Abstract
Cholesterol cholelithiasis is a multifactorial disease influenced by a complex interaction of genetic and environmental factors, and represents a failure of biliary cholesterol homeostasis in which the physical-chemical balance of cholesterol solubility in bile is disturbed. The primary pathophysiologic event is persistent hepatic hypersecretion of biliary cholesterol, which has both hepatic and small intestinal components. The majority of the environmental factors are probably related to Western-type dietary habits, including excess cholesterol consumption. Laparoscopic cholecystectomy, one of the most commonly performed surgical procedures in the US, is nowadays a major treatment for gallstones. However, it is invasive and can cause surgical complications, and not all patients with symptomatic gallstones are candidates for surgery. The hydrophilic bile acid, ursodeoxycholic acid (UDCA) has been employed as first-line pharmacological therapy in a subgroup of symptomatic patients with small, radiolucent cholesterol gallstones. Long-term administration of UDCA can promote the dissolution of cholesterol gallstones. However, the optimal use of UDCA is not always achieved in clinical practice because of failure to titrate the dose adequately. Therefore, the development of novel, effective, and noninvasive therapies is crucial for reducing the costs of health care associated with gallstones. In this review, we summarize recent progress in investigating the inhibitory effects of ezetimibe and statins on intestinal absorption and hepatic biosynthesis of cholesterol, respectively, for the treatment of gallstones, as well as in elucidating their molecular mechanisms by which combination therapy could prevent this very common liver disease worldwide.
Keywords: bile, bile flow, bile acid, cholesterol monohydrate crystal, cholesterol crystallization, mucin
Introduction
Cholesterol homeostasis is mainly maintained by balancing endogenous cholesterol biosynthesis and intestinal cholesterol absorption with excretion of biliary cholesterol and its metabolic products–bile acids [1]. In the normal physiological state, both cholesterol biosynthesis within the body and cholesterol excretion from the body via bile are precisely regulated to accommodate the varying amounts of cholesterol that are absorbed by the intestine at different times. Consequently, sufficient cholesterol is always available to meet the metabolic needs of the various tissues and there is little net accumulation of cholesterol in the body. However, under certain pathophysiological conditions, minor imbalances increase plasma cholesterol concentration and/or induce hepatic hypersecretion of biliary cholesterol. As a result, excess cholesterol causes atherosclerosis and cholesterol cholelithiasis. Indeed, some patients with hyperlipidemia, notably type IV, have increased cholelithiasis [2].
Hypercholesterolemia is an important risk factor for atherosclerosis, which has been the commonest cause of death in the US and other developed countries. After the first statin (lovastatin), a 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitor, was made available in 1987, statins have led to a substantial reduction in virtually all clinical manifestations of cardiovascular diseases and have prolonged the lives of millions of patients. In the late 1990s, a highly potent and selective cholesterol absorption inhibitor (ezetimibe) was discovered [3–6]. Co-administration of statins and ezetimibe enabled more patients to achieve the target LDL cholesterol levels recommended by the US National Cholesterol Education Program Adult Treatment Panel III [7–12]. Since excess cholesterol in serum and bile plays a crucial role in atherosclerosis and cholelithiasis, respectively, it is important to explore whether cholesterol gallstones could be prevented by combination therapy of statins and ezetimibe.
Cholesterol cholelithiasis is one of the most prevalent digestive diseases, leading to considerable financial and social burdens [13]. Presently, the commonest treatment for gallbladder gallstones is laparoscopic cholecystectomy, which is also one of the most commonly performed surgical procedures worldwide due to the overall high prevalence of gallstones. However, cholecystectomy is invasive and can cause surgical complications in terms of morbidity and mortality, and not all patients with symptomatic gallstones are candidates for surgery. Therefore, the development of novel, effective and noninvasive therapies will help reduce the costs of health care associated with gallstones. In this review, we will summarize recent progress in investigating the inhibitory effects of ezetimibe and statins on the treatment of cholesterol gallstones, as well as in elucidating their molecular mechanisms for preventing this very common liver disease in the US.
The pathogenesis of the formation of cholesterol gallstones
During the past 50 years, many human and animal studies have found that hepatic hypersecretion of biliary cholesterol is the primary pathophysiologic defect leading to cholesterol gallstone formation. This could be induced by multiple Lith genes, with insulin resistance as part of the metabolic syndrome working with cholelithogenic environmental factors to cause the phenotype [14–16]. As shown in Figure 1, five defects play important roles in cholesterol crystallization in bile and eventually in the formation of cholesterol gallstones [17]: (i) genetic factors and Lith genes; (ii) unphysiological supersaturation with cholesterol due to hepatic hypersecretion of biliary lipids and relative cholesterol hypersecretion may or may not be accompanied by normal, high, or low secretion rates of biliary bile acids or phospholipids; (iii) accelerated phase transitions of cholesterol; (iv) dysfunctional gallbladder motility accompanied with hypersecretion of mucins and accumulation of mucin gel in the gallbladder lumen, as well as immune-mediated gallbladder inflammation; and (v) increased amounts of cholesterol of intestinal origin due to high efficiency of cholesterol absorption and/or slow intestinal motility which aids “hydrophobe” absorption and augments “secondary” bile acid synthesis by the anaerobic microflora [16–18]. More recently, a large case-control study [19] has found that abnormal metabolic traits including increased hepatic biosynthesis and fecal excretion of cholesterol could precede cholesterol gallstone formation, which may be key features of some ethnic groups at high risk of gallstones. This important study strongly suggests that inhibiting both hepatic synthesis and intestinal absorption of cholesterol to reduce its biliary output may be envisioned for genetically defined subgroups of individuals at a high risk for gallstones [20].
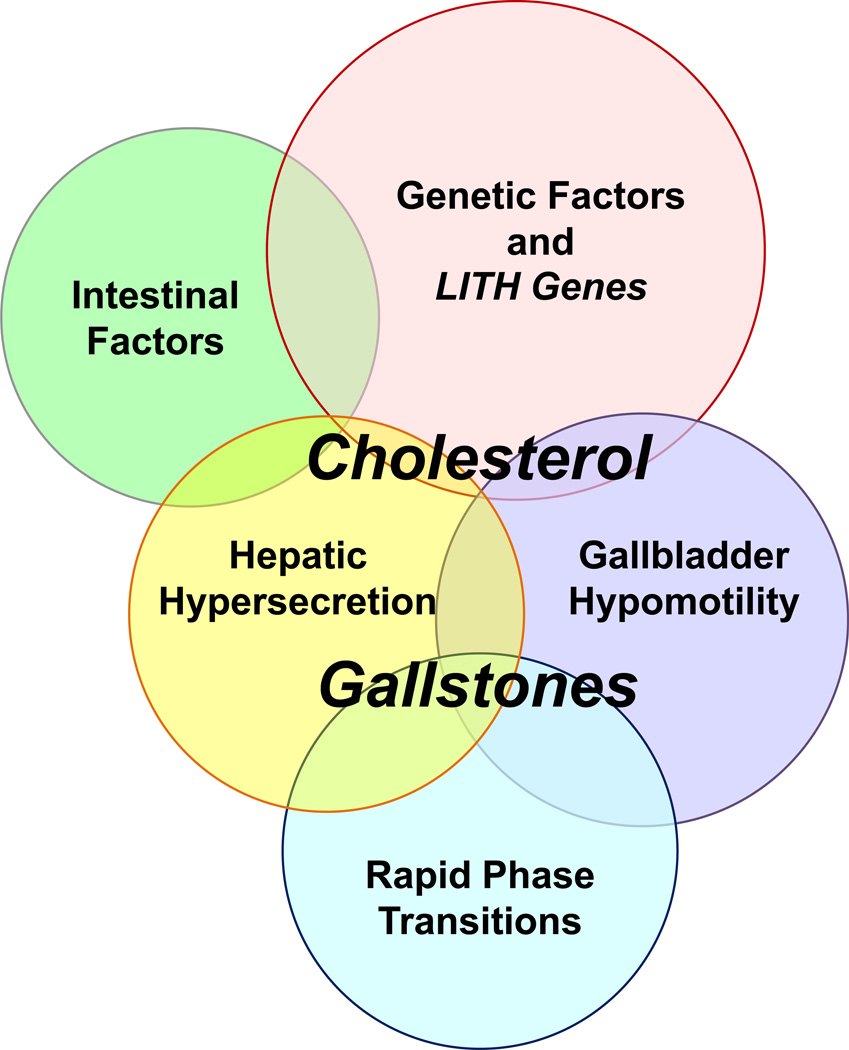
Figure 1.
Five defects play crucial roles in the pathogenesis of cholesterol gallstones: (i) genetic factors and LITH genes; (ii) hepatic hypersecretion; (iii) gallbladder hypomotility; (iv) rapid phase transitions; and (v) intestinal factors. Of note, hepatic hypersecretion of biliary cholesterol is the primary defect and is the outcome of a complex genetic predisposition. The downstream effects include gallbladder hypomotility and rapid phase transitions. Gallbladder hypomotility leads to alteration in the kinetics of the enterohepatic circulation of bile acids (intestinal factors), resulting in increased cholesterol absorption, and reduced bile acid absorption that causes abnormal enterohepatic circulation of bile acids and diminished bile acid pool size. Not only does gallbladder hypomotility facilitate cholesterol nucleation and crystallization, but it also allows the gallbladder to retain solid plate-like cholesterol monohydrate crystals. These crystals can be bound by mucin gel, inducing the formation of microlithiasis and facilitating their growth in bile. Lithogenic bile that is supersaturated with cholesterol is primarily induced by persistent hepatic hypersecretion of biliary cholesterol, which has both hepatic and small intestinal components. Therefore, investigations are needed to determine whether cholesterol gallstones can be prevented by combination therapy using 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors (statins) and a cholesterol absorption (NPC1L1) inhibitor (ezetimibe). Reproduced with modifications and with permission from Portincasa P, Wang DQ-H. Intestinal absorption, hepatic synthesis, and biliary secretion of cholesterol: where are we for cholesterol gallstone formation? Hepatology. 2012; 55: 1313–1316.
