Abstract
Summary
Both microbes and tumors activate innate resistance, tissue repair and adaptive immunity. Unlike acute infection, tumor growth is initially inapparent; however, inflammation and immunity affect all phases of tumor growth from initiation to progression and dissemination. Here, we discuss the shared features involved in the immune response to infection and cancer including modulation by commensal microbiota, reactive hematopoiesis, chronic immune responses and regulatory mechanisms to prevent collateral tissue damage. This comparative analysis of immunity to infection and cancer furthers our understanding of the basic mechanisms underlying innate resistance and adaptive immunity and their translational application to the design of new therapeutic approaches.
Introduction
Organisms resist infections by establishing barriers and activating different classes of innate resistance and adaptive immunity. The tissue in which the infection occurs regulates the strength, quality and type of the immune response for efficient pathogen eradication and tissue repair while limiting collateral tissue damage (Matzinger and Kamala, 2011). However, some microbes have evolved evasion mechanisms that thwart these host responses, resulting in uncontrolled or chronic infection associated with pathologic damage.
Unlike the traditional view of immunity as a response to foreign microbes and molecules, the primary trigger for innate resistance, which is followed by adaptive immunity in higher organisms, may be sensing of altered self, such as changes in tissue homeostasis or integrity (Matzinger, 2002; Medzhitov, 2008). Innate receptors respond to microbial associated molecular pattern (MAMPs) but also to endogenous mediators of inflammation released by damaged tissues [(damage associated molecular patterns (DAMPs)]. These tissue-intrinsic inflammatory responses are amplified by the recruitment of hematopoietic cells that express innate receptors for exogenous and endogenous ligands present in the inflamed tissue and set the stage, via antigen presentation and activation of T and B cells, for adaptive immunity.
Tumors may originate at the site of chronic inflammation that is either caused by infectious pathogens or is often aseptic. Nevertheless, tumors eventually establish an almost symbiotic relationship with their host that mimics chronic infections or cohabitation with commensal microorganisms and maintain their presence by suppressing excessive inflammation and anti-tumor immune responses. The molecular alterations in the transformed cells or the surrounding epithelial and stromal cells are associated with a pro-inflammatory wound healing microenvironment that, in addition to providing tumor growth and angiogenic factors, affects tissue remodeling and decreases the expression of molecules involved in cell-to-cell adhesion initiating the epithelial-mesenchimal progression, which can lead to tumor cell invasion and progression (Edme et al., 2002; Salcedo et al., 2013). Nucleic acids and other products released upon cell stress or death in more advanced tumors or following cytotoxic therapy are recognized by innate sensors, resulting in inflammation and activation of antigen presenting cells (APCs) (Ma et al., 2013b). Thus, like infection, tumors influence and are affected by inflammation and immunity from their early initiation to progression, dissemination, and eventually death of the host. Cancer-associated inflammation affects not only local tumor growth and dissemination but is also responsible for severe cancer-associated co-morbidities such as anorexia, cachexia and immunosuppression, making cancer a systemic rather than a localized disease (Trinchieri, 2012). Malignant growth originates within differentiated tissues and maintains some of the functional, morphologic and immune traits of the tissue of origin, representing a “caricature” of the original tissue (Pierce and Speers, 1988). The immune response elicited by the tumor will be in most cases unable to eradicate it and will establish a Darwinian environment selecting the genetically fittest cancer cells (tumor editing and escape) that evolve into aggressive malignant tumors or, in some cases, remain temporarily in equilibrium with non-malignant host cells (Schreiber et al., 2011).
Given the parallels between immunity to infection and cancer, this review focuses on the similarities and differences in these processes and how these two fields can help each other in advancing our understanding of innate resistance and immunity.
Inflammation and cancer
Inflammation and immunity are inherent characteristics of cancer, and “avoiding immune destruction” and “tumor promoting inflammation” are now among the hallmarks of cancer (Hanahan and Weinberg, 2011). Up to a quarter of human cancers are related to infection or infection-associated chronic inflammation. Helicobacter pylori, human papillomaviruses (HPVs), Epstein-Barr virus (EBV), and hepatitis B and C viruses are the most common etiopathogenic factors (Parkin, 2006). Some oncogenic pathogens directly transform the tumor-forming cells (e.g. EBV or HPV), whereas others (e.g. H. pylori) establish an inflammatory milieu favoring tumor generation. Other cancers initiate in tissues chronically inflamed for causes other than infection, e.g. tissue injury by physical or chemical insults or genetic diseases. Altered composition (dysbiosis) of the intestinal microbiota or its physical interaction with hematopoietic cells also regulate inflammation, and it has been shown to be a cause of cancer (Goldszmid and Trinchieri, 2012; Jobin, 2012; Rao et al., 2006). The class of inflammatory/immune response observed in tumors is determined by the characteristics of the originating tissue, the nature of tissue damage, pathogens, or commensals associated with carcinogenesis, and by the pro-inflammatory mediators released by the tumor cells or their stroma. Although most pro-inflammatory cytokines may have pleiotropic pro-tumor or anti-tumor effects, the classes of immune response with a predominant role in tissue repair and angiogenesis, e.g. the Th-2 response or the alternatively-activated M2 macrophages, are most likely tumor promoting, while tissue and tumor damaging responses, e.g. Th1 or classically activated M1 macrophages, are generally associated with antitumor effects.
Chronic inflammation promotes cancer through multiple mechanisms. Genomic instability and DNA damage, mediated in part by reactive oxygen species (ROS), may cause genetic and epigenetic mutations initiating cell transformation and cancer (Schetter et al., 2010). Inflammation also promotes tumor progression by inducing tissue remodeling, supporting angiogenesis, and providing growth factors (Schetter et al., 2010). In all tumors, regardless of their etiopathogenesis, cancer associated inflammation is present and maintains a tumor-promoting milieu as well as an immunosuppressive environment, allowing the tumor to escape immunity (Grivennikov et al., 2010).
