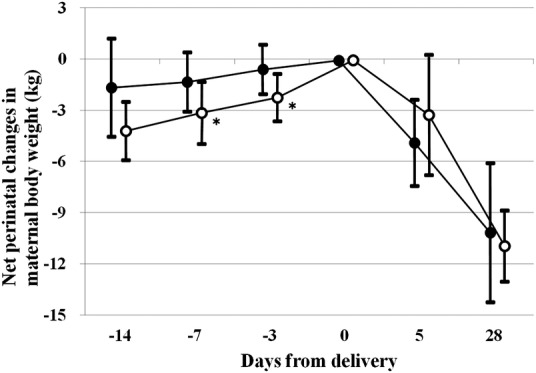
Net perinatal changes in maternal body weight. Open and closed circles indicate women with P-PE and O-PE, respectively. *p<0.05 versus women with O-PE. When data on body weight for the designated days were not available, data on the day before or after the designated days were used. Net antenatal maternal weight gain was 4.2±1.7 vs 1.6±2.8 kg (p=0.074) for the last antenatal 2 weeks, 3.1±1.8 vs 1.3±1.7 kg (p=0.023) for the last antenatal 1 week and 2.2±1.4 vs 0.5±1.4 kg (p=0.005) for the last antenatal 3 days in women with P-PE versus O-PE, respectively. Corrected weight loss during 4 weeks postpartum (maternal body weight just before delivery minus the sum of infant birth weight and maternal body weight at 4 weeks postpartum) was 9.4±2.0 vs 7.3±3.8 kg (p=0.109) in women with P-PE versus O-PE, respectively. O-PE, other pre-eclampsia; P-PE, proteinuria preceding pre-eclampsia.
Although most blood test results did not differ significantly between women with P-PE and O-PE, creatinine level was significantly higher and antithrombin activity and fibrinogen level tended to be lower in women with P-PE than in those with O-PE (table 2), suggesting that these parameters were associated with P/Cr levels. Indeed, the highest antenatal P/Cr levels were significantly inversely correlated with the lowest antenatal antithrombin activity and fibrinogen levels, but not with the lowest antenatal platelet counts or the highest antenatal levels of uric acid and creatinine (figure 3).
Table 2.
Lowest/highest antenatal levels of blood variables in two (three) groups
| Other types of PE |
||||
|---|---|---|---|---|
| P-PE | Overall | H-PE | S-PE | |
| Number of women | 10 | 18 | 8 | 10 |
| Platelet (×109/L) | 167±30 | 159±49 | 159±46 | 158±50 |
| <120 | 1 (10%) | 5 (27.8%) | 2 (25%) | 3 (30%) |
| Antithrombin activity | 68.1±7.3 | 73.8±10.1 | 71.4±8.0 | 75.7±11.6 |
| <65% | 3 (30%) | 4 (22.2%) | 2 (25%) | 2 (20%) |
| AST (IU/L) | 27.1±10.8 | 27.2±11.6 | 28.4±10.3 | 26.2±13.0 |
| >45 | 1 (10%) | 3 (16.7%) | 1 (12.5%) | 2 (20%) |
| LDH (IU/L) | 243±40 | 255±47 | 268±49 | 244±48 |
| >400 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Uric acid (mg/dL) | 6.6±1.1 | 6.1±1.1 | 6.3±0.8 | 6.0±1.3 |
| >6.0 | 8 (80%) | 11 (61.1%) | 5 (62.5%) | 6 (60%) |
| Fibrinogen (mg/dL) | 278±56 | 334±98 | 295±83 | 365±102* |
| <300 | 6 (60%) | 8 (44.4%) | 5 (62.5%) | 3 (30%) |
| Creatinine (mg/dL) | 0.69±0.09 | 0.57±0.10* | 0.61±0.12 | 0.53±0.04* |
| >0.7 | 4 (40%) | 2 (11.1%) | 2 (25%) | 0 (0%) |
Reference interval (not specific for pregnant women) was 80–130% for antithrombin activity.
*p<0.05 vs P-PE.
AST, aspartate aminotransferase; H-PE, hypertension preceding pre-eclampsia; LDH, lactate dehydrogenase; P-PE, proteinuria preceding pre-eclampsia; S-PE, simultaneous pre-eclampsia.
Figure 3.
Correlation between degree of antenatal proteinuria and antenatal blood variables. Open and closed circles indicate women with P-PE and O-PE, respectively. The highest antenatal P/Cr levels were significantly inversely correlated with either the lowest antenatal antithrombin activity levels or the lowest antenatal fibrinogen levels, but not with the lowest antenatal platelet counts, the highest antenatal uric acid levels or the highest antenatal creatinine levels. O-PE, other pre-eclampsia; P/Cr, protein-to-creatinine ratio; P-PE, proteinuria preceding pre-eclampsia.