Major sources of cholesterol for biliary secretion: the intestine, liver, and circulating lipoproteins
The small intestine is a unique organ providing both dietary and reabsorbed biliary cholesterol to the body [21]. The liver is a major organ for cholesterol synthesis and catabolism, a principal site of lipoprotein synthesis and metabolism, and the only excretory route for cholesterol from the body [1]. Furthermore, the dietary cholesterol that is absorbed daily through the small intestine provides the first major source for sterol in the body pool. The de novo cholesterol biosynthesis by the liver is the second major source contributing to the cholesterol pool in the body. Obviously, in a person consuming no dietary cholesterol, biliary cholesterol would be derived mainly from de novo synthesis. However, the biliary cholesterol reabsorbed by the small intestine is still delivered to the liver via enterolymphatic circulation for re-secretion into bile. In the past, the contribution of intestinally reabsorbed cholesterol to hepatic secretion was ignored. Increased biliary cholesterol secretion could result from increases in (i) intestinal absorption, (ii) hepatic biosynthesis, and (iii) hepatic uptake of HDL and LDL from plasma, as well as decreases in (iv) the conversion of cholesterol into bile acids, and (v) the esterification of cholesterol [1]. However, the contribution of each of these sources to hepatic secretion of biliary cholesterol is not yet fully known, especially in the lithogenic state. Moreover, any alterations in these factors may influence cholesterol saturation of bile. Although HDL cholesterol is a principal source of biliary cholesterol in the basal state [22–28], chylomicron remnant cholesterol appears to be a major contributor to biliary cholesterol hypersecretion during diet-induced gallstones in animal models [16]. Furthermore, hepatic cholesteryl ester stores are less involved in biliary cholesterol secretion. Thus, hepatic cholesterol from three identified sources can be utilized for biliary secretion: plasma lipoproteins, newly synthesized cholesterol, and intestinal absorption (Figure 2).
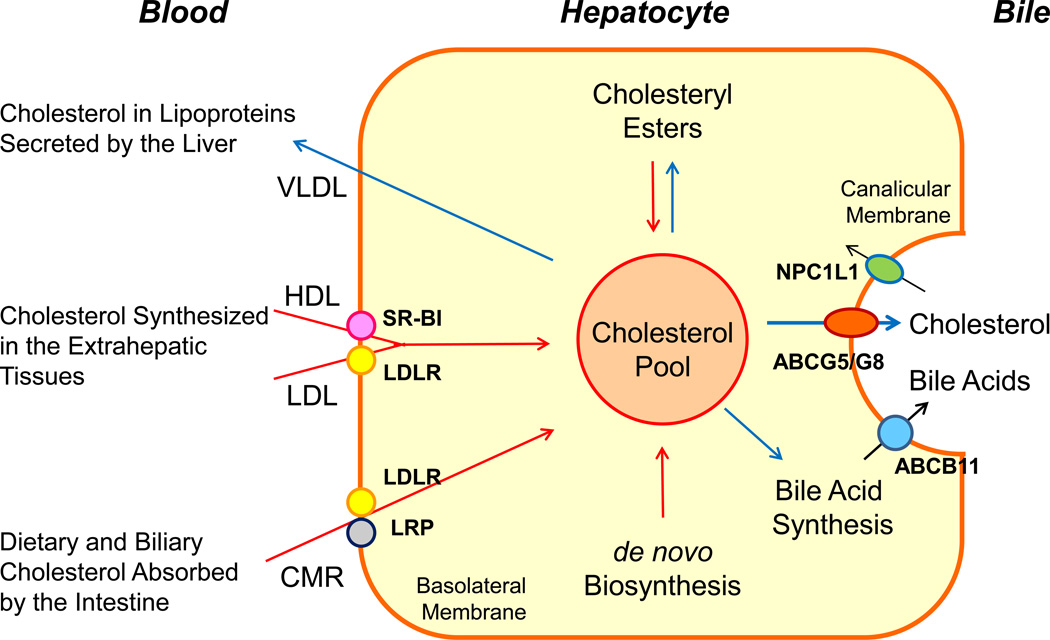
Figure 2.
This diagram shows cholesterol balance across the liver, indicating the major sources for cholesterol entering the hepatocyte (red arrows) and the main pathways for its disposition from the hepatocyte (blue arrows). Abbreviations: ABC, ATP-binding cassette (transporter); CMR, chylomicron remnants; LDLR, low-density lipoprotein receptor; LRP, low-density lipoprotein receptor-related protein; NPC1L1, Niemann-Pick C1-like 1 protein; SR-BI, scavenger receptor class B type I.
Human studies and in situ perfused rat liver experiments have found that plasma HDL rather than other lipoproteins such as VLDL and LDL preferentially provides unesterified cholesterol to the liver for secretion into bile [22–28]. HDL is crucial to ‘‘reverse cholesterol transport’’ because it promotes cholesterol transport from peripheral tissues to the liver where the unesterified cholesterol in HDL is secreted predominantly into bile [22]. The HDL receptor, scavenger receptor class B type I (SR-BI) plays a pivotal role in unloading HDL cholesterol to hepatocytes because it recognizes apolipoprotein (APO)-AI of HDL. Hepatic overexpression of SR-BI in transgenic mice lowered plasma HDL cholesterol and promoted rapid clearance of plasma HDL cholesterol with its appearance in bile [29, 30]. In contrast, clearance of HDL cholesterol and its biliary secretion were impaired in SR-BI knockout mice [31]. Yet, despite the importance of SR-BI on regulating plasma HDL and biliary cholesterol concentrations in the basal state, whether SR-BI influences gallstone formation under high dietary cholesterol loads was further investigated in SR-BI ‘‘att’’ mice having the partial (~50%) disruption of SR-BI expression. Secretion rate of biliary cholesterol, but not phospholipid and bile acids was significantly reduced by ~37% in chow-fed SR-BI att mice [32]. Consonant with this, cholesterol concentrations and cholesterol saturation index (CSI) were decreased markedly in gallbladder bile. However, feeding a lithogenic diet significantly increased biliary cholesterol secretion and induced gallstone formation, which are comparable between SR-BI att and control mice. These observations show that although HDL cholesterol is a principal source of biliary cholesterol in the basal state, hepatic uptake of cholesterol from chylomicron remnants is the major contributor to biliary cholesterol hypersecretion during diet-induced cholelithogenesis in mice. However, whether hepatic SR-BI may play a role in determining the relative risk for gallstone formation still needs to be investigated in individuals at a high risk for gallstones.
Because metabolism of chylomicrons (i.e., lipoproteins of intestinal origin) is so rapid, it is not easy to measure alterations in this pathway. However, a series of careful studies haves been performed to investigate chylomicron remnant metabolism and its role in biliary lipid secretion, underscoring the importance of chylomicron remnant cholesterol in murine cholelithogenesis [33–39]. Compared to resistant AKR mice, gallstone-susceptible C57L mice exhibited rapider removal of radiolabeled-cholesterol in chylomicron remnants from plasma, and by 12 hours, twofold higher radioactivities appeared in the bile [34]. These results are consistent with the findings that C57L mice are characterized by significantly higher biliary cholesterol secretion and gallstone prevalence compared to AKR mice, mostly attributed to the effect of Lith genes [40]. Furthermore, radiolabeled-cholesterol in chylomicrons was removed rapidly from plasma and the radioactivity appeared in bile within 15 minutes in rats [38]. Approximately 13% of the injected radioactive dose was secreted into bile in the first 3 hours after injection and 25% of the radioactivity was present as unesterified cholesterol. These studies indicate that the liver is very efficient at secreting lipoprotein cholesterol of intestinal origin into bile. Therefore, high dietary cholesterol distributed through the chylomicron pathway could provide an important source of excess cholesterol for inducing supersaturation of bile with cholesterol.
In the basal steady state, the contribution of de novo synthesis to biliary cholesterol secretion is approximately 15% in experimental animals and in humans [41–46]. However, in the lithogenic state, this pathway could provide the liver with more cholesterol for biliary secretion. The most consistent evidence is that bile is desaturated with cholesterol after long-term administration of statins [47–53].
The cholesterol synthesis rates in humans and animal models have been investigated by two methods [54–58]: (i) sterol balance analysis and (ii) measurement of the incorporation of [3H]water into sterols. By measuring sterol balance across the body, the rate of whole body cholesterol synthesis was found to be approximately 8–10 mg/day/kg body weight in humans [59]. Although the synthesis of cholesterol occurs in virtually all cells, the capacity is predominant in the liver, intestine, adrenal cortex, and reproductive tissues. Hepatic cholesterol synthesis contributes less than one-third of the cholesterol synthesized in the body each day; approximately 30% occurs in hamsters, and 20% in rabbits and guinea pigs [59]. In humans, the liver contributes approximately 10–15% of the newly synthesized cholesterol to the body [59]. Of note, when the amount of cholesterol in the diet is increased, these values are proportionately reduced because cholesterol synthesis is suppressed by the negative feedback regulatory mechanism in the liver. Furthermore, the liver responds to an increased amount of dietary cholesterol by (i) enhancing secretion of cholesterol into bile; (ii) converting cholesterol into bile acids, followed by their secretion into bile; (iii) down-regulating cholesterol biosynthesis; (iv) increasing cholesterol esterification and storage; and (v) enhancing lipoprotein secretion into the circulation [1]. Lammert and colleagues have found that on a lithogenic diet, C57L mice still display higher HMG-CoA reductase activity together with lower activity of both bile acid synthetic enzymes cholesterol 7α-hydroxylase and sterol 27-hydroxylase compared to AKR mice [60]. Moreover, higher HMG-CoA reductase activity has been found in gallstone patients compared with control subjects [61–66]. These studies highlight the importance of de novo synthesis to biliary cholesterol secretion, and its role in the formation of lithogenic bile in most cholelithogenic patients.
The importance of cholesterol of intestinal origin on the formation of supersaturated bile
Epidemiological studies have found that cholesterol gallstones are prevalent in cultures consuming a Western diet and the incidence of cholesterol gallstones in North and South American as well as European countries is higher than that in the developing nations [67–69]. Before the 1960s, cholesterol gallstones were rare in China, but over the past 50 years with a “westernization” of the traditional Chinese diet, the incidence has increased markedly [70–73]. Also, in Japan, several studies have found an association between the increasing incidence of cholesterol gallstones and the adoption of Western eating habits that include excessive consumption of high cholesterol food [74–76].
Biliary cholesterol secretion and saturation could be reduced by inhibiting intestinal cholesterol absorption, hepatic uptake of chylomicron remnants, or both. Direct evidence for the role of intestinal factors in murine cholelithogenesis came from an important study by Buhman and colleagues [33]. They found that the deficiency of cholesteryl ester synthesis in the intestine of ACAT2 knockout mice led to a marked reduction in intestinal cholesterol absorption and complete resistance to diet-induced gallstones. Furthermore, the absence of expression of intestinal APO-B48, but not hepatic APO-B100, significantly reduces biliary cholesterol secretion and cholelithogenesis by decreasing intestinal absorption and hepatic bioavailability [77]. These results imply that these mice fail to deliver cholesterol of intestinal origin to the liver for secretion into bile. Moreover, reduced biliary cholesterol secretion and gallstone prevalence in lithogenic diet-fed APO-E knockout mice may be explained by decreased availability of chylomicron-derived cholesterol to the liver for biliary cholesterol secretion [39]. Therefore, cholesterol derived from the intestine via the chylomicron pathway influences biliary cholesterol secretion, and high dietary cholesterol enhances cholelithogenesis through this pathway.
