Abstract
Adenosine triphosphate (ATP) plays a fundamental role in cellular communication, with its extracellular accumulation triggering purinergic signaling cascades in a diversity of cell types. While the roles for purinergic signaling in health and disease have been well established, identification and differentiation of the specific mechanisms controlling cellular ATP release is less well understood. Multiple mechanisms have been proposed to regulate ATP release with connexin (Cx) hemichannels and pannexin (Panx) channels receiving major focus. However, segregating the specific roles of Panxs and Cxs in ATP release in a plethora of physiological and pathological contexts has remained enigmatic. This multifaceted problem has arisen from the selectivity of pharmacological inhibitors for Panxs and Cxs, methodological differences in assessing Panx and Cx function and the potential compensation by other isoforms in gene silencing and genetic knockout models. Consequently, there remains a void in the current understanding of specific contributions of Panxs and Cxs in releasing ATP during homeostasis and disease. Differentiating the distinct signaling pathways that regulate these two channels will advance our current knowledge of cellular communication and aid in the development of novel rationally-designed drugs for modulation of Panx and Cx activity, respectively.
Keywords: connexin hemichannel, pannexin channel, purinergic, ATP, receptor
1. Introduction
ATP has emerged as a prominent extracellular signaling molecule influencing a diverse array of physiological and pathological processes. By binding to purinergic receptors at the plasma membrane of a cell, ATP and its metabolites (ADP, AMP and adenosine) control a variety of intracellular signaling cascades, imparting strict control over both intra- and intercellular communication in a number of tissues. The biological relevance for adenine nucleotides acting as autocrine/paracrine signaling molecules is evident by the medley of purinergic receptor subtypes that are differentially expressed across cell types, the extracellular expression of nucleotidases whose activity fine tunes the abundance of ATP outside of the cell and the ubiquitous presence of ATP in all cells in the body, providing a pool of agonist for the initiation of purinergic signaling cascades in all aspects of human physiology. Indeed, purinergic signaling has been implicated in numerous physiological processes, including vascular tone and blood pressure regulation [1–7], respiratory control [8–10], and neurotransmission [11–14], as well as a number of pathologies including inflammation [15–18], atherosclerosis [19,20], cancer [21–24], and neurological disorders [25,26]. Two broad families of purinergic receptors have been identified to date, termed P1 and P2 receptors (for an extensive review on purinergic receptors see [27]). P1 receptors are G-protein coupled receptors (GCPRs) that have a high binding affinity for the nucleoside adenosine and exist as 4 isoforms (A1, A2A, A2B and A3). These receptors differentially couple to Gs or Gi and their activation modulates the activity of adenylate cyclase and ultimately intracellular cAMP levels. In contrast, the P2 receptors have a high binding affinity for ATP and ADP (as well as the uridine nucleotides UTP and UDP) and are comprised of two subfamilies denoted P2X and P2Y. The P2X receptor family contains 7 isoforms (P2X1–7) which are classified as ligand-gated cation channels whose activation regulates cellular membrane potential and intracellular Ca2+ levels. The P2Y receptor family contains 8 isoforms (P2Y1,2,4,6,11–14) which are metabotropic GPCRs that couple to either Gq, Gs or Gi, in an isoform-specific manner, and their activation modulates intracellular inositol triphosphate (IP3), Ca2+ and cAMP levels. Due to the heterogeneity in G-protein coupling, agonist selectivity and cell and tissue specific expression profiles, purinergic receptors are poised to selectively regulate distinct signaling events both within and between cells throughout the body. Another regulatory component in the purinergic signaling axis is the expression and activity of extracellular enzymes that actively degrade purine nucleotides, or ecto-nucleotidases. Two of these enzymes fine tune the abundance of ATP and its metabolites outside of the cell, with ecto-apyrase (CD39) acting to degrade extracellular ATP and ADP to AMP and 5’-ectonucleotidase (CD73) converting AMP to adenosine [28]. It is now evident that the level of expression and activity of these ecto-enzymes can differentially regulate which family of purinergic receptor is activated when extracellular purine concentration rises. As the expression or activity of CD39 increases, there is a shift in favor of P1 receptor activation as adenosine levels rise. Conversely, decreases in CD73 activity will favor the activation of P2 receptors by shifting the extracellular purine pool from adenosine to ADP and ATP [29].