Hematopoietic response in infection and cancer
Hematopoietic stem cell (HSC) proliferation, formation of monocytes and granulocytes, extramedullary hematopoiesis and reactive increase of circulating neutrophils and inflammatory monocytes are well known responses to acute and chronic infections. The regulation of bone marrow (BM) HSCs is an integral part of the first response to inflammation or stress. HSCs express innate and chemokine/cytokine receptors, and their mobilization, proliferation and renewal is regulated by cytokines released during infection (King and Goodell, 2011). Leukocytosis, or an increase in white blood cells, has also been frequently associated with cancer and attributed to tumor cell production of colony stimulating factors, which induce HSC activation and differentiation (Ascensao et al., 1987). Additionally, the contribution of myeloid cells to tumor pathogenesis by promoting angiogenesis, tissue invasion and metastasis formation has been recently established (Shojaei et al., 2007; Yan et al., 2010). Also, both the tumor microenvironment and inflammation associated with infections endow some mobilized myeloid cells with immunosuppressive activity (Gabrilovich et al., 2012).
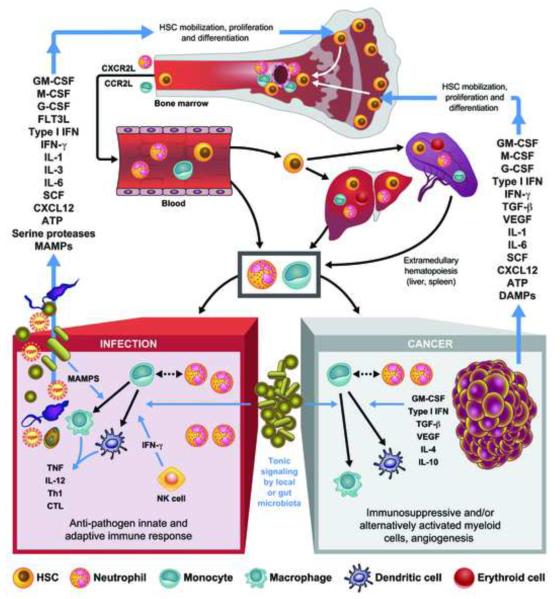
HSCs are present in BM specialized endosteal/arteriolar niches as quiescent or self-renewing cells (Kunisaki et al., 2013). The large majority of HSCs are dormant and divide a couple of times per year, whereas 5-10% of HSCs are active, divide once a month, and participate in their self-renewal while generating a continuous output of all the hematopoietic lineages to maintain homeostasis (Pietras et al., 2011). In response to injury as observed in both infections and cancer, quiescent HSCs are detached from their endosteal niche and induced into cell cycle and differentiation in vascular niches (Figure 1).
Figure 1.
Reactive hematopoiesis in infection and cancer. Factors produced by infected tissues or tumors and their stroma induce mobilization of HSCs from endosteal/arteriolar niches to vascular niches and promote their proliferation and differentiation. In response to chemotactic stimuli, HSCs, monocytes and neutrophils marginalize in the blood circulation. HSCs reach organs such as liver and spleen where extramedullary hematopoiesis occurs. Neutrophils and inflammatory monocytes enter infected tissues and tumor stroma, where monocytes differentiate into macrophages- or DC-like cells. In infections, these inflammatory myeloid cell types, in part in response to MAMPs and to IFN-γ secreted by NK cells, produce cytokines that favor the generation of effector Th cells, often Th1, and CTL. In tumors various mediators present in the microenvironment lead to the appearance of myeloid cells with immunosuppressive activity and favoring tumor growth and angiogenesis. In both infections and cancer, the differentiation of the myeloid cells in the inflamed tissue is modulated by the presence of the gut microbiota. Myeloid cells with immunosuppressive activities are also observed in infections where they may contribute to resolution of inflammation and immunity, while myeloid cells in the tumor can become immunostimulating if properly stimulated and the activity of suppressive factors such as IL-10 is inhibited.
The factors regulating reactive hematopoiesis include early acting cytokines such as interleukin (IL)-3, IL-6 and Fms-like tyrosine kinase 3 ligand (FLT3L) as well as myeloid differentiating-factors such as granulocyte colony-stimulating factor (G-CSF), macrophage (M)-CSF, and granulocyte-macrophage (GM)-CSF (King and Goodell, 2011). Acute exposure to either type I interferons (IFNs) or IFN-γ triggers activation and cycling of HSCs, and together with tumor necrosis factor (TNF) induce and skew the differentiation of monocytic lineages (Baldridge et al., 2011; de Bruin et al., 2012; Essers et al., 2009). However, chronic exposure to these factors induces a loss of HSC repopulation ability, possibly contributing to the hematopoietic defects observed in chronic infections or cancer (Baldridge et al., 2011). Other factors include neutrophil proteases that mobilize the HSCs from the endosteal niches and ATP that acts on purinergic receptors on HSCs to induce cell cycling (Rossi et al., 2012). Ligands for the innate immune Toll-like receptors (TLRs), such as endotoxin, DNA, and endogenous ligands, directly activate HSCs or act indirectly on mesenchimal and inflammatory cells (Boettcher et al., 2012; Boiko and Borghesi, 2012).
Tumor cells can affect hematopoiesis by producing some of these factors, such as IL-6, G-CSF or GM-CSF, or activating stromal cells to produce these cytokines. GM-CSF produced by pancreatic cancer cells regulates myeloid inflammation and is controlled by signaling through oncogenic Kras (Bayne et al., 2012). Similarly, GM-CSF is induced by oncogenic Hras in skin carcinogenesis in an IL-1-dependent manner (Salcedo et al., 2013). Low levels of type I IFN present in tumors are important for anti-tumor immunity and also centrally affect hematopoiesis (Fuertes et al., 2013). Additionally, damaged tumor cells release DAMPs involved in HSC mobilization. For example, mitochondrial damage releases DNA and formylpeptides, ligands for TLR9 and the chemoattractant formyl peptide receptors, respectively, and ATP is released by tumor cells damaged by anthracyclines, a class of anti-cancer chemotherapeutics (Ma et al., 2013a; Zhang et al., 2010).