However, studies on the effect of dietary cholesterol on biliary cholesterol metabolism in healthy humans yielded conflicting results, showing that high dietary cholesterol either increases or does not affect cholesterol saturation of bile. DenBesten and coworkers found that incrementing dietary cholesterol intake from 0, 100, 750, 1,000, to 2,000 mg per day markedly augmented the cholesterol content of bile [78]. Furthermore, they fed 10 healthy, normolipidemic men a eucaloric, cholesterol-free, liquid formula for 3 weeks. Cholesterol (750 mg daily) in the form of egg yolk then was consumed for another 3 weeks. Consequently, four subjects developed lithogenic bile and three formed cholesterol monohydrate crystals. Dam and colleagues investigated 9 healthy women before and 3 to 6 weeks after addition of egg yolk (1,000 or 2,000 mg cholesterol daily) to solid diets and found no increase in biliary cholesterol saturation [79]. Andersen and Hellstrom also found that there were no changes in biliary cholesterol saturation in 6 normolipidemic women and 6 hyperlipidemic patients without gallstones when dietary cholesterol was increased from 300 mg to 1,500 mg daily [80]. However, Lee et al. performed a careful investigation on the effect of high dietary cholesterol on biliary cholesterol saturation in 12 patients with asymptomatic gallstones (six men and six women) compared with 7 healthy women assigned diets containing 500, 750, and 1,000 mg of cholesterol daily for 3-week periods in random sequence [81]. They found an increase in biliary cholesterol saturation with modest increments in dietary cholesterol, regardless of whether or not these subjects had gallstones. Furthermore, women with gallstones had higher biliary cholesterol saturation than normal women at corresponding levels of cholesterol consumption, and six of the seven normal women formed lithogenic bile when ingesting a diet containing 1,000 mg of cholesterol. These discrepant results may be explained partly by differences in the populations, the specific diets used in different studies, and variations in absorption efficiency of intestinal cholesterol. Nevertheless, most clinical studies reveal that high dietary cholesterol is responsible for inducing cholesterol-supersaturated bile, which is consistent with epidemiological investigations showing that high dietary cholesterol is an important risk factor for gallstone formation.
Despite many putative sterol transporters influencing cholesterol absorption and physical-chemical factors affecting dietary cholesterol absorption have been extensively investigated, the importance of biliary cholesterol on the regulation of intestinal cholesterol absorption was recognized only recently [82]. Dietary and biliary cholesterol are the two major sources for intestinal absorption, which account for approximately 1/3 and 2/3, respectively. Physical-chemical studies of biliary lipids have found that in bile, cholesterol is solubilized as a mixture of vesicles, mixed micelles, simple micelles, and monomers, all in a dynamic equilibrium [83]. Because the physical forms of biliary cholesterol are different from those of dietary cholesterol, there could be two distinct fates of intestinal absorption of cholesterol from dietary and biliary sources [82]. In the lithogenic state, phase transitions take place from the liquid state to solid crystal formation and cholesterol monohydrate crystals often precipitate from bile [83]. Clinical investigations have found that solid cholesterol crystals are frequently detected in duodenal bile of healthy subjects [84–86]. Moreover, when obese subjects lose weight, cholesterol crystals are often discharged into the duodenum [87, 88]. Patients with cholesterol gallstones (15–20% of the general population) also frequently present with crystals in duodenal bile [89]. It is well known that micellization is one of the most important factors in enhancing the efficiency of intestinal cholesterol absorption [21]. However, these solid cholesterol crystals are less efficiently solubilized in the mixed micelles within the intestinal lumen. The unabsorbed cholesterol excreted in the feces is dramatically higher in mice infused with crystallized bile compared with supersaturated bile, indicating that solid crystalline cholesterol in bile is not absorbable [82].
In unsaturated bile, almost all cholesterol in bile is carried in the micellar phase. In supersaturated bile, excess cholesterol not dissolved in the micellar phase can be largely carried by vesicles and liquid crystals. This liquid crystalline phase provides an accessible source of cholesterol for continuous formation of mixed micelles in the presence of bile acids. These micelles function as a concentrated reservoir and transport vehicle for cholesterol across the unstirred water layer toward the brush border membrane of the enterocyte to facilitate uptake of monomeric cholesterol and subsequently its absorption by the enterocyte [21]. Therefore, the reabsorbed biliary cholesterol could greatly contribute hepatic bioavailability and enhance biliary cholesterol secretion. However, its importance on cholelithogenesis needs to be further investigated.
The efficiency of intestinal cholesterol absorption correlates positively and significantly with the prevalence of cholesterol gallstones in inbred strains of mice, and C57L mice display significantly higher cholesterol absorption efficiency than AKR mice [34]. However, it appears that the extent of intestinal cholesterol absorption is lower in gallstone patients compared with control subjects [90]. One possible explanation is that gallstone patients often secrete cholesterol-supersaturated bile that contains abundant solid cholesterol crystals. However, as discussed above, solid crystalline cholesterol cannot be absorbed by enterocytes and is excreted in feces, which leads to a significant reduction in the intestinal absorption of biliary cholesterol, as well as a much lower absorption efficiency of intestinal cholesterol in gallstone patients compared with control subjects. Nevertheless, animal studies have provided clear evidence that high dietary cholesterol and high efficiency of intestinal cholesterol absorption are two independent risk factors for gallstone formation [34].
Prevention and dissolution of cholesterol gallstones by ezetimibe
Cholesterol absorption is a multistep process that is regulated by multiple genes at the enterocyte level, and the efficiency of cholesterol absorption is determined by the net effect between influx and efflux of intraluminal cholesterol molecules crossing the brush border membrane of the enterocyte [21]. Therefore, the factors regulating intestinal membrane lipid transporters, lipid regulatory enzymes, intracellular lipid transporters, and lipid regulatory transcription factors could influence the amount of cholesterol of intestinal origin contributing to the liver for biliary secretion. Despite significant restrictions in dietary intake, a reduction of dietary cholesterol frequently does not decrease bile cholesterol levels appreciably. This is due in part to the continued presence of large amounts of biliary cholesterol in the intestine for its re-absorption, which constitutes two thirds of the total daily amount of cholesterol that is available for intestinal absorption. Therefore, pharmacological inhibition of cholesterol absorption is potentially an effective way of lowering bile cholesterol levels (Figure 3).
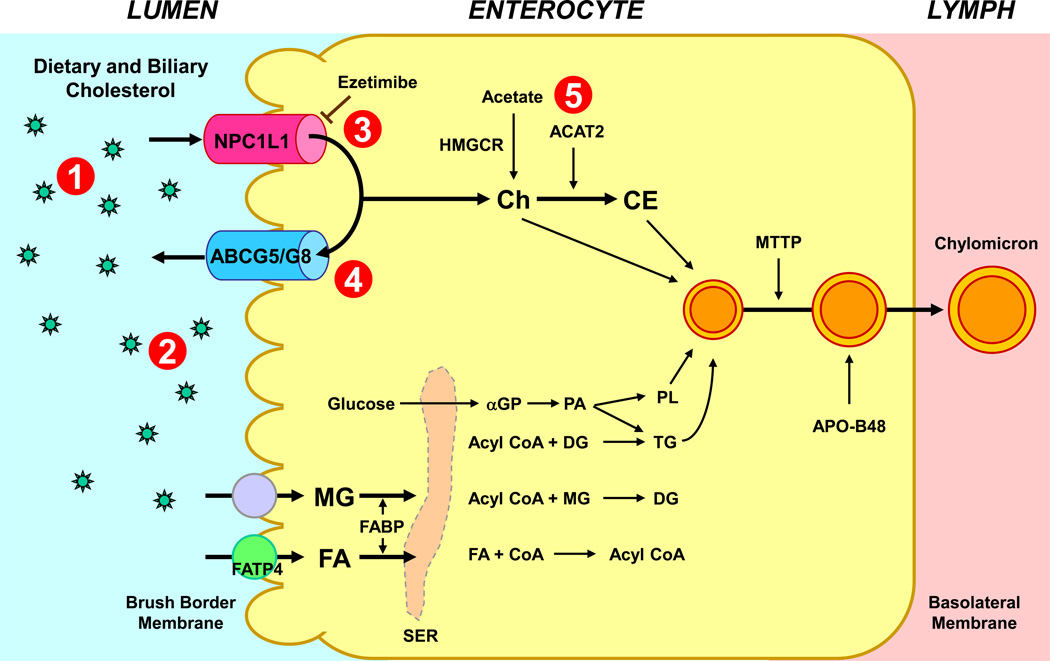
Figure 3.
Intestinal cholesterol absorption is a multistep process regulated by multiple genes. These intracellular events could also exert major influences on the regulation of cholesterol absorption. This provides multiple therapeutic targets for inhibiting cholesterol absorption by drugs: (1) the bile acid sequestrants; (2) specific lipase inhibitors; (3) intestinal NPC1L1 inhibitors; (4) intestinal ABCG5/G8 inhibitors; and (5) intestinal ACAT inhibitors. Ezetimibe is a highly selective intestinal cholesterol absorption inhibitor. It can effectively and potently prevent the absorption of cholesterol by inhibiting the uptake of dietary and biliary cholesterol across the brush border membrane of the enterocyte through the NPC1L1 pathway. Ezetimibe provides a novel strategy for the prevention of not only hypercholesterolemia, but also cholesterol gallstones. Abbreviations: ABC, ATP-binding cassette (transporter); ACAT2, acyl-CoA:cholesterol acyltransferase isoform 2; APO, apolipoprotein; DG, diacylglycerol; FA, fatty acids; FABP, fatty acid binding protein; FATP4, fatty acid transport protein 4; α-GP, α-glycerophosphate; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; MG, monoacylglycerol; MTTP, microsomal triglyceride transfer protein; NPC1L1, Niemann47 Pick C1-like 1 protein; PA, phosphatidic acid; PL, phospholipids; SER, smooth endoplasmic reticulum; TG, triacylglycerol. Reproduced with modifications and with permission from De Bari O, Neuschwander-Tetri BA, Liu M, Portincasa P, Wang DQ-H. Ezetimibe: its novel effects on the prevention and the treatment of cholesterol gallstones and nonalcoholic fatty liver disease. Journal of Lipids. 2012; 2012: 302847-63. See text for details.
