Abstract
Carbapenem-resistant Acinetobacter baumannii is an important cause of nosocomial infections, particularly in patients in the intensive care units. As chronic infections are difficult to treat, attempts have been made to discover new antimicrobials. Ceragenins, designed to mimic the activities of antimicrobial peptides, are a new class of antimicrobial agents. In this study, the in vitro activities of CSA-13 either alone or in combination with colistin (sulphate), tobramycin, and ciprofloxacin were investigated using 60 carbapenem-resistant A. baumannii strains isolated from bacteremia patients blood specimens. MICs and MBCs were determined by microbroth dilution technique. Combinations were assessed by using checkerboard technique. The MIC50 values (mg/L) of CSA-13, colistin, tobramycin, and ciprofloxacin were 2, 1, 1.25, and 80, respectively. The MIC90 (mg/L) of CSA-13 and colistin were 8 and 4. The MBCs were equal to or twice greater than those of the MICs. Synergistic interactions were mostly seen with CSA-13-colistin (55%), whereas the least synergistic interactions were observed in the CSA-13-tobramycin (35%) combination. No antagonism was observed. CSA-13 appears to be a good candidate for further investigations in the treatment of A. baumannii infections. However, future studies should be performed to correlate the safety, efficacy, and pharmacokinetic parameters of this molecule.
1. Introduction
Acinetobacter baumannii is a Gram-negative coccobacillus that recently has become one of the most common and highly antibiotic resistant pathogens throughout the world, and it is associated with high rates of morbidity and mortality [1, 2]. The most common clinical manifestations of A. baumannii infections in the intensive care units (ICUs) are ventilator associated pneumonia (VAP) and bacteremia, which are associated with morbidity and mortality rates as high as 52% [3, 4]. Invariably, one of the most alarming characteristics of this microorganism is its ability to manifest resistance to all available antibiotics including carbapenems, which is even higher than in other Gram-negative bacilli included in the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Enterobacter species) [5]. Multidrug-resistant (MDR) A. baumannii is a growing threat that leaves few therapeutic options and recently there has been a dramatic increase in carbapenem resistance in A. baumannii. The mechanisms of resistance to antimicrobials are principally acquired through its ability to exchange genetic material. This attribute makes the treatment of A. baumannii infections particularly difficult, especially in certain types of infections [6]. The lack of new antibiotics to treat MDR A. baumannii infections has led the Infectious Disease Society of America (IDSA) to describe A. baumannii as “an emblematic case of the mismatch between unmet medical needs and the current antimicrobial research and development pipeline” [7].
As chronic infections are difficult to treat, attempts have been made to discover new antimicrobial agents targeting novel sites that may circumvent resistance. One frequently studied target is the bacterial membrane. Most antimicrobial peptides display broad-spectrum antibacterial activities and target the bacterial membrane. However, many antimicrobial peptides are difficult to synthesize and purify due to their complexity and size [8]. In addition, antimicrobial peptides can be substrates for proteases, which limit their in vivo half-lives [9]. Consequently, development of nonpeptide mimics of antimicrobial peptides may provide a means of using the antimicrobial strategies evolved over eons without the disadvantages of peptide therapeutics.
Recently, a series of cationic derivatives of cholic acid have been synthesized and have been found to have properties that may make them useful antimicrobial agents. The ceragenins, designed to mimic the activities of antimicrobial peptides, are a new class of antimicrobial agent. Ceragenins are not peptide based, are not salt sensitive, and are relatively simple to prepare and purify on a large scale [10]. Among them, CSA-13, which stands for cationic steroidal antimicrobial, is a lead ceragenin and is highly active against Gram-positive and Gram-negative bacteria. MIC determinations against common Gram-positive and Gram-negative bacteria have demonstrated that CSA-13 displays a broad spectrum of activity. CSA-13 displays antimicrobial activity against vancomycin-resistant Staphylococcus aureus [11], Pseudomonas aeruginosa [12, 13], Helicobacter pylori [14], and periodontopathic bacteria such as Streptococcus mutans and Porphyromonas species [15]. CSA-13 is also active against vaccinia virus [16] and Trypanosoma cruzi [17]. In animal studies, CSA-13 shows low toxicity, supporting this compound's possible application in human treatment [18].
