Abstract
Most Candida spp. infections are associated with biofilm formation on host surfaces. Cells within these communities display a phenotype resistant to antimicrobials and host defenses, so biofilm-associated infections are difficult to treat, representing a source of reinfections. The present study evaluated the effect of eugenol on the adherence properties and biofilm formation capacity of Candida dubliniensis and Candida tropicalis isolated from the oral cavity of HIV-infected patients. All isolates were able to form biofilms on different substrate surfaces. Eugenol showed inhibitory activity against planktonic and sessile cells of Candida spp. No metabolic activity in biofilm was detected after 24 h of treatment. Scanning electron microscopy demonstrated that eugenol drastically reduced the number of sessile cells on denture material surfaces. Most Candida species showed hydrophobic behavior and a significant difference in cell surface hydrophobicity was observed after exposure of planktonic cells to eugenol for 1 h. Eugenol also caused a significant reduction in adhesion of most Candida spp. to HEp-2 cells and to polystyrene. These findings corroborate the effectiveness of eugenol against Candida species other than C. albicans, reinforcing its potential as an antifungal applied to limit both the growth of planktonic cells and biofilm formation on different surfaces.
1. Introduction
The prevalence of oral colonization by Candida spp. can vary among different population groups [1], and the presence of these fungi as commensals of human microbiota is one important predisposing factor for candidosis [2]. Adherence of the microorganisms to host cells and tissues is the first event required for initial colonization or establishment of infection. Moreover, the microbial surface contact can trigger various cellular behaviors, including biofilm formation [1], which is also strongly associated with candidosis [3].
Biofilms can be defined as irreversibly surface-attached communities of cells (sessile cells) embedded in a self-produced exopolymeric matrix, displaying a distinctive phenotype compared to their free-floating (planktonic cells) counterparts [4]. Remarkably, sessile cells are less susceptible to a variety of antifungal agents [5–7] and to host defenses [8]. Biofilms are thereby difficult to eradicate, representing a source of reinfections. Consequently, new antifungal agents are urgently needed, particularly those with antibiofilm activities, for effective management of Candida spp. infections.
Several researchers have shown the anti-Candida biofilm potential of plant-derived compounds such as flavonoids [9] and essential oils [10, 11]. Eugenol is the main active phenylpropanoid component of the essential oil from many aromatic plants [12]. The inhibitory effect of eugenol alone [13–17] and in combination with fluconazole and amphotericin B [18] against planktonic cells of Candida spp. has been previously reported. In addition, eugenol can interfere with the initial phases of biofilm formation, as well as with the viability of mature biofilm of Candida albicans [10, 11].
Although C. albicans continues to be the most common causative agent of candidosis in humans, other species of Candida have become a significant cause of such infections [19, 20]. Candida tropicalis and Candida dubliniensis have been regarded as high biofilm producers, and sessile cells within this community have been found to be resistant to antifungal agents [5–7]. Accordingly, we analyzed the effect of eugenol on the hydrophobicity and adhesion to human epithelial cells and polystyrene of planktonic cells of these species. Moreover, the inhibitory activity against biofilm formation on polystyrene and denture materials was also analyzed.
2. Materials and Methods
2.1. Candida spp. Isolates and Growth Conditions
The Candida species used in this study included three C. dubliniensis (strains 131, 219, and 248) and three C. tropicalis (strains 23, 150, and 176) isolated from the oral cavity of HIV-infected patients. The species identification of oral isolates was carried out by standard mycological methods [21, 22]. Species identification was confirmed by a PCR-based method using specific primers as previously described [23, 24]. C. tropicalis ATCC 28707 and C. dubliniensis ATCC MYA-646 type strains (kindly provided by FIOCRUZ, Rio de Janeiro, Brazil) were included as quality control. The isolates and strains were stored on Sabouraud dextrose (SD) agar and subcultured monthly. The yeasts were also maintained at −80°C. The study protocol was in accordance with the Ethics Committee of the Universidade Estadual de Londrina (CEP/UEL no. 036/10). Written informed consent was obtained from the patients for the publication of this report.
