Abstract
Background
Dietary fat is the most important energy source of all the nutrients. Fatty acids, stored as triacylglycerols in the body, are an important reservoir of stored energy and derive primarily from animal fats and vegetable oils.
Design
Although the molecular mechanisms for the transport of water-insoluble amphipathic fatty acids across cell membranes have been debated for many years, it is now believed that the dominant means for intestinal fatty acid uptake is via membrane-associated fatty acid-binding proteins, i.e., fatty acid transporters on the apical membrane of enterocytes.
Results
These findings indicate that intestinal fatty acid absorption is a multistep process that is regulated by multiple genes at the enterocyte level, and intestinal fatty acid absorption efficiency could be determined by factors influencing intraluminal fatty acid molecules across the brush border membrane of enterocytes. To facilitate research on intestinal, hepatic and plasma triacylglycerol metabolism, it is imperative to establish standard protocols for precisely and accurately measuring the efficiency of intestinal fatty acid absorption in humans and animal models. In this review, we will discuss the chemical structure and nomenclature of fatty acids and summarize recent progress in investigating the molecular mechanisms underlying the intestinal absorption of fatty acids, with a particular emphasis on the physical-chemistry of intestinal lipids and the molecular physiology of intestinal fatty acid transporters.
Conclusions
A better understanding of the molecular mechanism of intestinal fatty acid absorption should lead to novel approaches to the treatment and the prevention of fatty acid-related metabolic diseases that are prevalent worldwide.
Keywords: intestinal fatty acid transporter, chylomicrons, pancreatic lipase, micelles, cholesterol absorption, biliary lipids, bile acids, hyperlipidemia, hypolipidemic drugs
Introduction
Dietary fat is the most important source of energy of all the nutrients, supplying 9 kcal/g, about double that contributed by either protein or carbohydrate at 4 kcal/g [1]. Fatty acids, stored as triacylglycerols (also called triglycerides) in the body, are an important reservoir of stored energy. In a healthy person with a body weight of 70 kg, approximately 141,000 kcal are stored as fat as compared to 24,000 kcal as protein and 1,000 kcal as carbohydrate. In addition, dietary fat provides essential fatty acids and fat-soluble vitamins A, D, E and K to the body, and represents the only source of precursors of eicosanoids such as prostanoids, thromboxanes, leucotrienes and lipoxins. Because essential fatty acids cannot be synthesized by human beings, these or their precursors must be supplied in the diet. In general, dietary lipids account for ~42% of the calories ingested in the Western diet, whereas nutritional recommendations are 20–35% fat for adults [2]. In the Western diet, ~95% of dietary lipids are triacylglycerols, mainly composed of long-chain fatty acids, and the remaining are phospholipids (~4.5%) and cholesteryl esters (~0.5%). Dietary triacylglycerols derive principally from two sources: animal fats and vegetable oils. Animal fats contain a high proportion of saturated fatty acids. By contrast, vegetable oils have a higher proportion of unsaturated fatty acids than animal fats. In addition to food being a source of lipids absorbed by the intestines, bile also provides additional lipids for intestinal absorption, primarily in the form of phospholipids, unesterified cholesterol and bile acids. Approximately 10–15 g of biliary phospholipids enters the intestine daily and the major phospholipids are lecithin (phosphatidylcholine) in human bile. Of note, unesterified cholesterol, plant sterols and bile acids do not supply energy to the body.
The small intestine is a vital organ for triacylglycerol homeostasis. Indeed, increased quantities of dietary fat will cause plasma triacylglycerol concentrations to rise in most individuals. Because the high fat diet is an important risk factor for cardiovascular disease, obesity, diabetes, nonalcoholic fatty liver disease, the metabolic syndrome and cancer, many studies have focused mainly on identifying cellular, physical-chemical and genetic determinants of intestinal fatty acid absorption in humans and laboratory animals. Because triacylglycerols of the body originate mainly from both de novo lipogenesis and absorption from the diet, a better understanding of the mechanisms of intestinal fatty acid absorption should lead to novel approaches to the treatment and the prevention of these metabolic diseases that are prevalent worldwide.
In this review, we will discuss the chemical structure and nomenclature of fatty acids and summarize recent progress in investigating the molecular mechanisms underlying the intestinal absorption of fatty acids, with a particular emphasis on the physical-chemistry of intestinal lipids and the molecular physiology of intestinal fatty acid transporter system.
The chemical structure and nomenclature of fatty acids
Fatty acids are a class of molecules that contain a hydrocarbon chain and a terminal carboxyl group. They are combined with propan-1,2,3-triol (i.e., glycerol) to make triacylglycerol in fats and vegetable oils. Fatty acids also constitute a major component of phospholipids, which typically contain a diacylglycerol, a phosphate group, and a simple organic molecule such as choline. Similar to the nomenclature of other lipids, many common (trivial) names and shorthand nomenclatures of fatty acids have been widely used. To better understand the molecular metabolism elucidating intestinal absorption of fatty acids, the nomenclature of the International Union of Pure and Applied Chemists (IUPAC), common (trivial) names and shorthand (omega, ω) terminology of fatty acids will be briefly reviewed here although we have systematically discussed it elsewhere [3].
a. Nomenclature of fatty acids
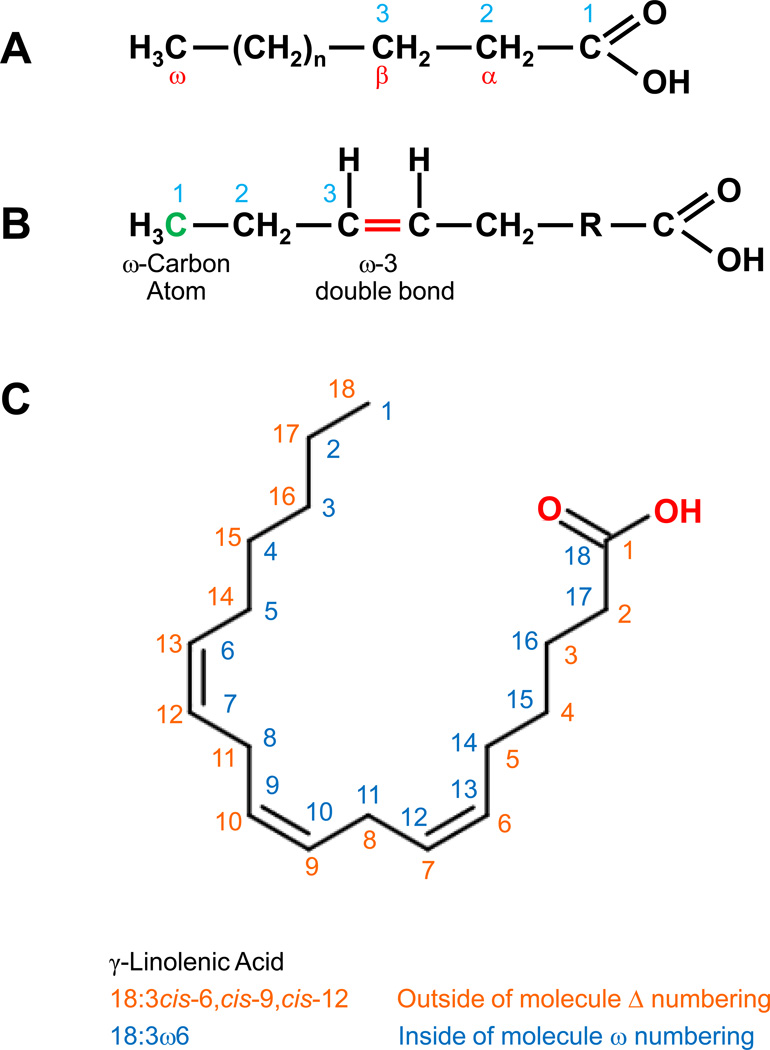
As shown in Figure 1A, fatty acid carbon atoms are often numbered starting at the carboxyl end. Carbon atoms 2 and 3 are usually referred to as α and β, respectively. The methyl carbon atom at the end of the chain is called the omega (ω) carbon. The position of the double bond is represented by the symbol (Δ) followed by a superscript number. Alternatively, the position of a double bond can be denoted by counting from the distal end, with the ω carbon atom (the methyl carbon) as number 1 (Figure 1B).
Figure 1.
(A) Fatty acid carbon atoms are numbered often starting at the carboxyl end. (B) The position of a double bond can be denoted by counting from the distal end, with the ω carbon atom (the methyl carbon) as number 1 (green). (C) The International Union of Pure and Applied Chemists (IUPAC) Δ and common ω numbering systems.
b. Standard IUPAC nomenclature of fatty acids
In standard IUPAC terminology, the systemic name for a fatty acid is derived from the name of its parent hydrocarbon by the substitution of oic for the final e. For example, the C18 saturated fatty acid is called octadecanoic acid because the parent hydrocarbon is octadecane. The C18 fatty acids with one, two, three and four double bonds are named octadecenoic, octadecadienoic, octadecatrienoic, and octadecatetraenoic acids, respectively. Moreover, the symbols 18:0, 18:1, 18:2, 18:3 and 18:4 denote C18 fatty acids with no double bonds, as well as one, two, three, and four double bonds, respectively.
The delta (Δ) configuration is usually used to indicate the locations of double bonds, which represents the distance from the carboxyl carbon. As shown in Figure 1C, γ-linolenic acid has three double bonds between C-6 and C-7, C-9 and C-10, and C-12 and C-13 from the carboxylic acid group and it is shown as Δ6, Δ9, and Δ12 bonds, respectively. The double-bond positions are shown with numbers before the fatty acid name (Δ6,Δ9,Δ12-octadecadienoic acid or simply 6,9,12-octadecadienoic acid).
Double-bond geometry is designated with the cis-trans nomenclature systems (also known as (E/Z)-isomerism), which indicates the positions of two carbon atoms or groups in the chain that are bound next to either side of the double bond. If atoms/groups lie on same (cis) or opposite (trans) sides of a reference plane in the molecule, they are cis or trans. For example, cis-Δ9 shows that there is a cis double bond between C-9 and C-10 while trans-Δ5 shows that there is a trans double bond between C-5 and C-6. The prefixes cis and trans are often abbreviated as c and t in structural formulas.
c. Common nomenclature of fatty acids
Of note, the use of common names is more convenient and popular because they are concise and clear compared to the above-mentioned standard IUPAC system. Also, the common names are the most frequent naming system used in literature. For example, oleic acid rather than cis-9-octadecenoic acid is often used. In general, the common names of most fatty acids originate from the first identified botanical or zoological origins. For example, myristic acid is found in seed oils from the Myristicaceae family (nutmeg is an example) and oleic acid is enriched in the fruit of the olive tree (Olea europaea).
d. Shorthand (ω) nomenclature of fatty acids
The shorthand nomenclature always consists of two numbers separated by a colon, i.e., the carbon number of the fatty acid chain followed by a colon, then the number of double bonds and the position of the double bond closest to the methyl side of the fatty acid molecule. For example, the symbol 16:0 denotes palmitic acid, a common saturated fatty acid, and 18:1 denotes oleic acid, a common monounsaturated fatty acid.
Both the shorthand nomenclatures and the systemic names can be combined to be used for showing the exact position and configuration of double bonds. For example, linoleic acid could be shown as cis(Δ-)9,cis(Δ-)12–18:2 or (cis,cis)9,12-octadecadienoic acid, which indicates that it is an 18-carbon (C18) fatty acid with cis double bonds between C-9 and C-12 from the carboxyl terminus. Occasionally, linoleic acid is also named as (cis,cis)n-6,9-octadecadienoic acid with the n donating that numbering is from the methyl terminus for cis double bonds between C-6 and C-9.
In addition, the ω can be replaced by the form (n-x), i.e., 18:2(n-6) instead of 18:2ω6. Although it has been recommended eliminating ω and using n- exclusively, both n- and ω are often used in the literature and are considered equivalent. Shorthand designations for polyunsaturated fatty acids are sometimes reported without the ω term (18:3). However, this notation is ambiguous since 18:3 could represent 18:3ω1, 18:3ω3, 18:3ω6, or 18:3ω9, and these fatty acids are completely different in their origins and nutritional significance. Two or more fatty acids with the same carbon and double-bond numbers are possible in many common oils. Therefore, the shorthand nomenclature should always be used with the ω term specified.