The ability of cells to orchestrate a variety of signaling cascades in response to ATP has been well established, but the mechanisms imparting control over the release of ATP from specific cell types has become more controversial. In a cell type and context-dependent manner, several mechanisms for ATP liberation have been proposed, including vesicular release and channel-dependent mechanisms, with connexin (Cx) hemichannels and pannexin (Panx) channels being the most highly studied (reviewed in [30,31]). These two families of membrane proteins form closed channels at the cell surface that when activated, allow for the diffusion of ions and small signaling molecules across the plasma membrane, notably ATP. While multiple lines of evidence have emerged implicating Cxs and Panxs in cellular ATP release, it has been difficult to clearly discriminate the contribution of each channel to this process in a myriad of cell types and tissues. These discrepancies have limited our understanding of the importance of Cx vs. Panx-dependent ATP release and have hindered the development of selective agonist and antagonists to modulate the contribution of these channels to purinergic signaling events. In order to fully elucidate the (patho)physiological roles of Cx hemichannels and Panx channels in correlation to ATP- driven cellular signaling processes, it is imperative to characterize the innate differences between these two channels and utilize this information for the development of rationally-designed drugs to selectively target each protein. To this end, this review is focused on the currently understood differences between Cxs and Panxs at the molecular and functional levels with an emphasis on the difficulties that are faced in segregating Cxs and Panxs in ATP release; including methodological differences between studies, the growing problem of pharmacology and the potential compensation by other protein isoforms. Additionally, emerging evidence that supports an intimate link between receptor-dependent activation of Panx channels vs. Cx hemichannels is addressed.
2. Similarities and differences between Cxs and Panxs
The connexins were first described in the early 1970’s as the principal proteins comprising intercellular gap junctions [32]. Today, more than 20 human isoforms have been identified, showing differential tissue distribution and functional properties. These gap junction proteins harbor four transmembrane domains with two extracellular loops, intracellular N- and C-termini and a single intracellular loop. Each extracellular loop contains three highly conserved cysteine resides which form intramolecular disulfide bridges that are thought to be critically important for stabilizing the secondary structure of these domains and gap junction formation [33,34]. Connexin monomers hexamerize to form half channels, or hemichannels, that are trafficked to the plasma membrane and readily dock with unapposed hemichannels on adjacent cells. This docking results in the formation of open gap junction channels, providing a direct conduit for the transfer of current, intracellular ions, second messengers and small molecules (up to 1 kD in size) between coupled cells, potentiating heterocellular communication in many organ systems; notably the heart, vasculature and brain [35–39]. As the concept of purinergic signaling gained support and the search for candidate pathways to mediate cellular ATP release ensued, it has been proposed that undocked connexin hemichannels may provide a mode for conducted ATP release from cells. Based on previous observations that ATP can traverse intact gap junction channels [40,41], and the strong concentration gradient of ATP between the cytosol and extracellular milieu, opening of connexin hemichannels at the non-junctional plasma membrane may favor ATP diffusion from the cell. Congruently, with the aid of ectopic expression systems, electrophysiology and bioluminescence-based assays for the quantification of ATP, several connexin isoforms have been reported to form hemichannels capable of releasing ATP [42–45]. In particular, the ubiquitous Cx43 isoform has been extensively studied in this regard. The physiological and pathological roles for Cx hemichannel activity have been reported in cell volume regulation, hearing, sensory processing in the retina, cardiac fibrosis, cerebral ischemia, and inflammation [44–55]. While these observations lend credence for an active role of Cx hemichannels outside of their primary function to form gap junctions, the experimental and biological conditions required to open these hemichannels has relied, in large part, on manipulating the cellular environment to more pathological conditions. For example, experimental interrogation of Cx hemichannel activity and ATP release has depended greatly on overexpressing connexins in unpaired cells and decreasing the extracellular [Ca2+] to nearly zero. The latter approach has stemmed from the observation that extracellular divalent cations impart negative regulation on Cx hemichannels [56,57]. In addition to decreasing [Ca2+]e below the