The small number of HSCs normally present in circulation is dramatically increased in response to inflammation and their homing to peripheral organs such as the liver, lung and, in experimental animals, spleen, establishes foci of extra medullary hematopoiesis. BM emargination of neutrophils depends on the expression of ligands for the chemokine receptor CXCR2 (Kohler et al., 2011), whereas signaling through TLRs induce the secretion of the chemokine ligand CCL2 that stimulates the egression of monocytes expressing the cognate chemokine receptor CCR2 into the peripheral blood (Serbina and Pamer, 2006). CCR2-positive inflammatory monocytes then migrate mostly in a CCR2-independent way, to inflamed tissues or tumors where they can differentiate into distinct subsets of activated myeloid cells sharing characteristics with macrophages and dendritic cells (DCs) (Serbina and Pamer, 2006).
Myeloid cells in infection and cancer
Myeloid-derived cells in cancer, infection or other stress conditions have been identified with different names including reactive neutrophils, inflammatory monocytes, macrophages, tumor-associated macrophages, DCs, immature myeloid cells, myeloid suppressor cells and others, often referring to cell subsets with identical or indistinguishable surface phenotypes. The unified terminology of myeloid-derived suppressor cells (MDSCs) has been proposed for myeloid cells present in the BM, spleen or tumor microenvironment and able to suppress T cell responses (Gabrilovich et al., 2007). Reactivity with RB6-8C5 antibody (anti-GR1) was initially used for identification of these cells. RB6-8C5 antibody recognizes the neutrophil-specific Ly6G antigen but also cross-reacts with Ly6C expressed on inflammatory monocytes. Based on Ly6C and Ly6G expression, two major subsets of monocytic MDSC-M and granulocytic MDSC-G, respectively, have been characterized (Gabrilovich et al., 2012).
Cells with the phenotypic characteristics of the so-called MDSCs in the BM represent a differentiation stage of the granulocytic/monocytic lineage, and their increase in inflammation probably reflects the reactive amplification of myelopoiesis over erythropoiesis and lymphopoiesis. The augmented frequency of these cells in the spleen could be due to homing of reactive myeloid cells or reflect extramedullary hematopoiesis in this organ. The immunosuppressive activity of reactive myeloid cells in spleen and tumors is not a property of subsets of normal mature or immature myeloid cells (Gabrilovich and Nagaraj, 2009), but instead appears to be endowed by the inflamed microenvironment. Their immunosuppressive mechanisms have been studied both in cancer and in infection. The major mechanism for MDSC-G is ROS production with high expression of the NOX2 NADPH oxidase that generates ROS, whereas MDSC-M express nitric oxide synthase 2 (NOS2) that produces Nitric Oxide (NO) (Gabrilovich et al., 2012). Other immunosuppressive mechanisms of tumor-associated myeloid cells are expression of arginases, surface galectin 9 (GAL9) and disintegrin and metalloproteinase domain-containing protein 17 (ADAM17), depletion of L-cysteine and production of transforming growth factor (TGF)-β, indoleamine-pyrrole 2,3-dioxygenase (IDO) and IL-10 (Gabrilovich et al., 2012).
The immunosuppression mediated by reactive myeloid cells in infection is critical for resolution of acute inflammation and exhaustion of immunity. In chronic lymphocyte choriomeningitis virus (LCMV) infection they accumulate and may play a role in its maintenance (Norris et al., 2013). Also, reactive myeloid cells may contribute to immunodepression in HIV infected patients (Qin et al., 2013). During Toxoplasma gondii infection in mice, monocyte-derived cells infiltrating the infected tissues are required for induction of a prototypic Th1 response by producing IL-12 that induces IFN-γ production and Th1 differentiation (Goldszmid et al., 2012). However, T. gondii acute infection also induces IL-6 in BM stromal cells, reactive hematopoiesis and expansion of regulatory myeloid cells that through NO production suppress the lung–associated immune system (Chou et al., 2012; Voisin et al., 2004). The cytokines regulating the protective Th1 response to T. gondii, IL-12 and IFN-γ, endow myeloid cells with NO-dependent immunosuppressive activity (Voisin et al., 2004). Similarly, the use of IL-12 to elicit antitumor immune responses in experimental cancer therapy resulted in a transient immunosuppression mediated by NO production from reactive myeloid cells (Koblish et al., 1998). However, in polymicrobial sepsis induced in mice by cecal ligation and puncture after an initial Th1-type inflammation, expansion of reactive myeloid cells producing IL-10 induces suppression of T cell responses and Th2 polarization, possibly reflecting the life-threatening immunosuppression following clinical sepsis (Delano et al., 2007). IL-10 production can be associated with Th2 response but IL-10 induction in IFN-γ producing Th1 cells can mediate the downregulation of the Th1 response in parasitic and viral infections (Jankovic and Trinchieri, 2007). The immunosuppressive mechanisms of tumor-associated myeloid cells are thus characteristics of both classical (M1) and alternative (M2) macrophage activation. During tumor progression reactive myeloid cells may mediate immunosuppression either by the self-limiting mechanism of Th1 inflammation resolution (e.g. NO, ROS, IL-10 production) or by switching to a wound repair and angiogenic pro-tumor Th2 inflammation with expression of arginase, TGF-β and IL-10. Repolarization of the tumor-infiltrating myeloid cells by blocking IL-10 removes the immunosuppressive brake and allows TLR ligands to induce a tumor-eradicating Th1 inflammation and cytotoxic T cell response (Guiducci et al., 2005).
Myeloid cell populations associated with infection and tumors are heterogeneous and often described as immature cells. However, the immunosuppressive activity in these cells is not due to their immaturity or expansion of specialized subsets. Their functions are very specialized and typical of differentiated cells rather than immature cells. Phenotypic and gene expression analysis of the reactive myeloid cells have shown differences from their unactivated counterparts (monocyte, macrophages, neutrophils) but did not identify them as immature myeloid cells (Table 1). In infection models, e.g. in T. gondii or Listeria monocytogenes infections, tissue-infiltrating monocytes differentiate into macrophages and DC subsets, important to sustain the protective Th1 response, that are morphologically and phenotypically equivalent to the immunosuppressive myeloid cells (Goldszmid et al., 2012). Differentiation of monocytes into Th1 promoting cells in T. gondii infection is controlled by NK-cell produced IFN-γ (Goldszmid et al., 2012). IFN-γ and TNF not only induce reactive myelopoiesis but also shift the myeloid differentiation toward monocyte/macrophages and act even on differentiated neutrophils to induce morphological and functional changes including the appearance of immature nuclear morphology (de Bruin et al., 2012). Subsets of neutrophils exist, with different morphological appearance and functions. Even terminally differentiated neutrophils can be induced to proliferate changing their morphological appearance and increasing their half-life (Beyrau et al., 2012). Thus, further studies are needed for a full understanding of the nature and mechanism of mobilization, differentiation and activation of immunosuppressive reactive myeloid cells.