Because the detergent nature of bile acids is obligatory for intestinal cholesterol absorption through micellar solubilization of intraluminal sterols [91], the bile acid sequestrants suppress cholesterol absorption, possibly via interruption of the enterohepatic circulation of bile acids [92–94]. Specific lipase inhibitors such as orlistat may also reduce cholesterol absorption by blocking the degradative process [95], which results in a decreased solubility of cholesterol during the critical stage of intestinal diffusion. Intestinal ACAT inhibitors [96] and cholesterol ester transfer protein inhibitors [97] are currently being evaluated in clinical trials, and the potential to alter ATP-binding cassette (ABC) transporter activity in the intestine is also being investigated. Because ezetimibe has been shown to markedly reduce bile cholesterol levels in gallstone patients [98], it will be the focus of the following sections.
Ezetimibe is a highly selective intestinal cholesterol absorption inhibitor by suppressing the uptake of dietary and biliary cholesterol across the brush border membrane of the enterocyte through the NPC1L1 pathway, possibly a transporter-facilitated mechanism (Figure 4) [4]. The high potency of this compound is evidenced by a 50% inhibition at dose of ranging from 0.0005 to 0.05 mg/kg in a series of different animal models [99–103]. Ezetimibe, administered either as monotherapy or in combination with statins, has been shown to be a safe and efficacious treatment for hypercholesterolemia, potentially enabling more patients to reach recommended LDL cholesterol goals. Therefore, the discovery and development of ezetimibe opens a new door to the treatment of not only hypercholesterolemia, but also cholesterol gallstones.
Figure 4.
Molecular structure of ezetimibe. The standard chemical formula (left panel), the perspective formula (middle panel), and the space-filling model (right panel) of ezetimibe are shown. Ezetimibe is a highly selective intestinal cholesterol absorption inhibitor through the Niemann-Pick C1-like 1 (NPC1L1) pathway. See text for details.
Because ezetimibe induces a significant dose-dependent reduction in intestinal cholesterol absorption efficiency [98, 103–105], this should diminish the cholesterol content of liver and the bioavailability of cholesterol for biliary secretion. Indeed, the inhibitory effect of ezetimibe was coupled with a significant dose-dependent decrease in biliary cholesterol output [98]. In addition, cholesterol-supersaturated bile facilitated gallbladder absorption of cholesterol and promoted the accumulation of excess cholesterol in the gallbladder wall. Because gallbladder absorptive cells apparently cannot assemble lipoproteins for lipid transport into plasma, a large amount of the absorbed cholesterol is converted to cholesteryl esters and stored in the mucosa and lamina propria. These changes diminish gallbladder contractility and impair gallbladder emptying because excess cholesterol in smooth muscle cells could stiffen sarcolemmal membranes and decouple the G-protein-mediated signal transduction that usually ensues when CCK binds to its receptor. Moreover, gallbladder stasis provides time for nucleation of cholesterol crystals and their aggregation into macroscopic stones, which is a frequent and distinctive feature in gallstone patients [106]. Because ezetimibe reduces biliary cholesterol content in bile, the lithogenic effects of cholesterol-supersaturated bile on gallbladder motility function can be deterred [98, 107]. As a result, cholesterol gallstones were prevented by ezetimibe in gallstone-susceptible C57L mice fed a lithogenic diet for 8 weeks [98]. Furthermore, after 30 days of treatment, ezetimibe at 20 mg/day significantly reduced cholesterol concentrations and CSI values of gallbladder bile in patients with gallstones [98]. Because cholesterol crystallization was retarded by ezetimibe, the detection time of cholesterol monohydrate crystals was significantly delayed [98].
Of note, the NPC1L1 gene is expressed in the liver of humans, but not in the liver of mice. Temel et al. have found that biliary cholesterol concentrations are increased markedly in mice transgenic for a human NPC1L1 gene [108]. These studies suggest that ezetimibe may rescue biliary cholesterol secretion and increase CSI values of bile by inhibiting the expression of hepatic NPC1L1. How could this explain the results of the above-mentioned human studies? Based on the molecular mechanism on the regulation of hepatic lipid secretion, the secretion efficiency of biliary cholesterol is most likely determined by the net effect between efflux and influx of cholesterol molecules across the canalicular membrane of the hepatocyte, which could be regulated by the ABCG5/G8-dependent and -independent pathways, as well as the NPC1L1 pathway. One possible explanation is that because biliary cholesterol secretion is a unique path for excretion of cholesterol from the body [1], hepatic ABCG5/G8 has a stronger effect on promoting biliary cholesterol secretion compared with hepatic NPC1L1 that absorbs bile cholesterol back into hepatocytes. Moreover, in the gut-liver axis, intestinal NPC1L1 plays a crucial role in providing dietary and reabsorbed biliary cholesterol to the body and inhibiting its function by ezetimibe can significantly reduce cholesterol absorption. Thus, the bioavailability of cholesterol from intestinal sources for biliary secretion is reduced dramatically. In contrast, the inhibition of the hepatic NPC1L1 by ezetimibe might produce a relatively weak effect on the regulation of biliary cholesterol secretion. Interestingly, like humans, hamsters also express NPC1L1 in the liver, and the profile of NPC1L1 expression in the liver and small intestine is similar between hamsters and humans, with expression levels of NPC1L1 being significantly higher in the small intestine than in the liver. Under ezetimibe treatment, hepatic secretion of biliary cholesterol is significantly reduced in hamsters fed a high cholesterol diet [104]. These findings confirm the inhibitory effect of ezetimibe on biliary cholesterol secretion in gallstone patients.
After binding to cholesterol, Niemann-Pick C2 protein (NPC2) is involved in intracellular cholesterol trafficking, allowing the exit of lysosomal cholesterol obtained via the lipoprotein endocytic pathway. Thus, this protein may play a crucial role in regulating hepatic cholesterol transport and secretion. Under conditions of feeding the lithogenic diet, biliary cholesterol secretion, gallbladder bile cholesterol saturation, and cholesterol crystal and gallstone formation were reduced in NPC2 hypomorph mice compared with wild-type mice [109].
The natural trihydroxy hydrophilic bile acid of rodents, β-muricholic acid can prevent diet-induced cholesterol gallstones and promote the dissolution of cholesterol gallstones in mice [110]. Moreover, β-muricholic acid and UDCA favor the formation of vesicles in bile so that the growth of liquid crystals on the cholesterol monohydrate surface and their subsequent dispersion might occur during gallstone dissolution. Liquid crystalline dissolution allows the transport of a great amount of cholesterol from stones. In contrast, the cholelitholytic mechanism of ezetimibe is different from that of hydrophilic bile acids. During ezetimibe treatment, the relative lipid composition of pooled gallbladder bile is progressively shifted down and to the left of the phase diagram and finally enters the favorable one-phase micellar zone [98]. As found by physical-chemical analysis of bile, the micellar zone contains abundant unsaturated micelles, but never solid cholesterol crystals or liquid crystals [16]. As a result, the micellar cholesterol solubility is dramatically increased in gallbladder bile, and the cholesterol molecules could be transferred from the cholesterol monohydrate surface into unsaturated micelles so that gallstones become smaller and are eventually dissolved. Thus, ezetimibe and hydrophilic bile acids promote gallstone dissolution by two distinct mechanisms: the formation of an unsaturated micelle and a liquid crystalline mesophase [98, 110].
The inhibitory effect of statins on hepatic de novo synthesis and the formation of lithogenic bile in animal models
Statins are effective in the treatment of hypercholesterolemia by competitively inhibiting HMG-CoA reductase, the rate-limiting enzyme for cholesterol synthesis (Figure 5). These drugs are orally absorbed, extracted on first pass through the liver, where they exert their primary effects, and are eliminated almost exclusively via biliary excretion. In clinical trials using both healthy volunteers and patients with hypercholesterolemia, long-term administration of statins leads to a significant reduction in serum total and LDL cholesterol and triglyceride concentrations. In addition, there is emerging evidence that statins can reduce biliary cholesterol concentrations and CSI values not only in animals, but also in healthy volunteers [111] and hypercholesterolemic patients [112]. Because statins produce conflicting results by increasing HMG-CoA reductase activity, as well as plasma and bile cholesterol levels in rodents such as rats and mice in comparison with humans [113], the prairie dog has been selected as a model for studying the inhibitory effect of statins on biliary cholesterol secretion and gallstone formation. Prairie dogs display similar biliary lipid composition and bile acid profiles compared to humans. They develop cholesterol gallstones after 3–4 weeks on a high (1.2%) cholesterol diet [114–118]. However, each statin displays different inhibitory effects on the formation of cholesterol gallstones in prairie dogs.
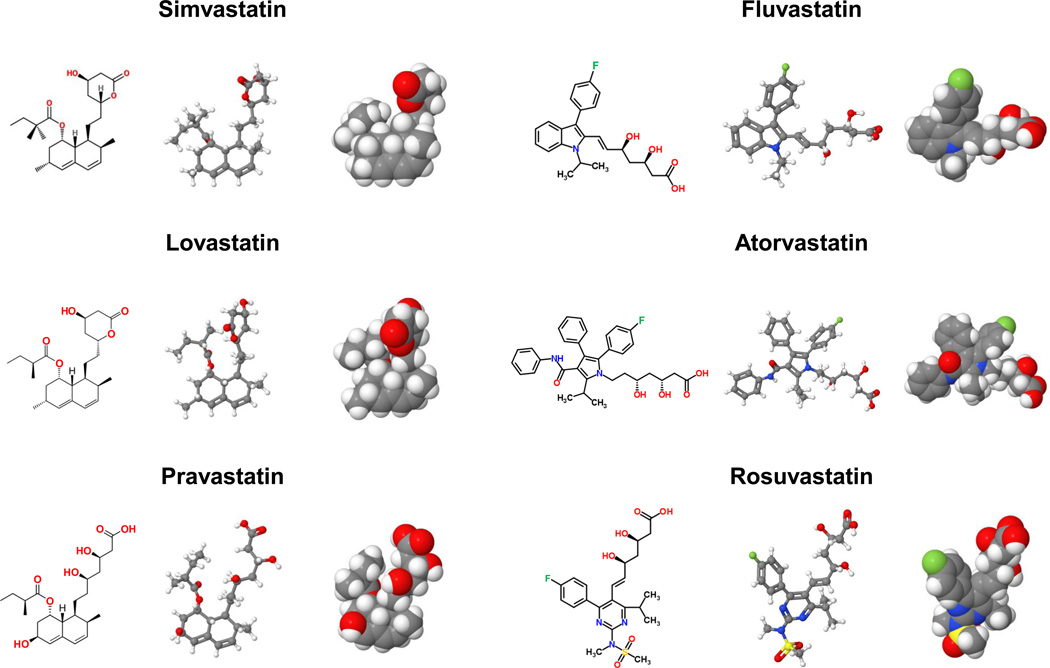
Figure 5.