physiological range, a number of studies have reported Cx hemichannel activity following strong depolarization beyond physiological membrane potentials (i.e. potentials >0 mV) [56,58–60]. As such, it is currently unclear whether these experimental conditions mimic the normal physiological context in which these hemichannels reside or whether their contribution to purinergic signaling is more relevant in disease. Nonetheless, several recent studies have identified Cx hemichannel activity in the presence of physiological [Ca2+]e and at resting membrane potentials, supporting the possibility that Cx hemichannels function to regulate physiological signaling cascades [61–66]. Specifically, recent evidence has indicated that endogenous Cx43 hemichannels activate in response to mechanical stimulation in isolated endothelial cells, which was reported to be tightly regulated by RhoA GTPase and inflammatory mediators [64]. In a separate study, Cx43 hemichannels were shown to be activated in response to increased fluid shear stress by a process requiring direct coupling of the hemichannel to α5β1 integrins o mechanical stress [63]. Additionally, Cx43 hemichannel activity increases in response to changes in extracellular glucose concentrations, potentially implicating their function in glucose sensing and downstream metabolic signaling [61]. Hemichannels formed by Cx26 have also been reported to open in response to increases in the polyunsaturated fat linoleic acid, suggesting a possible role for these channels in cell growth and audition [62]. Outside of Cx43 and Cx26, the lens-specific connexin isoforms, Cx46 and Cx50, have been shown to form functional hemichannels under physiological conditions[60,67,68]. While these recent studies are in support of Cx hemichannel function in a number of physiological process, it remains unclear whether their contribution involves providing a physiological conductive pathway for cellular ATP release. In addition, while these studies may support a role for Cx hemichannel activation under conditions of physiological [Ca2+]e and membrane potentials, it remains to be determined whether these functions are preserved in vivo. Outside their roles in physiological ATP release, Cx hemichannels may provide a patent conduit for cellular ATP release during a number of pathological conditions. Most notably, Cx37- and Cx43 hemichannel mediated ATP release has been observed from monocytes and activated neutrophils during inflammation and atherosclerosis [44,53,69]. Moreover, during ischemia, where there is a decrease in [Ca2+]e, Cx hemichannels in astrocytes open releasing ATP and glutamate, which potentiates neuronal excitotoxicity and cell death [70].
More recently, a novel family of channel forming proteins termed pannexins (Panx) has been identified [71]. Panxs are orthologs of the invertebrate gap junction proteins, innexins, but do not share significant sequence homology to the Cxs. Three Panx isoforms have been identified to date (Panx1, Panx2 and Panx3), with Panx1 being ubiquitously expressed, Panx2 expressed in the central nervous system and Panx3 expressed in skin, bone and cartilage [72–75]. Despite the lack of sequence homology to Cxs, Panxs share a similar membrane topology existing as tetraspan membrane proteins with intracellular N- and C-termini. Like the Cxs, the Panx1 and Panx3 isoforms oligomerize to form hexameric single membrane channels, whereas Panx2 is predicted to exist in higher order oligomers, notably heptamers and octomers [76,77]. Despite these similarities, Panxs do not form gap junction channels, but exist to solely function as single membrane channels [78]. Currently, it is thought that discrete differences between the extracellular loops of the Panxs, as compared to Cxs, might account for their inability to form cell-to-cell channels. While the Cxs contain 6 conserved cysteine residues in their extracellular loops, Panxs only harbor 4. The absence of two cysteines in these domains may impart an unfavorable secondary structure or decrease the stability of the extracellular loops on Panxs preventing gap junction docking. An alternative explanation for the absence of gap junction formation by Panxs may be due to the high amount of glycosylation of their extracellular loops, which may sterically hinder docking of Panx channels between cells [77,79,80]. Based on these observations, Panx channels have become a prominent candidate for cellular ATP release. Panx1 has been the most studied of the three isoforms and electrophysiological and biochemical analyses have revealed that Panx1 channels can activate and release ATP under normal physiological [Ca2+]e and favorable membrane potentials (i.e. <0mV), and in response to activation of a number of receptors making them prime candidates for regulated ATP release (discussed below) [7,81–88]. In this regard, Panx1 channels have been implicated in the regulation of vascular tone and blood flow, mucocilliary clearance in airway epithelia, cellular apoptosis, inflammasome activation and neuronal cell death during ischemia [7,81,82,89–92].