Table 1.
Myeloid cells associated with infection and cancer.
| Infection site | Tumor microenvironment | |||
|---|---|---|---|---|
| Ly6Chi
inflammatory monocytes |
PMN | MDSC-Mo | MDSC-G | |
| CD45 | + | + | + | + |
| CD11b | ++ | ++ | ++ | ++ |
| Gr-1 | ++ | +++ | ++ | +++ |
| Ly6C | +++ | ++ | +++ | ++ |
| Ly6G | − | +++ | − | +++ |
| F4/80 | + | − | + | − |
| CD68 | + | − | + | − |
| CD115 | ++ | +/− | ++ | +/− |
| CCR2 | ++ | + | ++ | + |
| CX3CR1 | + | − | + | − |
| CXCR2 | + | ++ | + | ++ |
| CXCR4 | + | + | + | + |
| CD124 | + | + | + | + |
| Ly6B (7/4) | +++ | ++ | +++ | ++ |
| CD117 | − | − | − | − |
| CD11C | +/− | − | +/− | − |
| MHC II | +/− | +/− | +/− | − |
| MHC I | + | + | + | + |
| CD49d | ++ | + | ++ | + |
| CD80 | + | + | + | + |
| RNS | ++ | + | ++ | + |
| ROS | + | ++ | + | ++ |
| Induce strong Th1 Pathogen killing |
Immunosuppressive Promote tumor growth |
|||
The table shows the surface phenotype and ability to produce reactive nitrogen and oxygen species (RNS, ROS) commonly reported for inflammatory monocytes and polymorphonuclear cells (PMN) at infection sites compared with tumor associated MDSC-M and MDSC-G. In infection, these myeloid cells are usually reported to induce a strong Th1 response and pathogen killing, although in some circumstance they may be immunosuppressive. Tumor-associated myeloid cells are usually found to be immunosuppressive.
Licensing of immunity by the commensal microbiota
At epithelial surfaces the microbiota modulates local inflammation and immunity. However, the commensal microbiota, particularly in the gut, also regulates systemic innate resistance and adaptive immunity. The development of the immune system after birth depends on exposure to the commensal microbiota. In addition, the microbiota modulates an inflammatory and immune tone necessary for protection against infections. Some translocation of microbes and their products through the skin/mucosal barriers takes place in physiological conditions and this permeability increases during infections or inflammation. In HIV patients, microbial translocation increases blood endotoxin and microbial DNA and it is considered to be a cause of the systemic immune activation important for T cell depletion and AIDS immunodeficiency (Qin et al., 2013).
The gut microbiota is required for the initiation of mouse lung immunity against infection with respiratory viruses, increasing the constitutive expression of the pro-IL-1β and pro-IL-18 genes involved in pro-inflammatory responses, as well as the ability to produce and respond to IFN (Abt et al., 2012; Ichinohe et al., 2011) (Figure 2). The commensal microbiota also poises inflammatory genes such as Ifnb1, Il6 and Tnf for transcription by inducing epigenetic chromatin modifications (Ganal et al., 2012). The importance of commensal-mediated innate immune signaling is illustrated by the fact that TLR ligands, administered locally in the lung or systemically, rescue anti-influenza immunity in antibiotics-treated mice (Ichinohe et al., 2011). Although the gut microbiota has systemic effects beyond intestinal mucosal immunity, the control of skin immunity is compartmentalized and resistance to cutaneous infection with Leishmania major requires the presence of skin microbiota (Naik et al., 2012).
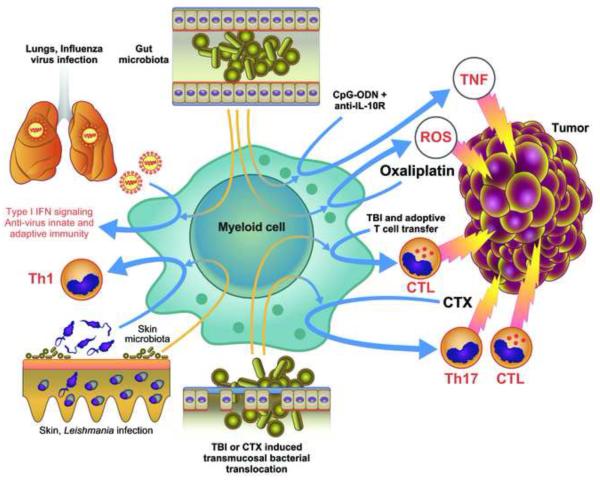
Figure 2.
Contribution of the commensal microbiota to the resistance to infection and anti-cancer therapy effectiveness. The presence of the gut microbiota is essential for resistance in the lungs to respiratory virus infection and for the success of anti-cancer therapies in subcutaneous tumors. The microbiota controls the efficacy of CpG-oligonucleotide (ODN) therapy combined with anti-IL-10R antibody treatment by enabling myeloid cells to produce TNF and the early genotoxic effect of oxaliplatin by enabling them to release ROS. Gut transmucosal translocation of bacteria induced by total body irradiation (TBI) or cyclophosphamide (CTX) treatment is required for the anti-cancer effectiveness of adoptive T cell transfer and for the induction of adaptive immunity following CTX-induced immunogenic cell death. The skin microbiota control skin immunity in a compartmentalized way and it is required for an efficient immune response against L. major infection.
