Abstract
Purpose
To assess the frequency of oral contrast coating of flat polyps, which may promote detection, and influencing factors within a screening CTC population.
Materials
From 7,426 individuals, 123 patients with 160 flat polyps were extracted. Flat polyps were defined as plaque-like, raised ≤ 3mm in height and reviewed for contrast coating. Factors including demographic variables such as age and sex, and polyp variables such as polyp size, location, and histology were analyzed for effect on coating.
Results
Of 160 flat polyps (mean size 9.4mm±3.6), 78.8% demonstrated coating. Mean coat thickness was 1.5mm±0.6; 23.8% (n=30), demonstrating a thin film of contrast. Large size (≥10 mm), and proximal colonic location (relative to splenic flexure) were predictive variables by univariate logistic regression [OR (odds ratio) 3.4(CI 1.3–8.9; p=0.011), 2.0(CI 1.2–3.5; p=0.011), respectively]. Adenomas (OR 0.37, CI: 0.14–1.02; p=0.054) and mucosal polyps or venous blebs (OR 0.07, CI: 0.02–0.25; p < 0.001) were less likely to coat than serrated/hyperplastic lesions. Age and sex were not predictive for coating (p=0.417, p= 0.499, respectively).
Conclusions
Surface contrast coating is common for flat polyps at CTC, promoted by large size, proximal location, and serrated/hyperplastic histology. Given the difficulty in detection, recognition may aid in flat polyp identification.
Introduction
The addition of oral contrast agents to the bowel preparation regimen at CT colonography (CTC) represents a past major advance in CT colonography and now is a standard part of any CTC bowel regimen.1,2 Contrast opacification of residual colonic fluid and admixing with residual stool allows for improved detection of polyps submerged in fluid pools and for improved differentiation between stool and true polyps. An unanticipated beneficial result has been the contrast coating of colorectal polyps by tagging agents.3 Here, the contrast tag adheres to the surface of the polyp yet washes away from the normal colonic mucosa. This phenomenon has allowed improved detection and characterization, as the contrast makes polyps more conspicuous on both 2D and 3D evaluation.
Flat polyp detection at CTC has been an area of controversy; some argue that detection is markedly decreased due to their lack of projection from the mucosal surface. Without the direct optical cues that may be present at colonoscopy, some assert that detection at CTC is nearly impossible. However, flat polyps have been identified in various CTC series, constituting up to 13.1% of non-diminutive polyps.4 CTC detection rates for flat polyps ≥ 6 mm range from 80% – 87.5% in two large series.5,6 Detection is possible because most flat polyps are typically not completely flat to the surrounding mucosal surface, but rather show a minimally raised profile.7 With growing clinical CTC experience, it has become evident to us that an additional important factor which can aid in flat polyp detection, is contrast coating of the lesional surface. The purpose of this paper is to assess the frequency of oral contrast coating of flat polyps, including the factors that promote such coating, for polyps detected within a large screening CTC population.
Materials and Methods
The Institutional Review Board at the University of Wisconsin School of Medicine and Public Health approved this Health Insurance Portability and Accountability Act (HIPAA)-compliant study. The need for informed consent was waived.
Study population
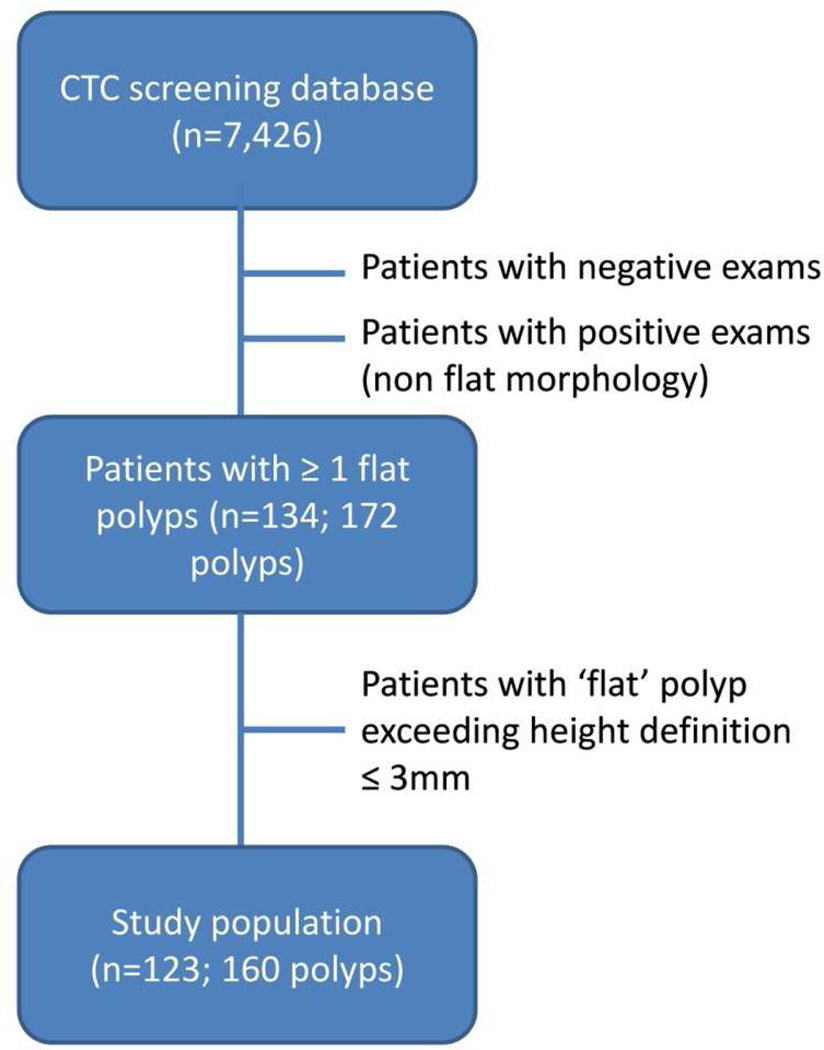
Patients harboring at least one detected non-diminutive polyp (≥ 6mm) of flat morphology were extracted from a database of 7,426 patients enrolled at a single institution CTC screening program. Patients are referred to this program for the indication of colorectal cancer screening and are either at average or mildly elevated risk. Patients with hereditary polyposis syndromes, inflammatory bowel disease, prior colorectal cancer or referred from incomplete optical colonoscopy evaluation, are not included in this screening database. Flat polyps detected at initial CTC screen or developed at a subsequent routine screen (5 years after an initial negative CTC screen) were included. All CTC-detected flat polyps were confirmed either at subsequent colonoscopy with polypectomy or by redemonstration at follow-up CTC surveillance (patients with one or two sub centimeter polyps are eligible for an imaging surveillance protocol).8 Any additional flat polyps seen at referred colonoscopy but not prospectively identified at CTC (i.e., CTC false negatives) were included. From this original group, 11 patients with 12 polyps were excluded upon re-measurement because the colonic polyps did not meet the strict definition of a flat polyp and were raised more than 3 mm from the colonic surface. Figure 1 outlines the study inclusion.