Classes of Fatty Acids
Fatty acids are composed of two major types: saturated and unsaturated fatty acids, and the presence or absence of carbon-carbon double bonds in the hydrocarbon chain is the only difference between them. Saturated fatty acids do not have double bonds in the hydrocarbon chain and unsaturated fatty acids contain at least one double bond. The latter are often divided into two subgroups: monounsaturated fatty acids with a single double bond and polyunsaturated fatty acids with two or more double bonds. The basic formulae of saturated and monounsaturated species are CH3(CH2)nCOOH and CH3(CH2)nCH=CH(CH2)nCOOH, respectively. In addition, based on chain length, they are often categorized as short-chain, medium-chain, or long-chain fatty acids. Short-chain fatty acids have aliphatic tails of fewer than eight carbons (<C8). Fatty acids with aliphatic tails of 8–12 carbons (C8-C12) are classified as medium-chain fatty acids, and long-chain fatty acids have aliphatic tails longer than 12 carbons (>C12). Sometimes, fatty acids with aliphatic tails longer than 22 carbons (>C22) are defined as very-long-chain fatty acids. In humans, nearly all fatty acids have an even number of carbon atoms in a straight chain. The number of carbon atoms in biological systems is typically between C14 and C24, with the C16 and C18 fatty acids being the commonest in humans.
Physical-chemical properties and compositions of intestinal lipids
Lipids within the lumen of the small intestine comprise triacylglycerols, phospholipids, cholesterol, plant sterols, and bile acids. Intestinal lipids originate from the diet, bile and cells sloughed from the lining of the small intestine. In bile, cholesterol, phospholipids and bile acids are three major lipid species. Although bile does not contain digestive enzymes, bile acids and phospholipids play critical roles in promoting intestinal lipid digestion and absorption by the enterocytes.
Triacylglycerols are the major source of dietary lipids and derive principally from two sources: animal fats and vegetable oils. They are triesters of one glycerol molecule with three fatty acids. Each of the three hydroxyl (-OH) groups of glycerol forms an ester group by reaction with the carboxyl (-COOH) group of a fatty acid molecule to form the triacylglycerol molecule. Triacylglycerols are also categorized as simple and complex. The former comprise three molecules of the same fatty acid that are esterified to glycerol. In the latter group, three fatty acids esterified with glycerol are different. In general, naturally occurring triacylglycerols are complex compounds. When all three positions have different acyl groups, each ester linkage is stereochemically distinct and a denotation by stereospecific number (sn)-1, -2, and -3 has been recommended. In general, saturated fatty acids tend to be esterified in the sn-1 or sn-2 positions of fats derived from animals. Most dietary triacylglycerols contain long-chain fatty acids, including the monounsaturated oleic acid (18:1) and the saturated palmitic acid (16:0). Animal fats differ from vegetable oils in the relative amounts of saturated and unsaturated fatty acids. The former usually contain less than 50 to 60% unsaturated fatty acids and the latter contain more than 80% unsaturated fatty acids. In the Western diet, dietary fat usually constitutes as much as 50% of total calories (i.e., 100–160 g/day). Of the dietary fat, triacylglycerols contribute as much as 90% of the total calories supplies by fat, with other fats such as phospholipids also yielding the remainder.
Phospholipids are composed of glycerol, fatty acids, phosphate and an organic base or polyhydroxy compound. The phosphate is always linked to the sn-3 position of the glycerol molecule. Approximately 10–15 g of biliary phospholipids enter the intestine daily, whereas the dietary contribution is only 1 to 2 g per day. The major phospholipid in human bile is lecithin (phosphatidylcholine), which accounts for more than 95% of total phospholipids. Lecithin consists of a zwitterionic phosphocholine head group and hydrophobic tails comprised of two long fatty acyl chains. The remainder is composed of cephalins (phosphatidylethanolamines) and trace amounts of sphingomyelins. The phospholipids comprise 15–25% of total lipids in bile. Similar to all naturally occurring phospholipids, biliary lecithin is a complex mixture of molecular species. The sn-1 position is esterified by the saturated fatty acyl chains 16:0 (~75%) and 18:0 (<20%), with small amounts of monounsaturated sn-1 16:1 or 18:1 comprising the remainder. The sn-2 position is esterified by unsaturated fatty acyl species, with 18:2, 18:1 and 20:4 fatty acids predominating. The major molecular species of lecithin in human bile are 16:0–18:2 (40–60%), 16:0–18:1 (5–25%), 18:0–18:2 (1–16%) and 16:0–20:4 (1–10%) [4].
Cholesterol is the most abundant steroid in animal tissues and in the intestinal lumen. It is poorly soluble in an aqueous environment. In addition to a double bond at C-5 and C-6 nucleus and a hydroxyl group on the third carbon of the cholestene nucleus, the angular methyl groups at C-10 and C-13, the hydrogen atom at C-8 and the side-chain at C-17 are in the β configuration. The hydrogen atoms at C-9 and C-14 are in the αconfiguration. Of note is that in bile, approximately 95% of the cholesterol molecule is in the free (esterified) form and the remaining 5% of the sterols are cholesterol precursors and dietary sterols. Moreover, the concentration of cholesteryl esters is negligible in human bile. The cholesteryl esters of dietary origin must be hydrolyzed to unesterified cholesterol and fatty acids for intestinal absorption.
Plant sterols (also called phytosterols) refer to sterols that originate from plants. These are abundant in the intestine as well. Plant sterols are naturally occurring and their chemical structures are very similar to cholesterol (i.e., a Δ5 double bond and a 3β-hydroxyl group, but with structural modifications of the side chain). Sitosterol and campesterol, which are 24-ethyl and the 24-methyl substituted variants of cholesterol, respectively, are the most abundant plant sterols. They are consumed in the diet and may be absorbed in the intestine. However, they are usually present only at very low plasma concentrations in humans due to a poor (<5%) net absorption rate by the small intestine. Sterols may also originate from shellfish, including brassicasterol and isofucosterol.
Bile acids are a family of closely related acidic sterols that are synthesized from cholesterol in the liver. They comprise approximately two thirds of the solute mass of normal human bile. Due to the presence of both hydrophilic and hydrophobic surfaces, bile acids are highly soluble, detergent-like amphiphilic molecules. At low concentrations in aqueous solution, bile acids exist as monomers; however, above a certain concentration, i.e., when a critical micellar concentration (CMC) is exceeded, they can spontaneously form negatively charged spherical aggregates called micelles. At this point, bile acids in bile can form simple micelles. Importantly, bile acids can solubilize other types of lipids such as cholesterol, fatty acids, phospholipids, monoacylglycerols and diacylglycerols by forming mixed micelles. This is a key function of bile acids in the small intestine where they emulsify dietary fat to facilitate their digestion and absorption. The potency of bile acids as detergents depends critically upon the distribution and orientation of hydroxyl groups around the steroid nucleus of the molecule, which is usually described as its hydrophobicity. The physical-chemical properties of bile acids depend upon the nature and ionization state of functional groups on the side chain. In general, the glycine conjugate is more hydrophobic than the taurine conjugate. In human bile, more than 95% of bile acids are 5β,C-24 hydroxylated acidic steroids amide-linked to taurine or glycine. These conjugates are present in an approximate ratio of 1:3 taurine:glycine in human bile.
Molecular physiology of intestinal fatty acid absorption
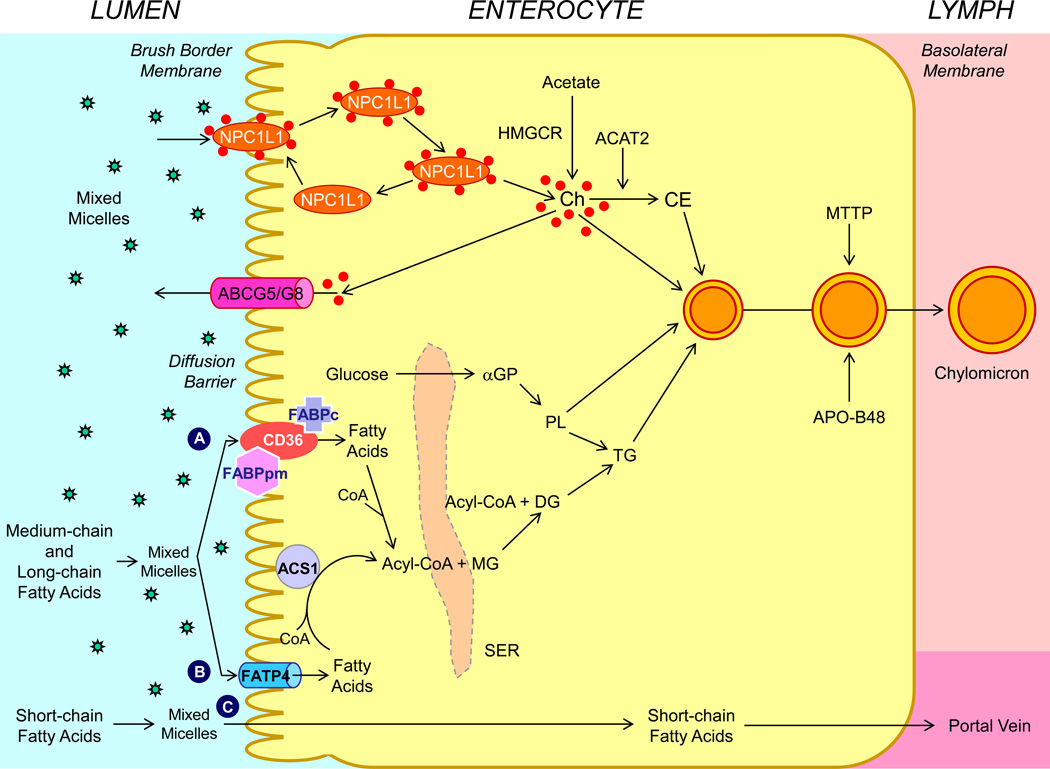
Conceptually, intestinal absorption of fatty acids is most precisely defined as the transfer of intraluminal fatty acids into intestinal or thoracic duct lymph. By contrast, intestinal uptake of fatty acids refers to its entry from the lumen into intestinal absorptive cells. As can be inferred from this distinction, intestinal fatty acid absorption is a multistep process that is regulated by multiple genes, as shown in Figure 2.
Figure 2.
Molecular and cellular mechanisms of intestinal lipid absorption. There are three putative pathways for uptake of fatty acids and their transport across the apical membranes of enterocytes. (A) Alternatively, CD36 (also referred to as fatty acid translocase; 88 kDa), alone or together with the peripheral membrane protein plasma membrane-associated fatty acid-binding protein (FABPpm; 43 kDa) accepts medium-chain and long-chain fatty acids at the cell surface to increase their local concentrations. This could help CD36 actively transport fatty acids across the apical membrane of the enterocyte. Once at the inner side of the membrane, these fatty acids are bound by cytoplasmic FABP (FABPc) before entering metabolic pathways. (B) Medium-chain and long-chain fatty acids are transported by fatty acid transport protein 4 (FATP4). These fatty acids may be rapidly activated by plasma membrane acyl-CoA synthetase 1 (ACS1) to form acyl-CoA esters. (C) Short-chain fatty acids may traverse the apical membrane by simple passive diffusion and may be absorbed into the mesenteric venous blood and then the portal vein. Monoacylglycerol (MG) may be taken up into enterocytes by facilitated transport. Acyl-CoA and monoacylglycerol are transported into the smooth endoplasmic reticulum (SER) where they are used for the synthesis of diacylglycerol (DG) and triacylglycerol (TG). Glucose is transported into the SER and contributes to the synthesis of phospholipids (PL) via α-glycerol phosphate (αGP). Within the intestinal lumen, the micellar solubilization of sterols facilitates movement through the diffusion barrier overlying the surface of the absorptive cells. In the presence of bile acids, mixed micelles deliver large amounts of the sterol molecules to the aqueous-membrane interface so that the uptake rate is greatly increased. The Niemann-Pick C1 like 1 protein (NPC1L1), a sterol influx transporter, is located at the apical membrane of the enterocyte, and can actively facilitate the uptake of cholesterol by promoting the passage of sterols across the brush border membrane of the enterocyte. NPC1L1 appears to mediate cholesterol uptake via vesicular endocytosis and ezetimibe may inhibit cholesterol absorption by suppressing the internalization of NPC1L1/cholesterol complex. By contrast, ABCG5 and ABCG8 promote active efflux of cholesterol and plant sterols from the enterocyte into the intestinal lumen for excretion. The combined regulatory effects of NPC1L1 and ABCG5/G8 play a critical role in modulating the amount of cholesterol that reaches the lymph from the intestinal lumen. Absorbed cholesterol, as well as some that is newly synthesized from acetate by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) within the enterocyte, is esterified to fatty acids by acyl-CoA:cholesterol acyltransferase isoform 2 (ACAT2) to form cholesteryl esters. All of these lipids participate in the formation of chylomicrons, which also requires the synthesis of apoB48 and the activity of microsomal triglyceride transfer protein (MTTP). As observed in lymph, the core of the secreted chylomicrons contains triacylglycerols and cholesteryl esters and the surface of the particles is a monolayer containing phospholipids, mainly phosphatidylcholine, unesterified cholesterol and apolipoproteins, including Apo-B48, Apo-AI, and Apo-AIV.