2.2. Biofilm Formation
Candida isolates were cultured in SD broth and incubated at 37°C for 18 h. The yeasts were harvested by centrifugation and washed twice with sterile 0.15 M phosphate-buffered saline, pH 7.2 (PBS), and the cells were counted in hemocytometric chamber (Neubauer Improved Chamber). A 20 μL SD broth suspension of 6 × 105 yeasts was placed into each well of flat-bottomed 96-well microtiter plates (Techno Plastic Products, Switzerland) containing 180 μL of SD broth. The plates were incubated at 37°C for 24 h. Afterwards, the wells were washed once with sterile distilled water, and the metabolic activity of the cells was quantified using the 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT)-reduction assay. A 100 μL aliquot of XTT-menadione (0.1 mg/mL XTT, 1 μM menadione, Sigma Chemical Co, USA) was added to each well, and the plates were incubated in the dark for 2 h at 37°C. The XTT formazan product was measured at 490 nm with a microtiter plate reader (Universal Microplate Reader ELx 800, Bio-Tek Instruments, USA) [6]. To analyze the biofilm formation on denture material surfaces, the wells were aseptically coated with polymethylmethacrylate (PMM, OrtoClass, Clássico, Brazil) and ceramic (Noritake, Shofu Dental Corp., USA) before the assay.
2.3. Antifungal Susceptibility Testing
The growth inhibitory effect of eugenol (SSWhite, Brazil) on planktonic cells of Candida isolates was determined by broth microdilution assays according to the Clinical and Laboratory Standards Institute [25]. A stock solution of eugenol was prepared in water containing 10% dimethylsulfoxide (DMSO v/v, Sigma Chemical Co., USA). The DMSO final concentration in the assays did not exceed 1.0%. The substance was serially diluted 2-fold in RPMI buffered with MOPS, pH 7.0 (3000–5.85 μg/mL eugenol). Quality control C. dubliniensis ATCC MYA-646 and C. tropicalis ATCC 28707 and fluconazole (512.0–0.5 μg/mL, Pfizer Central Research, United Kingdom) were included in each experiment. Two wells of each plate served as growth and sterility controls. The minimum inhibitory concentrations (MICs) were determined at total inhibition of visual growth after 24 h incubation compared to untreated planktonic cells. To determine antifungal susceptibilities of sessile cells, biofilms were formed on polystyrene, as described above. After 1 and 24 h of biofilm formation, the medium was aspirated off and each well was washed three times with sterile PBS. A 200 μL aliquot of RPMI 1640 medium containing serial 2-fold dilutions of eugenol and fluconazole was added, and the plates were further incubated for 24 h at 37°C. Controls included antifungal-free wells and biofilm-free wells. Sessile MICs were determined at 100% inhibition (SMIC100) compared to antifungal-free control wells using the XTT-reduction assay [6]. To evaluate the time-dependent effect of eugenol, 24 h biofilms of Candida species were formed in polystyrene and treated with SMIC100 of eugenol as described above. At determined time points (3, 6, 12, and 24 h), the metabolic activity of sessile cells was determined. All experiments were carried out in triplicate on three different occasions.
2.4. Cell Surface Hydrophobicity Determination
The hydrophobicity of untreated and eugenol-treated (0.5 × MIC for 1 h) planktonic cells was determined by the biphasic hydrocarbon/aqueous method according to Anil et al. [26]. Cell surface hydrophobicity (CSH) was expressed as the percentage decrease in optical density of the aqueous phase of the test as compared with the control, where the greater the change in absorbance of the aqueous phase, the more hydrophobic the yeast sample. Each assay was performed on three separate occasions with triplicate determinations each time.
2.5. Adhesion of Yeasts to HEp-2 Cells and Polystyrene
HEp-2 cells (human larynx epidermoid carcinoma) were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B in a humidified 5% CO2 atmosphere at 37°C. For adhesion assays, HEp-2 cells were seeded in 24-well plates at 4.0 × 105 cells per well and incubated for 18 h. The medium was removed and each well was washed three times with sterile PBS. The fresh culture medium minus the antimicrobials was added and the wells were inoculated with untreated and eugenol-treated Candida spp. (0.5 × MIC for 1 h) with approximately 2.0 × 106 cells, and the plates were incubated at 37°C for 2 h in a 5% CO2 atmosphere. Nonadherent yeasts were removed by washing with sterile PBS. Adherent yeasts were harvested by treatment of the cell monolayers with 1 mL 0.5% (v/v) Triton X-100 (Sigma Chemical Co.) for 10 min on ice. The viable yeasts were enumerated by dilution plating in SD agar. Experiments were carried out in duplicate on three different occasions. The percent adherence was calculated by the equation: % Adherence = (cfu120/cfu0) × 100, where cfu120 refers to adhered cells per mL after 2 h and cfu0 the initial number of inoculated cells. The adhesion on polystyrene surface was performed as described for biofilm formation with minor modifications. Briefly, untreated and eugenol-treated (0.5 × MIC for 1 h) planktonic cells were placed in each well, and the plates were incubated for 2 h. The metabolic activity of adherent cells was determined using the XTT-reduction assay as described above.
