Abstract
Objectives
Accumulating evidence suggests an adverse association between depressive symptoms and cognition, but a positive association between insulin like growth factor (IGF)-1 and cognition. The present study examined the influence of IGF-1 in the relationship between depressive symptoms and learning and memory.
Methods
A cross-sectional study of 94 healthy fit older adults. Blood was collected and plasma IGF-1 was measured. Depressive symptoms were assessed with the Geriatric Depression Scale (GDS), and learning and memory were assessed using the Rey Auditory Verbal Learning Test (AVLT).
Results
Among older adults with lower IGF-1 levels, higher depressive symptoms scores were associated with lower AVLT delayed recall and recognition. Older adults with higher IF-1 levels showed no associations between depressive symptoms and memory.
Conclusions
The association between depressive symptoms and cognition is stronger among older adults with lower levels of circulating IGF-1. Further validation studies on groups with depression or different stages of cognitive impairment are needed. IGF-1 may be a novel intervention target for slowing cognitive decline in older adults with depressive symptoms.
Keywords: IGF-1, memory, learning, depressive symptoms
Background
Cognitive decline is a major obstacle for successful aging. Depression or depressive symptoms are one of the most consistently identified risk factors for cognitive impairment in older adults (Steenland et al., 2012). However, a number of clinical trials failed to demonstrate that improving depression, for instance, using anti-depressants, also improves cognitive function (Thompson et al., 2007). Further, the efficacy of interventions aimed at improving cognitive function is reduced by the presence of depression or depressive symptoms (Portella et al., 2003). What is unclear is whether protective factors exist that can mitigate the influence of depressive symptomatology on cognitive function in older adults. If so, such factors may provide new avenues for interventions that protect cognitive function and well-being in older ages.
Insulin like growth factor (IGF-1), a primary endocrine agent mediating the action of growth hormone and cell growth and metabolism, also plays an important role in brain function. Circulating IGF-1 is able to cross the blood-brain barrier and bind to receptors in the brain (Reinhardt and Bondy, 1994). IGF-1 can also be synthesized directly in the brain. IGF-1 from both sources can promote synaptic function, neurogenesis, cerebrovascular function, and alleviate oxidative stress (Sonntag et al., 2013). Emerging studies suggest reduced circulating IGF-1 levels and loss of IGF-1 function contribute to the development of cognitive deficits (Aleman and Torres-Aleman, 2009). Receptors for IGF-1 are prominent in the prefrontal cortex, parahippocampus, hippocampus, and amygdala (van Dam and Aleman, 2004), brain regions that play a role in regulating learning and memory, as well as depressive symptomatology (Dere et al., 2010). Notably, in animal experiments, administering IGF-1 induced antidepressant-like behavioral effects (see review by (Paslakis et al., 2012); however, across a handful of published human studies, inconsistent relationships are observed between IGF-1 and depression, with some studies supporting a possible role for increased IGF-1 in alleviating depressive mood (Cassilhas et al., 2010), but others showing elevated IGF-1 levels in individuals with acute depression (Deuschle et al., 1997). Thus, direct associations between IGF-1 and depressive symptoms remain to be clarified. Nevertheless, given that cognition and depressive mood are similarly regulated by brain areas (Dere et al., 2010) that are also responsive to IGF-1 (van Dam and Aleman, 2004), it is plausible that IGF-1 may modify how depressive symptoms relate to cognition. The purpose of the present cross-sectional study was to examine the moderating role of IGF-1 in the association between depressive symptoms and cognitive function in a sample of healthy older adults.
Methods
Participants
One hundred and fourteen adults ages 50 years and older, recruited through community advertising, participated in a study of stress and memory (Heffner et al., 2012). Eligibility criteria are detailed elsewhere (Heffner et al., 2012). For the present study, participants were excluded if they had immune or endocrine-related health problems, were current smokers and alcoholic drinkers, showed cognitive impairment that would be consistent with Mild Cognitive Impairment or dementia, were more than 30% above ideal weight or more than 10% below, reported needle or blood phobias, reported use of psychotropic medications, or clinically depressed (taking anti-depressant or scored 20 or greater in the 30-item Geriatric Depression Scale (GDS)). The final sample included 94 older adults with IGF-1 measures available for analysis. The university affiliated internal review boards approved the study; all participants provided written informed consent prior to participation.