The commensal microbiota also affects inflammation-dependent carcinogenesis, tumor immunity, and response to therapy (For a more detailed discussion about the role of microbiota in colon cancer, see review in this issue by Sears and Garrett). Mice unable to produce, process or respond to IL-18 display dysbiosis and have an increased susceptibility to chemical colon and liver carcinogenesis that can be transferred to WT mice by co-housing (Elinav et al., 2013; Salcedo et al., 2010). Colonic infection with H. hepaticus, a bacterium in the intestinal microbiota, influences distant carcinogenesis by complex opposing mechanisms: it enhances the number of small intestine and colon polyps in the APCmin/+ mouse model of colon cancer and mammary carcinoma in APCmin/+/Rag2−/− mice; it increases chemical and viral transgenic liver carcinogenesis (Fox et al., 2010); but it also induces IL-10 producing T regulatory (T-reg) cells that protect against intestinal and mammary carcinogenesis (Rao et al., 1996). Furthermore, in the Atm−/− mouse model of ataxia-telengiectasia, natural or induced variation in intestinal microbiota modifies lymphoma incidence (Yamamoto et al., 2013). These profound effects of the gut microbiota on carcinogenesis in distant organs are mediated by modulation of the TNF-dependent systemic inflammatory tone, oxidative stress and leukocyte or epithelial cell genotoxicity (Fox et al., 2010; Westbrook et al., 2012; Yamamoto et al., 2013).
The gut microbiota has been shown in murine studies to modulate myeloid-derived cell functions in the tumor microenvironment required for response to immune and chemotherapy. Subcutaneous tumors of germ free (GF) or antibiotics-treated mice are impaired in their ability to respond to CpG-oligonucleotide immunotherapy and platinum chemotherapy (Iida et al., 2013). In these mice, tumor-infiltrating myeloid-derived cells have impaired production of various cytokines, including TNF, after CpG-oligonucleotide treatment and show deficient ROS production and cytotoxicity following chemotherapy. The presence of several Gram+ and Gram-bacterial spp correlates with the response of tumor myeloid cells to CpG-oligonucleotides, whereas certain commensal Lactobacillus spp were found to decrease the response (Iida et al., 2013). In addition to direct tumor cell cytotoxicity, some antineoplastic agents, e.g. cyclophosphamide (CTX), induce an adaptive anti-tumor immune response. In mice, CTX alters the composition of the intestinal microbiota and induces mucositis, or inflammation of the mucous membranes, associated with translocation of Gram+ bacteria into draining lymph nodes that enhance the generation of anti-tumor Th17 and memory Th1 immune responses (Viaud et al., 2013). Accordingly, GF or antibiotics-treated mice show relative resistance to CTX chemotherapy (Viaud et al., 2013). Thus, the activation of APCs and induction of an antitumor immune response by immunogenic death induced by chemotherapy is not only dependent on the release of endogenous DAMPs by damaged tissue but is also primed and/or enhanced by the contribution of MAMPs from commensal bacteria. With a somewhat analogous mechanism, total body irradiation by inducing mucosal damage and microbial translocation increase the efficacy of adoptively transferred tumor-specific CD8+ T cells (Paulos et al., 2007).
Type I Interferons: more than antiviral cytokines
Type I IFNs are secreted polypeptides produced by virally infected cells that induce in neighboring cells a cell-intrinsic anti-viral status preventing spread of the infection (Trinchieri, 2010). However, type I IFNs are not only induced by viral infections but also by MAMPs and DAMPs (Trinchieri, 2010). Many innate receptors activated by these ligands can trigger production of type I IFNs that play important roles in modulating the differentiation and activated gene expression profile in inflammatory cells. Notably, TLR3, TLR7, TLR8, and TLR9 sense endosomal nucleic acid whereas in the cytosol the RIG-I-like helicase receptors recognize RNA while DNA is sensed by stimulator of interferon gene (STING) directly or through a recently defined family of DNA sensors (Bhat and Fitzgerald, 2013). In addition to microbial nucleic acids, in tissues damaged by infection, tumor growth, or cytotoxic therapy both nuclear and mitochondrial DNA may be released and sensed by inflammatory and other cells resulting in the production of IFN.
Type I IFN also mediate several so-called “anti-cellular” activities including inhibition of cell proliferation, mobilization of HSCs, inhibition and skewing of hematopoietic cell differentiation, anti-angiogenesis, and immunomodulatory effects such as activation of NK cell functions, modulation of DC activation, migration, and antigen presentation functions, promotion of T cell response and memory, and differentiation of plasma cells (Trinchieri, 2010). Unlike their role in the innate response to acute viral infections, type I IFN have contrasting and often deleterious effects in the resistance to bacteria and play a role in the susceptibility to bacterial superinfection complicating many viral infections (Trinchieri, 2010). Type I IFN immunosuppressive effects also dominate and contribute to limiting the anti-viral effect of the IFN-γ-producing CD4+ T cells and, through NK cell activation, of the CD8+ cytotoxic T cells, thus contributing to chronic viral infections (Odorizzi and Wherry, 2013). The immunosuppression mediated by type I IFN in infection has been shown to be mediated in part by induction of IL-10 and the Programmed death 1 (PD1)-ligand PDL1, which initiates immune inhibitory signals (Odorizzi and Wherry, 2013).
Type I IFN induced during infection, if maintained for an extended time, may result in immunosuppression or autoimmune and hematologic pathology (Trinchieri, 2010). However, constitutive physiological low level IFN-β transcriptionally controlled by c-Jun and NF-κB rather than by interferon-regulatory factors (IRFs) is maintained by the commensal microbiota (Abt et al., 2012; Ganal et al., 2012; Gough et al., 2012). Constitutive IFN-β is required for maintaining hematopoietic homeostasis, optimal pro-inflammatory cytokine production by myeloid cells, and signaling in response to not only IFN but also unrelated cytokines (Abt et al., 2012; Gough et al., 2012). In the absence of tonic IFN-β signaling, animals are more susceptible to infection and cancer, have increased bone resorption and HSCs mobilization, and an IFN-γ-dependent inflammatory pathology (Gough et al., 2012).