In the setting of increasing resistance and diminishing therapeutic options, the “old” antibiotic colistin (polymyxin E) is now being used more extensively, especially in P. aeruginosa and carbapenem-resistant A. baumannii infections [19]. There are no current published studies evaluating the interactions between CSA-13 and colistin against carbapenem-resistant A. baumannii strains isolated from blood specimens. Therefore, the purpose of this study was to evaluate the in vitro activities of CSA-13 alone and in combination with colistin, tobramycin, and ciprofloxacin against 60 carbapenem-resistant A. baumannii strains isolated from bacteremia patients' blood specimens.
2. Materials and Methods
2.1. Bacterial Isolates
A total of sixty nonrepeat, bloodstream strains of carbapenem-resistant A. baumannii recovered from bacteremia patients in the year 2010-2011 admitted to the various hospitals in Turkey were included in the study. Thirty of these strains are obtained from the Department of Infectious Diseases and Clinical Microbiology, Medipol University, Istanbul, twenty of them are obtained from the Department of Infectious Diseases and Clinical Microbiology, Faculty of Medicine, Istanbul University, Istanbul, and the rest of them are obtained from Canakkale Onsekiz Mart University, Faculty of Medicine, Canakkale, Turkey. All strains were identified by the API 20 NE System (bioMerieux Vitek, Marcy l'Etoile, France). Isolates were defined as carbapenem-resistant strains using the disc diffusion and microdilution method. For the checkerboard experiments totally 20 strains from the three different institutions were used, since we carried out the combination experiments with susceptible strains. Escherichia coli ATCC 25922 (Rockville, Md., USA) was used as a quality control strain.
2.2. Antimicrobial Agents
CSA-13 was synthesized from a cholic acid scaffold technique as previously described (Figure 1) [20]. Colistin was obtained from Sigma Aldrich and tobramycin, ciprofloxacin, and meropenem were kindly provided from Bilim and Bayer Pharmaceuticals and Astra Zeneca, respectively. Stock solutions from dry powders were prepared in water and stored frozen at −80°C. Frozen solutions of antibiotics were used within 6 months.
Figure 1.
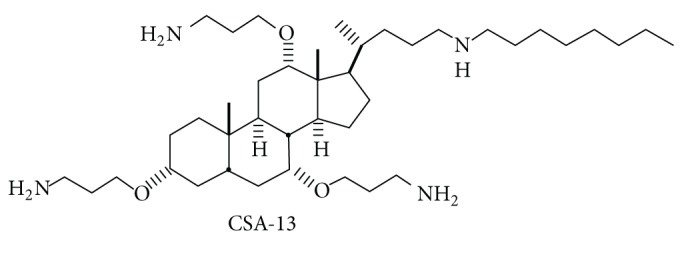
The chemical structure of ceragenin CSA-13 (molecular weight 822.94).
2.3. Media
Mueller-Hinton broth (MHB, Difco Laboratories, Detroit, MI) supplemented with divalent cations to a final concentration of 25 mg of Mg2+ and 50 mg of Ca2+ per liter (CSMHB) was used for all the experiments. Pour plates of Tryptic Soy agar (TSA, Difco Laboratories, Detroit, MI) were used for colony counts.
2.4. Determinations of MICs and MBCs
MICs were determined by the microbroth dilution technique as described by CLSI [21, 22]. Serial twofold dilutions ranging from 256 to 0.25 mg/L were prepared in CSMHB. The inoculum was prepared with a 4–6 h broth culture that gives a final concentration of 5 × 105 cfu/mL in the test tray. Experiments were performed in duplicate. MBCs were determined at the conclusion of the incubation period by removing two 0.01 mL samples from each well demonstrating no visible growth and plated onto TSA. The MBC was defined as the lowest concentration of antibiotic giving at least a 99.9% killing of the initial inoculums [23].
2.5. Determination of Fractional Inhibitory Concentration Index (FICI)
The effects of antibiotics in combination were assessed by using the microbroth checkerboard technique [24]. Each microtiter well containing the mixture of antibiotics was inoculated with a 4–6 h broth culture diluted to give a final concentration of approximately 5 × 105 cfu/mL. After incubation at 37°C for 18–20 h the fractional inhibitory concentration index (FICI) was determined as the combined concentration divided by the single concentration. The combination value was derived from the highest dilution of antibiotic combination permitting no visible growth. With this method, synergy was defined as a FICI of ≤0.5, no interaction as a FICI of >0.5–4, and antagonism as a FICI of 4.0 [25].