As our knowledge of the functional properties of Panx channels and Cx hemichannels continues to grow and new technologies are developed to aid in isolation of their respective functions in ATP release, the particular contribution of each channel to purinergic signaling in health and disease will become evident and provide novel therapeutic targets.
3. Current difficulties in segregating Cx- and Panx-dependent ATP release
Differentiating the roles of Cx hemichannels and Panx channels in cellular ATP release has been a difficult problem to overcome. These issues have been primarily associated with the lack of specificity of pharmacological blockers of the two channels, overlapping expression in a number of cell types and tissues, the use of different experimental approaches to assess channel function and the potential issue of compensation by other isoforms in studies employing genetic manipulation of Cx and Panx expression. Due to these limitations in segregating Cx- and Panx-dependent activity in relation to ATP release, it has become increasingly difficult to ascribe unequivocal function to one channel over the other in a number of physiological and pathological contexts. These limitations are discussed below in detail.
3.1. Limitations in pharmacological selectivity for Cx hemichannels and Panx channels
The study of Cx hemichannel and Panx channel function has relied intently on the use of pharmacological inhibitors of channel currents, dye uptake and ATP release. This review will focus on the most commonly used pharmacological inhibitors of Cxs and Panxs; for a comprehensive list of Panx and Cx inhibitors refer to [93–95]. Notably, a number of gap junction blockers including flufenamic acid (FFA), the long-chain alcohols octonol and heptanol, glycyrrhetinic acid (GA) derivatives (18α-GA and carbenoxolone (CBX)) and mimetic peptides have been employed to inhibit Cxs. Many of these substances have been reported to block both Cx hemichannel and gap junction activity, which has limited the interpretation of specific contribution of hemichannels vs. gap junctions in cell-to-cell communication. For example, FFA, a potent inhibitor of Cx based gap junctions, strongly reduces hemichannel currents in unpaired Xenopus oocytes overexpressing Cx50 or Cx46 [96]. In addition, the GA derivative 18α-GA which prevents dye coupling between cells paired by gap junctions, inhibits dye uptake by Cx43 hemichannels in astrocytes [97]. Nonetheless, several of these inhibitors have been assessed in their relative potency for inhibition of Cx hemichannels and Panx channels. In terms of Cx hemichannels, FFA, octonol and heptanol have been reported to block gap junction and Cx hemichannel activity with minimal effect on Panx1 channels [96,98–100]. Comparatively, FFA has been reported to inhibit Cx hemichannel activity at low micromolar concentrations, while analysis of the effects of this compound on Panx1 channels expressed in oocytes showed only modest inhibition of Panx1 currents at 300µM [100]. As Panxs have emerged as possible candidate channels for ATP release, a number of these inhibitors that were originally thought to selectively block Cx-based gap junctions and hemichannels are now known to also block Panx channels, in some cases to a much higher degree than Cx hemichannels. This is best exemplified by the GA derivative CBX which inhibits both Cx hemichannels and Panx1 channels. Pharmacological assessment of CBX potency has revealed a substantially greater affinity for Panx1 channel inhibition than Cx hemichannel inhibition (EC50 = ~5µM for Panx1 vs 10–100µM for Cx43 hemichannels [97,100]). Based on these observations, many previous studies employing CBX to block Cx hemichannel function likely also blocked Panx1 function and as a result, it has now become common to employ CBX as a Panx1 blocker. At the other end of the spectrum, a few compounds have been identified that are more specific for Panx channel inhibition than Cx hemichannel inhibition, notably the uricosuric drug probenecid. Probenecid has been reported to block Panx1 currents and dye uptake in oocytes expressing the channel, with no effect on currents carried by Cx46 or Cx32143 (a chimera of Cx32 containing the first extracellular loop of Cx43) [101]. In addition, probenecid reduced [Ca2+]i in response to histamine in subcutaneous fibroblasts which was dependent on Panx1-mediated ATP release [102].