Figure 1.
Study inclusion flow chart
All detected polyps are prospectively characterized at the time of the exam for linear size (mm), location, morphology, and diagnostic confidence. Flat polyps were defined as soft tissue polyps less than 3 cm in size with a plaque-like morphology raised 3 mm or less from the mucosal surface. Carpet or lateral spreading lesions (flat lesions ≥3 cm in size) were excluded from determination.9 Size was determined by longest linear measurement from a combination of 2D and 3D perspectives.10 Location was recorded by colonic segment (cecum, ascending, transverse, descending, sigmoid, and rectum). For the purposes of this study, location was condensed into proximal colon and distal colon as defined by the splenic flexure. Diagnostic confidence (for a true soft tissue polyp) was assigned by the interpreting radiologist at the time of the exam. A 3-point scoring system was utilized where a score of 3 represented a level of high confidence, 2 for intermediate confidence, and 1 for low confidence.11
CTC protocol
The bowel preparation consisted of a well-established CTC standard protocol involving both carthartic and tagging agents (Table 1). The specific protocol has been described in detail in previous publications.12–14 It begins one day prior to the scheduled examination; a dry osmotic cathartic agent is administered for fecal cleansing. Early in the program experience, sodium phosphate was the main cathartic agent. Double-dose magnesium citrate (296 ml single dose; Sunmark) was substituted beginning in 2008 once sodium phosphate was no longer available over the counter due to acute phosphate nephropathy concerns by the FDA. Following cleansing, a dual-contrast tagging regimen is undertaken consisting of oral administration of dilute 2.1% w/v barium sulfate (250 ml; Readi-cat, Bracco Diagnostics) and of diatrizoate meglumine (60 mL; MD-Gastroview, Tyco Healthcare/Mallinckrodt). It is thought that the primary action of the barium is to tag any residual solid stool and the diatrizoate to tag any residual colonic fluid. The hyperosmolar nature of diatrizoate also serves to create a final mini-catharsis to further cleanse the colon.
| Time | Prep |
|---|---|
| > Midnight | Liquid diet only |
| 11:00 am | Bisacodyl tabs |
| 3–5 pm | Mg citrate #1 |
| 6–8 pm | Mg Citrate #2 & Ba SO4 |
| 9–11 pm | Diatrizoate |
Colonic distention is undertaken with automated instillation of carbon dioxide under low pressure (PROTOCO2L; Bracco Diagnostics). Once equilibrium is reached and the colorectum is optimally distended, the patient is scanned in two positions (supine and prone) with additional decubitus position for areas of persistent collapse.15 No spasmolytics are given.
Examinations were acquired by a multi-detector CT scanner of 8, 16, or 64 rows (GE Medical Systems). The CTC technique consisted of 1.25 mm collimation with 1-mm reconstruction interval and a 500 msec rotation. Low dose technique was employed with either a fixed tube current of 50–75 mAs and 120 kVp or (more commonly) with tube current modulation using a noise index of 50 and mA range of 30–150. The data were networked to a central reading area with a CTC 3D workstation (V3D colon, Viatronix) for post-processing and interpretation.
Image interpretation
All flat polyps were retrospectively evaluated by one of three radiologists (DHK, MGL, JLH) utilizing the 2D source images in polyp windows (W: 2000 HU, L: 0 HU) and 3D reconstructions as needed. The previously recorded size (by 2D/3D assessment at CTC), morphology, and segmental location of each polyp were confirmed. In particular, each polyp was assessed to meet the strict criteria of flat morphology in regards to vertical height from mucosa. Any polyp greater than 3 mm in height despite a plaque-like morphology was excluded. For the purposes of this study, polyps were condensed into two size categories (small 6–9 mm and large ≥ 10 mm). In addition, the segmental colonic location was condensed into a proximal (relative to the splenic flexure) and distal colonic categories.
Additional prospective assessments were then made. These assessments were not undertaken during the original CTC interpretation. Each polyp was assessed for the presence or absence of an overlying adherent contrast coat. If present, the thickness of the contrast coat (in mm) was measured. The colon was also assessed for the presence of residual adherent tagged stool in the colonic segment where the polyp was located as well as the adjacent segments. As the elevation of the polyp from the mucosa was not prospectively reported, each polyp was also measured for the maximal height above the mucosa. For any flat polyps seen at colonoscopy but not called at initial CT colonography (i.e., CTC false negatives), the 2D and 3D images were reviewed in the colonic segment of concern to either retrospectively identify a soft tissue correlate at CTC or presence of a focal contrast plaque in the region that could represent a coated flat polyp where the soft tissue polyp was not seen.
Statistical analysis
Summary descriptive statistics were obtained for continuous variables; categorical variables were described with frequency counts and percentages. Fisher’s exact test was used to assess differences in the proportion of coated flat polyps between various groups, whereas Kruskal-Wallis tests were used to evaluate differences in the distribution of lesion size, lesion thickness, and coat thickness (all in mm) between groups. In the preceding, “groups” refers to lesion size (6–9 mm versus ≥ 10 mm), histology (adenomatous, serrated/hyperplastic, other), and lesion location (proximal versus distal). P < 0.05 (two sided) was the criterion for statistical significance. Statistical computations and graphics were obtained in R version 2.12.1.16
Logistic regression was used to model the probability of contrast coating as a function of age, sex, polyp size, colon segment, and histology. First, univariable models were fitted; polyp size was considered as a binary variable (6–9 mm vs. ≥ 10mm). Histology was recoded into three categories: (1) hyperplastic or serrated polyps; (2) tubular, tubulovillous and villous; (3) other (including mucosal polyps and venous blebs); a single case of adenocarcinoma was removed from this analysis. Hyperplastic/serrated histology was the referent category. Variables found to be significant at univariable analysis were entered into a multivariable analysis. First, a linear model was fitted, which included as main effects all the variables found to be significant at univariable analysis; then, pair-wise interactions were added, and their inclusion was tested via a Chi-squared likelihood ratio test. The multivariable models were only fitted once, so that non-significant variables were not omitted from the model.