Procedure
After informed consent and further screening, individuals eligible to continue returned approximately one week later for a study session. All study sessions started between 1:00 and 3:00 p.m. to control for diurnal variation in cognitive performance and biological indices. Participants completed questionnaires, had height and weight measured, and had blood drawn by a research nurse. Participants then sat quietly for a 30-minute adaptation period. Participants were then administered the neuropsychological tests of learning and memory.
IGF-1 Assays
Blood samples were kept on ice, centrifuged at 4°C, and plasma stored at −80°C within 30 minutes following collection. Plasma IGF-1 was assayed in duplicate using Human IGF-1 Quantikine ELISA kits (R&D Systems, Inc., Minneapolis, MN). Intra- and inter-assay coefficients of variation were < 10%.
Learning and Memory
The Rey Auditory Verbal Learning Test (AVLT) is a well-validated measure of verbal learning and memory, consisting of a 15-item word list (List A) practiced over five learning trials, immediate recall of the list after an intrusion list (List B), and a 30-minute delayed recall and recognition of the list. The following scores were used: total learning (the sum of the number of correct responses over the five learning trials), immediate recall (the number of correct responses from immediate recall of List A), delayed recall (the number of correct responses from delayed recall of List A), and recognition (the number of correct responses minus the number of false positives from delayed recall of List A). In addition, we also calculated the trajectory over the five learning trials to capture the learning curve.
Depressive symptoms and status
Depressive symptoms were assessed with the 30-item Geriatric Depression Scale (GDS), a reliable, well-validated instrument. Cronbach’s α for the present study was 0.91.
Demographic and health variables
Data on age, education, and gender were provided by self-report. Body mass index (BMI) was computed as weight (kg)/height (m)2. Anti-depressant use, estrogen replacement therapy (ERT), and antihypertensive medication use were extracted from participants’ medication list. Sleep quality was assessed with the Pittsburg Sleep Quality Inventory, a well-validated and reliable sleep quality measure, with higher scores indicative of worse sleep quality.
Data analysis
IBM SPSS 19.0 was used to analyze data. GDS data was computed as ln (GDS raw score + 1) due to the skewed distribution. IGF-1 was categorized into low versus high level using the median score (75ng/ml). To compare all variables by IGF-1 level, independent t-tests and χ2 tests were used for continuous and categorical variables, respectively; and analysis of covariance was used if any confounding factors need to be controlled.
To determine potential covariates for analyses for AVLT domains, preliminary correlational analyses were conducted between AVLT domains and demographic and health variables using Pearson’s r (for continuous variables) or Spearman’s ρ (for categorical variables). Variables that had correlations with AVLT domains at p value < .20 were considered covariates in later analyses (data was not shown). Based on the p values, age, gender, education, use of ERT, use of anti-hypertensive, and sleep quality were considered covariates in the following analyses.
To examine the association of IGF-1 and GDS with AVLT domains (except learning curve), Generalized Linear Model (GLM) was applied, setting high level IGF-1 as the reference group. The equation was: .
For AVLT learning curve, we modeled the effect of IGF-1, GDS, and their interaction on the five learning trials (time) using Generalized Estimating Equation (GEE) with AR(1) as the working correlation matrix. The equation was: .
If there were any significant interactions between IGF-1 and GDS, we next examined the association between GDS and AVLT domains using GLM or GEE models within the two levels of IGF-1 separately. Equations were similar as the ones described above without the terms of IGF-1 or IGF-1 × GDS, and included all covariates. To adjust for multiple comparisons, false discovery rate (FDR) corrected probability was applied (q value) when analyzing interaction. Otherwise, p value was used. Alpha was set at 0.05.
Results
Compared to the subgroup with lower IGF-1, the subgroup with a higher IGF-1 was significantly younger (M(SD) = 58.91 (8.09) vs. M(SD) = 62.36 (8.46), t = 2.02, p = .046); the two groups were similar in other demographic and health characteristics as well as AVLT domains (see Appendix Table).