The functions of type I IFN, including its immunomodulating, anti-angiogenic, anti-proliferative, and pro-apoptotic effects, have made it an obvious candidate for therapy in hematopoietic and solid tumors, although its use has now been superseded by targeted therapies (Trinchieri, 2010) due to severe toxicity that includes autoimmune, inflammatory, hematological and neurological side effects. High levels of type I IFNs such as those used in therapy have a direct antitumor effect by regulating cell proliferation, apoptosis, and autophagy. However, low level of type I IFNs are also present in tumors, either due to basal IFN-β production or active induction by DAMPs (Fuertes et al., 2013). Type I IFNs in the tumor microenvironment induce IL-10 from T-reg cells that control the pro-tumor and pro-angiogenic IL-17 (Stewart et al., 2013). It has been recently shown that endogenous type I IFN acting on hematopoietic cells retards the growth of mouse transplantable and carcinogen-induced tumors, in particular through activation of NK cells and CD8α+ DCs as well as inhibition of Th17 cells (Diamond et al., 2011; Fuertes et al., 2013; Stewart et al., 2013). Low levels of type I IFN are also required for the initial production of IL-12 by CD11b+ DCs while higher levels inhibit it (Gautier et al., 2005). Although radiotherapy directly mediate tumor cell DNA damage and cytotoxicity, ablative radiation of tumors induce type I IFN production that acts on immune cells and it is required for treatment efficacy (Burnette et al., 2011).
Lymphoid structures in infected tissues and tumors
Although progressing tumors fail to induce protective immunity, an ongoing chronic immune response to the tumor is often present and the extent and characteristics of tumor lymphocytic infiltration has prognostic significance (Bindea et al., 2013). Chronically inflamed tissues in infections, autoimmunity, and cancer develop ectopic lymphoid follicles named tertiary lymphoid organs (TLOs) (Neyt et al., 2012) (Figure 3). TLOs are similar to secondary lymphoid organs but lack the capsule surrounding the lymph nodes and resemble the organized lymphoid tissue of the gut. They are composed of B cells and T cells in continuous but separated anatomical structures, DCs and macrophages; they also have stromal elements characteristic of secondary lymphoid tissues (Link et al., 2011). As in secondary lymphoid organs, lymphoid tissue inducer (LTi) cells, a subset of innate lymphoid cells (ILCs), are important for TLO formation. LTi constitutively produce the cytokines lymphotoxin (LT)α and LTβ that together with LIGHT - another TNF family cytokine – are sufficient to transform mesenchimal stromal cells into lymphoid tissue organizer (LTo) cells expressing CXCL13 or CCL19 and CCL21 that recruit other cellular elements forming the TLOs (van de Pavert et al., 2009). CD11b+ DCs are essential for TLO maintenance via LTβ production (GeurtsvanKessel et al., 2009). However, IL-17- and IL-5-producing T-cells can also act on mesenchimal stromal cells leading to TLO formation in the absence of LTi cells (Rangel-Moreno et al., 2011).
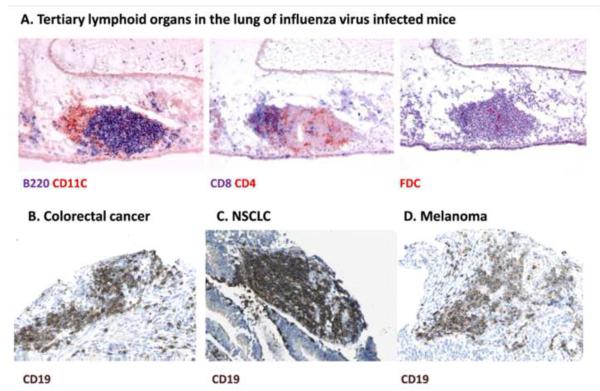
Figure 3.
Tertiary lymphoid organs (TLOs) in infection and cancer. A. In chronic infections such as in the lung of influenza virus infected mice shown here, TLOs are present and characterized by anatomically contiguous but separated T (CD4 or CD8 positive) and B cell areas (B220 positive) and the presence of conventional (CD11C positive) or follicular (FDC) DCs, suggesting localized sites of T and B cell responses (Reproduced with permission from ©2009 GeurtsvanKessel et al. Originally published in Journal of Experimental Medicine: 206:2339-2349. doi: 10.1084/jem.20090410). C-D. TLOs are often present in tumors as visualized by the B cell marker CD19 (from www.proteinatlas.org).
TLOs function as an additional line of immune defense, allowing local priming of naïve T-cells, and are necessary for effective immune responses (Yu et al., 2004). By examining CD19 expression as a marker for B cells in different cancer samples in public datasets (www.proteinatlas.org), we observed ectopic lymphoid tissues in almost all cancer types except gliomas. Other evidence has shown that the number of TLOs and presence of activated Th1-polarizing DCs in solid tumors positively correlate with patient survival (Dieu-Nosjean et al., 2008; Martinet et al., 2011). In mice, the induction of TLOs using LIGHT resulted in the rejection of established tumors (Yu et al., 2004). However, TLOs may also suppress anti-tumor immune response and accelerate tumor growth (Shields et al., 2010). It has been suggested that following immunogenic cell death induced by certain chemotherapy agents, antigen presentation may occur in the tumor bed independently of draining lymph nodes or TLOs (Ma et al., 2013a). Further in vivo imaging studies are required to directly identify the relationship between cellular components of TLOs and tumor. Overall, TLO generation could be considered a compensatory mechanism for chronically inflamed tissues that have decreased lymphatic drainage and APC migration (Thaunat et al., 2006).
Negative regulation of immunity in infection and cancer
The immune response is selectively controlled in different tissues to achieve a balance between an effective response and collateral damage until immune safe checkpoints are initiated (Matzinger and Kamala, 2011). The level of protection is particularly robust in organs with limited spontaneous regeneration, e.g. the central nervous system (Iliff et al., 2012), whereas in other tissues a more regulatory type of control is established. Many vital organs express high levels of TGF-β (and its activating enzyme MMP9) that directly suppress T and NK cell responses and induce T-reg cells. In the intestine, the cohabitation with commensal bacteria is facilitated by the presence of IL-10-producing T-reg cells that are induced by a subset of CD103 expressing DCs primed by the mucus forming protein Mucin 2 (MUC2) (Shan et al., 2013) and activated by environmental cues, such as bacterial short-chain fatty acids, which act via Free fatty acid receptor 2 (Ffar2) or the TLR2 agonist polysaccharide A found on the bacterial surface (Round et al., 2011; Smith et al., 2013). Many tumors hijack the mechanisms of tolerance induction, for instance by expressing high levels of TGF-β (Meulmeester and Ten Dijke, 2011) and IDO (Wainwright et al., 2012), or mimic the intestinal tolerant microenvironment by the presence of activated IL-10-producing T-reg cells (Stewart et al., 2013) (Figure 4). Tumor associated T-reg cells have opposite effects on tumor progression by regulating proangiogenic Th17 cells but also the anti-tumor T cell response; transient depletion of T-reg cells slow tumor progression and improve the efficacy of radiation therapy (Bos et al., 2013; Stewart et al., 2013).