Similar to cholesterol, triacylglycerols are totally water-insoluble. The solubility of long-chain fatty acids in aqueous solutions is extremely low, i.e., in the range of 1–10 nM. However, the digestion of dietary fat takes place in an aqueous environment within the gastrointestinal tract. Fortunately, pancreatic lipase is active only at an oil-water interface, which facilitates the digestion of triacylglycerols. Furthermore, biliary bile acids can form mixed micelles together predominantly with fatty acids, and to lesser degrees, with mono-, di- and triacylglycerols. These mixed micelles can function as a transport vehicle to deliver fatty acids to the apical membrane of enterocytes for the absorption. Because the efficiency (>90%) of intestinal fatty acids is significantly higher than that of cholesterol (~50%) in human beings, this indicates that fatty acids are much more efficient to be absorbed by the small intestine compared to cholesterol.
a. Intraluminal digestion of lipids
The digestion of fat begins in the stomach where dietary constituents are mixed together with lingual and gastric enzymes, resulting in partial fat digestion by preduodenal lipases and emulsification by peristalsis. The stomach also regulates the delivery of gastric chyme into the duodenum where it is mixed with bile and pancreatic juice. The major lipases and proteins secreted by the pancreas into the intestinal lumen in response to a meal include carboxyl ester lipase (CEL), pancreatic triglyceride lipase and the Group 1B phospholipase A2, as well as pancreatic lipase-related protein-1 and -2. However, the regulatory effects of the Group 1B phospholipase A2, as well as pancreatic lipase-related protein-1 and -2 on intestinal fatty acid absorption have not yet been defined. Of special note is that the key enzymes on the digestion of triacylglycerols include lingual lipase secreted by the salivary gland and gastric lipase secreted by the gastric mucosa. Humans express primarily gastric lipase, whereas rodents express mainly lingual lipase. Human gastric lipase and rodent lingual lipase share many characteristics of physiological functions. For example, both enzymes have a pH optimum ranging from 3 to 6, and hydrolyze medium-chain triacylglycerols better than long-chain triacylglycerols. These lipases preferentially hydrolyze fatty acids at the sn-3 position to produce diacylglycerols. However, they do not hydrolyze phospholipids or cholesteryl esters. Surprisingly, patients with cystic fibrosis can still absorb dietary cholesterol, despite a markedly or completely inhibition in the secretion of pancreatic lipase. This suggests that the digestion of triacylglycerols by both lingual and gastric lipase in the stomach also plays an important role in lipid absorption.
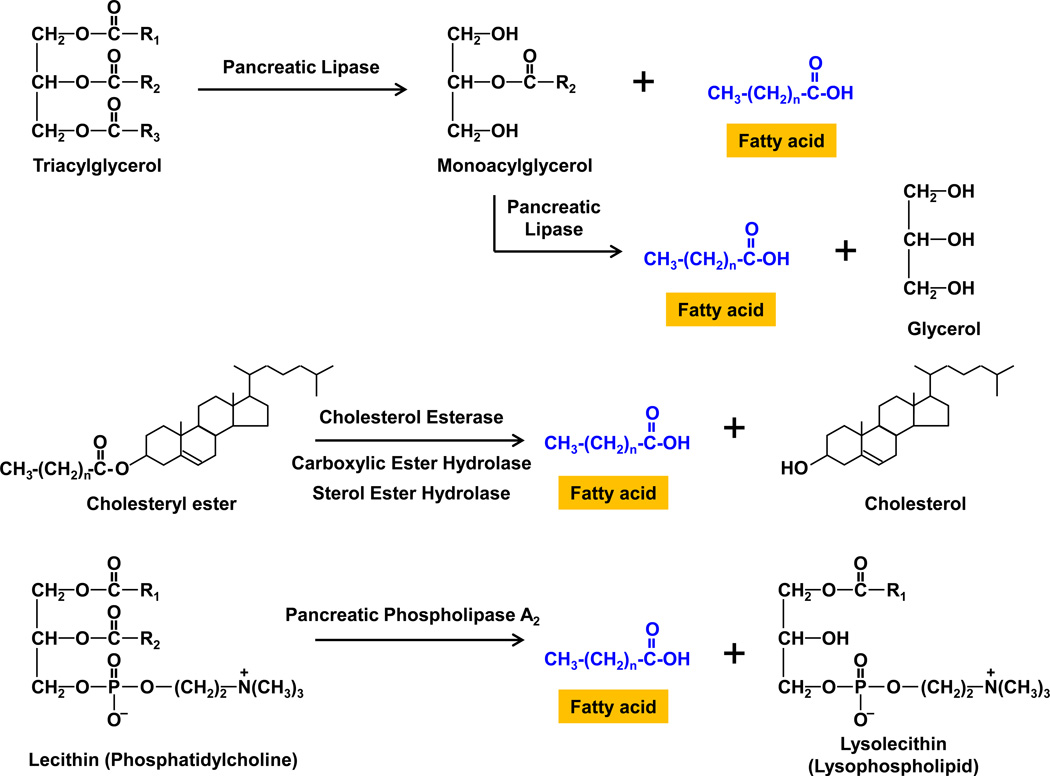
The stomach is also the major site for the mechanical emulsification of dietary fat, which is an important prerequisite for efficient hydrolysis by pancreatic lipase. Emulsification is facilitated by the diacyloglycerols and fatty acids produced as a result of the action of acid lipases in the stomach, as well as the phospholipids normally present in the diet. The lipid emulsion enters the small intestine as fine lipid droplets with diameters of less than 500 nm. The combined actions of bile and pancreatic juice markedly alter the chemical composition of the lipid emulsion in the upper part of the small intestine. As shown in Figure 3, pancreatic lipase functions at the interface between oil and aqueous phases, and hydrolyzes mainly the sn-1 and sn-3 positions of the triacylglycerol molecules to release monoacylglycerols and fatty acids. Further hydrolysis of monoacylglycerols by pancreatic lipase results in the formation of glycerols and fatty acids. When fat digestion is observed by polarizing light microscopy in vitro, it is appreciated that at least three phases are present: an oil phase (mainly triacylglycerols, partial diacylglycerols, and fatty acids), a calcium soap phase (Ca2+ ions and protonated long-chain fatty acids), and a viscous isotropic phase (monoacylglycerols and fatty acids) [5].
Figure 3.
In the small intestine, pancreatic lipase catalyses the hydrolysis of dietary triacylglycerols to 2-monoacylglycerols by removing fatty acids from the sn-1 and sn-3 positions. The 2-monoacylglycerol molecule is further hydrolyzed to release the last fatty acid from the sn-2 position, leaving a glycerol. Cholesteryl esters from the diet are hydrolyzed by the action of cholesterol esterase, carboxylic ester hydrolase (also called carboxylic ester lipase), and sterol ester hydrolase. Phospholipids, i.e., mainly lecithin (phosphatidylcholine), from bile and the diet are hydrolyzed by pancreatic phospholipase A2, which removes the fatty acid at the sn-2 position, leaving a lysolecithin (lysophospholipid), a powerful detergent. The fatty acids released and the lysolecithins are incorporated into mixed micelles and transported into the apical membrane of enterocyte for uptake and appear in chylomicrons. R = hydrocarbon chain.
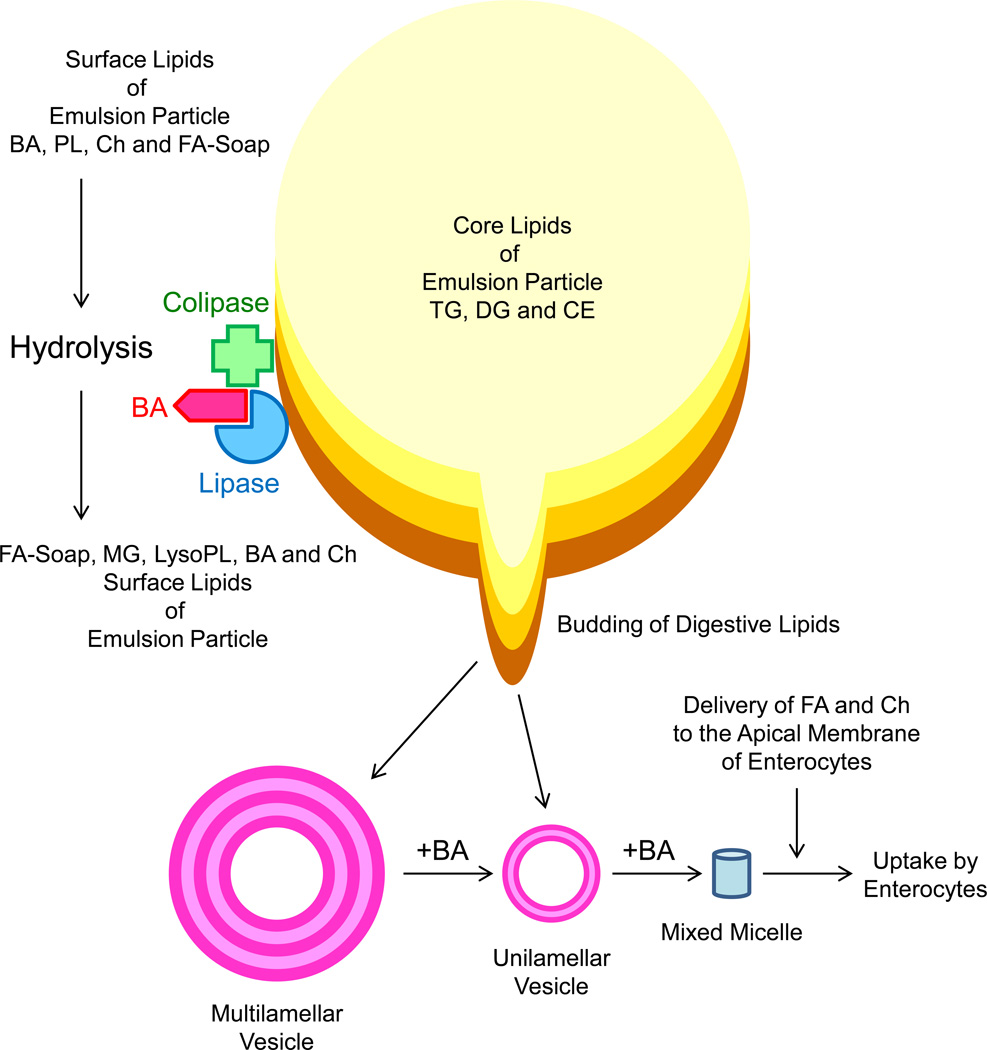
Pancreatic lipase is present only in pancreatic juice. Its high concentration in pancreatic secretions together with its great catalytic efficiency ensures the complete digestion of dietary fat in the small intestine. The very high capacity for fat digestion is highlighted by the findings that the symptom of fat malabsorption is present in patients only with severe pancreatic lipase deficiency. It is surprising to find that purified pancreatic lipase is inefficient at hydrolyzing triacylglycerols in a model lipid mixture; however, it is highly efficient when present in pancreatic juice. These findings led to the discovery that the colipase is required for pancreatic lipase to produce its physiological functions in the intestine (Figure 4). Further studies found that colipase is also secreted by the pancreas as a procolipase. After entering the small intestinal lumen, the procolipase is activated by the cleavage of a pentapeptide from the N-terminus. Whereas lipid droplets of triacylglycerols surrounded by bile acids are not accessible to pancreatic lipase, the binding of the colipase to the triacylglycerol/aqueous interface allows the binding of the lipase molecule to the lipid/aqueous interface, which greatly facilitates digestion of dietary fat.
Figure 4.