Discussion
In this study, 36% of all the 28 women with PE during the study period developed proteinuria first and hypertension later, confirming that a considerable number of women with PE develop proteinuria first and subsequently develop hypertension. This phenomenon was first described in the literature in 20085 followed by reports that the appearance of IGP as determined by dipstick test is associated with established risk factors for PE, such as higher pre-pregnancy body mass index, nulliparity, twin pregnancy and elevation of blood pressure in late pregnancy,8 supporting the hypothesis that IGP is part of the disease spectrum of PE. In addition, women with IGP defined by repeated positive dipstick test results in two successive antenatal visits show increased likelihood of developing PE.12
The P/Cr levels have been validated as a proxy for 24 h collection in non-pregnant patients, and determination of P/Cr is the preferred method for evaluation of the amount of protein loss in the urine outside of obstetrics.13 A P/Cr level around 0.27 is a reasonable cut-off level to exclude or detect proteinuria of 0.3 g/day,14 although the prognostic value of different quantities of urinary protein is unclear and further studies to identify diagnostic thresholds of proteinuria that are accurate for predicting clinically important outcomes is needed.10 A recent study examining the occurrence of adverse maternal and perinatal outcomes with different thresholds of proteinuria (0.3–0.49 and >0.5 g/day, respectively) in women with PE confirmed that 0.3 g/day is an appropriate threshold.15 It may be clinically beneficial to be aware of physiological changes in the amount of protein loss in the urine of pregnant women. Among otherwise healthy women who do not develop PE, mean P/Cr level increases with advancing gestation in singleton and twin pregnancies, and women with twins are significantly more likely to have high P/Cr level than those with singleton pregnancies16: the mean P/Cr of 0.06 and 0.10 for singletons and twins, respectively, at GW 16–20 increased to 0.09 and 0.12 at GW 20–28 and to 0.15 and 0.22 at GW 34–38, respectively. In the 10 women with P-PE in this study, the mean P/Cr level continued to increase until delivery after showing P/Cr ≥0.27 (figure 1). These results suggested that the P/Cr increases linearly in some pregnant women in whom it exceeds a cut-off level of 0.27 in the absence of hypertension.
As mentioned above, P/Cr increases with advancing gestation. The P/Cr at birth was significantly greater in women with P-PE than in O-PE, perhaps reflecting the significantly longer antenatal duration of proteinuria in the former than in the latter group (figure 1). However, the time interval after onset of proteinuria until delivery in women with P-PE did not differ from that after onset of hypertension until delivery in women with P-PE (16.1±10.3 vs 10.9±9.8 days, respectively). In addition, the time intervals after diagnosis of PE until delivery were similar in both groups (6.1±8.2 vs 5.9±6.8 days, respectively). These observations suggested that IGP should be considered as an important clinical sign equal to hypertension.
In the present study, women with P-PE showed significantly greater maternal weight gain in the last antenatal 1 week compared to those with O-PE. The net weight gains of 4.2 and 3.1 kg in the last 2 weeks and 1 week in women with P-PE accounted for approximately 34% and 25% of the total weight gain of 12.2 kg in pregnancy, respectively, while respective values of 1.6 and 1.3 kg in women with O-PE accounted for 12% and 9.6% of the total weight gain of 13.6 kg. As the mean±SD weekly weight gain, defined as (maternal weight at delivery−pre-pregnancy maternal weight)/(GW at delivery−2), is 0.26±0.12 kg for Japanese women with singleton pregnancies not complicated with pregnancy-induced hypertension,17 and weight gain in the last antenatal 2 weeks is 0.9±0.9 kg for otherwise healthy Japanese women with singleton pregnancies18; these results indicated that although oedema due to the accumulation of excess water in the interstitial space occurred in late pregnancy in P-PE and O-PE, its degree was greater in women with P-PE than in those with O-PE.
Excessive weight gain during the final stage of pregnancy is suggested to be a risk factor for eclampsia.18 The daily protein loss in the urine was greater in women with P-PE than in those with O-PE, and the degree of proteinuria was inversely correlated with reductions of antithrombin activity and fibrinogen level in this study. A decrease in antithrombin activity has also been suggested to be a risk factor for eclampsia.18 As enhanced coagulation–fibrinolysis is a characteristic laboratory finding in women with PE,19 20 the degree of reduction in fibrinogen level may reflect the severity of PE. The circulating plasma volume was reduced in women with PE,21 22 and serum creatinine level was significantly higher in women with P-PE than in those with O-PE in the present study, suggesting a greater decrease in circulating plasma volume in women with P-PE. Taken together, these results suggest that P-PE constitutes a more serious condition than other types of PE.
Study limitations
IGP developed at GW 30.0±3.0, while GH developed at GW 32.9±3.8 in this study (table 1), suggesting that IGP occurs earlier than GH. However, this may not be a general characteristic and may have been distorted by incidental bias due to the small size of the study population. Although the definition of proteinuria differed between the present study and our previous study,5 IGP occurred at a mean GW of 31.1 in 19 women with IGP preceding PE, while GH occurred at mean GW of 28.2 in 5 women with GH preceding PE in the previous study.5 In another study to determine the fraction of women who developed PE among those with GH,9 GH developed at GW 33±4 in 26 women with GH preceding PE. As IGP and GH may occur at any time point on or after GW 20, and as there have been no reports regarding the number of women who develop GH and IGP according to GW, the mean GW at the onset of IGP and GH in the present study and previous reports may have been subject to incidental bias due to the limited number of study patients.