Molecular structures of statins. The standard chemical formulae (left panel), the perspective formulae (middle panel), and the space-filling models (right panel) of statins are shown. Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, the rate-limiting enzyme for cholesterol biosynthesis. Each statin displays different inhibitory effects on the formation of cholesterol gallstones in prairie dogs. See text for details.
After 3 weeks on a high cholesterol diet, all (100%) prairie dogs formed solid cholesterol crystals and 83% animals formed gallstones [119]. In contrast, no gallstones were detected in prairie dogs treated with lovastatin (8 mg/kg, twice per day). Also, lovastatin markedly reduced cholesterol, but not phospholipid and bile acid concentrations in hepatic bile. Moreover, lovastatin induced a reduction of 20% to 30% in plasma total and LDL cholesterol and triglyceride concentrations in cholesterol-fed prairie dogs compared with control animals receiving no drug. These results indicate that lovastatin can prevent diet-induced cholesterol gallstones in prairie dogs.
To investigate whether lovastatin alone or combination therapy of lovastatin and UDCA could promote the dissolution of cholesterol gallstones, prairie dogs were first induced gallstone formation by feeding a high cholesterol diet for 5 weeks [120]. Subsequently, these animals were fed a chow diet supplemented with lovastatin (3.3 mg/g diet), UDCA (10 mg/g diet), or both for 10 weeks. Lovastatin treatment led to complete dissolution of gallstones in 68% animals. In contrast, combination therapy of lovastatin and UDCA failed to improve the dissolution of gallstones, with a 12.5% success rate, although UDCA alone induced complete dissolution of gallstones in 33% animals. To further study the effect of statins on gallstone dissolution in prairie dogs that have formed gallstones, these animals were fed lovastatin at 8 mg, UDCA at 50 mg, or both drugs twice daily by way of orogastric tube for 4 weeks [121]. Combination therapy of lovastatin and UDCA induced an augmented response in completely dissolving gallstones (56%), while monotherapy of lovastatin or UDCA induced complete dissolution of gallstones in 28% animals. These discrepancies in dissolution rates of gallstones could be explained by differences in drug doses, feeding methods, duration of drug administration, and daily dietary cholesterol intake.
To examine the effects of pravastatin on gallstone formation, prairie dogs were fed 1% cholesterol with or without 0.05% (w/w) pravastatin for 4 weeks [122]. Pravastatin produced a preventive effect on diet-induced gallstone formation. However, other studies observed that gallstones were formed in 50% prairie dogs treated with simvastatin (2.5 mg, twice per day) and on a 1.2% cholesterol diet for 3 weeks compared with 60% in control animals receiving no drugs [123]. Moreover, serum cholesterol concentrations were reduced by 37% in simvastatin-treated animals compared with controls. By contrast, simvastatin induced a 42% elevation in serum triglycerides. A positive association between high serum triglyceride concentrations and gallstone formation has been suggested [124]. This may explain in part why simvastatin produces a relatively weak effect on the prevention of gallstones although it reduces bile cholesterol concentrations.
Potential therapeutic effect of statins on cholesterol gallstones in humans
In humans, most research groups have reported that statins reduce the cholesterol content in bile, prolong the detection time of cholesterol crystals, and promote gallstone dissolution [50, 53, 112, 125, 126], whereas a few groups did not find evidence for such an effect [127–129]. Despite these conflicting results, statins indeed can reduce biliary cholesterol output by inhibiting hepatic cholesterol biosynthesis, thus leading to diminished biliary cholesterol concentrations and cholesterol saturation of bile.
Simvastatin (20 or 40 mg/day) was reported to reduce CSI values of gallbladder bile in 10 patients with hypercholesterolemia after 7 to 13 weeks of treatment [112]. Also, simvastatin (20 mg/day) was observed to decrease plasma and biliary cholesterol levels primarily by curbing cholesterol synthesis in 31 gallstone patients after 3 weeks of medication [49]. As a result, CSI values of gallbladder and hepatic bile were noticeably lower in simvastatin-treated patients compared with control subjects. Also, CSI values of gallbladder bile were reduced markedly by lovastatin (40 mg, twice per day) and pravastatin (40 mg/day) [51, 53, 130, 131], and their therapeutic effects on CSI values of bile were dose-dependent [53, 132]. After 3 weeks of pravastatin (40 mg/day) treatment, biliary cholesterol and phospholipid, but not bile acid concentrations were significantly reduced in 33 patients having radiolucent gallstones compared with control group [133]. However, CSI values and the detection time of cholesterol crystals in gallbladder bile were comparable between both groups. In addition, many studies have found that lovastatin and pravastatin do not alter fractional turnover, synthesis, absorption, enterohepatic cycling, or pool sizes of bile acids [51, 128, 131, 133].
To investigate whether long-term administration of statins could reduce the risk of gallstone disease followed by cholecystectomy, Bodmer and co-workers performed a large case-control study using the UK-based General Practice Research Database in a total of 27,035 patients with cholecystectomy and 106,531 matched controls, including 2,396 patients and 8,868 controls who had statin use between 1994 and 2008 [134]. Long-term use of statins was associated with decreased risk of a subsequent diagnosis of gallstone disease requiring cholecystectomy. These findings are consistent with results from Erichsen and colleagues who performed a population-based case-control study in 32,494 patients with gallstones occurring from 1996 to 2008 [48]. Also, long-term (1–2 years) statin administration reduced the risk of gallstone disease in both men and women compared with nonusers. Tsai et al. performed a large cohort retrospective analysis from the Nurses’ Health Study with a history of gallstones in 2,479 American women who had undergone cholecystectomy between 1994 and 2004 [47]. They concluded that long-term statin use is associated with reduced risk of cholecystectomy in women. However, in a French cross-sectional study of 830 patients, Caroli-Bosc et al. did not find evidence for such an effect [135].
Smit et al. observed successful gallstone dissolution after pravastatin treatment (40 mg/day) for 3 months in a hypercholesterolemic male patient who had a large (12 mm in diameter) solitary cholesterol gallstone [126]. However, most studies did not find that statin monotherapy led to complete dissolution of gallstones [50]. Because UDCA has been shown to promote gallstone dissolution by reducing intestinal absorption and biliary secretion of cholesterol, as well as the hydrophobicity index of the bile acid pool, it was used with statins to dissolve cholesterol gallstones. Long-term (12 months) co-administration of simvastatin (10 mg/day) and UDCA (600 mg/day) dissolved gallstones more effectively than UDCA monotherapy in patients with multiple gallstones [136]. However, no significant difference in dissolution rates was observed between two therapeutic approaches in patients with solitary gallstone. Several studies found that combination therapy of pravastatin (20 mg, twice per day) and UDCA (500 mg, twice per day) did not produce a stronger effect on reducing biliary cholesterol concentrations and CSI values than UDCA monotherapy in patients with gallstones [127]. Taken together, more detailed studies on long-term administration of statins with or without UDCA are needed to carefully determine their therapeutic effects on gallstone dissolution, including stone size, number and composition in both genders of patients.
New perspectives on the prevention and the treatment of cholesterol gallstones
Because the prevalence of gallstones is rising due to the worldwide epidemic of obesity and insulin resistance, both key features of the metabolic syndrome, it is imperative to find potential ways to prevent this common liver disease. Growing evidence from pathophysiological, physical-chemical, and genetic studies has clearly shown that unphysiological supersaturation, predominantly from sustained hepatic hypersecretion of biliary cholesterol, is crucial for the formation of cholesterol gallstones. Similar to atherosclerosis, the risk for cholesterol cholelithiasis increases with aging, dyslipidemia, hyperinsulinemia, obesity, diabetes, and sedentary lifestyle. As statins and ezetimibe are widely used in primary and secondary prevention of cardiovascular diseases, they would have a potential benefit in preventing or treating cholesterol cholelithiasis as well. Furthermore, preventive therapy of cholesterol gallstones can be considered as primary or secondary. The goal of primary prevention is to prevent the formation of cholesterol gallstones both in the general population at high risk for gallstones and in some epidemiologically identified high-risk ethnic groups. The goal of secondary prevention is to prevent the recurrence of gallstones after dissolution therapy. Of note, many gallstones are silent although one third eventually causes symptoms and complications. Thus, secondary prevention can also be used to prevent the development of symptoms and complications in such a subgroup of patients.
Indeed, statins and ezetimibe can prevent diet-induced gallstones and promote gallstone dissolution in mice and prairie dogs. Also, their therapeutic effects have been proved in part in patients with cholesterol gallstones. To evaluate treatment time, response rate and overall cost-benefit analysis, a more detailed, long-term human study is needed. If effective primary and secondary prevention of cholesterol gallstones becomes a reality, then the potential could exist for the prevalence of gallstones and the frequency of gallbladder surgery such as open and laparoscopic cholecystectomies to substantially decline in the next few decades.
Acknowledgments
We are greatly indebted to Tony Y. Wang for preparing figures. This work was supported in part by a research grant DK73917 (D.Q.-H.W.) from the National Institutes of Health (US Public Health Service), the Saint Louis University Liver Center Seed Grant Award (D.Q.-H.W.), the Saint Louis University President’s Research Fund (D.Q.-H.W.), and Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) FIRB2003 and RBAU01RANB002, Fondazione Cassa di Risparmio di Puglia (Ricerca Scientifica e Tecnologica) (P.P.).