Table 1 shows the effect of IGF-1 and GDS on AVLT domains from the GLM. There was a significant interaction of IGF-1 and GDS on AVLT delayed recall and recognition (Model c), but there was no significant main effect of IGF-1 or GDS (Model a and Model b).
Table 1.
Relationships between IGF-1, GDS, and AVLT Domains (n = 94)
| Model a | Model b | Model c | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| B (SE) | q value (p value) | B (SE) | q value (p value) | B (SE) | q value (p value) | |
| Total learning | ||||||
| GDS‡ | −1.62 (1.00) | .44 (.11) | −1.60 (1.00) | .44 (.11) | 0.23 (1.41) | .87 (.87) |
| IGF-1 † | 0.87 (1.62) | .79 (.59) | 4.34 (2.49) | .16 (.082) | ||
| IGF-1† × GDS‡ | −3.23 (1.79) | .071 (.071) | ||||
| Immediate recall | ||||||
| GDS‡ | −0.09 (0.34) | .80 (.80) | −0.09 (0.34) | .79 (.79) | 0.57 (0.48) | .32 (.24) |
| IGF-1 † | −0.07 (0.55) | .90 (.90) | 1.18 (0.85) | .16 (.16) | ||
| IGF-1† × GDS‡ | −1.16 (0.61) | .071 (056) | ||||
| Delayed recall | ||||||
| GDS‡ | −0.34 (0.36) | .80 (.71) | −0.35 (0.36) | .60 (.33) | 0.79 (0.49) | .24 (.11) |
| IGF-1 † | −0.57 (0.58) | .66 (.33) | 1.58 (0.87) | .16 (.068) | ||
| IGF-1† × GDS‡ | −2.01 (0.62) | .004 (.001) | ||||
| Recognition | ||||||
| GDS‡ | −0.36 (0.51) | .80 (.48) | −0.38 (0.51) | .60 (.45) | 1.09 (0.70) | .24 (.12) |
| IGF-1 † | −1.00 (0.82) | .66 (.23) | 1.79 (1.23) | .16 (.15) | ||
| IGF-1† × GDS‡ | −2.60 (0.88) | .006 (.003) | ||||
Note. Confounding factors included age, gender, education, use of ERT, use of antihypertensive, and sleep quality.
IGF-1 high level was taken as referent.
GDS was recoded as ln(GDS raw score +1).
Applying GEE, after controlling for all covariates, participants showed a significant increase in the number of words they learned over learning trials (B = 1.46, SE = 0.08, p < .001), but no significant main effect of IGF, GDS, or interaction between IGF and GDS.
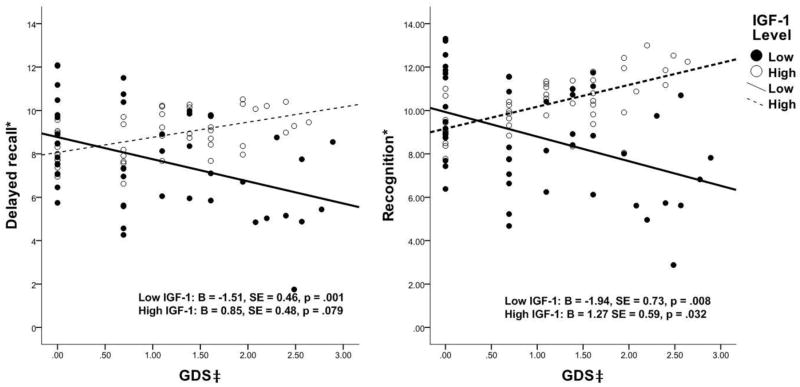
We next examined the relationship between GDS and AVLT delayed recall and recognition within two IGF-1 levels controlling for all covariates. In GLM models, there were significant associations between higher GDS and lower delayed recall and recognition in participants with lower IGF-1 levels, as well as higher GDS and higher recognition in participants with higher IGF-1 levels, but no association between GDS and delayed recall in higher IGF-1 levels (see Figure 1).
Figure 1.
Relationships between GDS and AVLT Domains by IGF-1 Level (Outcomes below were controlled for age, gender, education, use of ERT, use of anti-hypertensive, and sleep quality. * standardized using Z-score for comparison; ‡ GDS was recoded as ln(GDS raw score +1).