Results
123 patients with 160 non-diminutive flat polyps comprise the study population with an overall prevalence of 1.7% (123/7,426). Of the positive patients with at least one CTC-detected polyp of any morphology in the database (n=1,061), this constitutes 11.6% of the positive cohort. The demographics of the flat polyp, positive fraction, and entire CTC database are presented in Table 2.
| Variable | Flat cohort | Positive cohort |
Entire population |
|---|---|---|---|
| (n) | 123 | 1,061 | 7,426 |
| Mean Age-yr | 59.5 ± 8.2 | 58.5 ± 8.8 | 56.8 ± 7.3 |
| Male (%) | 63 (51.2%) | 584 (55.0%) | 3,420 (46.1%) |
| Female (%) | 60 (48.8%) | 477 (45.0%) | 4,006 (53.9%) |
Of the 160 flat polyps in this series, the mean size 9.4 mm ± 3.6 with a vertical elevation of 1.9 mm ± 0.6 from the surrounding mucosal surface. A single 6 mm flat polyp was detected that was even with the mucosa with a measured vertical height of 0 mm but showed surface coating with contrast, allowing for detection. This polyp was confirmed at colonoscopic resection as a tubular adenoma. Overall, 78.8% (126/160) of flat polyps demonstrated coating by oral tagging agents whereas 21.2% (34/160) did not. Of the polyps demonstrating tagging, 23.8% (30/126) demonstrated only a thin film overlying the flat polyp measuring less than 1 mm. Excluding these polyps, the mean measured thickness of the contrast coat was 1.5 mm ± 0.6 (range 0.4–3.9) (Fig. 2). The measured coat thickness was independent of lesion geometry, as neither lesion size nor height (p=0.4, 0.5 respectively) correlated with contrast coat thickness. Diagnostic confidence at CTC was increased when contrast coating was present. A confidence score 3 corresponded with an 89.9% (62/69) rate of lesions coating while 73.7% (56/76) of confidence score 2 polyps and 62.5% (5/8) of score 1 polyps showed contrast coating (p=0.02). There were 7 cases where confidence scores were not recorded. There was no significant difference in percentage of coated lesions whether residual tagged stool and/or fluid was seen in the segment or adjacent segments (p=0.2)
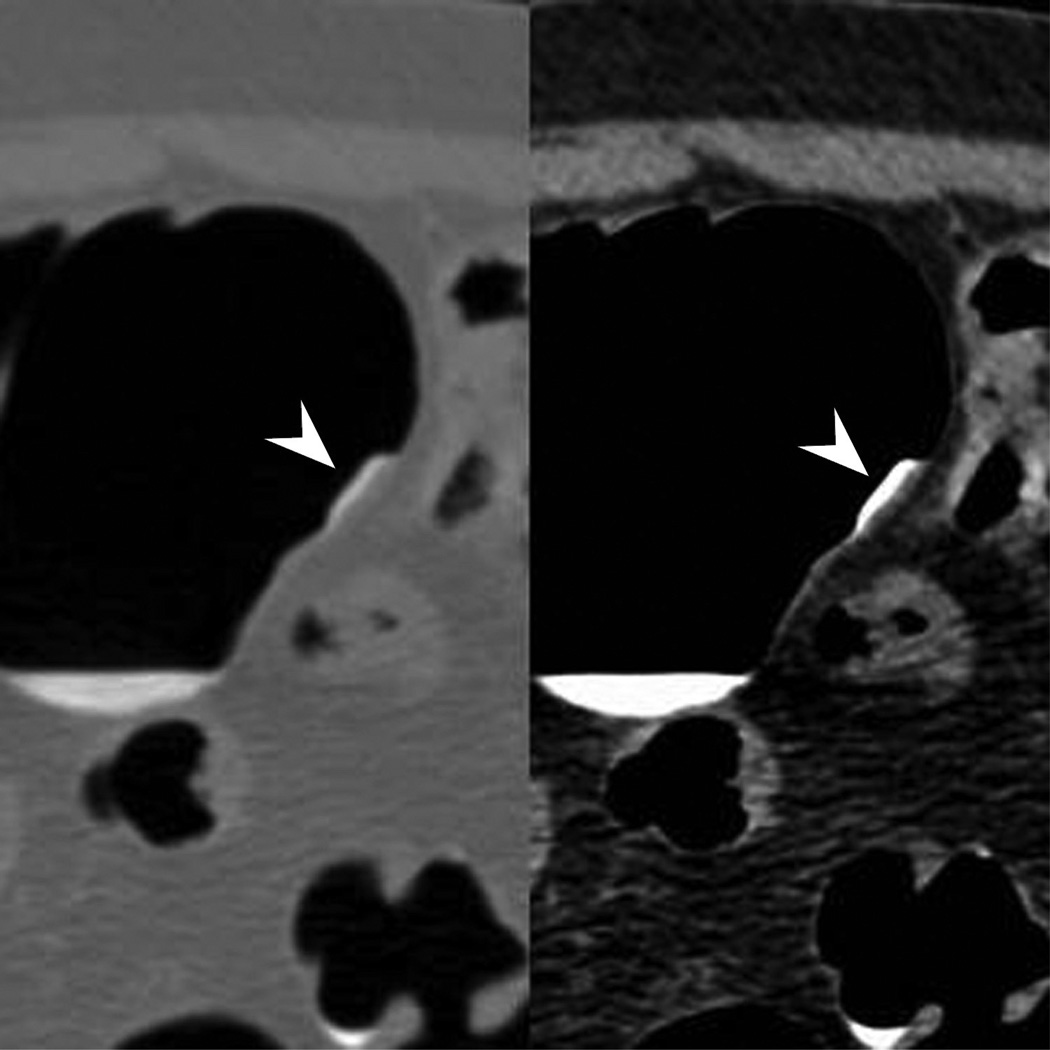
Figure 2.