2.6. Scanning Electron Microscopy (SEM)
Discs (0.8 cm diameter) of PMM and ceramic were aseptically placed in wells of 24-well tissue culture plates (Techno Plastic Products, Switzerland). A standard inoculum of 3.0 × 106 cells, from overnight culture of the yeast, was prepared in 1 mL of RPMI 1640 medium, pH 7.0, and used to form biofilm on these surfaces. The discs were then immersed in these cell suspension and incubated statically at 37°C for 24 h. Afterwards, nonadherent organisms were removed by washing gently three times with PBS. One milliliter of RPMI containing eugenol (SMIC100) was added and the plates were further incubated for 24 h. Biofilms formed on these strips were fixed with 2.5% (v/v) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) at room temperature. After fixation, the cells were dehydrated with a series of ethanol washes (15, 30, 50, 70, 80, 90, 95, and 100%), critical-point dried in CO2, coated with gold and examined with a SHIMADZU SS-550 scanning electron microscope.
2.7. Statistical Analysis
The results were evaluated by Student's t-test using the software GRAPHPAD PRISM version 5.0 (GRAPHPAD Software, San Diego, CA). P values less than 0.05 were considered significant.
3. Results
3.1. Biofilm Formation on Abiotic Surfaces
Biofilm formation by Candida species on polystyrene, PMM, and ceramic was monitored using the XTT-reduction assay. All isolates were able to form biofilms on these different substrate surfaces within 24 h, as assessed by the metabolic activity of sessile cells (Table 1). No significant differences in metabolic activities were observed between the strains and isolates in each substrate analyzed. However, a significant difference (P < 0.05) was observed between the substrates, where the highest biofilm formation was detected on polystyrene surface, followed by PMM and ceramic. The mean OD490 nm (optical density at 490 nm ± standard deviation) was 1.032 ± 0.090 for the polystyrene, 0.777 ± 0.037 for PMM, and 0.598 ± 0.051 for ceramic surfaces.
Table 1.
Metabolic activities of biofilm formed by Candida dubliniensis and Candida tropicalis on different substrate surfaces.
| Isolate | Metabolic activity (OD)a | ||
|---|---|---|---|
| Polystyrene | PMM | Ceramic | |
| Candida dubliniensis | |||
| ATCC MYA-646 | 0.855 ± 0.029 | 0.711 ± 0.056 | 0.499 ± 0.055 |
| 131 | 1.045 ± 0.032 | 0.795 ± 0.058 | 0.628 ± 0.056 |
| 219 | 0.989 ± 0.033 | 0.751 ± 0.054 | 0.566 ± 0.057 |
| 248 | 1.094 ± 0.034 | 0.810 ± 0.055 | 0.637 ± 0.056 |
| Candida tropicalis | |||
| ATCC 28707 | 0.978 ± 0.029 | 0.745 ± 0.058 | 0.559 ± 0.056 |
| 23 | 1.136 ± 0.032 | 0.815 ± 0.056 | 0.638 ± 0.057 |
| 150 | 1.100 ± 0.034 | 0.801 ± 0.056 | 0.635 ± 0.057 |
| 176 | 1.056 ± 0.031 | 0.786 ± 0.057 | 0.624 ± 0.054 |
|
| |||
| Mean ± SD | 1.032 ± 0.090* | 0.777 ± 0.037# | 0.598 ± 0.051¥ |
aMetabolic activity of sessile cells was determined by the XTT-reduction assay. The XTT formazan product was measured at 490 nm.
∗,#,¥Means not sharing a symbol differ significantly (P < 0.05) between the abiotic surfaces.