As the problem of pharmacological selectivity for Cx hemichannels and Panx channels has grown, novel methods for targeting each respective channel have been developed with the mimetic peptides receiving major focus. Cx mimetic peptides were first developed to block gap junction formation and subsequent intercellular communication and were widely used in model systems where cellular conduction is essential including the heart [103], lung epithelium [104], and vascular smooth muscle and endothelial cells [105] [106]. These peptides have been designed to mimic the primary amino acid sequence of varying regions of Cx isoforms, with the majority mimicking the extracellular loop regions. Following the observation that Cx mimetic peptides could inhibit gap-junctional communication in coupled cells, presumably by preventing docking of Cx hemichannels between cells, their ability to selectively block Cx hemichannels was assessed. Of particular note, the Cx mimetics Gap26 and Gap27 which mimic regions of the first and second extracellular domains of Cx43, respectively, can inhibit currents carried by Cx43 hemichannels expressed in HeLa cells [107,108]. Moreover, Gap26 has been reported to inhibit ATP release from corneal endothelial cells in response to mechanical stress and reduce basal ATP release from vascular endothelial cells in culture [109,110]. While these studies initially advanced the repertoire of pharmacological agents to selectively block Cx hemichannels and gap junctions, recent evidence has emerged indicating cross-inhibition of Panx1 channels by several Cx mimetic peptides. Most notably, the Gap26 and Gap27 peptides were shown to inhibit Panx1 currents in oocytes as well as those carried by Cx43 [111]. Moreover, the Cx mimetic peptide 32Gap24, which mimics a sequence in the Cx32 intracellular loop domain, had no effect on hemichannel currents from cells expressing the Cx32143 chimera but drastically reduced Panx1 currents [111]. These observations may suggest that the mode of action of these mimetic peptides is not dependent on the mimicked sequence, as Panx1 does not share sequence homology in either its extracellular loop or intracellular domains with Cxs. Based on the cross-inhibition observed with many of the currently employed connexins mimetic peptides, new efforts have been made to develop novel peptide antagonists that may specifically inhibit Cx hemichannels without affecting gap junction channels or Panx channels. Recently, a novel Cx mimetic peptide termed Gap19 was developed, mimicking a sequence in the intracellular loop of Cx43. Gap19 has been reported to specifically prevent Cx43 hemichannel activity in HeLa cell expressing exogenous Cx43 as well as endogenous Cx43 currents in isolated cardiac myocytes through a mechanism that disrupts intramolecular interactions between the intracellular loop and C-tail of Cx43 [112]. This inhibition was selective for Cx43 hemichannels without affecting Panx1 channel currents or Cx40 hemichannel currents. In addition to Gap19, a second mimetic peptide designed to interrupt intracellular loop:C-tail interactions called TAT-L2 has been reported to specifically inhibit endogenous Cx43 hemichannel activity in endothelial cells and hemichannel activity in ectopic expression systems [113]. These new peptides have shed light on the gating of Cx43 hemichannels which appears to be tightly regulated by interactions between the intracellular domains of Cx43. Due to the increasing success of mimetic peptides in targeting Cx hemichannls, a mimetic peptide of the first extracellular loop of Panx1 termed 10panx1 was developed and has subsequently been shown to significantly inhibits channel currents, dye uptake and ATP release mediated by Panx1 in a number of cell types [114–116]. The Panx mimetic peptide has not escaped the issue of cross-inhibition, however, as it was recently shown that the 10panx1 peptide also blocks Cx46 hemichannel currents [111].