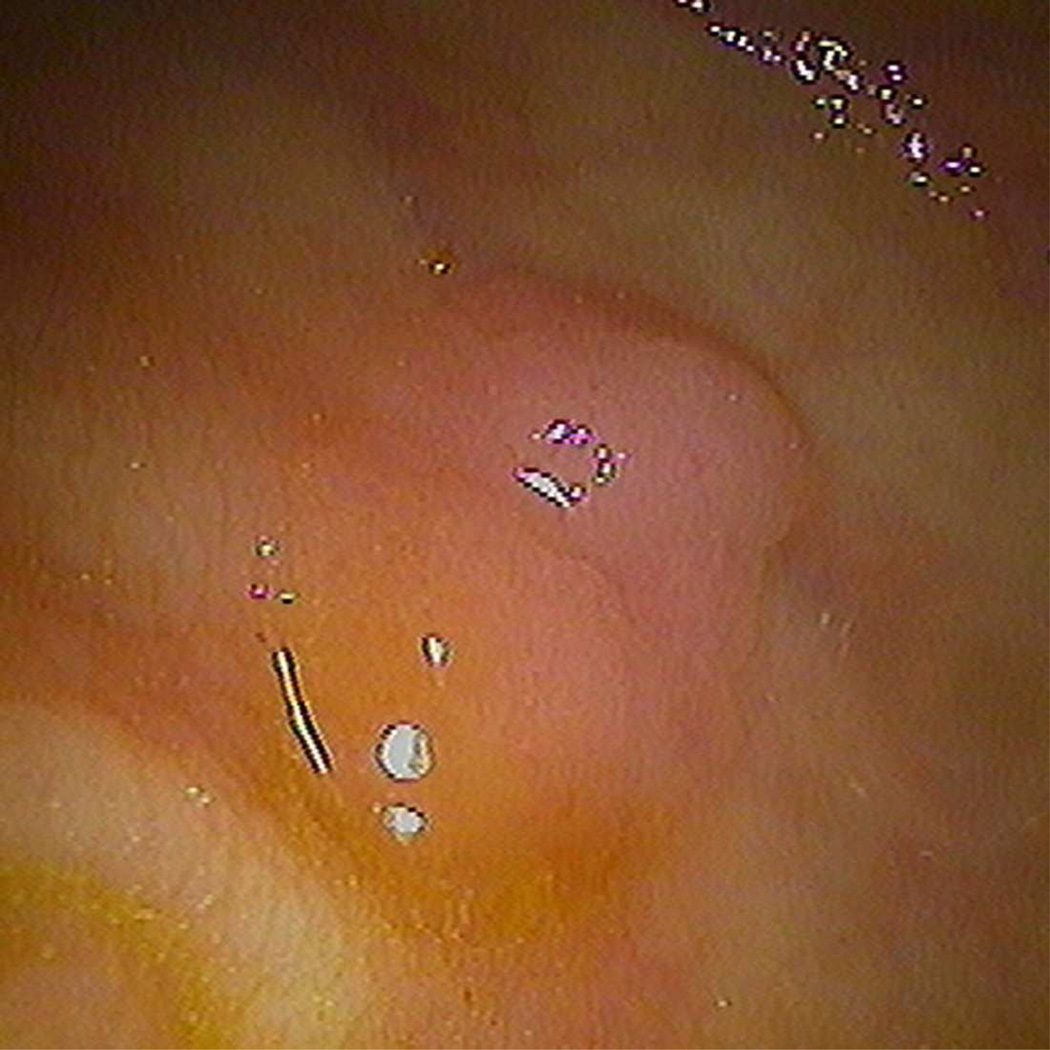
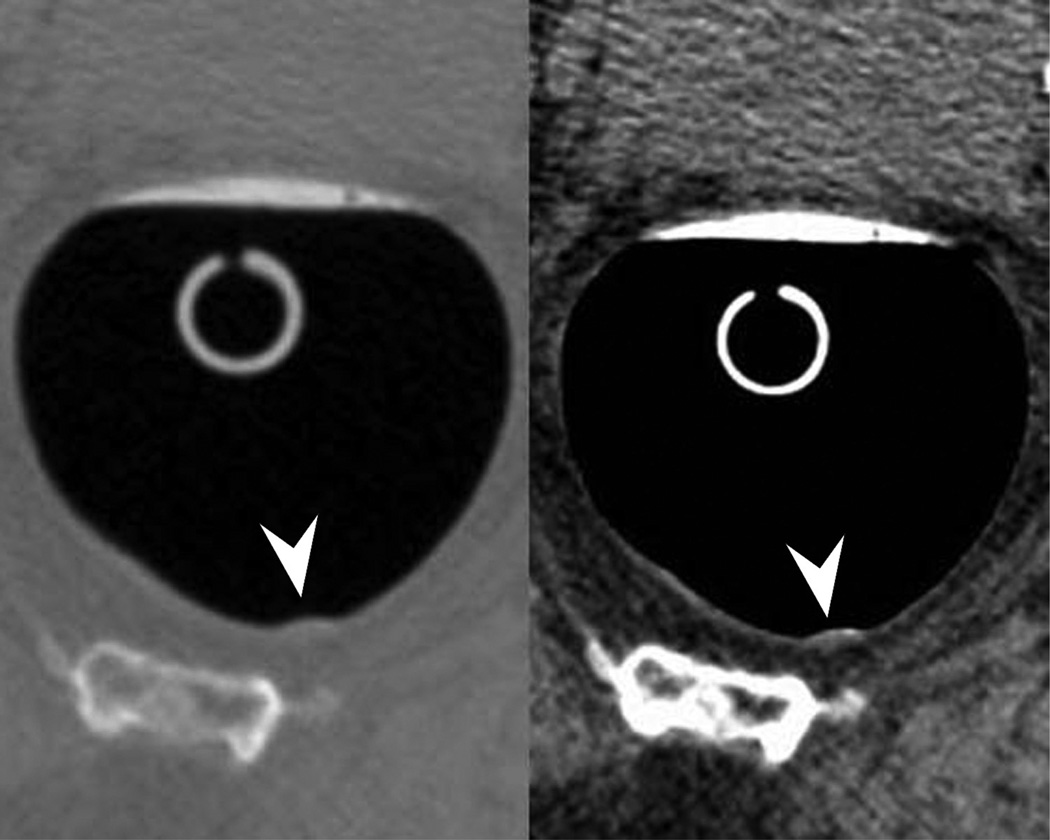
Typical appearance of coated flat polyp. 2D supine images (A) in polyp (2000/0) and soft tissue (400/50) windows show a typical coated polyp (arrowhead) in the cecum. Note the subtle soft tissue thickening deep to the contrast coat, which is more evident on the soft tissue windows. 2D prone images (B) again demonstrates a focal contrast plaque of typical thickness overlying the polyp (arrowhead). The polyp is more prominent at 3D (C) as it represents a combination of polyp and contrast from this perspective. Optical colonoscopy (D) confirms the polyp which was hyperplastic in nature. 2D image (E) in another patient shows a thin film on contrast (seen in a quarter of coated polyps) (arrowhead). Note the polyp is less prominent on the 3D view (F).
No studied demographic variable was seen predictive for coating at univariate analysis (Table 3). In contrast, large flat polyps (≥ 10 mm) were more likely to coat than small flat polyps (6–9 mm); 88.3% (53/60) of large polyps demonstrated coating while only 72.0% (72/100) of 6–9 mm lesions were tagged with contrast (p=0.009). Odds ratio for coating of large flat polyps was 3.4 (CI 1.3–8.9) as compared to flat polyps of the small size category (Table 3). Tagging occurred more often in flat lesions located in the proximal colon (i.e., cecum, ascending, and transverse colon) (85.6%; 83/97) than compared to distal flat lesions (ie, descending, sigmoid, or rectum) (68.3%; 43/63) (p=0.01). Odds ratio for coating of proximal polyps was 2.0 (CI 1.2–3.5) as compared with distal lesions (Table 3).