3.2. Antifungal Activity against Planktonic and Sessile Cells
The MICs and SMICs of eugenol and fluconazole for the Candida spp. isolates and type strains are reported in Table 2. Planktonic cells of all isolates and the type strain of C. dubliniensis were susceptible to fluconazole. However, variation in fluconazole susceptibility was observed for C. tropicalis, where the reference type strain (ATCC 28707) and isolate 176 were resistant, isolate 150 dose-dependently susceptible, and isolate 23 susceptible to fluconazole, according to the CLSI [25] interpretative breakpoints. The biofilm of these Candida species exhibited high resistance to fluconazole. The SMIC100 of this compound for all isolates and type strains was higher than 512 μg/mL. The MIC values of eugenol for C. dubliniensis and C. tropicalis planktonic cells ranged from 375 to 750 μg/mL. Trailing growth was observed when fluconazole was tested against all C. tropicalis strains, while eugenol completely inhibited the growth of planktonic cells. Eugenol also exhibited an inhibitory effect against mature biofilms of Candida species, which appeared to be dose dependent (Figure 1(a)). There was a more than 80% reduction in metabolic activity of 24 h sessile cells with eugenol at concentrations of 187.5 to 750 μg/mL. No metabolic activity was detected at concentrations ranging from 375 to 1500 μg/mL, and these values were considered the SMIC100. The inhibitory effect of eugenol against 24 h sessile cells was also time dependent (Figure 1(b)). The reduction in metabolic activity ranged from 11.1 to 31.6%, 76.6 to 85.5%, and 90.6 to 93.5% after incubation in the presence of SMIC100 eugenol for 3, 6, and 12 h, respectively. No detectable metabolic activity was observed after 24 h treatment. Eugenol also interfered with biofilm formation, since treatment of 1 h adherent cells resulted in dose-dependent reduction of their metabolic activity (data not shown). The SMIC100 for 1 h adherent cells ranged from 375 to 750 μg/mL (Table 2).
Table 2.
Antifungal concentrations of eugenol and fluconazole against planktonic and sessile cells of Candida dubliniensis and Candida tropicalis.
| Yeast | Eugenol | Fluconazole | |||
|---|---|---|---|---|---|
| MICa | SMIC-1b | SMIC-24c | MICd | SMICc | |
| Candida dubliniensis | |||||
| ATCC MYA-646 | 375 | 375 | 375 | 8 | >512 |
| 131 | 750 | 750 | 1,500 | 4 | >512 |
| 219 | 375 | 750 | 1,500 | 8 | >512 |
| 248 | 375 | 750 | 750 | 4 | >512 |
| Candida tropicalis | |||||
| ATCC 28707 | 375 | 375 | 375 | 128 | >512 |
| 23 | 375 | 750 | 750 | 8 | >512 |
| 150 | 750 | 750 | 1,500 | 32 | >512 |
| 176 | 375 | 750 | 1,500 | 64 | >512 |
aMinimum inhibitory concentration of the antifungal which resulted in total inhibition of visible planktonic cell growth; bMinimum inhibitory concentration of the antifungal which resulted in total reduction in metabolic activity of sessile cells, using the XTT-reduction assay, after 1 h of adhesion; cMinimum inhibitory concentration of the antifungal which resulted in total reduction in metabolic activity of sessile cells, using the XTT-reduction assay, after 24 h of biofilm formation; dMIC was defined according to CLSI (2008) guidelines for fluconazole broth microdilution assays; The results are expressed as μg/mL.
Figure 1.
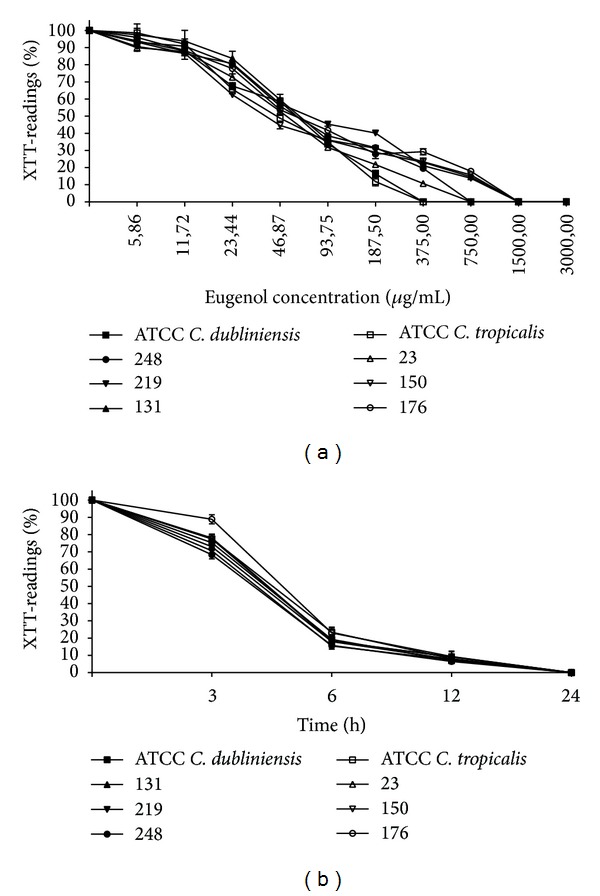
Effect of eugenol on viability of mature biofilm of Candida dubliniensis and Candida tropicalis. (a) The mature biofilms (24 h) were incubated in the presence of different concentrations (3000.0–5.86 μg/mL) of eugenol for 24 h at 37°C. (b) The mature biofilms were incubated with SMIC100 concentrations of eugenol at 37°C and the metabolic activity of sessile cells was assessed at determined time points (3–24 h). Values are expressed as the average percentage of optical density (OD) of wells containing treated biofilms compared to that of control wells (considered to be 100%) for the XTT assays.