Abbreviations
- ABC
ATP-binding cassette transporter
- ACAT
acyl-CoA:cholesterol acyltransferase
- APO
apolipoprotein
- CSI
cholesterol saturation index
- HMG-CoA
3-hydroxy-3-methylglutaryl-CoA
- NPC1L1
Niemann-Pick C1-like 1
- SR-BI
scavenger receptor class B type I
- UDCA
ursodeoxycholic acid
Footnotes
There is no conflict of interest to disclose for all authors
References
- 1.Wang DQ, Neuschwander-Tetri BA, Portincasa P. The Biliary System. 1st ed. Princeton, New Jersey: Morgan & Claypool Life Sciences; 2012. pp. 1–146. [Google Scholar]
- 2.Einarsson K, Hellstrom K, Kallner M. Gallbladder disease in hyperlipoproteinaemia. Lancet. 1975;1:484–487. doi: 10.1016/s0140-6736(75)92831-7. [DOI] [PubMed] [Google Scholar]
- 3.Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- 4.Rosenblum SB, Huynh T, Afonso A, Davis HR, Jr, Yumibe N, Clader JW, Burnett DA. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- 5.Clader JW, Burnett DA, Caplen MA, Domalski MS, Dugar S, Vaccaro W, Sher R, et al. 2-Azetidinone cholesterol absorption inhibitors: structure-activity relationships on the heterocyclic nucleus. J Med Chem. 1996;39:3684–3693. doi: 10.1021/jm960405n. [DOI] [PubMed] [Google Scholar]
- 6.van Heek M, Farley C, Compton DS, Hoos L, Alton KB, Sybertz EJ, Davis HR., Jr Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129:1748–1754. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24:e149–e161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 8.Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, Knopp RH, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–1230. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 9.Knopp RH, Dujovne CA, Le Beaut A, Lipka LJ, Suresh R, Veltri EP. Evaluation of the efficacy, safety, and tolerability of ezetimibe in primary hypercholesterolaemia: a pooled analysis from two controlled phase III clinical studies. Int J Clin Pract. 2003;57:363–368. [PubMed] [Google Scholar]
- 10.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 11.Feldman T, Koren M, Insull W, Jr, McKenney J, Schrott H, Lewin A, Shah S, et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol. 2004;93:1481–1486. doi: 10.1016/j.amjcard.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krawczyk M, Wang DQ, Portincasa P, Lammert F. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin Liver Dis. 2011;31:157–172. doi: 10.1055/s-0031-1276645. [DOI] [PubMed] [Google Scholar]
- 14.Mendez-Sanchez N, Chavez-Tapia NC, Motola-Kuba D, Sanchez-Lara K, Ponciano-Rodriguez G, Baptista H, Ramos MH, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653–1657. doi: 10.3748/wjg.v11.i11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nervi F, Miquel JF, Alvarez M, Ferreccio C, Garcia-Zattera MJ, Gonzalez R, Perez-Ayuso RM, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol. 2006;45:299–305. doi: 10.1016/j.jhep.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50(Suppl):S406–S411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HH, Portincasa P, Afdhal NH, Wang DQ. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol Clin North Am. 2010;39:185–207. vii–viii. doi: 10.1016/j.gtc.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Wang DQ, Afdhal NH. Gallstone Disease. In: Feldman M, Friedman LS, Brandt L, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9 ed. Philadelphia: Elsevier Saunders; 2010. pp. 1089–1120. [Google Scholar]
- 19.Krawczyk M, Lutjohann D, Schirin-Sokhan R, Villarroel L, Nervi F, Pimentel F, Lammert F, et al. Phytosterol and cholesterol precursor levels indicate increased cholesterol excretion and biosynthesis in gallstone disease. Hepatology. 2012;55:1507–1517. doi: 10.1002/hep.25563. [DOI] [PubMed] [Google Scholar]
- 20.Portincasa P, Wang DQ. Intestinal absorption, hepatic synthesis, and biliary secretion of cholesterol: where are we for cholesterol gallstone formation? Hepatology. 2012;55:1313–1316. doi: 10.1002/hep.25604. [DOI] [PubMed] [Google Scholar]
- 21.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz CC, Halloran LG, Vlahcevic ZR, Gregory DH, Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978;200:62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- 23.Robins SJ, Fasulo JM. High density lipoproteins, but not other lipoproteins, provide a vehicle for sterol transport to bile. J Clin Invest. 1997;99:380–384. doi: 10.1172/JCI119170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robins SJ, Fasulo JM. Delineation of a novel hepatic route for the selective transfer of unesterified sterols from high-density lipoproteins to bile: studies using the perfused rat liver. Hepatology. 1999;29:1541–1548. doi: 10.1002/hep.510290518. [DOI] [PubMed] [Google Scholar]
- 25.Robins SJ, Fasulo JM, Leduc R, Patton GM. The transport of lipoprotein cholesterol into bile: a reassessment of kinetic studies in the experimental animal. Biochim Biophys Acta. 1989;1004:327–331. doi: 10.1016/0005-2760(89)90080-5. [DOI] [PubMed] [Google Scholar]
- 26.Esnault-Dupuy C, Chanussot F, LaFont H, Chabert C, Hauton J. The relationship between HDL-, LDL-, liposomes-free cholesterol, biliary cholesterol and bile salts in the rat. Biochimie. 1987;69:45–52. doi: 10.1016/0300-9084(87)90270-7. [DOI] [PubMed] [Google Scholar]
- 27.Bravo E, Cantafora A. Hepatic uptake and processing of free cholesterol from different lipoproteins with and without sodium taurocholate administration. An in vivo study in the rat. Biochim Biophys Acta. 1990;1045:74–80. doi: 10.1016/0005-2760(90)90205-c. [DOI] [PubMed] [Google Scholar]
- 28.Bravo E, Botham KM, Mindham MA, Mayes PA, Marinelli T, Cantafora A. Evaluation in vivo of the differential uptake and processing of high-density lipoprotein unesterified cholesterol and cholesteryl ester in the rat. Biochim Biophys Acta. 1994;1215:93–102. doi: 10.1016/0005-2760(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 29.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 31.Mardones P, Quinones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res. 2001;42:170–180. [PubMed] [Google Scholar]
- 32.Wang DQ, Carey MC. Susceptibility to murine cholesterol gallstone formation is not affected by partial disruption of the HDL receptor SR-BI. Biochim Biophys Acta. 2002;1583:141–150. doi: 10.1016/s1388-1981(02)00194-4. [DOI] [PubMed] [Google Scholar]
- 33.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 34.Wang DQ, Zhang L, Wang HH. High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim Biophys Acta. 2005;1733:90–99. doi: 10.1016/j.bbalip.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Smit MJ, Kuipers F, Vonk RJ, Temmerman AM, Jackle S, Windler EE. Effects of dietary cholesterol on bile formation and hepatic processing of chylomicron remnant cholesterol in the rat. Hepatology. 1993;17:445–454. [PubMed] [Google Scholar]
- 36.Robins SJ, Fasulo JM, Robins VF, Patton GM. Response of serum triglycerides of endogenous origin to the administration of triglyceride-rich lipid particles. Am J Physiol. 1989;257:E860–E865. doi: 10.1152/ajpendo.1989.257.6.E860. [DOI] [PubMed] [Google Scholar]
- 37.Botham KM, Bravo E. The role of lipoprotein cholesterol in biliary steroid secretion. Studies with in vivo experimental models. Prog Lipid Res. 1995;34:71–97. doi: 10.1016/0163-7827(94)00007-9. [DOI] [PubMed] [Google Scholar]
- 38.van Dijk MC, Pieters M, van Berkel TJ. Kinetics of biliary secretion of chylomicron remnant cholesterol (esters) in the rat. Eur J Biochem. 1993;211:781–787. doi: 10.1111/j.1432-1033.1993.tb17609.x. [DOI] [PubMed] [Google Scholar]
- 39.Amigo L, Quinones V, Mardones P, Zanlungo S, Miquel JF, Nervi F, Rigotti A. Impaired biliary cholesterol secretion and decreased gallstone formation in apolipoprotein E-deficient mice fed a high-cholesterol diet. Gastroenterology. 2000;118:772–779. doi: 10.1016/s0016-5085(00)70147-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang DQ, Lammert F, Paigen B, Carey MC. Phenotypic characterization of lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice. Pathophysiology Of biliary lipid secretion. J Lipid Res. 1999;40:2066–2079. [PubMed] [Google Scholar]
- 41.Quarfordt SH, Greenfield MF. Estimation of cholesterol and bile acid turnover in man by kinetic analysis. J Clin Invest. 1973;52:1937–1945. doi: 10.1172/JCI107378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Normann PT, Norum KR. Newly synthesized hepatic cholesterol as precursor for cholesterol and bile acids in rat bile. Scand J Gastroenterol. 1976;11:427–432. [PubMed] [Google Scholar]
- 43.Long TT, 3rd, Jakoi L, Stevens R, Quarfordt S. The sources of rat biliary cholesterol and bile acid. J Lipid Res. 1978;19:872–878. [PubMed] [Google Scholar]
- 44.Schwartz CC, Berman M, Vlahcevic ZR, Halloran LG, Gregory DH, Swell L. Multicompartmental analysis of cholesterol metabolism in man. Characterization of the hepatic bile acid and biliary cholesterol precursor sites. J Clin Invest. 1978;61:408–423. doi: 10.1172/JCI108952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turley SD, Dietschy JM. Regulation of biliary cholesterol output in the rat: dissociation from the rate of hepatic cholesterol synthesis, the size of the hepatic cholesteryl ester pool, and the hepatic uptake of chylomicron cholesterol. J Lipid Res. 1979;20:923–934. [PubMed] [Google Scholar]
- 46.Robins SJ, Brunengraber H. Origin of biliary cholesterol and lecithin in the rat: contribution of new synthesis and preformed hepatic stores. J Lipid Res. 1982;23:604–608. [PubMed] [Google Scholar]
- 47.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Statin use and the risk of cholecystectomy in women. Gastroenterology. 2009;136:1593–1600. doi: 10.1053/j.gastro.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erichsen R, Froslev T, Lash TL, Pedersen L, Sorensen HT. Long-term statin use and the risk of gallstone disease: A population-based case-control study. Am J Epidemiol. 2011;173:162–170. doi: 10.1093/aje/kwq361. [DOI] [PubMed] [Google Scholar]