3. Results
3.1. Susceptibility
The in vitro activities of the studied antibiotics against 60 A. baumannii strains are summarized in Table 1. Susceptibility testing demonstrated that the MIC ranges for CSA-13, colistin, tobramycin, and ciprofloxacin were 1–16, 0.06–32, 0.3–160, and 0.3–80 mg/L and MBC ranges for those antibiotics were 1–32, 0.06–32, 0.3–160, and 0.6–160 mg/L, respectively. As seen from the results, CSA-13 showed a similar pattern of MIC and MBC ranges as colistin. In addition, the highest MIC and MBC values of CSA-13 were just onefold higher of the MIC90 and MBC90 values. However, 14%, 55%, and 95% of the strains were found resistant to colistin, tobramycin, and ciprofloxacin, respectively. All the strains were resistant to meropenem. CSA-13 MICs (and also MBCs) of the colistin-resistant strains are at the same value or twofold greater than those of the colistin-resistant strains. There was no major difference between bactericidal and inhibitory endpoints. The MBCs were generally equal to or twofold greater than those of the MICs.
Table 1.
Comparative in vitro activity of antimicrobial agents against 60 isolates of A. baumannii.
| Antibiotics | mg/L | Percent inhibited at CLSI breakpointsa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | MBC range | MBC50 | MBC90 | Susceptible | M.S.b | Resistant | |
| CSA-13 | 1–16 | 2 | 8 | 1–32 | 2 | 16 | — | — | — |
| Colistin | 0.06–32 | 1 | 4 | 0.06–32 | 2 | 8 | 86 | 0 | 14 |
| Tobramycin | 0.3–160 | 1.25 | 80 | 0.3–160 | 2.5 | 160 | 45 | 0 | 55 |
| Ciprofloxacin meropenem |
0.3–80 16–128 |
80 32 |
160 64 |
0.6–160 16–256 |
80 64 |
160 128 |
5 0 |
0 0 |
95 100 |
aCLSI breakpoints for susceptible and resistant to colistin ≤2 mg/L and ≥4 mg/L; tobramycin ≤4 mg/L and ≥16 mg/L; ciprofloxacin ≤1 mg/L and ≥4 mg/L and meropenem ≤4 mg/L and ≥16 mg/L, respectively.
bM.S.: moderately susceptible.
3.2. Checkerboard
The results of combination studies are shown in Table 2. With a FIC index of ≤0.5 as borderline, synergistic interactions were mostly seen with CSA-13-colistin combination (synergism was observed with 55% of the strains tested), whereas the least synergistic interactions were observed with the CSA-13-tobramycin combination (synergism was observed with 35% of the strains tested). No antagonism was observed with any combination.
Table 2.
In vitro activity of CSA-13 and colistin combined with studied antibiotics against A. baumannii strains.
| Antibiotic combinations | n | Number (%) of synergistic effects |
|---|---|---|
| CSA-13 + colistin | 20 | 11 (55) |
| CSA-13 + tobramycin | 20 | 7 (35) |
| CSA-13 + ciprofloxacin | 20 | 8 (40) |
| colistin + tobramycin | 20 | 9 (45) |
| colistin + ciprofloxacin | 20 | 9 (45) |
4. Discussion
Ceragenins are a group of cholic acid derivatives that have potent activities against various microorganisms [10]. Here, we report an MIC50 of 2 mg/L for CSA-13 against 60 carbapenem-resistant A. baumannii strains. As seen from the results, MIC90 value of CSA-13 was equal to two dilutions higher of the MIC50 value, which is parallel to colistin results (Table 1). These results indicate that CSA-13 shows an activity with similar MIC values independent of whether or not the bacteria are resistant to other antibiotics. Probably, this situation could be attributed to its ability to permeabilize both outer and cytoplasmic membrane of the bacteria and its resistance to protease degradation [26]. These results support the idea that development of resistance to CSA-13 might be rare if it is used in the treatment. Our study also shows that CSA-13 has an MIC50/MBC50 ratio of 1, suggesting that the bactericidal activity is close to the inhibitory concentration. Indeed, varying CSA-13 concentrations at, below, and above the MIC demonstrated rapid bactericidal antimicrobial activity, even when the strains were resistant to colistin, ciprofloxacin, and/or tobramycin, similar to our previous work [13].