| Univariable Model | Multivariable Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI limits | 95% CI limits | ||||||||
| Variable | OR | lower | upper | P-value | OR | lower | upper | P-value | |
| Age | 0.982 | 0.938 | 1.026 | 0.417 | -- | -- | -- | -- | |
| Sex | Female (ref) | 1 | -- | -- | -- | -- | -- | -- | -- |
| Male | 0.769 | 0.360 | 1.645 | 0.499 | -- | -- | -- | -- | |
| Polyp size | < 10 mm (ref) | 1 | -- | -- | -- | -- | -- | -- | -- |
| ≥ 10 mm | 3.435 | 1.328 | 8.886 | 0.011 | 2.404 | 0.795 | 7.271 | 0.042 | |
| Location | Distal (ref) | 1 | -- | -- | -- | -- | -- | -- | -- |
| Proximal | 2.031 | 1.173 | 3.517 | 0.011 | 2.126 | 1.019 | 4.437 | 0.044 | |
| Histology | Serrated/HP (ref) | 1 | -- | -- | -- | -- | -- | -- | -- |
| TA/TV/Villous | 0.374 | 0.138 | 1.015 | 0.054 | 0.324 | 0.113 | 0.927 | 0.036 | |
| MP/venous bleb | 0.071 | 0.020 | 0.246 | <0.001 | 0.051 | 0.013 | 0.205 | <0.001 | |
Box and whisker plots and Kruskal-Wallis calculations showed that the sample distributions of flat lesions size were skewed within colon location, with larger lesions located in the more proximal colon (p<0.001). Despite the uneven distribution of lesions by size, no pair-wise interactions resulted in an improvement in fit (p ≥ 0.0631). Polyp size (p=0.04), location (p = 0.04), and histology (p < 0.001), preserved their significance in the additive model (Table 3).
Of those lesions with pathologic examination at colonoscopic resection (n=140/160; 87.5%), there appeared to be three discrete groups related to coating. Coating was more prevalent for serrated/hyperplastic histologies (68/76; 89.5%), than for adenomatous polyps (tubular, tubulovillous, villous) (35/47; 74.5%) and for miscellaneous non-neoplastic lesions such as mucosal polyps or venous blebs (6/16; 37.5%). Using serrated/hyperplastic lesions as the referent category, the odds ratio was 0.37 (CI 0.14–1.02; p=0.054) compared against adenomatous polyps and 0.07 (CI 0.02–0.25; p<0.001) compared against other non-neoplastic lesions. Histology remained an independent variable in the multi-variate model (p<0.001) (Table 3).
Seven additional flat polyps not prospectively detected at CTC were seen at therapeutic colonoscopy. These represented cases sent for CTC detected flat polyps where additional missed lesions were present. This group comprised 4.4% of the flat polyps in this series. In contrast to the initially detected CTC flat lesions, only 3/7 or 42.9% demonstrated contrast coating.
Discussion
This study highlights important aspects regarding the property of oral contrast tagging of colorectal polyps with flat morphology. First and foremost is the recognition that contrast adherence occurs with polyps at CT colonography and that it occurs in the large majority of polyps with flat morphology. In our series, 80% of flat polyps demonstrated overlying adherent contrast. Thus, it is important to critically assess an apparent flat plaque of contrast at CTC for the sometimes subtle soft tissue thickening deep to the contrast coat. A lack of awareness of this phenomenon could lead to decreased detection of flat polyps where they are discounted as adherent plaques of tagged stool.