Discussion
In the present cross-sectional correlational study, the adverse association of depression with memory, specifically delayed recall and recognition, was only observed in the group with low, but not high, IGF-1. Our findings provide preliminary evidence that IGF-1 may modulate the relationship between memory and depression. Although IGF-1 and depressive symptoms were not directly related, they may regulate memory via similar pathways. First, while not conclusive, circulating IGF-1 may influence levels of IGF-1 in brain regions (e.g., hippocampus) that regulate emotional symptoms, including depression, and organize learning and memory (Yan et al., 2011). Second, current theories of learning and memory suggest that the storage of new information is regulated by the strength of synaptic connections between neurons of cortical networks (also called long-term potentiation, LTP). Occurrence of depression may be attributed to an imbalance of excitation and inhibition of LTP, while IGF-1 has a major role in regulating or reversing LTP (Deak and Sonntag, 2012). Third, a substantial proportion of cognitive decline in the aging process can be explained by the degeneration of cerebrovascular system. Depressive symptoms in old age are believed to be vascular-related, while IGF-1 is related to lower oxidative stress and better cerebrovascular function (Ungvari and Csiszar, 2012). Future studies need to directly examine all these potential mechanisms. Of note, IGF-1 moderated the association between depressive symptoms and delayed recall and recognition (retrieval of items) but not the learning process (acquisition and encoding of items). Retrieval and encoding of items are two highly correlated but slightly different cognitive processes that may be regulated by different regions in the hippocampus, although this distinction remains debated (Mitchell and Johnson, 2009). Notably, depression has a stronger influence on encoding than retrieval processes (Storbeck and Clore, 2011). Replication and future study are necessary to determine whether indeed IGF-1’s potentially protective role may be limited to particular domains of cognitive function.
In a cognitively homogeneous, and generally non-depressed sample, we did find a strong relationship between depressive symptoms and memory in adults with lower but not higher levels of IGF-1. The advantage of examining these associations in a sample of healthy and fit older adults is that the variability in cognitive capacity is less likely due to chronic morbidity (e.g., vascular diseases); thus, findings may underscore important mechanisms pertaining to aging-related changes in cognitive function rather than morbidity-related changes, which are often difficult to disentangle. However, our healthy sample of older adults limits the generalizability of our findings. Prospective studies of older adults across the range of cognitive and emotional health are needed to clarify multiple issues raised by these and more recent findings regarding IGF-1. First the protective effect of IGF-1 on cognition in an elderly group with diagnosed major depression remains to be determined. Second, recent research shows IGF-1 resistance appears early in the Alzheimer’s disease process (Talbot et al., 2012), thus, it will be necessary to examine the moderating role of IGF-1 on cognition and mood links in different stages of cognitive impairment. Third, it remains to be clarified whether IGF-1 is a mediator regulating depressive symptoms and cognitive deficits, or even whether depressive symptoms mediates the relationship between IGF-1 and cognitive deficits.
Findings from the current study suggest that IGF-1 may protect memory against the ill effects of depression. Increasing IGF-1, perhaps indirectly (e.g., through exercise, (Gatti et al., 2012), may provide a new intervention target to prevent cognitive decline in older adults with depressive symptoms.
Acknowledgments
Role of the Funding Resource
Data collection of this research project was supported in part by National Institute on Aging grants R03 AG030029-01, R24 AG031089-01 to K. Heffner. The manuscript development was supported by the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F. Lin.
Appendix Table 1.