Based on the sum of these observations, the study of Cx hemichannel and Panx channel function remains plagued by the overlapping pharmacology. As we continue to accumulate new information on the relative potency of current and new drugs for blockade of Panxs and Cxs, it will be important to not rely solely on one inhibitor but to utilize a gamut of blockers at appropriate concentrations to define the contributions of Cx hemichannels and Panx channels to purinergic signaling events.
3.2. Experimental methodologies in the study of Cx and Panx function
In addition to the limitations in segregating Cx hemichannel and Panx channel function pharmacologically, there is a discourse in the methods utilized to interrogate each channel’s function as it relates to ATP release. Currently, three techniques are commonly employed to measure Cx and Panx activity: dye uptake or release, electrophysiological preparations to measure channel currents and strategies aimed at directly quantifying ATP liberation from cells. Dye uptake assays have been used as a parameter of cell permeability in response to a range of stimuli that evoke Cx hemichannel or Panx channel opening. These assays rely on the ability of a dye present in the extracellular milieu to cross the plasma membrane and become concentrated inside of the cell and quantified by fluorescence microscopy. Some commonly used fluorescent molecules include Lucifer yellow, ethidium bromide, propidium iodide, DAPI, YoPro-1 and ToPro-1 (e.g., [86,117,118]). Each of these molecules has very distinct characteristics including differences in charge, size and conformation which may specifically affect their respective permeabilities through Cx hemichannels or Panx channels. In agreement, differences in the permeability of several of these dyes through gap junctions and Cx hemichannels have been assessed in HeLa cells expressing a number of different Cx isoforms (Cx26, Cx31, Cx32, Cx40, Cx43 and Cx45) [119,120]. The results of tthese studies indicate that specific Cx isoforms harbor differential permeabilities to specific fluorescent molecules, likely based on the relative charge or size of the permeant and conductance properties of the gap junction or hemichannel. Based on these observations, the selection of molecules for dye uptake experiments should be considered carefully depending on the particular isoform being studied [119]. Notwithstanding, dye uptake experiments have provided a strong output for quantifying the permeability of cells as it relates to hemichannel or channel activation but this methodology should not be solely relied on for the assessment of ATP release as one is not indicative of the other.
Electrophysiology has been the workhorse for assessing Cx hemichannel and Panx channel activity. These methods, coupled with ectopic expression systems to isolate the channel of interest, have provided great insight into the properties of both Cxs and Panxs and aided in discerning the relative selectivity of pharmacological blockers for the two channels as discussed above. Whole-cell and singe-channel recordings have identified a number of unique properties between Cx hemichannels and Panx channels. Cx43 hemichannels expressed in oocytes show a conductance of ~220pS whereas Panx1 conductance has been initially reported to be ~500pS [84,121]. Cx hemichannels have been shown to be tightly regulated by membrane potential, with strong depolarizations (~+60mV) activating the channel while Panx1 channels can open under hyperpolarizing potentials in the physiological range (>−20mV) [121,122]. Moreover, electrophysiological analyses have revealed that both Cx43 hemichannels and Panx1 channels are permeable to ATP. Utilizing an ATP gradient between the patch pipette and bath solution, Bao et al. demonstrated the direct permeability of ATP through single Panx1 channels in excised patches [84]. Similarly, whole cell and excised patch clamp techniques have indicated conductance of ATP through hemichannels composed of Cx43 [43]. These latter studies also employed dual electrophysiological recordings and bioluminescence for simultaneous photo-detection of ATP in inside-out patches from Cx43 expressing oocytes, directly coupling the electrophysiological recording of hemichannel activity to ATP permeability. While the implement of electrophysiology in the study of Cx hemichannel and Panx channel function has significantly advanced our knowledge on the unique properties of these two families of channels, there remain limitations in extrapolating these functional studies to the physiological setting. As mentioned above, many of the observations implicating Cx hemichannels and Panx channels activity in ATP release have relied on ectopic expression systems to isolate the channel from contaminating signals. Thus, these channels are placed in an artificial setting that may not reflect their endogenous environment. Given these considerations, future studies aimed at assigning Cx hemichannel or Panx channel activity to ATP release should make effort to analyze these channel properties in native cells. Nonetheless, the knowledge we have gained on the biophysical properties of Cxs and Panxs through the use of electrophysiological techniques has made great strides in differentiating between the two families of proteins.