The idea that surface coating with oral contrast improves polyp detection makes intuitive sense and becomes clearly evident in clinical practice. Because the tagging agent washes away from the colonic mucosa but adheres to the polyp surface, the contrast draws attention to the polyp where a focal ‘white’ structure is present on the background of the gray untagged colonic mucosa (during 2D review). Similarly, at 3D review, detection is improved because the polyps look larger and more raised since the appearance represents a combination of polyp and overlying contrast (Fig. 3). Albeit small in number, the CTC false negatives (FN) in our series further support this hypothesis where a substantially higher percentage of the missed polyps (CTC FN) were not coated in contrast as compared with the CTC-detected ones. In the same vein, the results of an older Korean series which demonstrated sensitivities less than 50% for flat polyps adds credence to the effect of polyp coating, as this study was conducted at a time when contrast tagging was not widely used (and not used in that series).17 Besides improved detection, contrast tagging also appears to aid interpretative assessment. Diagnostic confidence scores point to higher reader confidence for flat polyps that exhibit contrast adherence from those characterized by only minimal elevation from the mucosal surface without any contrast tagging.
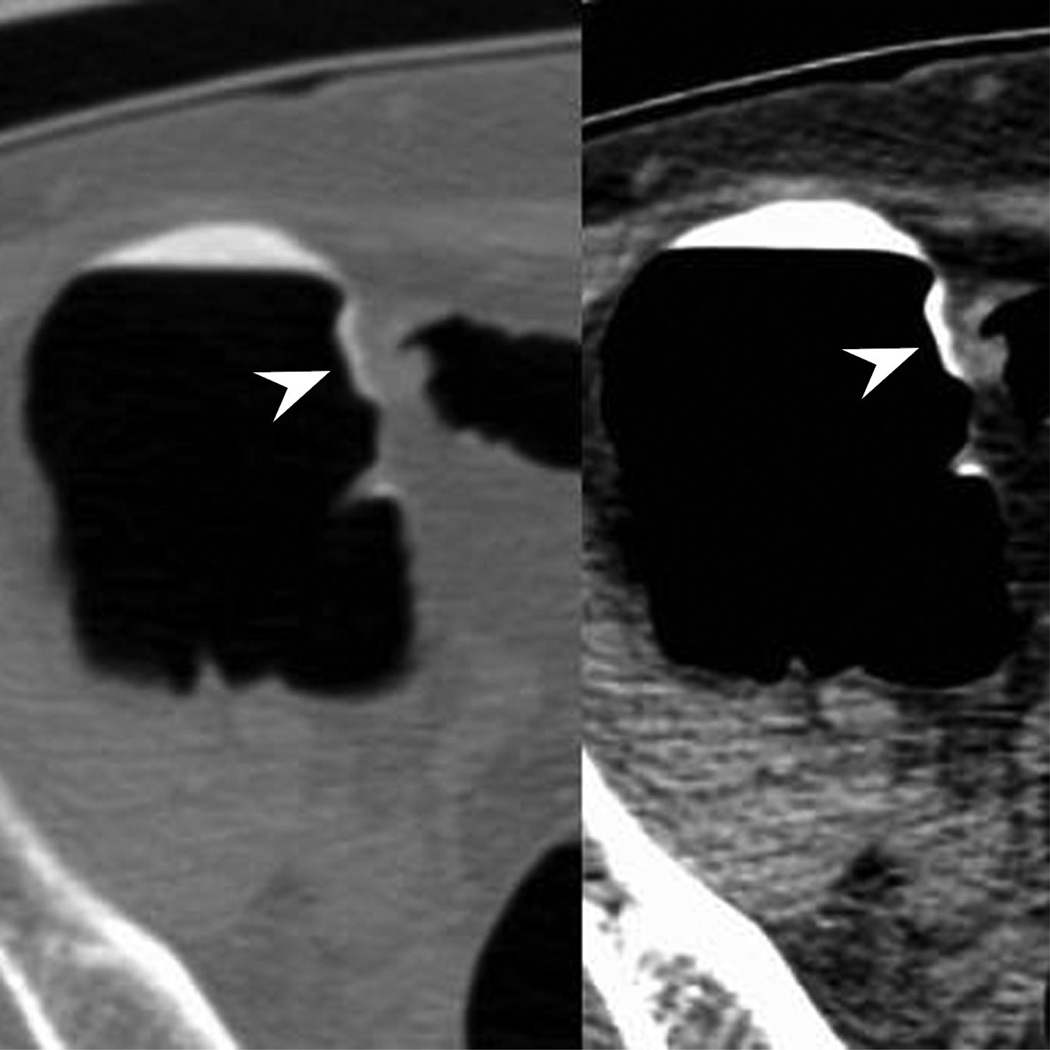
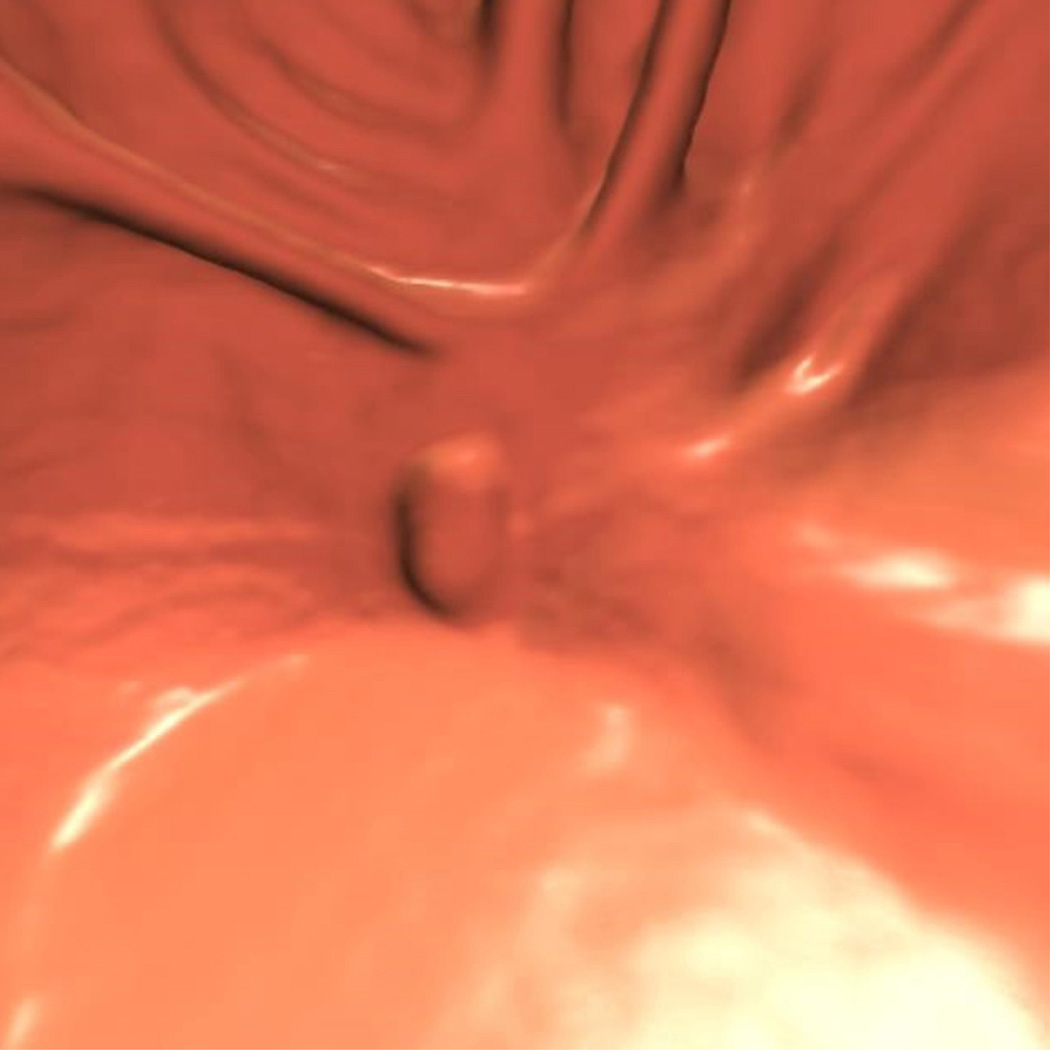
Figure 3.
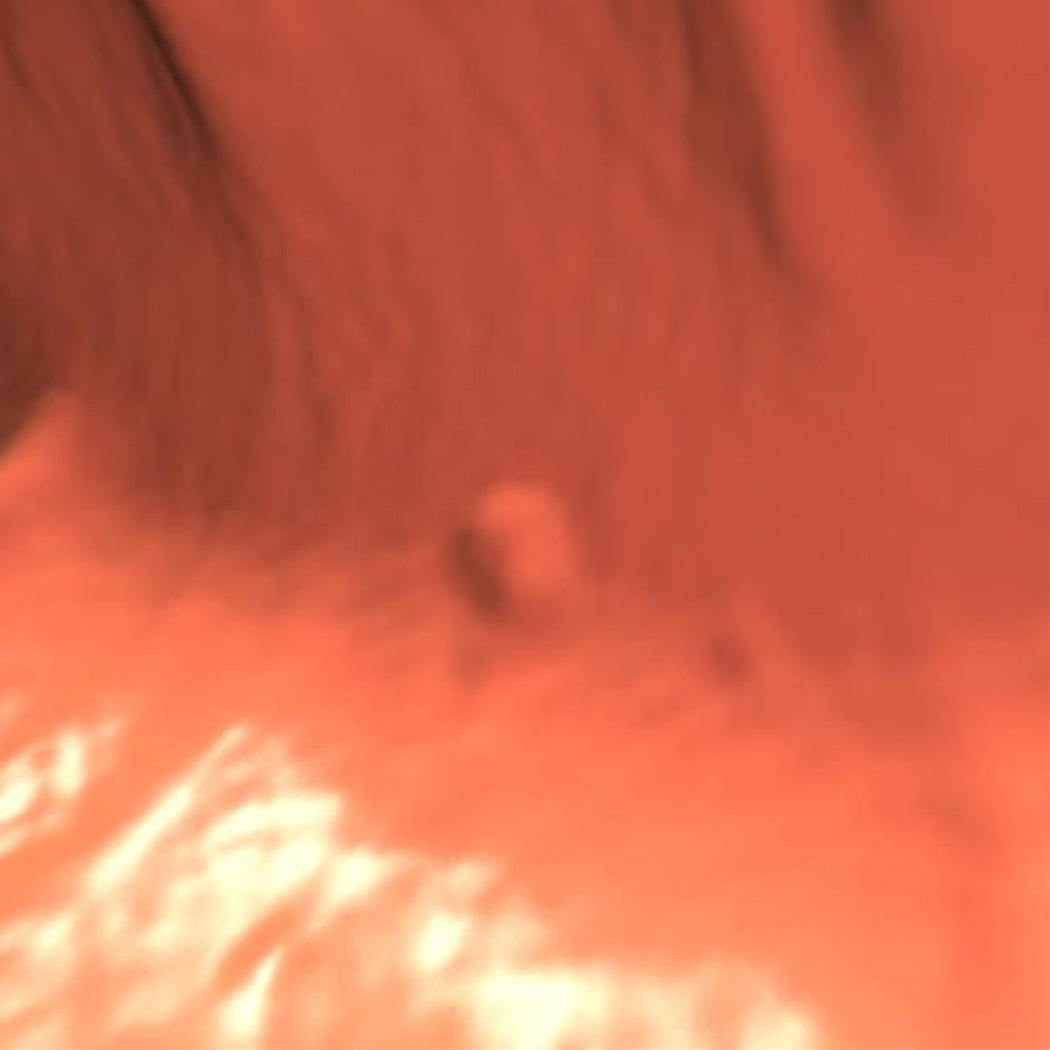
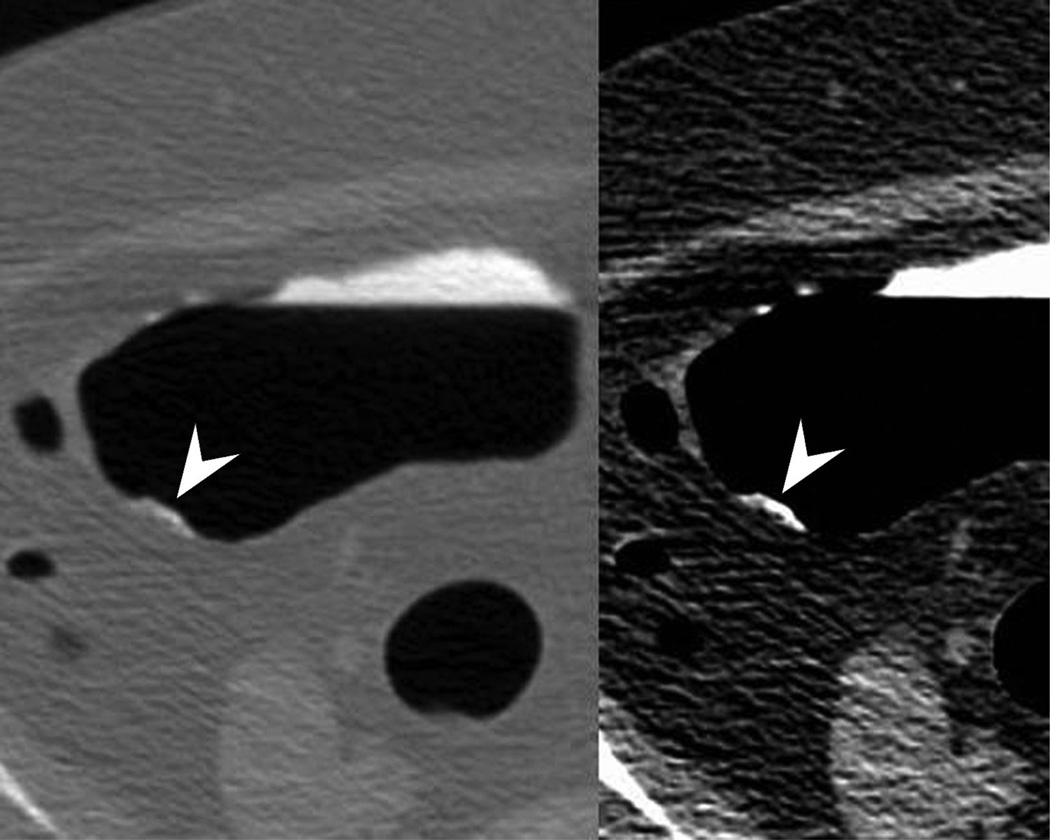
Ascending flat tubular adenoma. 2D prone images (A) in polyp and soft tissue windows again show a coated polyp (arrowhead). Like most coated polyps, it is large and proximal in location. Note how the presence of adherent tag draw attention to this portion of the cecum and that it is washed away from the rest of the colonic mucosa. 3D endoluminal (B) shows the flat polyp as almost sessile in appearance due to the additive effects of the added contrast. Colonoscopic image (C) in snare shows the balled up flat polyp which was a tubular adenoma at polypectomy.
Thus, polyp coating is an unintended consequence of bowel preparation that likely improves the ability to identify flat lesions at CTC and may account for the unexpectedly high detection rates for flat polyps seen in the literature.5,6 Outcomes data from a large negative screening cohort (n=1,011) further support the idea that flat lesions are not missed at CTC. The crude cancer incidence of this group was calculated at 0.2 cancers/1000 patient follow up years.12 If the majority of flat lesions were missed at CTC, presumably a large number of interval cancers would occur in the patients deemed previously negative at cancer screening.