Illustration of the physical-chemical event of lipid digestion and absorption in the upper part of small intestine. The core of crude emulsion particles includes predominately triacylglycerols (TG), followed by diacylglycerols (DG) and cholesteryl esters (CE). Because of gastric lipase hydrolysis, fatty acids (FA) in emulsion particles migrate to the emulsion-water interfaces and are built for the surfaces of emulsion particles together with biliary bile acids (BA) and phospholipids (PL). After entering the small intestinal lumen, the procolipase is activated by the cleavage of a pentapeptide from the N-terminus. Whereas lipid droplets of triacylglycerols surrounded by bile acids are not accessible to pancreatic lipase, the binding of the colipase to the emulsion-water interface allows the binding of the lipase molecule to the emulsion-water interface, which greatly facilitates digestion of dietary fat. The pancreatic lipase hydrolyzes the emulsion particles, building up a multilamellar liquid crystalline layer of FA, MG, lysophospholipids (LysoPL), BA and cholesterol (Ch). The budding off as multilamellar liquid crystalline layers is transformed by BA to form multilamellar vesicles and then unilamellar vesicles. Dissolution of unilamellar vesicles by BA leads to the formation of mixed micelles that contain FA and Ch. More importantly, mixed micelles function as transport vehicles for FA and Ch across the unstirred water layer toward the apical membrane of enterocytes, which facilitates the uptake of FA and Ch molecules by the enterocytes.
Phospholipids are the second main source of fat in the intestine although their concentrations are much lower compared to dietary triacylglycerols. The digestion of phospholipids from bile and the diet also takes place in the small intestinal lumen. These phospholipids, i.e., principally lecithin (phosphatidylcholine), are hydrolyzed by pancreatic phospholipase A2 at the sn-2 position to yield fatty acids and lysolecithin (lysophosphatidylcholine) molecules (Figure 3). The fatty acids are released and the lysophospholipids are incorporated into micelles and transported into mucosal cells and appear in the intestinal lymph in chylomicrons. Moreover, in concentrated gallbladder bile, phospholipids are solubilized primarily in mixed micelles together with bile acids and cholesterol. In the intestinal lumen, phospholipids are largely solubilized in mixed micelles, but also participate in the emulsification of triacylglycerols.
Because only unesterified cholesterol is incorporated into simple and mixed micelles and transported to the brush border of enterocytes for uptake, a critically important step is lipase-mediated hydrolysis of cholesteryl esters. The enzymes for the hydrolysis of cholesteryl esters include cholesterol esterase, carboxylic ester hydrolase (also called carboxylic ester lipase), and sterol ester hydrolase (Figure 3). However, the contribution of unesterified cholesterol (mainly biliary) to intestinal cholesterol is much greater than the dietary esterified cholesterol. As a result, inhibition or loss of some of the pancreatic lipolytic enzyme activities would be unlikely to result in an appreciable reduction of cholesterol absorption. This may partly explain why targeted disruption of the carboxylic ester lipase (Cel) gene in mice has little or only a slight inhibitory effect on intestinal cholesterol absorption [6, 7]. In human beings, cholesterol esterase has a broad specificity, with the capacity to hydrolyze triacylglycerols, cholesteryl esters and phospholipids [8, 9]. Cholesterol esterase activity is greatly enhanced by the presence of bile acids, particularly the trihydroxy bile acid cholate. As entering the small intestine, dietary cholesterol is typically mixed in a lipid emulsion with triacylglycerols and phospholipids. The digestion of phospholipids on the surface of the lipid emulsion particles and triacylglycerols in the core, is required to liberate the dietary cholesterol. This cholesterol molecule is transferred to phospholipid vesicles and then bile acid micelles for its transport to the brush border of enterocyte. Therefore, the cholesterol molecules often have to be incorporated into disk-shaped micelles and liquid crystalline vesicles prior their uptake by enterocytes [10, 11]. Interestingly, a lack of triacylglycerol hydrolytic activity in the intestinal lumen in pancreatic triglyceride lipase knockout mice reduces dietary cholesterol absorption substantially, without impairing digestion and absorption of triacylglycerols [12].
Due to their critical detergent properties, biliary bile acids are crucial to the uptake of fatty acids and cholesterol by the enterocytes because they coordinate micellar solubilization of intraluminal fatty acids and cholesterol [13]. Simple bile acid micelles (3 nm in diameter) are small, thermodynamically stable aggregates that can solubilize only minimal amounts of cholesterol. By contrast, phospholipids, monoacylglycerides, and fatty acids are highly soluble in simple bile acid micelles. As a result, when combined together with ionized and non-ionized fatty acids, monoacylglycerides and lysophospholipids, bile acids form mixed micelles. Mixed micelles can solubilize much greater amounts of cholesterol compared with simple micelles. Bile acids also form mixed micelles with fat-soluble vitamins, diacylglycerols and triacylglycerols in the small intestinal lumen. Mixed micelles are larger, thermodynamically stable aggregates and their sizes (4–8 nm in diameter) vary depending on the relative proportion of bile acids and phospholipids. More importantly, mixed micelles function as transport vehicles for fatty acids and cholesterol across the unstirred water layer toward the brush border of enterocytes, where they facilitate uptake of fatty acid and cholesterol molecules by the enterocytes [14–16].
In the small intestinal lumen, excess triacylglycerols and cholesteryl esters that are not dissolved in mixed micelles can exist as a stable emulsion that is mainly composited by bile acids, phospholipids, monoacylglycerides and fatty acids (Figure 4). During the fat hydrolysis, liquid crystals composed of multilamellar products of lipid digestion form at the surface of the emulsion droplets [10, 11]. These liquid crystals give rise to vesicles, which are unilamellar spherical structures and contain phospholipids and cholesterol, with little, if any, bile acids. Vesicles (40–100 nm in diameter) are substantially larger than either simple or mixed micelles, but much smaller than liquid crystals (~500 nm in diameter) that are composed of multilamellar spherical structures. Liquid crystals and vesicles each provide an accessible source of fatty acids, cholesterol and other lipids for continuous formation and modification of mixed micelles in the presence of bile acids. Within the intestinal lumen, the presence of hydrophilic bile acids may reduce the solubility of cholesterol by favoring the formation of liquid crystals and vesicles at the expense of mixed micelles [17]. As a result, cholesterol molecules are poorly absorbed by the enterocytes when incorporated into liquid crystals or vesicles. By contrast, hydrophobic bile acids markedly increase micellar solubility of cholesterol and thereby augment cholesterol absorption [17, 18]. This suggests that the hydrophobic bile acids are more effective at promoting cholesterol absorption than the hydrophilic bile acids. However, the hydrophilic-hydrophobic index of bile acid pool seems not to markedly influence intestinal fatty acid absorption. Luminal bile acids are derived from hepatic secretion and reabsorbed from the intestine (mainly the ileum) through an active bile acid transporter mechanism and returned to the liver via portal blood to complete the enterohepatic circulation.
b. Intestinal uptake of lipids
The mixed micelles in the small intestinal lumen promote the absorption of fatty acids and cholesterol by facilitating transport of these lipids across the unstirred water layer adjacent to the surface of the apical membrane of enterocytes. The micelle particle itself does not penetrate the cell membrane. Rather, it facilitates passage across a diffusion barrier that is located at the intestinal lumen-membrane interface for the uptake by the enterocytes. Mucous coating the intestinal mucosa is also diffusion-limiting barrier, especially since cholesterol molecules may be extensively bound to surface mucins prior to transfer into the enterocyte. Physiological quantities of epithelial mucin encoded by the MUC1 gene are necessary for normal intestinal uptake and absorption of cholesterol in mice, as evidenced by a reduction of cholesterol absorption efficiency by 50% in MUC1 knockout mice [19]. However, it is likely that MUC1 loss does not impair intestinal fatty acid uptake in mice.
The molecular mechanisms for the transport of water-insoluble amphipathic fatty acids across membranes have been debated for many years: by a simple passive diffusion mechanism [20–24], a protein-mediated mechanism [25–30], or both. A protein-mediated fatty acid uptake system is now believed to be the dominant means by which fatty acids are taken up by membrane-associated fatty acid-binding proteins, i.e., fatty acid transporters on the apical membrane of enterocytes [31]. As shown in Figure 2, a large number of fatty acid transporters have been identified, which facilitate the cellular uptake of fatty acids and these include cluster determinant 36 (CD36, also called fatty acid translocase), plasma membrane-associated fatty acid-binding protein (FABPpm), and a family of fatty acid transport proteins 1–6 (FATP1–6). Fatty acid binding protein (FABP) is present in the small intestine and may play an important role in the intracellular transport of the absorbed fatty acids [25, 28, 29]. This assertion is based on the higher concentration of FABP in villi compared with crypts, in the jejunum compared with the ileum, and in intestinal mucosa of animals fed a high-fat diet compared with those fed a low-fat diet. The prevalent view is that these fatty acid transporters could act as acceptors for fatty acids and subsequently the fatty acids make their way through the cell membrane possibly by simple diffusion [31–35]. At the inner side of the membrane, the transmembrane proteins may provide a docking site for a cytoplasmic fatty acid-binding protein (FABPc) that is abundantly present in the soluble cytoplasm of enterocytes [31, 36, 37]. Thus, these proteins may function to sequester fatty acids in the membrane, and help organize them within specific membrane domains so as to make the fatty acids readily available for subsequent aqueous transport [38]. It is still unclear whether there is a difference in the intestinal uptake among saturated, monounsaturated and polyunsaturated fatty acids and among short-chain, middle-chain, and long-chain fatty acids.
A major advance in the effort to identify intestinal sterol transporters was the discovery that mutations in the genes encoding human ABCG5 and ABCG8 transporters constituted the molecular basis of sitosterolemia in patients [39, 40]. Studies in genetically engineered mice and in vitro experiments further found that ABCG5 and ABCG8 are localized in the apical brush border membrane of enterocyte and in the canalicular membrane of hepatocyte [41–43], and appear to function as an efficient efflux pump system for both cholesterol and plant sterols. Consistent with this hypothesis, there is a negative correlation between the efficiency of cholesterol absorption and the expression levels of ABCG5 and ABCG8 in the jejunum and ileum but not in the duodenum [44]. This suggests that under normal physiological conditions, the jejunal and ileal ABCG5 and ABCG8 play a major regulatory role in modulating the amount of cholesterol that is absorbed from the intestine. The expression levels of these genes may explain, in part, why cholesterol absorption occurs selectively, and plant sterols and other non-cholesterol sterols are absorbed poorly or not at all.
The discovery of ezetimibe as a specific and potent inhibitor of intestinal cholesterol absorption helped identify a putative sterol influx transporter at the brush border membrane of enterocyte [45]. Radiolabeled ezetimibe is localized to the brush border membrane of enterocyte and appears to directly inhibit the uptake activity of this cholesterol transporter [45]. Using a genomic-bioinformatics approach, the transcripts containing expression patterns and structural characteristics anticipated in cholesterol transporters were identified [46, 47]. This led to the discovery of the Niemann-Pick C1 like 1 (NPC1L1) protein and established it as a strong candidate for the ezetimibe-sensitive cholesterol transporter. Moreover, similarities in cholesterol absorption characteristics between ezetimibe-treated mice and NPC1L1 knockout mice support the likelihood that NPC1L1 is the ezetimibe-inhibitable cholesterol transporter [46]. The Npc1l1 gene is expressed predominantly in the small intestine in mice, with peak expression in the proximal jejunum [46, 47], paralleling the efficiency of cholesterol absorption along the gastrointestinal axis. Moreover, NPC1L1 has been found to mediate cholesterol uptake mostly via vesicular endocytosis and ezetimibe inhibits cholesterol absorption possibly by blocking the internalization of the NPC1L1/cholesterol complex [48].