3.3. Scanning Electron Microscopy of Candida tropicalis Biofilm on Denture Materials
The effect of eugenol on C. tropicalis (isolate 150, MIC of fluconazole = 32 μg/mL) biofilms formed on PMM and ceramic surfaces was monitored by SEM (Figure 2). Mature biofilms of untreated cells of this isolate consisted of a dense network of cells, composed of a heterogeneous layer of yeast, pseudohyphae, and hyphae (Figures 2(a), 2(c), 2(e), and 2(g)). The treatment of biofilms with eugenol drastically reduced the amount of sessile cells of C. tropicalis on the denture materials surfaces (Figures 2(b), 2(d), 2(f), and 2(h)).
Figure 2.
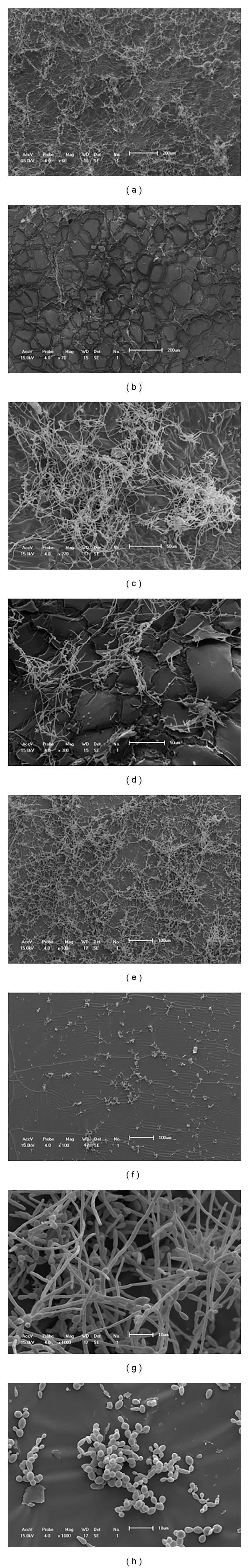
Scanning electron microscopy images of the effect of eugenol on Candida tropicalis mature biofilm formed on the surface of polymethylmethacrylate ((a)–(d)) and ceramic ((e)–(h)). Untreated mature biofilms ((a), (c), (e), and (g)) and treated biofilms with eugenol-SMIC100 for 24 h ((b), (d), (f), and (h)).
3.4. Effect of Eugenol on Cell Surface Hydrophobicity and Adhesion to HEp-2 Cells and Polystyrene
To evaluate the effect of eugenol on CSH and adhesion to mammalian cells and polystyrene, planktonic cells of Candida species were exposed to eugenol at a subinhibitory (0.5 × MIC) concentration for 1 h before the assays. Most Candida spp. isolates showed hydrophobic behavior as determined by the biphasic hydrocarbon/aqueous method, and the mean relative CSH was 60.82 ± 20.79 ranging from 29.48 ± 2.97 to 84.59 ± 4.32. Except for C. tropicalis 176, a significant difference (P < 0.005) in CSH of Candida species was observed after exposure of planktonic cells to eugenol for 1 h (Table 3). There was a range of 42.3 to 75.1% reduction in the CSH of eugenol-treated cells as compared to untreated counterpart cells. Eugenol also caused a significant reduction in adhesion of most Candida species to HEp-2 cells (P < 0.005) and to polystyrene (P < 0.05). There was no significant difference in the adhesion percentage of isolate 176 of C. tropicalis to either surface, although a 20% reduction in adhered cells to mammalian cells was seen after eugenol exposure. The other Candida isolates showed a range of 46.9 to 68.9% and 27.4 to 67.8% reduction in adhesion to HEp-2 cells and polystyrene, respectively.
Table 3.