The factors of size and location both positively impact on the likelihood of polyp coating. While most flat polyps regardless of size or location coat with contrast, polyps that are ≥ 10 mm in size or located in the proximal colon are more likely to do so. In our series, the odds ratios for coated large and proximal flat polyps were substantially increased. Ultimately, over 90% of flat polyps over the size of 10 mm demonstrated overlying adherent contrast, as well as approximately 85% of flat polyps in a proximal location in the colon (Fig. 3). Carpet lesions, which are flat colorectal masses (≥ 3 cm), were excluded from consideration herein due to a lesion height > 3 mm. However, it has been shown that these lesions also demonstrate this phenomenon of contrast coating at CTC.
These observations involving contrast coating of flat polyps bode well for CTC use in colorectal screening. In recent years, a second precursor polyp type, the serrated polyp (also known as a serrated adenoma), has been increasingly recognized. Whereas all hyperplastic polyps have traditionally been considered innocuous without future potential for malignant transformation, it is now known that a small subset may progress to cancer. This subset of hyperplastic polyps have been reclassified as sessile serrated adenomas (or sessile serrated polyps) and traditional serrated adenomas, both of which have future malignant potential.18 The nomenclature is confusing as these lesions are of a hyperplastic lineage and not of an adenomatous origin. As opposed to the changes seen in the adenoma pathway within genes including APC, k-ras, and p53, these polyps undergo genetic alterations in the B-RAF oncogene (and in some cases, k-ras) with subsequent various epigenetic events. Ultimately, DNA mismatch repair genes are involved leading to micro-satellite instability.19 This minor pathway accounts for approximately 15% of sporadic cancers.20 The serrated lesions of consequence are typically large (≥ 10 mm), right-sided (i.e., proximal colon), and flat in morphology.21 These lesions are precisely the polyps that are most likely to be coated at CT colonography. In our series, histologic examination of the resected flat cohort demonstrated that over 90% of serrated and hyperplastic polyps coated with contrast. Conversely, it may be these lesions that are difficult to detect at colonoscopy, leading to the deceased protective effects of this test seen for right sided cancers.22
The mechanism of contrast coating of polyps remains speculative. However, it is well recognized that polyps, particularly serrated and hyperplastic polyps, can elaborate an overlying mucin coat.23,24 At optical colonoscopy, this is commonly seen at clinical practice. One hypothesis is that the negatively charged mucin coat attracts the positively charged barium within the tagging regimen to create the adherent contrast coat seen at CTC. It is unknown whether this phenomenon is affected by differing bowel preparations including differences in cathartic agents and iodine-only based tagging regimens. It is our theory that an osmotic cathartic which leads to low residual fluid and overall ‘dry’ colon such as magnesium citrate promotes this action as compared to a wet preparation such as polyethylene glycol which results in larger amounts of residual colonic fluid.25,26 Diatrizoate then causes a final catharsis (in addition to tagging the fluid) due to its hypertonic nature to wash away the barium from normal mucosa but not from the polyp.