Carbapenem resistance rates are increasing to such an extent to threaten the world and this situation is becoming a routine phenotype for the A. baumannii. Therefore, in order to take the microorganism under control, selection of the antimicrobial agents is extremely important [27]. Increase of carbapenem resistance raises the fact that the reuse of old antibiotics like polymyxin E. (colistin) has a lower rate of mortality than carbapenems in treatment of multidrug resistance infections [19]. Colistin is frequently used to treat infections caused by carbapenem-resistant A. baumannii, due to its efficacy [28]. However, recently colistin resistance is reported worldwide, especially in Europe [29, 30]. In our country, Ergin et al. reported colistin resistance as 2% in A. baumannii [31]. According to our study, 86% of the strains were found to be colistin susceptible. Moreover, we demonstrated that of all the studied antibiotics, colistin was active at the lowest MIC (MIC50 = 1 μg/mL) value against the strains. This may arise from the fact that colistin has been recently used for clinical use in Turkey.
Results of our study showed that the highest resistance rate is obtained with ciprofloxacin (95%). This was in accordance with one multicentric study [32]. High percentages of strains belonging to A. baumannii were resistant to ciprofloxacin, ofloxacin, and cefotaxime (79, 76, and 54%, resp.) by agar dilution method.
Management of the infections caused by carbapenem-resistant A. baumannii is difficult and combination therapy for the treatment of carbapenem-resistant A. baumannii has increasingly been used [28]. Therefore, in our study, in vitro interactions of CSA-13 in combination with colistin, tobramycin, and ciprofloxacin against carbapenem-resistant A. baumannii strains were assessed by using the microbroth checkerboard technique since it provides fast results and interpretation of these results is simple [24]. The results of this in vitro trial provide evidence that, with a FICI of ≤0.5 as borderline, synergistic interactions were detected in all combinations (Table 2). Synergistic interactions were mostly seen with CSA-13-colistin combination (55% of tested strains), whereas the least synergistic interactions were observed with the CSA-13-tobramycincombination (35% of tested strains).
Consequently, ceragenins are novel molecules resistant to proteolysis and promise opportunities in treatment of bacterial, fungal, and even viral infections. According to the results of this in vitro study, CSA-13 may have important therapeutic implications for infections caused by carbapenem-resistant A. baumannii strains. So, these molecules should be evaluated carefully and must be reserved for the most important necessity. Possible success for the combination therapy of ceragenins and colistin or other antibiotics depends on the pharmacokinetics and pharmacodynamics of these molecules in vivo. Therefore, future studies should be performed to correlate the safety, efficacy, and pharmacokinetic parameters of these combinations.
Acknowledgment
This work was supported by a grant from the Research Fund of Istanbul University (Project no. BYP 27662).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. Extensively drug-resistant Acinetobacter baumannii . Emerging Infectious Diseases. 2009;15(6):980–982. doi: 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii . The Journal of Antimicrobial Chemotherapy. 2010;65(2):233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 3.Cisneros JM, Reyes MJ, Pachón J, et al. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clinical Infectious Diseases. 1996;22(6):1026–1032. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 4.Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii: clinical features, epidemiology, and predictors of mortality. Medicine. 1995;74(6):340–349. doi: 10.1097/00005792-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Llaca-Díaz JM, Mendoza-Olazarán S, Camacho-Ortiz A, Flores S, Garza-González E. One-year surveillance of ESKAPE pathogens in an intensive care unit of Monterrey, Mexico. Chemotherapy. 2012;58(6):475–481. doi: 10.1159/000346352. [DOI] [PubMed] [Google Scholar]
- 6.Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Current Opinion in Infectious Diseases. 2010;23(4):332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 7.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clinical Infectious Diseases. 2008;46(8):1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 8.Savage PB, Li C, Taotafa U, Ding B, Guan Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiology Letters. 2002;217(1):1–7. doi: 10.1111/j.1574-6968.2002.tb11448.x. [DOI] [PubMed] [Google Scholar]
- 9.Hadley EB, Hancock REW. Strategies for the discovery and advancement of novel cationic antimicrobial peptides. Current Topics in Medicinal Chemistry. 2010;10(18):1872–1881. doi: 10.2174/156802610793176648. [DOI] [PubMed] [Google Scholar]