Effect of eugenol on cell surface hydrophobicity, and adhesion to human epithelial cells and polystyrene.
| Isolate | CSHa | Adhesion to HEp-2 cellsb | Adhesion to polystyrenec | |||
|---|---|---|---|---|---|---|
| Untreated | Treatedd | Untreated | Treatedd | Untreated | Treatedd | |
| Candida dubliniensis | ||||||
| 131 | 67.97 ± 5.61* | 39.22 ± 6.97 | 92.00 ± 5.60# | 35.00 ± 5.27 | 0.450 ± 0.001′′ | 0.302 ± 0.001 |
| 219 | 29.48 ± 2.97* | 15.58 ± 3.16 | 45.00 ± 4.16# | 14.00 ± 4.53 | 0.405 ± 0.002′′ | 0.209 ± 0.001 |
| 248 | 69.20 ± 9.10* | 16.00 ± 6.11 | 90.00 ± 5.21# | 30.00 ± 4.73 | 0.384 ± 0.001′′ | 0.216 ± 0.002 |
| Candida tropicalis | ||||||
| 23 | 72.00 ± 8.22* | 21.00 ± 5.63 | 92.00 ± 5.12# | 46.00 ± 4.33 | 0.397 ± 0.004′′ | 0.288 ± 0.003 |
| 150 | 41.66 ± 4.72* | 23.75 ± 5.21 | 81.00 ± 5.06# | 43.00 ± 5.84 | 0.335 ± 0.002′′ | 0.108 ± 0.001 |
| 176 | 84.59 ± 4.32 | 81.16 ± 3.19 | 45.00 ± 3.12 | 36.00 ± 3.21 | 0.395 ± 0.005 | 0.393 ± 0.002 |
aPercentage of cell surface hydrophobicity (CSH) determined by the difference in the optical density (OD) of the aqueous phase between test and control. The greater the change in OD of the aqueous phase, the more hydrophobic the yeast sample is. bThe percent adherence was calculated by the equation: % Adherence = (cfu120/cfu0) × 100, where cfu120 refers to adhered bacterial cells per mL after 2 h and cfu0 the initial number of inoculated cells. cThe metabolic activity of cells was determined by the XTT-reduction assay after 2 h of adhesion on polystyrene surface. dPlanktonic cells were eugenol-treated for 1 h with 0.5 × MIC before the assay. Significant differences in CSH (∗), adhesion to HEp-2 cells (#) and to polystyrene (′′) properties when compared to eugenol-treated counterpart cells (∗,# P < 0.005; ′′P < 0.05).
4. Discussion
Eugenol has been widely used in medicine and dentistry due to its antiseptic, antimicrobial, anesthetic, analgesic, antioxidant, anti-inflammatory, and cardiovascular properties [27, 28]. This phenylpropanoid compound has been reported to have antimicrobial activity against planktonic cells of C. albicans, C. dubliniensis, C. glabrata, C. guilliermondii, C. krusei, C. parapsilosis, and C. tropicalis [13–17, 29]. Moreover, this compound shows in vitro synergy with fluconazole and amphotericin B against C. albicans [11, 18]. As previously reported [14–17], our results showed that eugenol has fungicidal activity against planktonic cells of C. tropicalis, including those classified as fluconazole-resistant and dose-dependent yeasts, and this effect was also observed for C. dubliniensis.
Previous studies reported in the literature have focused on determining the antibiofilm activity of eugenol against C. albicans. He et al. [10] showed a dose-dependent reduction in metabolic activity of 48 h biofilm formed on a polystyrene surface and treated with eugenol for another 48 h. In the presence of 500 μg/mL and 2000 μg/mL eugenol, 50% (SMIC50) and over 80% (SMIC80) reduction were detected, respectively. Khan and Ahmad [11] evaluated the effect of phytocompounds (eugenol, cinnamaldehyde, citral, and geraniol) against 48 h biofilm of C. albicans, and their results also showed a dose- and time-dependent inhibitory activity for eugenol. The SMIC80 after treatment with the compounds for 48 h ranged from 100 to 400 μg/mL. The results obtained in this study showed that eugenol displayed inhibitory activity against biofilms of C. dubliniensis and C. tropicalis, which, not surprisingly, were highly resistant to fluconazole. Eugenol inhibited biofilm formation, as well as reducing metabolic activity of mature biofilms formed on polystyrene, in a dose-dependent manner. SEM analysis further revealed the reduction in biofilm formed on denture materials (PMM and ceramic).
The mechanisms by which eugenol induces death in Candida spp. are not completely understood. This compound caused profound changes in the morphology of planktonic cells and leakage of cytoplasmic constituents, indicating an action on the cell envelope [13, 14]. In fact, several authors have shown that the fungicidal concentration of eugenol against C. albicans causes a significant reduction in ergosterol content of the cell [15, 16, 30] and interferes with H+-ATPase activity [31]. In addition, the extensive damage to the cell membrane [15, 30] may be attributed to oxidative stress mediated by reactive oxygen species [29].