These studies indicate that intestinal uptake and absorption of fatty acids and cholesterol is determined by two different transporter-mediated systems. Both intestinal fatty acid and cholesterol absorption are a multistep process that is regulated by multiple genes at the enterocyte level. Moreover, the efficiency of cholesterol absorption is determined by the net effect between influx and efflux of intraluminal cholesterol molecules crossing the brush border membrane of the enterocyte [1].
c. Intracellular metabolism of absorbed lipids
Another potential step for sorting/regulation is the incompletely characterized intracellular pathway whereby the absorbed fatty acid molecules reach the endoplasmic reticulum and are re-esterified to triacylglycerols. After entering the enterocytes, monoacylglycerols and fatty acids are largely reconstituted to form triacylglycerols mainly via the consecutive actions of monoacylglycerol and diacylglycerol acyltransferases. The locations of these enzymes on the cytoplasmic surface of the endoplasmic reticulum, suggest that triacylglycerols are formed in the cytoplasmic surface of the endoplasmic reticulum and gain entry into the cisternae of the endoplasmic reticulum. The second pathway present in intestinal mucosa for the formation of triacylglycerols is the α-glycerophosphate pathway that involves the stepwise acylation of glycerol-3-phosphate to form phosphatidic acid. In the presence of phosphatidate phosphohydrolase, phosphatidic acid is hydrolyzed to form diacylglycerols, which are then converted to triacylglycerols. The relative importance of these two pathways depends greatly on the supply of monoacylglycerols and fatty acids. During normal lipid absorption, the monoacylglycerol pathway is the predominant route for the synthesis of triacylglycerols, because monoacylglycerols and fatty acids are very efficiently converted to triacylglycerols. Monoacylglycerols also inhibit the α-glycerophosphate pathway. By contrast, when monoacylglycerols are insufficient, the α-glycerophosphate pathway becomes the major route for the formation of triacylglycerols. Some of the absorbed lysophosphatidylcholines are reacylated to form phospholipids, and the others are hydrolyzed to form glycerol-3-phosphorylcholine, which may be transported to the liver via the portal vein. The liberated fatty acids are then used for triacylglycerol synthesis. Finally, some lysophosphatidylcholine molecules are combined to form one molecule of phospholipid and one molecule of glycerol-3-phosphorylcholine.
The absorbed cholesterol enters a cholesterol pool within the enterocyte. This contains cholesterol derived from the diet, from non-dietary sources (biliary cholesterol and cholesterol from cells shed from the intestinal mucosa), from newly synthesized cholesterol within the enterocyte, and from plasma lipoproteins. The enterocyte may treat these various sources of cholesterol differently. For example, in a fasting state, very little newly synthesized cholesterol is transported into lymph. By contrast, during active lipid absorption, significantly more of the newly synthesized cholesterol is transported into lymph following incorporation into chylomicrons. Cholesterol transported by the lymphatic system is almost exclusively esterified, and the rate of esterification of cholesterol may regulate lymphatic transport. In the small intestine, acyl-CoA:cholesterol acyltransferase 2 (ACAT2) is highly specific for cholesterol and does not appreciably esterify plant sterols. This also explains, in part, very low absorption rate of plant sterols. Re-esterification of the absorbed cholesterol with fatty acids within the enterocyte would enhance the diffusion gradient from the lumen to the enterocyte, favoring cholesterol absorption. In this connection, it has been observed that pharmacological inhibition of ACAT significantly reduces transmucosal transport of cholesterol in rats [49], and that deletion of the Acat2 gene decreases intestinal cholesterol absorption in mice [50]. Moreover, the inhibition of intestinal 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase by pharmacological treatment with statins also reduces intestinal cholesterol absorption in laboratory animals and in humans. Finally, cholesteryl esters are incorporated into nascent chylomicrons. This process allows nascent chylomicrons to mature and exit the endoplasmic reticulum for eventual secretion as chylomicron particles into the lymph [51].
Together with cholesterol, cholesteryl esters and phospholipids, the triacylglycerols synthesized in the enterocytes are used as the major lipid components for the assembly of the surface and core of chylomicrons.
d. Assembly and secretion of intestinal lipoproteins
Triacylglycerols are reformed within the enterocytes from the absorbed medium-chain and long-chain fatty acids and monoglycerides and are then incorporated into chylomicrons. The small intestine secretes chylomicrons (75–450 nm in diameter, Sf≥60, d<0.93 g/ml) into the intestinal lymph, which are predominantly triacylglycerol-rich lipoproteins. These large, spherical particles are lipid-enriched: triacylglycerols (85–90%), phospholipids (7–9%), cholesteryl esters (3–5%), unesterified cholesterol (1–3%), and apolipoproteins (1–2%). The chemical composition of chylomicrons indicates their primary roles as triacylglycerol transport vehicles. The core of chylomicrons contains triacylglycerols and cholesteryl esters, whereas the surface of the particles comprises a monolayer of phospholipids, unesterified cholesterol and apolipoproteins [52, 53]. The major apolipoproteins of chylomicrons are apolipoprotein-B48, apolipoprotein-AI, and apolipoprotein-AIV. Traces of apolipoprotein-E and apolipoprotein-C detected in chylomicrons are added to the surface after interactions of chylomicrons with other plasma lipoproteins. Fatty acid compositions of triacylglycerols in intestinal lipoproteins reflect the dietary fatty acids [54, 55]. By contrast, the fatty acid compositions of phospholipids (phosphatidylcholines, phosphatidylethanolamines and sphingomyelins) and cholesteryl esters present in chylomicrons are not representatives of the fatty acids present in the diet. Although the size and composition of the secreted chylomicrons are dependent on the rate of fat absorption and the type of fat absorbed, the molecular basis for the trafficking of different lipids for incorporation into chylomicrons by the enterocyte remains unclear.
The assembly of chylomicrons is a characteristic property of the enterocytes during the postprandial state. The small intestine also produces a large number of very low density lipoproteins (VLDL)-sized particles (30–80 nm in diameter, Sf=20–60, 0.93<d<1.006 g/ml), and to lesser degrees, high density lipoproteins (HDL). The VLDLs are constitutively synthesized and are the primary lipoproteins secreted during the fasting state. VLDL may transport lipids derived from the bile and sloughed enterocytes, and fatty acids derived from the plasma.
The assembly of chylomicrons requires apolipoprotein-B48 that is translated from a common apolipoprotein B mRNA following posttranscriptional editing in which enzymatic deamination of cytosine by the apolipoprotein B editing complex creates a uracil at nucleotide 6666. The nucleotide conversion results in truncation of apolipoprotein B codon 2153, so that the mature protein is 48% as long as apolipoprotein B-100 in units of amino acids [56, 57]. The assembly of chylomicrons involves three independent events [58, 59]. First, the incorporation of preformed phospholipids and synthesis of smaller lipoproteins results in the formation of primordial lipoproteins. Second, triacylglycerol-rich lipid droplets of various sizes are synthesized during the postprandial state. Third, the fusion of primordial lipoproteins with triacylglycerol-rich lipid droplets results in the formation of various lipoproteins (VLDL-sized particles, small chylomicrons, and large chylomicrons), which is a process called core expansion. Core expansion appears to render lipid droplets “secretion-competent”. This notion is supported by the observation that the smooth endoplasmic reticulum contains lipid droplets without apolipoprotein-B48 synthesis. In the absence of apolipoprotein-B48, no lipid droplets are found in the Golgi and the intercellular space [60, 61]. However, after apolipoprotein-B48 is synthesized, lipoprotein particles are detected in the Golgi and intercellular spaces. Moreover, triacylglycerols and apolipoprotein-B48 are transported together from the endoplasmic reticulum to the Golgi complex. Therefore, the core expansion may be a crucial step for the regulation of the assembly and secretion of large numbers of triacylglycerol-rich particles.
Intestinal microsomal triglyceride transfer protein (MTTP) transfers neutral lipids into newly-synthesized chylomicrons in the endoplasmic reticulum [62]. MTTP mutations constitute the genetic basis for patients with abetalipoproteinemia, which is characterized by severe steatorrhoea, neurological symptoms, liver steatosis, and very low plasma cholesterol levels. Interestingly, the deletion of the Cel gene in mice induces a significant decrease in the number of chylomicron particles produced by the enterocytes after a lipid meal, with most of the intestinal lipoproteins produced by CEL knockout mice being VLDL-sized particles [6]. Although the exact mechanism by which CEL participates in chylomicron assembly is unknown at this time, indirect evidence suggests that CEL may have an important effect on intracellular lipid trafficking. Intestinal apolipoproteins AI/CIII/AIV have been proposed to play a role in the regulation of cholesterol absorption [63]. However, the regulatory effects of these proteins remain to be defined. Nevertheless, these collective observations concerning chylomicron assembly suggest that the later steps in the absorption process of fatty acids and cholesterol are indeed critically important.
Following secretion into intestinal lymph, chylomicrons enter the blood through the thoracic duct. As they circulate, the triacylglycerols of chylomicrons undergo hydrolysis by lipoprotein lipase, an enzyme located on the surface of capillary endothelial cells of muscle and adipose tissues. Circulating chylomicrons have a half-life of 5–10 minutes. The hydrolysis of chylomicrons leads to release of fatty acids and glycerol from the core of chylomicrons, as well as unesterified cholesterol from the surface coat of these particles. The delipidation occurs predominantly in the adipose, muscle and heart tissues which take up and oxidize or store the fatty acids released by lipoprotein lipase. After the delipidation of chylomicrons by intravascular lipolysis, chylomicron remnants are released back into the circulation and transform into remnant particles. The chylomicron remnants are cleared rapidly by the liver after further delipidation by intravascular hepatic lipase and then binding to the LDL receptor and endocytosis.
Fecal excretion of intestinal lipids
Fatty acids, cholesterol and bile acids that escape intestinal absorption are excreted as fecal fatty acids, as well as neutral and acidic sterols, respectively. This constitutes the major route for sterol elimination from the body. In addition, transintestinal cholesterol excretion may be a new pathway for removal of cholesterol from the body, independent from the classical biliary excretion [64], and proprotein convertase subtilisin kexin type 9 (PCSK9) and ABCB1a/b may be involved in this pathway in mice [65]. Moreover, transintestinal cholesterol excretion may contribute approximately 30% to total neutral sterols excreted from the body in mice [64]. In the fasting state or on fat-free diets, fecal sterol excretion in humans ranges from 0.7–1 g/day, emphasizing that fecal sterols do not consist only of dietary constituents. Likewise, fecal sterol excretion in patients with steatorrhea exceeds dietary intake, indicating that some of endogenous lipids entering the small intestinal lumen are excreted in feces.
Genetic analysis of intestinal fatty acid absorption
There is no evidence showing that genetic factors play a critical role in the regulation of intestinal fatty acid absorption because no significant inter-individual differences and inter-strain variations in the efficiency of intestinal fatty acid absorption in humans and in laboratory animals have been found yet. Although the observations strongly suggest that intestinal fatty acid absorption is regulated by multiple genes (Figure 2), what is not clear is which cellular step(s) could play a critical role in the absorption process of intestinal fatty acids.
As discussed above, the identity of the genes responsible for protein-mediated fatty acid transport has been intensively investigated over the past twenty years. Among these candidates, CD36, FABP2, FATP4 and caveolin-1 have been proposed as potential intestinal fatty acid transporters (Table 1). Some in vitro experiments suggest that these proteins may have an effect on the regulation of intestinal fatty acid absorption [66–68]. However, to date, none of these candidates has been proven indispensable for physiological intestinal fatty acid transport in vivo [69–72]. One possible explanation is that intestinal fatty acid absorption may be regulated by two or more than two genes so that one gene is inactivated and the other could compensate its function. As a result, intestinal fatty acid absorption is not impaired in the above-mentioned single gene knockout mice. Thus, it is crucial to investigate the interaction between these transporters on intestinal fatty acid absorption in mice. Recent mouse studies found that ezetimibe treatment or NPC1L1 loss reduces intestinal absorption of saturated fatty acids and cholesterol [73]. Several experiments also show that NPC1L1 may mediate the metabolism of dietary fatty acids and cholesterol [74, 75]. Nevertheless, further studies are needed to explore these hypotheses by using genetically engineered mouse models with double-knockout or knock-in of these potential fatty acid transporter genes.
Table 1.
The effect of potential fatty acid transporters on the regulation of intestinal fatty acid absorption
| Gene Symbol |
Gene Name | Effects on Fatty Acid Absorption |
Mouse Chra |
cM | Human Ortholog |
Reference |
|---|---|---|---|---|---|---|
| CD36 | Cluster determinant 36 (also called fatty acid translocase) | Enhancing intestinal uptake and absorption of fatty acids | 5 | 8.11 | 7q11.2 | [67,68,71,72] |
| FABP2 (also called FABPi, or I-FABP) | Fatty acid-binding protein 2 (also called fatty acid-binding protein 2, intestinal) | Enhancing intestinal uptake and absorption of fatty acids | 3 | 53.74 | 4q28–q31 | [25,69] |
| FATP4 (also called SLC27A4) | Fatty acid transport protein 4 (also called long-chain fatty acid transport protein 4, or solute carrier family 27 (fatty acid transporter), member 4) | Enhancing intestinal uptake and absorption of fatty acids | 2 | 20.64 | 9q34.11 | [27,30,70] |
| CAV1 | Caveolin-1 | Enhancing intestinal uptake and absorption of fatty acids | 6 | 7.73 | 7q31.1 | [66] |
| NPC1L1 | Niemann-Pick C1-like 1 protein | Inhibiting intestinal uptake and absorption of cholesterol and fatty acids | 11 | 3.93 | 7p13 | [73–75] |
Abbreviations: Chr, Chromosome; cM, centimorgan; p, short arm of the Chr; q, long arm of the Chr.