Assessment of the contribution of Cx hemichannels and Panx channels to cellular ATP release should not depend on dye uptake and electrophysiological recordings alone, but employ assays for the direct detection of ATP liberated from the cell. The current gold-standard for ATP quantification relies on luciferin:luciferase chemistry where the enzyme luciferase catalyzes the oxidation of ATP and luciferin ultimately resulting in the generation of a photon which can be readily quantified by luminometry. Luciferin:luciferase bioluminescence has subsequently been implemented in a number of models to directly test the accumulation of extracellular ATP in response to Cx hemichannel and Panx channel activation [43,81,82,88,109,123].
The aforementioned methods currently used for assessing Panx channel and Cx hemichannel activity as it relates to ATP release and purinergic signaling have shed light on the properties of these two channels and have provided direct evidence that both Cx hemichannels and Panx channels are permeable to ATP. It is important to note that not all studies are created equally, with experimental conditions and outputs differing between groups. Based on these points, future studies geared towards assigning function to one channel over another should strive to implement a combination of these methods.
3.3. Potential for compensation in genetic knockdown and knockout models
The advent of RNA interference and knockout animal models has led to great advances in the study of Cx hemichannels and Panx channels. In combination with pharmacological characterization of channel activity and ATP release, the targeted reduction or deletion of Cx and Panx isoforms has revealed specific contributions of each channel to physiological and pathological ATP release. In particular, genetic knockout of Cx43 from murine neutrophils was shown to suppress hemichannel-dependent ATP release from these cells under inflammatory conditions [44]. Similarly, deletion of the Cx37 isoform in monocytes abolishes ATP release and promotes atherosclerotic plaque development [53]. In these studies, hemichannel activity was clear as these inflammatory cells do not form gap junctions. Since the discovery of the pannexin family, a number of genetic knockout mouse models have been developed to assess the role of these channels in various physiological and pathological conditions, with much focus placed on ischemic injury. Due to the increasingly evident role for Panx1 channels in neuronal ischemia, global Panx1 knockout mice have been assessed for their susceptibility to ischemic damages that accompany stroke. Interestingly, a study by Bargiotas et al. reported that mice lacking both Panx1 and Panx2 isoforms are protected from ischemic brain injury and have reduced Panx channel activity in their cortical neurons [124]. In another study, both global and neuron-specific deletion of Panx1 prevented ischemic insult to retinal ganglion cells by eliminating Panx1-dependent membrane permeation and interleukin-1β processing [125].
It is evident from these studies that genetic manipulation of Cxs and Panxs in vivo can have dramatic effects on a number of pathologies. However, in some cases, the phenotype of genetically modified animals does not reflect what has been established using other model systems. One potential reason for this discrepancy is the possibility of altering the expression of other related isoforms that may either compensate for the function of the lost protein or, conversely, contribute to the resulting phenotype. This has been well exemplified in the vasculature where genetic deletion of endothelial Cx37 caused a related decrease in Cx40 expression in the mouse aortic endothelium [126]. A similar effect was observed in mice lacking Cx40 expression, where Cx37 was also downregulated. In contrast, Cx40 knockout mice exhibited a dramatic increase in Cx37 and Cx43 expression in the medial smooth muscle cells [126]. In a related study, Cx40 knockout mice showed a redistribution and decrease of Cx43 in aortic endothelial cells, suggesting that these two isoforms are regulated in a similar fashion [127].