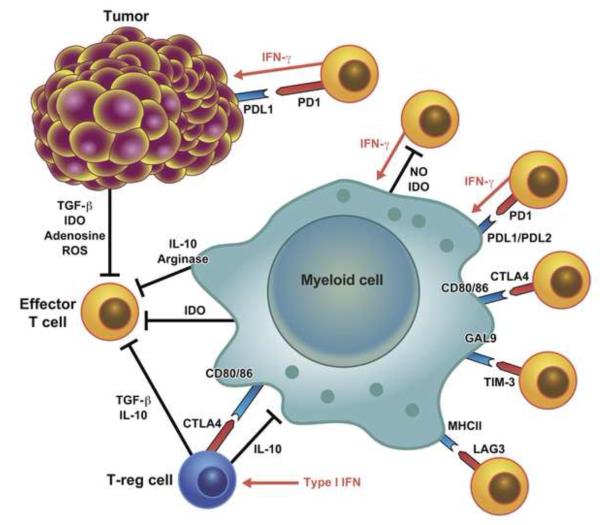
Figure 4.
Main immune checkpoints negatively regulating the immune response in infection and cancer. T cells in the tumor environment have an exhausted phenotype and their proliferation and activation is restrained both by tissue intrinsic tolerogenic mechanisms that limit excessive inflammatory and immune response to infection as well as by the mechanisms responsible for the contraction of the immune response to infection.
During acute infection, the number of antigen-specific T cells rapidly increases, peaking around days 7-10. Once the pathogen is cleared, the majority of T-cells undergo apoptosis, leaving a small number of memory cells able to rapidly respond to secondary challenge. In chronic infection, the immune response is suppressed by various mechanisms to prevent tissue damage, and T cells acquire an exhausted phenotype characterized by the loss of effector molecules (IFN-γ, TNF, granzymes, perforin) and upregulation of inhibitory molecules, such as cytotoxic T lymphocyte-associated antigen 4 (CTLA4 or CD152), PD-1 (or CD279), lymphocyte-activation gene 3 (LAG3 or CD223), T cell immunoglobulin and mucin protein 3 (TIM-3), adenosine receptor (A2AR), killer-cell immunoglobulin-like receptors (KIRs) and several others. Although tumors rarely induce an acute immune response, T cells in the tumor environment have an exhausted phenotype and are restrained both by tissue intrinsic tolerogenic mechanisms as well as mechanisms responsible for the contraction of the immune response to infections (Crespo et al., 2013). T cell exhaustion is reversible by the removal of inhibitory signals. CTLA4 competes with CD28 for binding to CD80/86 on DCs and is an important negative regulator in infection and cancer (Kaufmann and Walker, 2009; Leach et al., 1996). CTLA4 also scavenges CD80 from the surface of DCs and induces IDO production, mechanisms that may explain how CTLA4-expressing T-reg cells inhibit T-cell responses (Fallarino et al., 2005; Wing et al., 2008). PD-1 has T-cell inhibitory properties in chronic infections and cancer (Barber et al., 2006; Zha et al., 2004). LAG-3 and TIM-3 are also highly expressed on the surface of exhausted T-cells (Anderson, 2012; Blackburn et al., 2009; Norde et al., 2012).
T-cells also directly sense the environment and modify the strength of TCR signaling, e.g. by assessing nutrient availability and oxygen levels. Hypoxia-inducible factor-I (HIF-I) activated in the hypoxic conditions observed in chronically inflamed tissues and tumors determines the activity of cytotoxic CD8 T-cells by inducing increased production of effector molecules (e.g. perforin and granzymes) as well as increasing the levels of inhibitory molecules, such as CTLA4 and LAG3 (Doedens et al., 2013). Nutrient availability, due to low blood supply or altered cell metabolism, mostly sensed by mammalian target of rapamycin (mTOR) and general control nonrepressed 2 (GCN2), also affects T-cell responses (McGaha et al., 2012). Myeloid, stromal and tumor cells similarly modulate mTOR activity and strength of TCR signaling in T-cells by producing enzymes that deplete essential aminoacids such as IDO, which regulate tryptophan, in immediate T-cell environment.
Conclusions
Unlike vaccines against pathogens, cancer vaccines have so far obtained only limited clinical success most likely due to the persistency of an immunosuppressive tumor microenvironment . As discussed above, the immune environment created by the tumor recapitulates that present in chronic infections in which homeostatic mechanisms aimed at preventing excessive inflammation and tissue damage establish immunological checkpoints that prevent an effective immune response able to completely eliminate the pathogens or destroy the tumor. Thus, immunoinhibitory mechanisms responsible for chronic infection, such as local production of IL-10, type I IFN, IDO, TGF-β and ligands for inhibitory co-receptors are also present in the tumor microenvironment (Diamond et al., 2011; Guiducci et al., 2005; Odorizzi and Wherry, 2013; Pardoll, 2012; Stewart et al., 2013). It is therefore not surprising that the effectiveness of procedures simply directed to induce a new response to tumor antigens or to enhance the natural response is prevented by the same mechanisms that limit the ability of immunosurveillance to prevent the growth of the tumors.