Some limitations of this study include the issue of clustering. Patients could harbor multiple polyps (the unit of analysis) and thus multiple polyps from a few patients could bias the results. However, we feel that this is a limited effect as the majority of patients (n=28) with multiple polyps typically had only two polyps (82.1%) or three polyps (14.3%). And secondly, because not all patients went to colonoscopy (i.e., negative CTC cohort in routine screening), the polyp coating percentages could be skewed upwards related to inclusion bias as it is possible that CTC false negative polyps would more likely not coat (and thus be more difficult to detect).
In conclusion, polyp coating by tagging agents is an unintended consequence of the CTC bowel preparation regimen that occurs in a high percentage of flat polyps. It occurs more often in larger and right sided polyps which describes the newly discovered serrated polyp precursors for colon cancer. This phenomenon may be a major factor why flat polyps can be detected at CT colonography. Physicians who interpret CTC should be aware of this tagging property to increase flat polyp detection.
Key Points.
Surface contrast coating of most flat colorectal polyps at CT colonography.
Large size, proximal colonic location, and serrated/hyperplastic histology increase polyp coating.
Contrast coating increases diagnostic confidence for flat polyps.
Contrast coating may help in flat polyp detection at CTC.
Acknowledgements
The scientific guarantor of this publication is David H. Kim, MD. The authors of this manuscript declare relationships with the following companies: D Kim is a consultant for Viatronix, co-founder of VirtuoCTC, and is on the medical advisory board for Digital Artforms; P. Pickhardt is a consultant for Viatronix, Mindways, and Braintree, and a co-founder of VirtuoCTC. This study has received funding in part by 1R01CA144835-01 (NIH/NCI). One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Some study subjects or cohorts have been previously reported in Pickhardt PJ et al. Acad Radiol 2010;17:784-790.
Methodology: retrospective, observational, performed at one institution.
References
- 1.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 2.Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Van Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: Initial results-polyp detection and patient acceptance. Radiology. 2002;224:393–403. doi: 10.1148/radiol.2241011222. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. J Comput Assist Tomogr. 2006;30:51–57. doi: 10.1097/01.rct.0000191686.35968.f1. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Kim DH, Robbins JB. Flat (Nonpolypoid) Colorectal Lesions Identified at CT Colonography in a US Screening Population. Acad Radiol. 2010;17:784–790. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: Implications for screening with CT virtual colonoscopy. Am J Roentgenol. 2004;183:1343–1347. doi: 10.2214/ajr.183.5.1831343. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto T, Mitsuzaki K, Utsunomiya D, et al. Detection of flat colorectal polyps at screening CT colonography in comparison with conventional polypoid lesions. Acta Radiologica. 2012;53:714–719. doi: 10.1258/ar.2012.110685. [DOI] [PubMed] [Google Scholar]
- 7.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. Jama. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Kim DH, Pooler BD, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol. 2013;14:711–720. doi: 10.1016/S1470-2045(13)70216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Lam VP, Weiss JM, Kennedy GD, Kim DH. Carpet lesions detected at CT colonography: clinical, imaging, and pathologic features. Radiology. doi: 10.1148/radiol.13130812. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology. 2005;236:872–878. doi: 10.1148/radiol.2363041534. [DOI] [PubMed] [Google Scholar]
- 11.Pickhardt PJ, Choi JR, Nugent PA, Schindler WR. The effect of diagnostic confidence on the probability of optical colonoscopic confirmation of potential polyps detected on CT colonography: Prospective assessment in 1,339 asymptomatic adults. Am J Roentgenol. 2004;183:1661–1665. doi: 10.2214/ajr.183.6.01831661. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Pooler BD, Weiss JM, Pickhardt PJ. Five year colorectal cancer outcomes in a large negative CT colonography screening cohort. Eur Radiol. 2012;22:1488–1494. doi: 10.1007/s00330-011-2365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL. CT Colonography: Performance and Program Outcome Measures in an Older Screening Population. Radiology. 2010;254:493–500. doi: 10.1148/radiol.09091478. [DOI] [PubMed] [Google Scholar]
- 14.Pickhardt PJ. Screening CT colonography: How I do it. Am J Roentgenol. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 15.Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdom Imaging. 2011;36:538–544. doi: 10.1007/s00261-010-9666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing R Foundation of Statistical Computing. Vienna, Austria: 2009. ISBN 3-900051-07-0 http://www.R-project.org. [Google Scholar]
- 17.Park SH, Ha HK, Kim AY, et al. Flat polyps of the colon: Detection with 16-MDCT colonography - Preliminary results. Am J Roentgenol. 2006;186:1611–1617. doi: 10.2214/AJR.04.1889. [DOI] [PubMed] [Google Scholar]
- 18.Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VLJ. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287–302. doi: 10.1007/s00535-012-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterol Clin N Am. 2007;36:947–968. doi: 10.1016/j.gtc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of Colonoscopy and Death From Colorectal Cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 23.Ishigooka S, Nomoto M, Obinata N, et al. Evaluation of magnifying colonoscopy in the diagnosis of serrated polyps. World J Gastroenterol. 2012;18:4308–4316. doi: 10.3748/wjg.v18.i32.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson JA, Hahn HP, Shahsafaei A, Odze RD. MUC Expression in Hyperplastic and Serrated Colonic Polyps: Lack of Specificity of MUC6. Am J Surg Pathol. 2011;35:742–749. doi: 10.1097/PAS.0b013e31821537a2. [DOI] [PubMed] [Google Scholar]
- 25.Macari M, Lavelle M, Pedrosa I, et al. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218:274–277. doi: 10.1148/radiology.218.1.r01ja31274. [DOI] [PubMed] [Google Scholar]
- 26.Conry BG, Jones S, Bartram CI. The Effect of Oral Magnesium-Containing Bowel Preparation Agents on Mucosal Coating by Barium-Sulfate Suspensions. Br J Radiol. 1987;60:1215–1219. doi: 10.1259/0007-1285-60-720-1215. [DOI] [PubMed] [Google Scholar]