Microbial adherence on the surface of substrates is the initial event of biofilm formation, and the cell envelope mediates the first interaction between the microorganism and the environment. CSH, a nonspecific factor, is considered an important feature that contributes to adherence of Candida spp. on different surfaces [32, 33]. Moreover, it has been shown that CSH of planktonic cells of C. albicans isolated from different sources correlates positively with biofilm formation on polystyrene [34]. In this study, the presence of eugenol (0.5 × MIC) caused a significant reduction in CSH and adhesion to polystyrene and HEp-2 cells of almost all planktonic cells of C. tropicalis and C. dubliniensis. These results suggest that eugenol may interfere with the adhesion properties of Candida species. It was previously reported that C. albicans adhesion to polystyrene [35] and epithelial cells [36] was reduced after in vitro exposure to subinhibitory concentrations of fluconazole, an antifungal that interferes with ergosterol biosynthesis.
Altogether, the findings reported here corroborate the effectiveness of eugenol against planktonic and sessile cells of Candida species other than C. albicans, reinforcing the potential of this compound as an antifungal, indicating that this phenylpropanoid may have additional beneficial effect in the treatment of local candidiasis. Accordingly, initial in vivo studies have demonstrated the safety and efficacy of the topical use of eugenol for the treatment of vaginal [37] and oral [14] candidosis in rats. Further studies are warranted to confirm its efficacy in the prophylaxis and/or treatment of biofilm-associated candidosis in human.
5. Conclusion
The results obtained in this study showed that besides having fungicidal activity, eugenol is capable of changing the CSH and adhesion capacity of planktonic cells of C. dubliniensis and C. tropicalis. In addition, this phenylpropanoid compound inhibited biofilm formation and mature biofilm formed on polystyrene and denture materials of both Candida species.
Acknowledgments
This study was supported by grants from Programa de Pesquisa para o SUS: Gestão Compartilhada em Saúde (PPSUS)/Fundação Araucária/SESA-PR/MS/CNPq and Programa de Pós-Graduação em Microbiologia da Universidade Estadual de Londrina. Suelen Balero de Paula was funded by a student scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors thank Dr. A. Leyva for English editing of the manuscript. This work is part of the MS dissertation of Suelen Balero de Paula.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annual Review of Microbiology. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clinical Microbiology Reviews. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rautemaa R, Ramage G. Oral candidosis Clinical challenges of a biofilm disease. Critical Reviews in Microbiology. 2011;37(4):328–336. doi: 10.3109/1040841X.2011.585606. [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage G, Walle KV, Wickes BL, López-Ribot JL. Biofilm formation by Candida dubliniensis . Journal of Clinical Microbiology. 2001;39(9):3234–3240. doi: 10.1128/JCM.39.9.3234-3240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bizerra FC, Nakamura CV, De Poersch C, et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Research. 2008;8(3):442–450. doi: 10.1111/j.1567-1364.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Medical Mycology. 2011;49(3):253–262. doi: 10.3109/13693786.2010.530032. [DOI] [PubMed] [Google Scholar]
- 8.Katragkou A, Kruhlak MJ, Simitsopoulou M, et al. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. Journal of Infectious Diseases. 2010;201(12):1941–1949. doi: 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Dai B, Wang Y, et al. In Vitro activity of baicalein against Candida albicans biofilms. International Journal of Antimicrobial Agents. 2008;32(1):73–77. doi: 10.1016/j.ijantimicag.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 10.He M, Du M, Fan M, Bian Z. In Vitro activity of eugenol against Candida albicans biofilms. Mycopathologia. 2007;163(3):137–143. doi: 10.1007/s11046-007-0097-2. [DOI] [PubMed] [Google Scholar]
- 11.Khan MS, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. Journal of Antimicrobial Chemotherapy. 2012;67(3):618–621. doi: 10.1093/jac/dkr512.dkr512 [DOI] [PubMed] [Google Scholar]