Map position is based on conserved homology between mouse and human genomes and assigned indirectly from localization in other species. Information on homologous regions was retrieved from the mouse/human homology databases maintained at the Jackson Laboratory (http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=markerQF) and the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/HomoloGene).
Factors influencing intestinal fatty acid absorption efficiency
Because intestinal fatty acid absorption is a multistep process, any factors that can modulate the transport of fatty acids from the intestinal lumen to the lymph may influence the efficiency of intestinal fatty acid absorption. Table 2 lists dietary, pharmacological, biliary, cellular, and luminal factors that could potentially influence absorption of intestinal fatty acids.
Table 2.
Possible factors influencing intestinal fatty acid absorption
|
Abbreviations: APO, apolipoprotein; NR, nuclear receptor.
It has been found that consumption of high dietary fat increases intestinal fatty acid absorption, thereby excess amounts of triacylglycerols being stored in the body. When dietary conditions are controlled, biliary factors have been shown to exert a major influence on the efficiency of intestinal fatty acid absorption. Significant reduction in biliary phospholipid output impairs intestinal fatty acid absorption and chylomicron secretion in mice with the deletion of the Abcb4 gene that encodes a phospholipid transporter on the canalicular membrane of hepatocytes [76]. By contrast, administration of phosphatidylcholine-cholesterol liposomes partially reconstitutes intestinal fat absorption in chronically bile-diverted rats [77]. It is well-known that phospholipids are a critical lipid component for the assembly of the surface of chylomicrons. Although bile acids play a critical role in micellar solubilization of intraluminal fatty acids and cholesterol as well as a stable emulsion, intestinal fatty acid absorption is comparable between bile fistula rats and control rats with intact biliary secretion as studied by intestinal absorption and lymphatic transport of radioisotope-labeled fatty acids [78]. This suggests that bile acids can influence intestinal absorption of cholesterol, but not fatty acids.
It has been found that slow small intestinal transit rate increases the efficiency of cholesterol absorption in humans [79]. Mice with deletion of the cholecystokinin-1 receptor (Cck-1r) gene also absorb cholesterol at higher rates, which correlate with slower small intestinal transit rates [80]. Likely, increased contact time of intestinal lipids with the cholesterol transporters on the brush border membrane of enterocytes might explain such findings. However, it is unclear whether altering small intestinal transit influences intestinal fatty acid absorption.
Before fatty acid and cholesterol molecules in the small intestinal lumen can interact with their possible transporters for uptake and absorption, they must pass through a diffusion barrier, which may modify the kinetics of nutrient assimilation. This barrier includes an unstirred water layer and a surface mucous coat, which is located at the intestinal lumen-membrane interface. Intestinal uptake and absorption of cholesterol but not palmitic acid are significantly reduced in Muc1 (−/−) mice compared with the wild-type mice [19]. This study shows that physiological levels of the epithelial mucin produced by the Muc1 gene are necessary for normal intestinal uptake and absorption of cholesterol in mice. However, the lack of epithelial Muc1 mucin does not impair intestinal uptake of fatty acids.
Establishment of standard protocols for measurement of the efficiency of intestinal fatty acid absorption
Accurate and precise measurements of intestinal fatty acid absorption are one of the basic requirements for quantitation of triacylglycerol homeostasis in the body. Studies on investigation of the efficiency of intestinal fatty acid absorption and of factors influencing intestinal fatty acid absorption have been lagging behind those of intestinal cholesterol absorption. The lack of an appropriate fat-soluble nonabsorbable substance as a reference marker for a precise and accurate measurement of the efficiency of intestinal fatty acid absorption is one of the most important reasons. Due to significant differences in the chemical structures and the physical-chemical properties among saturated, monounsaturated and polyunsaturated fatty acids and among short-chain, medium-chain and long-chain fatty acids, this also makes it more difficult to find an ideal reference marker.
Over the past half a century, the following methods have been used to investigate intestinal fatty acid absorption in humans and/or animal models.
(i) Fat mass balance assay. In a metabolic steady state, the fat mass balance method estimates the mass absorption of exogenous dietary fat based on the difference between dietary fat and its fecal excretion. Therefore, the analysis of consumed and excreted fat for the fat mass balance assay is an accepted method for measuring intestinal fat absorption. The validity of this method is based on negligible chemical alteration of fatty acids during transit through the colon because intestinal bacteria can metabolize fatty acids [81]. The advantage of this method is noninvasive and does not need radioisotopes. Although the concept of “fat intake minus fat output” technique is straightforward, its practice is difficult because an accurate measurement of diet composition and consumption is required.
(ii) Fecal radioisotope method. Because fat mass balance measurements are cumbersome and often inaccurate, other techniques have been used to estimate intestinal fatty acid absorption. The most frequently employed method in the literature is the fecal radioisotope method, which is based on the administration of a single oral dose of radiolabled fatty acids and a nonabsorbed radiolabled fat-like compound as reference marker. The measurement is critically dependent on an accurate assessment of the ratios of excreted radioactivities of the two isotopes and their bacterial metabolites in feces. Undoubtedly, for fecal radioisotope method, nonabsorbable radioisotope-labeled reference markers are critical for the measurement of dietary lipid absorption [82]. Cholesterol absorption has been measured with radiolabeled dietary plant sterols such as sitostanol or sitosterol as nonabsorbable reference markers [82], and dietary triacylglycerol absorption by simultaneous feeding of [133I]-triolein and [75Se]-glyceryl triether [83]. A saturated mixed-chain glycerol triether, 1-hexadecyl-2,3-didodecyl glycerol (1-hexadecoxy-2,3-didodecoxypropane), is synthesized with [3H] at positions 9 and 10 or [14C] at position 1 of the hexadecyl moiety [84, 85]. Because less than 0.2% of the triether is absorbed based on lymph and fecal recoveries, and radioactivity is present exclusively as triether in feces, this shows that it is not degraded by digestive or bacterial enzymes. Also, it is nonabsorbable and does not influence the absorption of dietary fat. When the triether is partitioned between an oil phase of triolein or fatty acid and monoacylglycerol, and an aqueous micellar phase, it remains exclusively in the oil phase. The triether appears to be an ideal nonabsorbable oil-phase marker for use in the study of intestinal fatty acid absorption [84, 85].
In addition, intake of radioisotope-labeled or stable isotope-labeled triolein followed by assay of expired labeled CO2 has been used to give a relative measure of fat absorption. This technique does not give an absolute fractional absorption measurement and requires radioactivity or mass spectroscopy.
(iii) Intestinal absorption and lymphatic transport of fatty acids [78, 82, 86–92]. The lymph fistula animal models (i.e., mice, rats, rabbits, hamsters and dogs) have traditionally been used to study the intestinal absorption of nutrients such as cholesterol and fatty acids. The small intestine is responsible not only for the digestion and transport of dietary lipids such as triacylglycerols, cholesterol and phospholipids, but also for the formation of chylomicrons. With assistance of radioisotope-labeled fatty acids and cholesterol, this method can be used for a direct measurement of absorption efficiency of intestinal fatty acids and cholesterol. Also, it allows investigating the lipid and lipoprotein compositions of chylomicrons and their physical structures and sizes. However, because it is invasive, this method cannot be used for chronic experiments on the investigation of intestinal fatty acid absorption. It is impossible to be used for human studies.
(iv) Fecal non-radioisotope method [93]. Because the fecal method is useful in the studies of intestinal fatty acid absorption, it is desirable to use a non-radioisotope rather than a radioactive compound as a reference marker. Although the polyester made from the esterification of sucrose and behenic acid (“sucrose polybehenate”; SPB) is not absorbed, it has the physical properties of dietary fat and is readily measured by gas chromatography analysis of its hydrolysis product, behenic acid. Thus, SPB is a potential non-radioactive marker. SPB is part of olestra, a blend of long-chain fatty acid esters of sucrose that is commercially used in snack foods. The physical properties of olestra are virtually identical to those of analogous triacylglycerols, but it is not hydrolyzed by pancreatic lipase and not absorbed from the intestine. More importantly, it does not interfere with the absorption of dietary triacylglycerols. Another advantage of the use of SPB is the low level of behenic acid in the diet. Behenic acid is not present in most dietary fats with the exception of peanut oil, in which it comprises 3% of the fatty acids [93]. To measure intestinal fatty acid absorption, dietary fat containing 5% sucrose polybehenate is fed in a semisynthetic diet to rats and mice. At day 2 or 3 on the diet containing test fats, and fecal samples are collected. Fecal samples of approximately 10 mg (single fecal pellet from mice) are assayed. Intestinal fatty acid absorption is calculated from the ratios of behenic acid to other fatty acids in the diet and feces as analyzed by gas chromatography of fatty acid methyl esters. This method is noninvasive, does not require isotope analyses, and can be carried out as part of an animal’s normal feeding regimen. The safety and availability of SPB suggest that it should be applicable in intestinal fat absorption measurements in humans.
In summary, to facilitate research on fatty acid metabolism and lipid-related diseases in humans, it is imperative to establish a standard protocol(s) for measuring the efficiency of intestinal fatty acid absorption in animal models and humans.
Inhibitors of intestinal fatty acid absorption
Over the past 50 years, prevalence of lipid-related metabolic diseases has significantly increased and overconsumption of the Western diet containing high-fat and high-cholesterol content is one of the most important risk factors. As a result, restriction of dietary calories, saturated fat and cholesterol is a rational primary therapeutic intervention for the treatment of patients with dyslipidemia. Despite significant restrictions in dietary intake, a reduction of dietary fat and cholesterol frequently does not reduce plasma triacylglycerol concentrations and circulating LDL cholesterol levels appreciably. This is due in part to the continued presence of large amounts of biliary cholesterol in the intestine and de novo synthesis of triacylglycerols. Therefore, pharmacological inhibition of intestinal fatty acid and cholesterol absorption is potentially an effective way of lowering plasma triacylglycerol and LDL cholesterol levels. Because intestinal fatty acid absorption is a complex process (Figure 2), multiple potential therapeutic targets would be expected to be applicable to the management of patients with hypertriglyceridemia. For example, specific lipase inhibitors such as orlistat not only reduce intestinal fatty acid absorption by inhibiting the hydrolysis of triacylglycerols, but also suppress cholesterol absorption by interfering with the digestive processes within the gastrointestinal lumen, leading to decreased solubilization of cholesterol. However, few drugs are currently available to treat patients with hypertriglyceridemia by inhibiting intestinal fatty acid absorption. Because these hypolipidemic agents have been shown to lower plasma triacylglycerol levels in humans, orlistat, fibrates, nacin, lomitapide and ezetimibe will be the focus of the following sections.
a. Orlistat is the saturated derivative of lipstatin, a potent natural gastric and pancreatic lipase inhibitor that is isolated from the bacterium Streptomyces toxytricini. After an oral administration, it inhibits both gastric and pancreatic lipases, the key enzymes that break down triacylglycerols in the small intestine. When lipase activity is blocked, triacylglycerols from the diet are not hydrolyzed into absorbable unesterified fatty acids, and are excreted undigested instead. As a result, orlistat prevents the intestinal absorption of about 30% of fat from the diet, thereby reducing caloric intake. In addition, orlistat is a drug designed to treat obesity. The effectiveness of orlistat in inducing weight loss is definite, though modest.
b. Fibrates. The fibrates are a class of amphipathic carboxylic acids. They have been in clinical use since the late 1960s, with clofibrate as the first member followed by the approval of fenofibrate, bezafibrate, gemfibrozil and ciprofibrate. Fibrates are indicated for hypertriglyceridemia. Fibrates typically lower triacylglycerols by 20% to 50%. Although clofibrate and gemfibrozil primarily reduce plasma triacylglycerols along with modest reductions in total cholesterol, the newer-generation fibrates (fenofibrate, bezafibrate and ciprofibrate) have greater reductions in total cholesterol and LDL-cholesterol levels. In addition, level of the good cholesterol HDL is also increased. Similar to statins, there is a risk of severe muscle damage (myopathy and rhabdomyolysis) with fibrates.