Conclusions
We compared the clinical features of 10 consecutive women who first developed IGP and later developed hypertension with those of 18 consecutive women who developed hypertension first or hypertension and proteinuria simultaneously and were diagnosed with PE. The P/Cr level at birth and net maternal weight gain in the last antenatal 1 week were greater in the former than the latter group. The P/Cr levels at birth were significantly inversely correlated with the lowest antenatal antithrombin activities and fibrinogen levels among women with PE. All of these results suggested that women with PE along with IGP followed by hypertension constitute a more severe PE group.
Supplementary Material
Footnotes
Contributors: RA was involved in drafting the manuscript, data collection and data analysis. TY was involved in study design, data collection and data analysis. MM was involved in study design and data collection. RN was involved in data collection. HM was involved in study design and supervision of this study.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Ethics approval: The Institutional Review Board of Hokkaido University Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Steegers EAP, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet 2010;376:631–44 [DOI] [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74 [DOI] [PubMed] [Google Scholar]
- 3.Kamiya K. Maternal and child health statistics of Japan. Tokyo: Japan Mothers’ & Children's Health Organization, 2007 and 2012 [Google Scholar]
- 4.Chesley LC. Diagnosis of preeclampsia. Obstet Gynecol 1985;65:423. [PubMed] [Google Scholar]
- 5.Morikawa M, Yamada T, Yamada T, et al. Pregnancy outcome of women who developed proteinuria in the absence of hypertension after mid-gestation. J Perinat Med 2008;36:419–24 [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, Yamada T, Morikawa M, et al. Isolated proteinuria as an initial sign of severe preeclampsia. Open J Obstet Gynecol 2011;1:13–16 [Google Scholar]
- 7.Morikawa M, Yamada T, Minakami H. Outcome of pregnancy in patients with isolated proteinuria. Curr Opin Obstet Gynecol 2009;21:491–5 [DOI] [PubMed] [Google Scholar]
- 8.Macdonald-Wallis C, Lawlor DA, Heron J, et al. Relationship of risk factors for pre-eclampsia with patterns of occurrence of isolated gestational proteinuria during normal term pregnancy. PLoS ONE 2011;6:e22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saudan P, Brown MA, Buddle ML, et al. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol 1998;105:1177–84 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy (clinical guideline 107). 2010. http://wwwniceorguk/CG107.2010
- 11.Minakami H, Hiramatsu Y, Koresawa M, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J Obstet Gynaecol Res 2011;37:1174–97 [DOI] [PubMed] [Google Scholar]
- 12.Chiba K, Yamada T, Kawaguchi S, et al. Clinical significance of proteinuria determined with dipstick test, edema, and weekly weight gain ≥500 g at antenatal visit. Pregnancy Hypertens 2013;3:161–5 [DOI] [PubMed] [Google Scholar]
- 13.Maynard SE, Thadhani R. Pregnancy and the kidney. J Am Soc Nephrol 2009;20:14–22 [DOI] [PubMed] [Google Scholar]
- 14.Côté A-M, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot protein: creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ 2008;336:1003–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramham K, Poli-de-Figueiredo CE, Seed PT, et al. Association of proteinuria threshold in pre-eclampsia with maternal and perinatal outcomes: a nested case control cohort of high risk women. PLoS ONE 2013;8:e76083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith NA, Lyons JG, McElrath TF. Protein: creatinine ratio in uncomplicated twin pregnancy. Am J Obstet Gynecol 2010;203:381.e1–4 252 [DOI] [PubMed] [Google Scholar]
- 17.Morikawa M, Yamada T, Akaishi R, et al. Gestational weight gain according to number of fetuses in Japanese women. J Perinat Med 2013;13:1–6 [DOI] [PubMed] [Google Scholar]
- 18.Yamada T, Kuwata T, Matsuda H, et al. Risk factors of eclampsia other than hypertension: pregnancy-induced antithrombin deficiency and extraordinary weight gain. Hypertens Pregnancy 2012;31:268–77 [DOI] [PubMed] [Google Scholar]
- 19.de Boer K, ten Cate JW, Sturk A, et al. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol 1989;160:95–100 [DOI] [PubMed] [Google Scholar]
- 20.Cadroy Y, Grandjean H, Pichon J, et al. Evaluation of six markers of the haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br J Obstet Gynaecol 1993;100:416–20 [DOI] [PubMed] [Google Scholar]
- 21.Silver HM, Seebeck MA, Carlson R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurement of red blood cell and plasma volumes. Am J Obstet Gynecol 1998;179:87–93 [DOI] [PubMed] [Google Scholar]
- 22.Scholten RR, Sep S, Peeters L, et al. Prepregnancy low-plasma volume and predisposition to preeclampsia and fetal growth restriction. Obstet Gynecol 2011;117:1085–93 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