- 10.Lai X-Z, Feng Y, Pollard J, et al. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Accounts of Chemical Research. 2008;41(10):1233–1240. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- 11.Chin JN, Rybak MJ, Cheung CM, Savage PB. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2007;51(4):1268–1273. doi: 10.1128/AAC.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa . Journal of Antimicrobial Chemotherapy. 2008;61(2):365–370. doi: 10.1093/jac/dkm457. [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt-Guzel C, Savage PB, Gerceker AA. In vitro activities of the novel ceragenin CSA-13, alone or in combination with colistin, tobramycin, and ciprofloxacin, against Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Chemotherapy. 2012;57(6):505–510. doi: 10.1159/000335588. [DOI] [PubMed] [Google Scholar]
- 14.Leszczyska K, Namiot A, Fein DE, et al. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiology. 2009;9, article 187 doi: 10.1186/1471-2180-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isogai E, Isogai H, Takahashi K, Okumura K, Savage PB. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral Microbiology and Immunology. 2009;24(2):170–172. doi: 10.1111/j.1399-302X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 16.Howell MD, Streib JE, Kim BE, et al. Ceragenins: a class of antiviral compounds to treat orthopox infections. Journal of Investigative Dermatology. 2009;129(11):2668–2675. doi: 10.1038/jid.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lara D, Feng Y, Bader J, Savage PB, Maldonado RA. Anti-trypanosomatid activity of ceragenins. Journal of Parasitology. 2010;96(3):638–642. doi: 10.1645/GE-2329.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Savage PB, Bal M. Enhancement of the efficacy of erythromycin in multiple antibiotic-resistant gram-negative bacterial pathogens. Journal of Applied Microbiology. 2008;105(3):822–828. doi: 10.1111/j.1365-2672.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- 19.Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Clinical Microbiology and Infection. 2012;18(1):18–29. doi: 10.1111/j.1469-0691.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 20.Guan Q, Li C, Schmidt EJ, et al. Preparation and characterization of cholic acid-derived antimicrobial agents with controlled stabilities. Organic Letters. 2000;2(18):2837–2840. doi: 10.1021/ol0062704. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Seventh Edition. Wayne, Pa, USA: CLSI; 2006. (Approved Standard M7-A7). [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty second Informational Supplement. Wayne, Pa, USA: CLSI; 2012. (M100-S22). [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents—Approved Guideline. Wayne, Pa, USA: NCCLS; 1999. (M26-A). [Google Scholar]
- 24.Pillai SK, Moellering RC, Jr., Eliopoulos GM. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2005. pp. 365–440. [Google Scholar]
- 25.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. Journal of Antimicrobial Chemotherapy. 2003;52(1, article 1) doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 26.Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrobial Agents and Chemotherapy. 2010;54(9):3708–3713. doi: 10.1128/AAC.00380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogue JM, Mann T, Barber KE, Kaye KS. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Review of Anti-infective Therapy. 2013;11(4):383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- 28.Carmeli Y, Akova M, Cornaglia G, et al. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clinical Microbiology and Infection. 2010;16(2):102–111. doi: 10.1111/j.1469-0691.2009.03115.x. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. Journal of Antimicrobial Chemotherapy. 2012;67(7):1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 30.Giannouli M, Cuccurullo S, Crivaro V, et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a tertiary care hospital in Naples, Italy, shows the emergence of a novel epidemic clone. Journal of Clinical Microbiology. 2010;48(4):1223–1230. doi: 10.1128/JCM.02263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ergin A, Hascelik G, Eser OK. Molecular characterization of oxacillinases and genotyping of invasive Acinetobacter baumannii isolates using repetitive extragenic palindromic sequence-based polymerase chain reaction in Ankara between 2004 and 2010. Scandinavian Journal of Infectious Diseases. 2013;45(1):26–31. doi: 10.3109/00365548.2012.708782. [DOI] [PubMed] [Google Scholar]
- 32.Jones RN, Pfaller MA, Marshall SA, Hollis RJ, Wilke WW. Antimicrobial activity of 12 broad-spectrum agents tested against 270 nosocomial blood. Stream infection isolates caused by non-enteric gram-negative bacilli: occurrence of resistance, molecular epidemiology, and screening for metallo-enzymes. Diagnostic Microbiology and Infectious Disease. 1997;29(3):187–192. doi: 10.1016/s0732-8893(97)81808-1. [DOI] [PubMed] [Google Scholar]