Because the fibrates are synthetic ligands that bind to peroxisome proliferator-activated receptor α (PPARα), they are known to clinically reduce plasma triacylglycerol levels through the PPARα-mediated mechanisms, which include (i) increased fatty acid uptake by inducing fatty acid transport protein and increased fatty acid β-oxidation, which reduces the substrate needed for the formation of VLDL; and (ii) increased transcription of lipoprotein lipase and repressed transcription of apolipoprotein C-III, which inhibits lipoprotein lipase activity [94, 95]. In addition, fibrates increase fatty acid uptake and conversion to acyl-CoA by the liver due to the induction of fatty acid transporter protein and acyl-CoA synthetase activity. Induction of the β-oxidation pathway with a concomitant reduction in fatty acid synthesis by fibrates leads to a lower availability of fatty acids for triacylglycerol synthesis, a process that is amplified by the inhibition of hormone-sensitive lipase in adipose tissue by fibrates.
c. Niacin (also known as vitamin B3 or nicotinic acid). Like fibrates, it is also well-suited for lowering plasma triacylglycerol levels by 20–50% [96]. It reduces plasma LDL by 5–25% and increases plasma HDL by 15–35%. Niacin may cause hyperglycemia and liver damage. The niacin derivative acipimox is also associated with a modest decrease in plasma LDL.
d. Lomitapide is a microsomal triglyceride transfer protein (MTTP) inhibitor which is necessary for VLDL assembly and secretion in the liver. It is a new hypolipidemic drug that was approved in 2012 by the FDA to as an adjunct to a low-fat diet and other lipid-lowering treatments in patients with homozygous familial hypercholesterolemia. However, it is unclear whether it could reduce intestinal absorption of fatty acids and cholesterol through inhibiting intestinal MTTP activity. Thus, further studies are needed to test this hypothesis.
e. Ezetimibe, 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone is a highly selective intestinal cholesterol absorption inhibitor, which effectively and potently prevents the absorption of intestinal cholesterol by inhibiting the uptake of dietary and biliary cholesterol across the brush border membrane of the enterocyte. Because NPC1L1 promotes cholesterol uptake probably via vesicular endocytosis, ezetimibe could inhibit cholesterol absorption by suppressing the internalization of NPC1L1/cholesterol complex [48]. The high potency of ezetimibe is evidenced by a 50% inhibition at dose of ranging from 0.0005 to 0.05 mg/kg in a series of different animal models [45, 97]. Following oral administration, ezetimibe undergoes rapid glucuronidation in the enterocyte during its first pass [97–99]. Both ezetimibe and its glucuronide undergo enterohepatic cycling. As a result, there is repeated delivery back to the site of action in the intestine, resulting in multiple peaks of the drug and a long elimination half-life of approximately 22 hours [100]. Because ezetimibe is a relatively small molecular structure and is effective at low concentrations, it does not appear to alter the physical-chemistry of lipids within the intestinal lumen. Ezetimibe does not affect the enterohepatic circulation of bile acids and the absorption of fat-soluble vitamins. Furthermore, ezetimibe neither inhibits pancreatic lipolytic enzyme activities in the intestinal lumen, nor does it disrupt bile acid micelle solubilization of cholesterol [101]. During ezetimibe treatment, there is a marked compensatory increase in cholesterol synthesis in the liver, but not in the peripheral organs, and an accelerated loss of cholesterol in the feces with little or no change in the rate of conversion of cholesterol to bile acids. Thus, the combination of ezetimibe with HMG-CoA reductase inhibitors (statins) has proven to be a potent therapeutic approach to reducing plasma LDL cholesterol levels [102, 103]. It is interesting to find that ezetimibe protects against diet-induced nonalcoholic fatty liver disease in mice, rats and hamsters [104–106]. Although the molecular mechanisms underlying the effect of ezetimibe on the prevention of fatty liver in mice on a high-fat and high-cholesterol diet remain elusive, it is likely that ezetimibe may reduce intestinal absorption of saturated fatty acids in a graded manner that correlated with chain length [73]. Of note is that NPC1L1 deletion also prevents high-fat diet-induced fatty liver in mice [107]. One reasonable explanation is that ezetimibe treatment or NPC1L1 deletion may influence the assembly and secretion of chylomicrons because cholesterol and cholesteryl esters are the major lipid components for the surface and core of chylomicrons, respectively [108]. Thus, intestinal fatty acid absorption may be reduced indirectly by ezetimibe.
Conclusions
Although the research on the molecular mechanism underlying intestinal fatty acid absorption lagged behind that of intestinal cholesterol absorption, a significant progress has been made in the past thirty years. Because the efficiency (>90%) of intestinal fatty acids is significantly higher compared to cholesterol absorption (~50%), this clearly shows that fatty acids can be efficiently absorbed by the small intestine in human beings. The protein-mediated fatty acid uptake system has been proposed to be the dominant means by which fatty acids are taken up by membrane-associated fatty acid-binding proteins, i.e., fatty acid transporters on the apical membrane of enterocytes. These findings show that intestinal fatty acid absorption is a multistep process, which is regulated by multiple genes at the enterocyte level, and the efficiency of intestinal fatty acid absorption could be determined by factors influencing intraluminal fatty acid molecules across the brush border membrane of the enterocyte. However, it remains elusive whether there are differences in the absorption efficiency of intestinal fatty acids due to their chain length and bond saturation. To facilitate research on intestinal, hepatic and plasma triacylglycerol metabolism, it is urgent to establish a standard protocol(s) for precisely and accurately measuring the efficiency of intestinal fatty acid absorption in humans and animal models. This will also promote the research and development of hypolipidemic drugs by inhibiting intestinal fatty acid absorption because the Western diet is prevalent worldwide. It is still unclear whether or not there is a significant inter-individual difference in fatty acid absorption efficiency in humans although multiple potential genes are involved in the regulation of intestinal fatty acid absorption. If such a difference exists, it will provide an opportunity to apply state-of-the-art genetic techniques to identify the responsible genes, and such a gene(s) may be used as a therapeutic target for treating patients with hypertriglyceridemia. A better understanding of the molecular mechanisms whereby fatty acids are absorbed in the small intestine will no doubt lead to more molecular targets for hyperlipidemic patients who require aggressive triacylglycerol- and cholesterol-lowering therapy.
Acknowledgment
This work was supported in part by research grants DK73917 (to D.Q.-H.W.) and DK92779 (to M.L.) from the National Institutes of Health (US Public Health Service) and research grant MRAR08P011-2012 (to P.P.) from Italian Agency of Drug (AIFA).
Footnotes
Conflicts of interest: None of the authors has any financial or any conflict of interest related to the contents of this manuscript.
References
- 1.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Agriculture and U.S. Dietary Guidelines for Americans. Gardiner, Maine: Northern House Media; 2011. Department Health and Human Services; pp. 1–95. [Google Scholar]
- 3.Wang DQ, Portincasa P, Neuschwander-Tetri BA. Steatosis in the liver. Comprehensive Physiology. 2013 doi: 10.1002/cphy.c130001. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Hay DW, Cahalane MJ, Timofeyeva N, Carey MC. Molecular species of lecithins in human gallbladder bile. J Lipid Res. 1993;34:759–768. [PubMed] [Google Scholar]
- 5.Patton JS, Carey MC. Watching fat digestion. Science. 1979;204:145–148. doi: 10.1126/science.432636. [DOI] [PubMed] [Google Scholar]
- 6.Kirby RJ, Zheng S, Tso P, Howles PN, Hui DY. Bile salt-stimulated carboxyl ester lipase influences lipoprotein assembly and secretion in intestine: a process mediated via ceramide hydrolysis. J Biol Chem. 2002;277:4104–4109. doi: 10.1074/jbc.M107549200. [DOI] [PubMed] [Google Scholar]
- 7.Weng W, Li L, van Bennekum AM, Potter SH, Harrison EH, Blaner WS, Breslow JL, et al. Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry. 1999;38:4143–4149. doi: 10.1021/bi981679a. [DOI] [PubMed] [Google Scholar]
- 8.Lombardo D, Fauvel J, Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. I. Action on carboxyl esters, glycerides and phospholipids. Biochim Biophys Acta. 1980;611:136–146. doi: 10.1016/0005-2744(80)90049-2. [DOI] [PubMed] [Google Scholar]
- 9.Lombardo D, Guy O. Binding of human pancreatic carboxylic ester hydrolase to lipid interfaces. Biochim Biophys Acta. 1981;659:401–410. doi: 10.1016/0005-2744(81)90066-8. [DOI] [PubMed] [Google Scholar]
- 10.Staggers JE, Hernell O, Stafford RJ, Carey MC. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry. 1990;29:2028–2040. doi: 10.1021/bi00460a011. [DOI] [PubMed] [Google Scholar]
- 11.Hernell O, Staggers JE, Carey MC. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29:2041–2056. doi: 10.1021/bi00460a012. [DOI] [PubMed] [Google Scholar]
- 12.Huggins KW, Camarota LM, Howles PN, Hui DY. Pancreatic triglyceride lipase deficiency minimally affects dietary fat absorption but dramatically decreases dietary cholesterol absorption in mice. J Biol Chem. 2003;278:42899–42905. doi: 10.1074/jbc.M303422200. [DOI] [PubMed] [Google Scholar]
- 13.Siperstein MD, Chaikoff IL, Reinhardt WO. C14-Cholesterol. V. Obligatory function of bile in intestinal absorption of cholesterol. J Biol Chem. 1952;198:111–114. [PubMed] [Google Scholar]
- 14.Borgstrom B. The micellar hypothesis of fat absorption: must it be revisited? Scand J Gastroenterol. 1985;20:389–394. doi: 10.3109/00365528509089669. [DOI] [PubMed] [Google Scholar]
- 15.Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976;58:97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson FA, Sallee VL, Dietschy JM. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science. 1971;174:1031–1033. doi: 10.1126/science.174.4013.1031. [DOI] [PubMed] [Google Scholar]
- 17.Wang DQ, Tazuma S, Cohen DE, Carey MC. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol. 2003;285:G494–G502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 18.Wang DQ, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am J Physiol. 1999;276:G751–G760. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- 19.Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Lack of the intestinal Muc1 mucin impairs cholesterol uptake and absorption but not fatty acid uptake in Muc1−/− mice. Am J Physiol. 2004;287:G547–G554. doi: 10.1152/ajpgi.00097.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton JA, Johnson RA, Corkey B, Kamp F. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16:99–108. doi: 10.1385/JMN:16:2-3:99. discussion 151-107. [DOI] [PubMed] [Google Scholar]
- 21.Trigatti BL, Gerber GE. The effect of intracellular pH on long-chain fatty acid uptake in 3T3-L1 adipocytes: evidence that uptake involves the passive diffusion of protonated long-chain fatty acids across the plasma membrane. Biochem J. 1996;313(Pt 2):487–494. doi: 10.1042/bj3130487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton JA. How fatty acids bind to proteins: the inside story from protein structures. Prostaglandins Leukot Essent Fatty Acids. 2002;67:65–72. doi: 10.1054/plef.2002.0400. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton JA, Guo W, Kamp F. Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol Cell Biochem. 2002;239:17–23. [PubMed] [Google Scholar]
- 24.Kamp F, Guo W, Souto R, Pilch PF, Corkey BE, Hamilton JA. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J Biol Chem. 2003;278:7988–7995. doi: 10.1074/jbc.M206648200. [DOI] [PubMed] [Google Scholar]
- 25.Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP) Eur J Biochem. 1996;238:368–373. doi: 10.1111/j.1432-1033.1996.0368z.x. [DOI] [PubMed] [Google Scholar]
- 26.Meunier-Durmort C, Poirier H, Niot I, Forest C, Besnard P. Up-regulation of the expression of the gene for liver fatty acid-binding protein by long-chain fatty acids. Biochem J. 1996;319:483–487. doi: 10.1042/bj3190483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faergeman NJ, DiRusso CC, Elberger A, Knudsen J, Black PN. Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J Biol Chem. 1997;272:8531–8538. doi: 10.1074/jbc.272.13.8531. [DOI] [PubMed] [Google Scholar]
- 28.Abumrad N, Harmon C, Ibrahimi A. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J Lipid Res. 1998;39:2309–2318. [PubMed] [Google Scholar]
- 29.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 30.Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, Daniels T, et al. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem. 2003;278:49512–49516. doi: 10.1074/jbc.M309759200. [DOI] [PubMed] [Google Scholar]