Demographic and Health Information as a Total Sample and by IGF-1 Level (N = 94)
| Total
|
IGF-1 Level
|
||||
|---|---|---|---|---|---|
| Low (≤ 75 ng/ml) N = 48 |
High (> 75 ng/ml) N = 46 |
t or χ2 test or F test (p value) | df | ||
|
|
|
||||
| log IGF-1, Mean (SD) | −0.32 (0.35) | −0.59 (0.23) | −0.03 (0.20) | −12.43(< .001) | 92 |
| Age, Mean (SD) | 60.68 (8.42) | 62.36 (8.46) | 58.91 (8.09) | 2.02 (.046) | 92 |
| Male, n (%) | 39 (41.5%) | 22 (45.8%) | 17 (37.0%) | 0.83 (.41) | |
| Years of education, Mean (SD) | 15.03 (3.39) | 14.48 (3.51) | 15.61 (3.19) | −1.63 (.11) | 92 |
| BMI, Mean (SD) | 26.80 (4.55) | 26.70 (5.14) | 26.92 (3.89) | −0.23 (.82) | 92 |
| Use of ERT, n (%) | 4 (4.3%) | 3 (6.3%) | 1 (2.2%) | 0.33 (.62) | 1 |
| Use of anti-hypertensive, n (%) | 18 (19.1%) | 11 (22.9%) | 7 (15.2%) | 0.34 (.44) | 1 |
| Sleep quality, Mean (SD) § | 4.87 (2.90) | 4.57 (2.77) | 5.18 (3.03) | −1.00 (.32) | 90 |
| GDS ‡ | 1.05 (0.87) | 0.98 (0.92) | 1.12 (0.12) | −0.79 (.43) | 92 |
| AVLT total learning, Mean (SD) | 44.22 (8.62) | 8.86 (1.28) | 8.37 (1.23) | 0.20 (.66) a | 91, 1 |
| AVLT immediate recall, Mean (SD) | 8.83 (2.82) | 8.46 (3.06) | 9.22 (2.52) | 1.17 (.28) a | 91, 1 |
| AVLT delayed recall, Mean (SD) | 8.26 (3.06) | 7.67 (3.37) | 8.87 (2.59) | 3.71 (.057) a | 91, 1 |
| AVLT recognition, Mean (SD) | 9.48 (4.13) | 8.63 (4.73) | 10.37 (3.21) | 3.71 (.057) a | 91, 1 |
Note.
two persons’ data were missing.
GDS was recoded as ln(GDS raw score +1).
controlled for age.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Torres-Aleman I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89:256–265. doi: 10.1016/j.pneurobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Antunes HK, Tufik S, de Mello MT. Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept Mot Skills. 2010;110:265–276. doi: 10.2466/PMS.110.1.265-276. [DOI] [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 2010;215:162–171. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Strasburger CJ, Schweiger U, Weber B, Korner A, Standhardt H, Gotthardt U, Schmider J, Pflaum CD, Heuser I. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology. 1997;22:493–503. doi: 10.1016/s0306-4530(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Gatti R, De Palo EF, Antonelli G, Spinella P. IGF-I/IGFBP system: metabolism outline and physical exercise. J Endocrinol Invest. 2012;35:699–707. doi: 10.3275/8456. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Ng HM, Suhr JA, France CR, Marshall GD, Pigeon WR, Moynihan JA. Sleep Disturbance and Older Adults’ Inflammatory Responses to Acute Stress. Am J Geriatr Psychiatry. 2012;20:744–752. doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslakis G, Blum WF, Deuschle M. Intranasal insulin-like growth factor I (IGF-I) as a plausible future treatment of depression. Med Hypotheses. 2012;79:222–225. doi: 10.1016/j.mehy.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Portella MJ, Marcos T, Rami L, Navarro V, Gasto C, Salamero M. Residual cognitive impairment in late-life depression after a 12-month period follow-up. Int J Geriatr Psychiatry. 2003;18:571–576. doi: 10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31:265–275. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storbeck J, Clore GL. Affect influences false memories at encoding: evidence from recognition data. Emotion. 2011;11:981–989. doi: 10.1037/a0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Herrmann N, Rapoport MJ, Lanctot KL. Efficacy and safety of antidepressants for treatment of depression in Alzheimer’s disease: a metaanalysis. Can J Psychiatry. 2007;52:248–255. doi: 10.1177/070674370705200407. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur J Pharmacol. 2004;490:87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Yan H, Mitschelen M, Bixler GV, Brucklacher RM, Farley JA, Han S, Freeman WM, Sonntag WE. Circulating IGF1 regulates hippocampal IGF1 levels and brain gene expression during adolescence. J Endocrinol. 2011;211:27–37. doi: 10.1530/JOE-11-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]