- 49.Smith JL, Roach PD, Wittenberg LN, Riottot M, Pillay SP, Nestel PJ, Nathanson LK. Effects of simvastatin on hepatic cholesterol metabolism, bile lithogenicity and bile acid hydrophobicity in patients with gallstones. J Gastroenterol Hepatol. 2000;15:871–879. doi: 10.1046/j.1440-1746.2000.02231.x. [DOI] [PubMed] [Google Scholar]
- 50.Chapman BA, Burt MJ, Chisholm RJ, Allan RB, Yeo KH, Ross AG. Dissolution of gallstones with simvastatin, an HMG CoA reductase inhibitor. Dig Dis Sci. 1998;43:349–353. doi: 10.1023/a:1018862507469. [DOI] [PubMed] [Google Scholar]
- 51.Duane WC. Effects of lovastatin and dietary cholesterol on bile acid kinetics and bile lipid composition in healthy male subjects. J Lipid Res. 1994;35:501–509. [PubMed] [Google Scholar]
- 52.Hanson DS, Duane WC. Effects of lovastatin and chenodiol on bile acid synthesis, bile lipid composition, and biliary lipid secretion in healthy human subjects. J Lipid Res. 1994;35:1462–1468. [PubMed] [Google Scholar]
- 53.Tazuma S, Takizawa I, Kunita T, Mizuno T, Watanabe T, Teramen K, Horikawa K, et al. Effects of long-term treatment with low-dose pravastatin on biliary lipid and bile acid composition in patients with nonfamilial hyperlipoproteinemia. Metabolism. 1995;44:1410–1412. doi: 10.1016/0026-0495(95)90138-8. [DOI] [PubMed] [Google Scholar]
- 54.Andersen JM, Dietschy JM. Regulation of sterol synthesis in 16 tissues of rat. I. Effect of diurnal light cycling, fasting, stress, manipulation of enterohepatic circulation, and administration of chylomicrons and triton. J Biol Chem. 1977;252:3646–3651. [PubMed] [Google Scholar]
- 55.Dietschy JM, Spady DK. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984;25:1469–1476. [PubMed] [Google Scholar]
- 56.Spady DK, Dietschy JM. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983;24:303–315. [PubMed] [Google Scholar]
- 57.Grundy SM, Ahrens EH., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969;10:91–107. [PubMed] [Google Scholar]
- 58.Grundy SM, Ahrens EH, Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969;10:304–315. [PubMed] [Google Scholar]
- 59.Turley SD, Dietschy JM. The metabolism and excretion of cholesterol by the liver. In: Arias IM, Jakoby WB, Popper H, DSASD, editors. The Liver: Biology and Pathobiology. 2 ed. New York: Raven Press; 1988. pp. 617–641. [Google Scholar]
- 60.Lammert F, Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: integrated activities of hepatic lipid regulatory enzymes. J Lipid Res. 1999;40:2080–2090. [PubMed] [Google Scholar]
- 61.Salen G, Nicolau G, Shefer S, Mosbach EH. Hepatic cholesterol metabolism in patients with gallstones. Gastroenterology. 1975;69:676–684. [PubMed] [Google Scholar]
- 62.Key PH, Bonorris GG, Marks JW, Chung A, Schoenfield LJ. Biliary lipid synthesis and secretion in gallstone patients before and during treatment with chenodeoxycholic acid. J Lab Clin Med. 1980;95:816–826. [PubMed] [Google Scholar]
- 63.Grundy SM, Metzger AL, Adler RD. Mechanisms of lithogenic bile formation in American Indian women with cholesterol gallstones. J Clin Invest. 1972;51:3026–3043. doi: 10.1172/JCI107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nervi FO, Covarrubias CF, Valdivieso VD, Ronco BO, Solari A, Tocornal J. Hepatic cholesterogenesis in Chileans with cholesterol gallstone disease. Evidence for sex differences in the regulation of hepatic cholesterol metabolism. Gastroenterology. 1981;80:539–545. [PubMed] [Google Scholar]
- 65.Maton PN, Ellis HJ, Higgins MJ, Dowling RH. Hepatic HMGCoA reductase in human cholelithiasis: effects of chenodeoxycholic and ursodeoxycholic acids. Eur J Clin Invest. 1980;10:325–332. doi: 10.1111/j.1365-2362.1980.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 66.Coyne MJ, Bonorris GG, Goldstein LI, Schoenfield LJ. Effect of chenodeoxycholic acid and phenobarbital on the rate-limiting enzymes of hepatic cholesterol and bile acid synthesis in patients with gallstones. J Lab Clin Med. 1976;87:281–291. [PubMed] [Google Scholar]
- 67.Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20:1–19. [PubMed] [Google Scholar]
- 68.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169. vii. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Kratzer W, Mason RA, Kachele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound. 1999;27:1–7. doi: 10.1002/(sici)1097-0096(199901)27:1<1::aid-jcu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X, Zhang S, Huang Z. The trend of the gallstone disease in China over the past decade. Zhonghua Wai Ke Za Zhi. 1995;33:652–658. [PubMed] [Google Scholar]
- 71.Huang YC, Zhang XW, Yang RX. Changes in cholelithiasis in Tianjin in the past 30 years. Chin Med J (Engl) 1984;97:133–135. [PubMed] [Google Scholar]
- 72.Zhao Y, Zhang R, Hu Y, Li R, Liang L, Gang Y. [An epidemiological survey of gallstones with gray-scale ultrasound] Hua Xi Yi Ke Da Xue Xue Bao. 1990;21:217–220. [PubMed] [Google Scholar]
- 73.Huang ZQ. Characteristic features of cholelithiasis in China. A nationwide survey of 11342 surgical cases 1983–1985. Zhonghua Wai Ke Za Zhi. 1987;25:321–329. [PubMed] [Google Scholar]
- 74.Nakayama F, Miyake H. Changing state of gallstone disease in Japan. Composition of the stones and treatment of the condition. Am J Surg. 1970;120:794–799. doi: 10.1016/s0002-9610(70)80052-6. [DOI] [PubMed] [Google Scholar]
- 75.Nagase M, Tanimura H, Setoyama M, Hikasa Y. Present features of gallstones in Japan. A collective review of 2,144 cases. Am J Surg. 1978;135:788–790. doi: 10.1016/0002-9610(78)90165-4. [DOI] [PubMed] [Google Scholar]
- 76.van der Linden W, Nakayama F. Gallstone disease in Sweden versus Japan. Clinical and etiologic aspects. Am J Surg. 1973;125:267–272. doi: 10.1016/0002-9610(73)90039-1. [DOI] [PubMed] [Google Scholar]
- 77.Wang HH, Wang DQ. Reduced susceptibility to cholesterol gallstone formation in mice that do not produce apolipoprotein B48 in the intestine. Hepatology. 2005;42:894–904. doi: 10.1002/hep.20867. [DOI] [PubMed] [Google Scholar]
- 78.DenBesten L, Connor WE, Bell S. The effect of dietary cholesterol on the composition of human bile. Surgery. 1973;73:266–273. [PubMed] [Google Scholar]
- 79.Dam H, Prange I, Jensen MK, Kallehauge HE, Fenger HJ. Studies on human bile. IV. Influence of ingestion of cholesterol in the form of eggs on the composition of bile in healthy subjects. Z Ernahrungswiss. 1971;10:178–187. doi: 10.1007/BF02020929. [DOI] [PubMed] [Google Scholar]
- 80.Andersen E, Hellstrom K. The effect of cholesterol feeding on bile acid kinetics and biliary lipids in normolipidemic and hypertriglyceridemic subjects. J Lipid Res. 1979;20:1020–1027. [PubMed] [Google Scholar]
- 81.Lee DW, Gilmore CJ, Bonorris G, Cohen H, Marks JW, Cho-Sue M, Meiselman MS, et al. Effect of dietary cholesterol on biliary lipids in patients with gallstones and normal subjects. Am J Clin Nutr. 1985;42:414–420. doi: 10.1093/ajcn/42.3.414. [DOI] [PubMed] [Google Scholar]
- 82.Wang DQ, Lee SP. Physical chemistry of intestinal absorption of biliary cholesterol in mice. Hepatology. 2008;48:177–185. doi: 10.1002/hep.22286. [DOI] [PubMed] [Google Scholar]
- 83.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 84.Marks JW, Bonorris G. Intermittency of cholesterol crystals in duodenal bile from gallstone patients. Gastroenterology. 1984;87:622–627. [PubMed] [Google Scholar]
- 85.Susann PW, Sheppard F, Baloga AJ. Detection of occult gallbladder disease by duodenal drainage collected endoscopically. A clinical and pathologic correlation. Am Surg. 1985;51:162–165. [PubMed] [Google Scholar]
- 86.Neoptolemos JP, Davidson BR, Winder AF, Vallance D. Role of duodenal bile crystal analysis in the investigation of 'idiopathic' pancreatitis. Br J Surg. 1988;75:450–453. doi: 10.1002/bjs.1800750517. [DOI] [PubMed] [Google Scholar]
- 87.Shiffman ML, Shamburek RD, Schwartz CC, Sugerman HJ, Kellum JM, Moore EW. Gallbladder mucin, arachidonic acid, and bile lipids in patients who develop gallstones during weight reduction. Gastroenterology. 1993;105:1200–1208. doi: 10.1016/0016-5085(93)90968-i. [DOI] [PubMed] [Google Scholar]
- 88.Broomfield PH, Chopra R, Sheinbaum RC, Bonorris GG, Silverman A, Schoenfield LJ, Marks JW. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med. 1988;319:1567–1572. doi: 10.1056/NEJM198812153192403. [DOI] [PubMed] [Google Scholar]
- 89.Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology. 1986;6:118–122. doi: 10.1002/hep.1840060123. [DOI] [PubMed] [Google Scholar]
- 90.McMurry MP, Connor WE, Lin DS, Cerqueira MT, Connor SL. The absorption of cholesterol and the sterol balance in the Tarahumara Indians of Mexico fed cholesterolfree and high cholesterol diets. Am J Clin Nutr. 1985;41:1289–1298. doi: 10.1093/ajcn/41.6.1289. [DOI] [PubMed] [Google Scholar]
- 91.Wang DQ, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am J Physiol. 1999;276:G751–G760. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- 92.Davidson MH, Dillon MA, Gordon B, Jones P, Samuels J, Weiss S, Isaacsohn J, et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159:1893–1900. doi: 10.1001/archinte.159.16.1893. [DOI] [PubMed] [Google Scholar]
- 93.Hunninghake D, Insull W, Jr, Toth P, Davidson D, Donovan JM, Burke SK. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis. 2001;158:407–416. doi: 10.1016/s0021-9150(01)00437-3. [DOI] [PubMed] [Google Scholar]
- 94.Insull W, Jr, Toth P, Mullican W, Hunninghake D, Burke S, Donovan JM, Davidson MH. Effectiveness of colesevelam hydrochloride in decreasing LDL cholesterol in patients with primary hypercholesterolemia: a 24-week randomized controlled trial. Mayo Clin Proc. 2001;76:971–982. doi: 10.4065/76.10.971. [DOI] [PubMed] [Google Scholar]