- 31.Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids. 2010;82:149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Kampf JP, Kleinfeld AM. Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda) 2007;22:7–14. doi: 10.1152/physiol.00011.2006. [DOI] [PubMed] [Google Scholar]
- 33.Bonen A, Chabowski A, Luiken JJ, Glatz JF. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda) 2007;22:15–29. doi: 10.1152/physiologyonline.2007.22.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Chabowski A, Gorski J, Luiken JJ, Glatz JF, Bonen A. Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins Leukot Essent Fatty Acids. 2007;77:345–353. doi: 10.1016/j.plefa.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:355–361. doi: 10.1016/j.plefa.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 37.Masson CJ, Plat J, Mensink RP, Namiot A, Kisielewski W, Namiot Z, Fullekrug J, et al. Fatty acid- and cholesterol transporter protein expression along the human intestinal tract. PLoS One. 2010;5:e10380. doi: 10.1371/journal.pone.0010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niot I, Poirier H, Tran TT, Besnard P. Intestinal absorption of long-chain fatty acids: evidence and uncertainties. Prog Lipid Res. 2009;48:101–115. doi: 10.1016/j.plipres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 40.Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(−/−) mice. Hepatology. 2007;45:998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan LP, Wang HH, Wang DQ. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J Lipid Res. 2004;45:1312–1323. doi: 10.1194/jlr.M400030-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.van Heek M, Farley C, Compton DS, Hoos L, Alton KB, Sybertz EJ, Davis HR., Jr Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129:1748–1754. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 47.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 48.Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Clark SB, Tercyak AM. Reduced cholesterol transmucosal transport in rats with inhibited mucosal acyl CoA:cholesterol acyltransferase and normal pancreatic function. J Lipid Res. 1984;25:148–159. [PubMed] [Google Scholar]
- 50.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 51.van Greevenbroek MM, Robertus-Teunissen MG, Erkelens DW, de Bruin TW. Participation of the microsomal triglyceride transfer protein in lipoprotein assembly in Caco-2 cells: interaction with saturated and unsaturated dietary fatty acids. J Lipid Res. 1998;39:173–185. [PubMed] [Google Scholar]
- 52.Miller KW, Small DM. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J Biol Chem. 1983;258:13772–13784. [PubMed] [Google Scholar]
- 53.Miller KW, Small DM. Triolein-cholesteryl oleate-cholesterol-lecithin emulsions: structural models of triglyceride-rich lipoproteins. Biochemistry. 1983;22:443–451. doi: 10.1021/bi00271a030. [DOI] [PubMed] [Google Scholar]
- 54.Zilversmit DB. The composition and structure of lymph chylomicrons in dog, rat, and man. J Clin Invest. 1965;44:1610–1622. doi: 10.1172/JCI105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redgrave TG, Dunne KB. Chylomicron formation and composition in unanaesthetised rabbits. Atherosclerosis. 1975;22:389–400. doi: 10.1016/0021-9150(75)90019-2. [DOI] [PubMed] [Google Scholar]
- 56.Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, Silberman SR, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238:363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 57.Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987;50:831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 58.Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1–15. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- 59.Field FJ, Mathur SN. Intestinal lipoprotein synthesis and secretion. Prog Lipid Res. 1995;34:185–198. doi: 10.1016/0163-7827(95)00005-k. [DOI] [PubMed] [Google Scholar]
- 60.Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998;39:1543–1557. [PubMed] [Google Scholar]
- 61.Young SG, Cham CM, Pitas RE, Burri BJ, Connolly A, Flynn L, Pappu AS, et al. A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J Clin Invest. 1995;96:2932–2946. doi: 10.1172/JCI118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon DA, Jamil H, Gregg RE, Olofsson SO, Boren J. Inhibition of the microsomal triglyceride transfer protein blocks the first step of apolipoprotein B lipoprotein assembly but not the addition of bulk core lipids in the second step. J Biol Chem. 1996;271:33047–33053. doi: 10.1074/jbc.271.51.33047. [DOI] [PubMed] [Google Scholar]
- 63.Ordovas JM, Schaefer EJ. Genetic determinants of plasma lipid response to dietary intervention: the role of the APOA1/C3/A4 gene cluster and the APOE gene. Br J Nutr. 2000;83(Suppl 1):S127–S136. doi: 10.1017/s0007114500001069. [DOI] [PubMed] [Google Scholar]
- 64.Tietge UJ, Groen AK. Role the TICE?: advancing the concept of transintestinal cholesterol excretion. Arterioscler Thromb Vasc Biol. 2013;33:1452–1453. doi: 10.1161/ATVBAHA.113.301562. [DOI] [PubMed] [Google Scholar]
- 65.Le May C, Berger JM, Lespine A, Pillot B, Prieur X, Letessier E, Hussain MM, et al. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler Thromb Vasc Biol. 2013;33:1484–1493. doi: 10.1161/ATVBAHA.112.300263. [DOI] [PubMed] [Google Scholar]
- 66.Siddiqi S, Sheth A, Patel F, Barnes M, Mansbach CM., 2nd Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim Biophys Acta. 2013;1831:1311–1321. doi: 10.1016/j.bbalip.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, Besnard P. CD36 as a lipid sensor. Physiol Behav. 2011;105:36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 68.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 69.Vassileva G, Huwyler L, Poirier K, Agellon LB, Toth MJ. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 2000;14:2040–2046. doi: 10.1096/fj.99-0959com. [DOI] [PubMed] [Google Scholar]
- 70.Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, et al. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res. 2009;50:491–500. doi: 10.1194/jlr.M800400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, et al. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res. 2009;50:999–1011. doi: 10.1194/jlr.P700032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, et al. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol. 2008;295:G776–G783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walters JW, Anderson JL, Bittman R, Pack M, Farber SA. Visualization of lipid metabolism in the zebrafish intestine reveals a relationship between NPC1L1-mediated cholesterol uptake and dietary fatty acid. Chem Biol. 2012;19:913–925. doi: 10.1016/j.chembiol.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Anderson JL, Carten JD, Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011;101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Werner A, Havinga R, Perton F, Kuipers F, Verkade HJ. Lymphatic chylomicron size is inversely related to biliary phospholipid secretion in mice. Am J Physiol. 2006;290:G1177–G1185. doi: 10.1152/ajpgi.00127.2005. [DOI] [PubMed] [Google Scholar]
- 77.Nishioka T, Having R, Tazuma S, Stellaard F, Kuipers F, Verkade HJ. Administration of phosphatidylcholine-cholesterol liposomes partially reconstitutes fat absorption in chronically bile-diverted rats. Biochim Biophys Acta. 2004;1636:90–98. doi: 10.1016/j.bbalip.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Morgan RG, Borgstrom B. The mechanism of fat absorption in the bile fistula rat. Q J Exp Physiol Cogn Med Sci. 1969;54:228–243. doi: 10.1113/expphysiol.1969.sp002021. [DOI] [PubMed] [Google Scholar]
- 79.Ponz de Leon M, Iori R, Barbolini G, Pompei G, Zaniol P, Carulli N. Influence of small-bowel transit time on dietary cholesterol absorption in human beings. N Engl J Med. 1982;307:102–103. doi: 10.1056/NEJM198207083070207. [DOI] [PubMed] [Google Scholar]
- 80.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest. 2004;114:521–528. doi: 10.1172/JCI16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howard FA, Henderson C. Hydrogenation of polyunsaturated fatty acids by human colonic bacteria. Lett Appl Microbiol. 1999;29:193–196. doi: 10.1046/j.1365-2672.1999.00616.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang DQ, Carey MC. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J Lipid Res. 2003;44:1042–1059. doi: 10.1194/jlr.D200041-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Hoving J, Wilson JH, Valkema AJ, Woldring MG. Estimation of fat absorption from single fecal specimens using 131I-triolein and 75Se-triether. A study in rats with and without induced steatorrhea. Gastroenterology. 1977;72:406–412. [PubMed] [Google Scholar]
- 84.Morgan RG, Hofmann AF. Use of 3H-labeled triether, a nonabsorbable oil-phase marker, to estimate fat absorption in rats with cholestyramine-induced steatorrhea. J Lipid Res. 1970;11:231–236. [PubMed] [Google Scholar]
- 85.Morgan RG, Hofmann AF. Synthesis and metabolism of glycerol-3H triether, a nonabsorbable oil-phase marker for lipid absorption studies. J Lipid Res. 1970;11:223–230. [PubMed] [Google Scholar]
- 86.Nilsson A. Intestinal absorption of lecithin and lysolecithin by lymph fistula rats. Biochim Biophys Acta. 1968;152:379–390. doi: 10.1016/0005-2760(68)90047-7. [DOI] [PubMed] [Google Scholar]
- 87.Scow RO, Stein Y, Stein O. Incorporation of dietary lecithin and lysolecithin into lymph chylomicrons in the rat. J Biol Chem. 1967;242:4919–4924. [PubMed] [Google Scholar]
- 88.Sylven C, Borgstrom B. Intestinal absorption and lymphatic transport of cholesterol in the rat: influence of the fatty acid chain length of the carrier triglyceride. J Lipid Res. 1969;10:351–355. [PubMed] [Google Scholar]
- 89.Tso P, Lindstrom MB, Borgstrom B. Factors regulating the formation of chylomicrons and very-low-density lipoproteins by the rat small intestine. Biochim Biophys Acta. 1987;922:304–313. doi: 10.1016/0005-2760(87)90053-1. [DOI] [PubMed] [Google Scholar]
- 90.Wang DQ, Paigen B, Carey MC. Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J Lipid Res. 2001;42:1820–1830. [PubMed] [Google Scholar]
- 91.Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, D'Alessio DA, et al. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol. 2005;288:G943–G949. doi: 10.1152/ajpgi.00303.2004. [DOI] [PubMed] [Google Scholar]
- 92.Kohan AB, Yoder SM, Tso P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 94.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 95.Watts GF, Dimmitt SB. Fibrates, dyslipoproteinaemia and cardiovascular disease. Curr Opin Lipidol. 1999;10:561–574. doi: 10.1097/00041433-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 96.Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2005;45:185–197. doi: 10.1016/j.jacc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 97.Sudhop T, von Bergmann K. Cholesterol absorption inhibitors for the treatment of hypercholesterolaemia. Drugs. 2002;62:2333–2347. doi: 10.2165/00003495-200262160-00002. [DOI] [PubMed] [Google Scholar]
- 98.Clader JW, Burnett DA, Caplen MA, Domalski MS, Dugar S, Vaccaro W, Sher R, et al. 2-Azetidinone cholesterol absorption inhibitors: structure-activity relationships on the heterocyclic nucleus. J Med Chem. 1996;39:3684–3693. doi: 10.1021/jm960405n. [DOI] [PubMed] [Google Scholar]
- 99.Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- 100.Ezzet F, Krishna G, Wexler DB, Statkevich P, Kosoglou T, Batra VK. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther. 2001;23:871–885. doi: 10.1016/s0149-2918(01)80075-8. [DOI] [PubMed] [Google Scholar]
- 101.van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001;134:409–417. doi: 10.1038/sj.bjp.0704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melani L, Mills R, Hassman D, Lipetz R, Lipka L, LeBeaut A, Suresh R, et al. Efficacy and safety of ezetimibe coadministered with pravastatin in patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Eur Heart J. 2003;24:717–728. doi: 10.1016/s0195-668x(02)00803-5. [DOI] [PubMed] [Google Scholar]
- 103.Feldman T, Koren M, Insull W, Jr, McKenney J, Schrott H, Lewin A, Shah S, et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol. 2004;93:1481–1486. doi: 10.1016/j.amjcard.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 104.Zheng S, Hoos L, Cook J, Tetzloff G, Davis H, Jr, van Heek M, Hwa JJ. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur J Pharmacol. 2008;584:118–124. doi: 10.1016/j.ejphar.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 105.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol. 2008;295:G813–G822. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, et al. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581:5664–5670. doi: 10.1016/j.febslet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 107.Jia L, Ma Y, Rong S, Betters JL, Xie P, Chung S, Wang N, et al. Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. J Lipid Res. 2010;51:3135–3144. doi: 10.1194/jlr.M006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Bari O, Neuschwander-Tetri BA, Liu M, Portincasa P, Wang DQ. Ezetimibe: its novel effects on the prevention and the treatment of cholesterol gallstones and nonalcoholic Fatty liver disease. J Lipids. 2012;2012:302847. doi: 10.1155/2012/302847. [DOI] [PMC free article] [PubMed] [Google Scholar]