Many of the mechanisms regulating the immune responses to infections and cancer discussed in this review are derived from studies in experimental animals. Although it is important to avoid an automatic extrapolation from mouse studies to humans due to the differences in the immune systems between the two species and the limitations of the experimental models of cancer, the recent success in cancer immunotherapy indicates that the information from mouse studies can be can be useful for the design of clinical approaches. Thus, in both chronic infectious disease and tumor immunotherapy, much effort has been devoted to developing strategies to reverse immunosuppression, e.g. better adjuvants or blockers of immunosuppressive factors, such as TFG-β, IDO, or IL-10. The most promising advances have been obtained with antibodies that block co-inhibitory molecules. The monoclonal antibody to CTLA4 (ipilimumab), approved for use in patients with metastatic melanoma, increases patients’ survival likely by amplifying the endogenous anti-tumor T cell response. Antibodies against PD1 receptor or its ligands PD-L1 induce significant clinical responses in patients with melanoma or other solid tumors such as lung cancer (Pardoll, 2012). The effectiveness of these new drugs suggests that in a proportion of patients the endogenous immune response to cancer is sufficient to induce tumor regression. Thus, there is an optimistic expectation that the combination of immunocheckpoint inhibitors with therapies such as cancer vaccines or certain chemotherapies that amplify the anti-tumor immune response might offer better chances of therapeutic success. Recent studies have also identified in viral infections and tumors the existence of memory T cells with stem cell-like properties that maintain the ability to develop in potent effector T cells; expansion of these cells or reprogramming terminally differentiated tumor-infiltrating lymphocytes to confer stem cell-like properties might be used to augment immunotherapies against cancer (Gattinoni et al., 2012). The promise of these new therapeutic approaches proves the usefulness of comparative studies in infectious and cancer immunity for a better understanding of their basic mechanisms and translational application for the development of new therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Ascensao JL, Oken MM, Ewing SL, Goldberg RJ, Kaplan ME. Leukocytosis and large cell lung cancer. A frequent association. Cancer. 1987;60:903–905. doi: 10.1002/1097-0142(19870815)60:4<903::aid-cncr2820600431>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N, Fitzgerald KA. Recognition of Cytosolic DNA by cGAS and other STING-dependent sensors. Eur J Immunol. 2013 doi: 10.1002/eji.201344127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, Heikenwalder M, Manz MG. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J Immunol. 2012;188:5824–5828. doi: 10.4049/jimmunol.1103253. [DOI] [PubMed] [Google Scholar]
- Boiko JR, Borghesi L. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2012;57:1–8. doi: 10.1016/j.cyto.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DB, Sworder B, Bouladoux N, Roy CN, Uchida AM, Grigg M, Robey PG, Belkaid Y. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during Toxoplasma gondii infection. J Leukoc Biol. 2012;92:123–131. doi: 10.1189/jlb.1011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119:1543–1554. doi: 10.1182/blood-2011-07-367706. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edme N, Downward J, Thiery JP, Boyer B. Ras induces NBT-II epithelial cell scattering through the coordinate activities of Rac and MAPK pathways. J Cell Sci. 2002;115:2591–2601. doi: 10.1242/jcs.115.12.2591. [DOI] [PubMed] [Google Scholar]
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Orabona C, Vacca C, Bianchi R, Gizzi S, Asselin-Paturel C, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells. Int Immunol. 2005;17:1429–1438. doi: 10.1093/intimm/dxh321. [DOI] [PubMed] [Google Scholar]
- Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, Williams M, McFaline JL, Essigmann JM, Schauer DB, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;59:88–97. doi: 10.1136/gut.2009.183749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003748. 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat Immunol. 2007;8:1281–1283. doi: 10.1038/ni1207-1281. [DOI] [PubMed] [Google Scholar]
- Jobin C. Colorectal cancer: CRC--all about microbial products and barrier function? Nat Rev Gastroenterol Hepatol. 2012;9:694–696. doi: 10.1038/nrgastro.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblish HK, Hunter CA, Wysocka M, Trinchieri G, Lee WM. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J Exp Med. 1998;188:1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, De Filippo K, Hasenberg M, van den Brandt C, Nye E, Hosking MP, Lane TE, Mann L, Ransohoff RM, Hauser AE, et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117:4349–4357. doi: 10.1182/blood-2010-09-308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Link A, Hardie DL, Favre S, Britschgi MR, Adams DH, Sixt M, Cyster JG, Buckley CD, Luther SA. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. The American journal of pathology. 2011;178:1662–1675. doi: 10.1016/j.ajpath.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013a;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013b;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, Mellor AL. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. 2012;249:135–157. doi: 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Meulmeester E, Ten Dijke P. The dynamic roles of TGF-beta in cancer. The Journal of pathology. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norde WJ, Hobo W, van der Voort R, Dolstra H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012;120:728–736. doi: 10.1182/blood-2012-02-412510. [DOI] [PubMed] [Google Scholar]
- Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity. 2013;38:309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi PM, Wherry EJ. Immunology. An interferon paradox. Science. 2013;340:155–156. doi: 10.1126/science.1237568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol. 2012;13:1129–1132. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988;48:1996–2004. [PubMed] [Google Scholar]
- Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013;87:1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JB, Chamberlain RS, Bronte V, Carroll MW, Irvine KR, Moss B, Rosenberg SA, Restifo NP. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996;156:3357–3365. [PMC free article] [PubMed] [Google Scholar]
- Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM. The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012;120:2365–2375. doi: 10.1182/blood-2012-04-422378. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Cataisson C, Hasan U, Yuspa SH, Trinchieri G. MyD88 and its divergent toll in carcinogenesis. Trends Immunol. 2013;34:379–389. doi: 10.1016/j.it.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O'Huigin C, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, Leighty RM, Roers A, Karp CL, Muller W, Trinchieri G. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123:4859–4874. doi: 10.1172/JCI65180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaunat O, Kerjaschki D, Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27:441–445. doi: 10.1016/j.it.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Hopken UE, Lipp M, Niederreither K, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin MB, Buzoni-Gatel D, Bout D, Velge-Roussel F. Both expansion of regulatory GR1+ CD11b+ myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infection and immunity. 2004;72:5487–5492. doi: 10.1128/IAI.72.9.5487-5492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook AM, Wei B, Hacke K, Xia M, Braun J, Schiestl RH. The role of tumour necrosis factor-alpha and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity. Mutagenesis. 2012;27:77–86. doi: 10.1093/mutage/ger063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Yamamoto ML, Maier I, Dang AT, Berry D, Liu J, Ruegger PM, Yang JI, Soto PA, Presley LL, Reliene R, et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 2013;73:4222–4232. doi: 10.1158/0008-5472.CAN-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- Zha Y, Blank C, Gajewski TF. Negative regulation of T-cell function by PD-1. Crit Rev Immunol. 2004;24:229–237. doi: 10.1615/critrevimmunol.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]