- 95.Mittendorfer B, Ostlund RE, Jr, Patterson BW, Klein S. Orlistat inhibits dietary cholesterol absorption. Obes Res. 2001;9:599–604. doi: 10.1038/oby.2001.79. [DOI] [PubMed] [Google Scholar]
- 96.Insull W, Jr, Koren M, Davignon J, Sprecher D, Schrott H, Keilson LM, Brown AS, et al. Efficacy and short-term safety of a new ACAT inhibitor, avasimibe, on lipids, lipoproteins, and apolipoproteins, in patients with combined hyperlipidemia. Atherosclerosis. 2001;157:137–144. doi: 10.1016/s0021-9150(00)00615-8. [DOI] [PubMed] [Google Scholar]
- 97.Davidson MH, Maki K, Umporowicz D, Wheeler A, Rittershaus C, Ryan U. The safety and immunogenicity of a CETP vaccine in healthy adults. Atherosclerosis. 2003;169:113–120. doi: 10.1016/s0021-9150(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 98.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 100.Sudhop T, von Bergmann K. Cholesterol absorption inhibitors for the treatment of hypercholesterolaemia. Drugs. 2002;62:2333–2347. doi: 10.2165/00003495-200262160-00002. [DOI] [PubMed] [Google Scholar]
- 101.van Heek M, Compton DS, Davis HR. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol. 2001;415:79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- 102.van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001;134:409–417. doi: 10.1038/sj.bjp.0704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Heek M, Austin TM, Farley C, Cook JA, Tetzloff GG, Davis HR. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 2001;50:1330–1335. doi: 10.2337/diabetes.50.6.1330. [DOI] [PubMed] [Google Scholar]
- 104.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol. 2008;295:G813–G822. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zuniga S, Molina H, Azocar L, Amigo L, Nervi F, Pimentel F, Jarufe N, et al. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28:935–947. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 106.Portincasa P, Di Ciaula A, Wang HH, Palasciano G, van Erpecum KJ, Moschetta A, Wang DQ. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47:2112–2126. doi: 10.1002/hep.22204. [DOI] [PubMed] [Google Scholar]
- 107.Mathur A, Walker JJ, Al-Azzawi HH, Lu D, Swartz-Basile DA, Nakeeb A, Pitt HA. Ezetimibe ameliorates cholecystosteatosis. Surgery. 2007;142:228–233. doi: 10.1016/j.surg.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balboa E, Morales G, Aylwin P, Carrasco G, Amigo L, Castro J, Rigotti A, et al. Niemann-Pick C2 protein expression regulates lithogenic diet-induced gallstone formation and dietary cholesterol metabolism in mice. Lipids. 2012;47:13–25. doi: 10.1007/s11745-011-3625-2. [DOI] [PubMed] [Google Scholar]
- 110.Wang DQ, Tazuma S. Effect of beta-muricholic acid on the prevention and dissolution of cholesterol gallstones in C57L/J mice. J Lipid Res. 2002;43:1960–1968. doi: 10.1194/jlr.m200297-jlr200. [DOI] [PubMed] [Google Scholar]
- 111.Logan GM, Duane WC. Lovastatin added to ursodeoxycholic acid further reduces biliary cholesterol saturation. Gastroenterology. 1990;98:1572–1576. doi: 10.1016/0016-5085(90)91092-k. [DOI] [PubMed] [Google Scholar]
- 112.Duane WC, Hunninghake DB, Freeman ML, Pooler PA, Schlasner LA, Gebhard RL. Simvastatin, a competitive inhibitor of HMG-CoA reductase, lowers cholesterol saturation index of gallbladder bile. Hepatology. 1988;8:1147–1150. doi: 10.1002/hep.1840080531. [DOI] [PubMed] [Google Scholar]
- 113.Yamauchi S, Linscheer WG, Beach DH. Increase in serum and bile cholesterol and HMG-CoA reductase by lovastatin in rats. Am J Physiol. 1991;260:G625–G630. doi: 10.1152/ajpgi.1991.260.4.G625. [DOI] [PubMed] [Google Scholar]
- 114.Brenneman DE, Connor WE, Forker EL, DenBesten L. The formation of abnormal bile and cholesterol gallstones from dietary cholesterol in the prairie dog. J Clin Invest. 1972;51:1495–1503. doi: 10.1172/JCI106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DenBesten L, Safaie-Shirazi S, Connor WE, Bell S. Early changes in bile composition and gallstone formation induced by a high cholesterol diet in prairie dogs. Gastroenterology. 1974;66:1036–1045. [PubMed] [Google Scholar]
- 116.Holzbach RT, Corbusier C, Marsh M, Naito HK. The process of cholesterol cholelithiasis induced by diet in the prairie dog: a physicochemical characterization. J Lab Clin Med. 1976;87:987–998. [PubMed] [Google Scholar]
- 117.Lee SP, LaMont JT, Carey MC. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981;67:1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singhal AK, Cohen BI, Mosbach EH, Une M, Stenger RJ, McSherry CK, May-Donath P, et al. Prevention of cholesterol-induced gallstones by hyodeoxycholic acid in the prairie dog. J Lipid Res. 1984;25:539–549. [PubMed] [Google Scholar]
- 119.Saunders KD, Cates JA, Abedin MZ, Rege S, Festekdjian SF, Howard W, Roslyn JJ. Lovastatin inhibits gallstone formation in the cholesterol-fed prairie dog. Ann Surg. 1991;214:149–154. doi: 10.1097/00000658-199108000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abedin MZ, Narins SC, Park EH, Smith PR, Kirkwood KS. Lovastatin alters biliary lipid composition and dissolves gallstones: a long-term study in prairie dogs. Dig Dis Sci. 2002;47:2192–2210. doi: 10.1023/a:1020174908650. [DOI] [PubMed] [Google Scholar]
- 121.Saunders KD, Cates JA, Abedin MZ, Roslyn JJ. Lovastatin and gallstone dissolution: a preliminary study. Surgery. 1993;113:28–35. [PubMed] [Google Scholar]
- 122.Tazuma S, Hatsushika S, Aihara N, Sagawa H, Yamashita G, Sasaki M, Sasaki H, et al. Inhibitory effects of pravastatin, a competitive inhibitor of hydroxymethylglutaryl coenzyme A reductase, on cholesterol gallstone formation in prairie dogs. Digestion. 1992;51:179–184. doi: 10.1159/000200894. [DOI] [PubMed] [Google Scholar]
- 123.Davis KG, Wertin TM, Schriver JP. The use of simvastatin for the prevention of gallstones in the lithogenic prairie dog model. Obes Surg. 2003;13:865–868. doi: 10.1381/096089203322618678. [DOI] [PubMed] [Google Scholar]
- 124.Smelt AH. Triglycerides and gallstone formation. Clin Chim Acta. 2010;411:1625–1631. doi: 10.1016/j.cca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 125.Kallien G, Lange K, Stange EF, Scheibner J. The pravastatin-induced decrease of biliary cholesterol secretion is not directly related to an inhibition of cholesterol synthesis in humans. Hepatology. 1999;30:14–20. doi: 10.1002/hep.510300119. [DOI] [PubMed] [Google Scholar]
- 126.Smit JW, van Erpecum KJ, Stolk MF, Geerdink RA, Cluysenaer OJ, Erkelens DW, van Berge Henegouwen GP. Successful dissolution of cholesterol gallstone during treatment with pravastatin. Gastroenterology. 1992;103:1068–1070. doi: 10.1016/0016-5085(92)90045-z. [DOI] [PubMed] [Google Scholar]
- 127.Hillebrant CG, Nyberg B, Gustafsson U, Sahlin S, Bjorkhem I, Rudling M, Einarsson C. Effects of combined treatment with pravastatin and ursodeoxycholic acid on hepatic cholesterol metabolism. Eur J Clin Invest. 2002;32:528–534. doi: 10.1046/j.1365-2362.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 128.Hillebrant CG, Axelson M, Bjorkhem I, Wang FH, Nyberg B, Einarsson C. Effects of short-term treatment with pravastatin on the hepatic synthesis of cholesterol and bile acids in gallstone patients. Eur J Clin Invest. 1998;28:324–328. doi: 10.1046/j.1365-2362.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- 129.Sharma BC, Agarwal DK, Baijal SS, Saraswat VA. Pravastatin has no effect on bile lipid composition, nucleation time, and gallbladder motility in persons with normal levels of cholesterol. J Clin Gastroenterol. 1997;25:433–436. doi: 10.1097/00004836-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 130.Duane WC. Effects of lovastatin in humans on biliary lipid composition and secretion as a function of dosage and treatment interval. J Pharmacol Exp Ther. 1994;270:841–845. [PubMed] [Google Scholar]
- 131.Hoogerbrugge-vd Linden N, de Rooy FW, Jansen H, van Blankenstein M. Effect of pravastatin on biliary lipid composition and bile acid synthesis in familial hypercholesterolaemia. Gut. 1990;31:348–350. doi: 10.1136/gut.31.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nitsche R, Schlage J, Ramin V, Kirchhoff S, Folsch UR, Creutzfeldt C, Creutzfeldt W. Influence of the HMG-CoA-reductase inhibitor lovastatin on cholesterol saturation index and nucleation time of duodenal bile. Z Gastroenterol. 1991;29:242–245. [PubMed] [Google Scholar]
- 133.Smit JW, van Erpecum KJ, Renooij W, Stolk MF, Edgar P, Doornewaard H, Vanberge-Henegouwen GP. The effects of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor pravastatin on bile composition and nucleation of cholesterol crystals in cholesterol gallstone disease. Hepatology. 1995;21:1523–1529. [PubMed] [Google Scholar]
- 134.Bodmer M, Brauchli YB, Krahenbuhl S, Jick SS, Meier CR. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA. 2009;302:2001–2007. doi: 10.1001/jama.2009.1601. [DOI] [PubMed] [Google Scholar]
- 135.Caroli-Bosc FX, Le Gall P, Pugliese P, Delabre B, Caroli-Bosc C, Demarquay JF, Delmont JP, et al. Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation: epidemiological study in an unselected population. Dig Dis Sci. 2001;46:540–544. doi: 10.1023/a:1005643014395. [DOI] [PubMed] [Google Scholar]
- 136.Tazuma S, Kajiyama G, Mizuno T, Yamashita G, Miura H, Kajihara T, Hattori Y, et al. A combination therapy with simvastatin and ursodeoxycholic acid is more effective for cholesterol gallstone dissolution than is ursodeoxycholic acid monotherapy. J Clin Gastroenterol. 1998;26:287–291. doi: 10.1097/00004836-199806000-00015. [DOI] [PubMed] [Google Scholar]