- 12.De Vincenzi M, Silano M, Stacchini P, Scazzocchio B. Constituents of aromatic plants: I. Methyleugenol. Fitoterapia. 2000;71(2):216–221. doi: 10.1016/s0367-326x(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura CV, Ishida K, Faccin LC, et al. In Vitro activity of essential oil from Ocimum gratissimum L. against four Candida species. Research in Microbiology. 2004;155(7):579–586. doi: 10.1016/j.resmic.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Chami N, Bennis S, Chami F, Aboussekhra A, Remmal A. Study of anticandidal activity of carvacrol and eugenol in vitro and in vivo . Oral Microbiology and Immunology. 2005;20(2):106–111. doi: 10.1111/j.1399-302X.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 15.Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. Journal of Medical Microbiology. 2009;58(11):1454–1462. doi: 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad A, Khan A, Manzoor N, Khan LA. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida . Microbial Pathogenesis. 2010;48(1):35–41. doi: 10.1016/j.micpath.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Marcos-Arias C, Eraso E, Madariaga L, Quindós G. In Vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complementary and Alternative Medicine. 2011;11:p. 119. doi: 10.1186/1472-6882-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan MS, Malik A, Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans . Medical Mycology. 2012;50(1):33–42. doi: 10.3109/13693786.2011.582890. [DOI] [PubMed] [Google Scholar]
- 19.Nucci M, Queiroz-Telles F, Tobón AM, Restrepo A, Colombo AL. Epidemiology of opportunistic fungal infections in latin America. Clinical Infectious Diseases. 2010;51(5):561–570. doi: 10.1086/655683. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008-2009) International Journal of Antimicrobial Agents. 2011;38(1):65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141(7):1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzman CP, Fell JW. The Yeasts. A Taxonomic Study. 4th edition. New York, NY, USA: Elsevier; 1998. [Google Scholar]
- 23.Donnelly SM, Sullivan DJ, Shanley DB, Coleman DC. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology. 1999;145(8):1871–1882. doi: 10.1099/13500872-145-8-1871. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Khan Z, Mustafa AS, Khan ZU. Seminested PCR for diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for species identification. Journal of Clinical Microbiology. 2002;40(7):2483–2489. doi: 10.1128/JCM.40.7.2483-2489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute (CLSI) Document. M27-A3. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2008. Clinical and Laboratory Standards Institute: reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd ed. [Google Scholar]
- 26.Anil S, Ellepola ANB, Samaranayake LP. The impact of chlorhexidine gluconate on the relative cell surface hydrophobicity of oral Candida albicans . Oral Diseases. 2001;7(2):119–122. [PubMed] [Google Scholar]
- 27.Pauli A, Kubeczka K. Antimicrobial properties of volatile phenylpropanes. Natural Product Communications. 2010;5(9):1387–1394. [PubMed] [Google Scholar]
- 28.Pramod K, Ansari SH, Ali J. Eugenol: a natural compound with versatile pharmacological actions. Natural Product Communications. 2010;5(12):1999–2006. [PubMed] [Google Scholar]
- 29.Khan A, Ahmad A, Akhtar F, et al. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Research. 2011;11(1):114–122. doi: 10.1111/j.1567-1364.2010.00697.x. [DOI] [PubMed] [Google Scholar]
- 30.Khan MS, Ahmad I, Cameotra SS. Phenyl aldehyde and propanoids exert multiple sites of action towards cell membrane and cell wall targeting ergosterol in Candida albicans . AMB Express. 2013;3(1):p. 54. doi: 10.1186/2191-0855-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad A, Khan A, Yousuf S, Khan LA, Manzoor N. Proton translocating ATPase mediated fungicidal activity of eugenol and thymol. Fitoterapia. 2010;81(8):1157–1162. doi: 10.1016/j.fitote.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Klotz SA, Drutz DJ, Zajic JE. Factors governing adherence of Candida species to plastic surfaces. Infection and Immunity. 1985;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazen KC, Brawner DL, Riesselman MH, Jutila MA, Cutler JE. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infection and Immunity. 1991;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borghi E, Sciota R, Biassoni C, et al. Cell surface hydrophobicity: a predictor of biofilm production in Candida isolates? Journal of Medical Microbiology. 2011;60(5):689–690. doi: 10.1099/jmm.0.026898-0. [DOI] [PubMed] [Google Scholar]
- 35.Imbert C, Rodier M-H, Daniault G, Jacquemin J-L. Influence of sub-inhibitory concentrations of conventional antifungals on metabolism of Candida albicans and on its adherence to polystyrene and extracellular matrix proteins. Medical Mycology. 2002;40(2):123–129. doi: 10.1080/mmy.40.2.123.129. [DOI] [PubMed] [Google Scholar]
- 36.Ellepola AN, Samaranayake LP. The postantifungal effect (PAFE) of antimycotics on oral C. albicans isolates and its impact on candidal adhesion. Oral Diseases. 1998;4(4):260–267. doi: 10.1111/j.1601-0825.1998.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 37.Chami F, Chami N, Bennis S, Trouillas J, Remmal A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. Journal of Antimicrobial Chemotherapy. 2004;54(5):909–914. doi: 10.1093/jac/dkh436. [DOI] [PubMed] [Google Scholar]