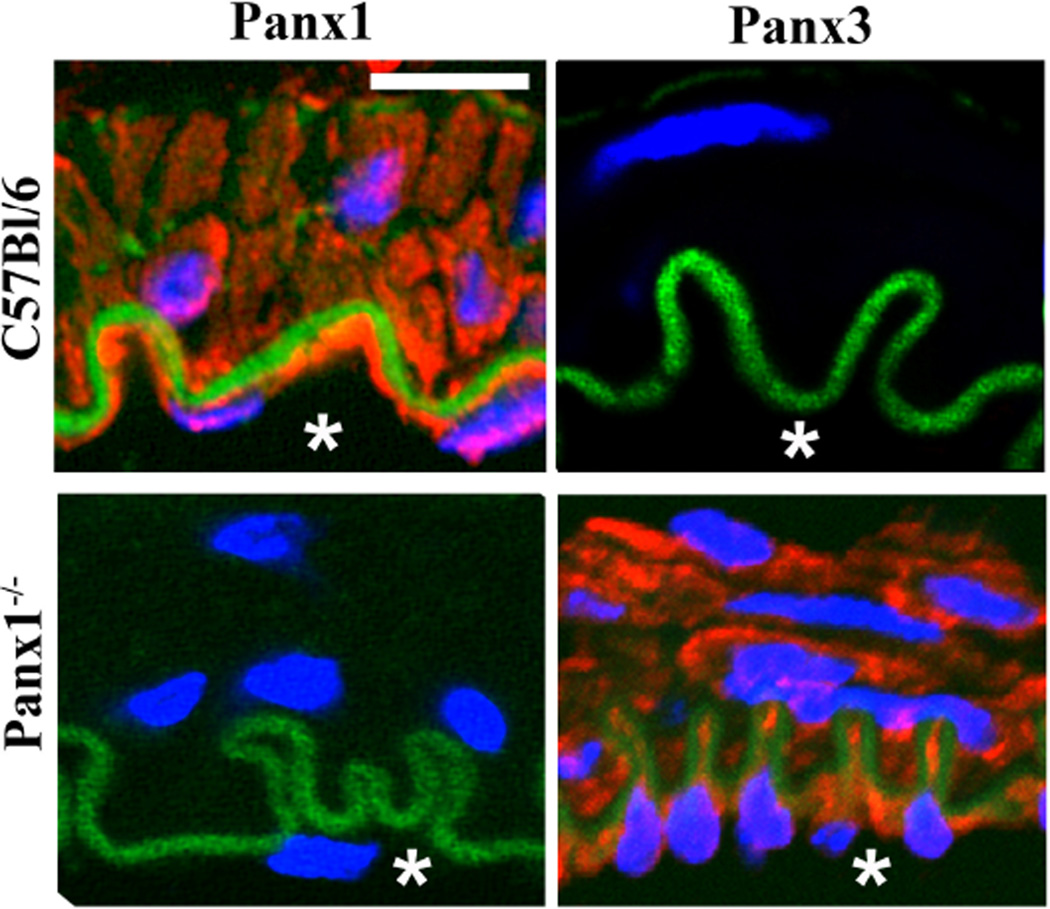
To assess the possible role for compensation of Panx isoforms in the vasculature, we have performed immunocytochemical labeling for Panx1, Panx2 and Panx3 on isolated thoracodorsal arteries from a global Panx1 knockout mouse generated by Taconic. In this study, Panx1 expression was undetectable in the endothelial and smooth muscle layers of Panx1 knockout mice as compared to the robust expression observed in wild type mice. Examination of the effect of global Panx1 knockout on the other two Panx isoforms revealed that Panx3, which is not normally expressed by the cells in the blood vessel wall was dramatically upregulated and readily detected in both smooth muscle and endothelium (Fig. 1). These puzzling findings may point towards a compensatory mechanism by which Panx3 is up-regulated to fulfill the role of Panx1. Comparison of the sequences between the three Panx isoforms has also indicated that Panx1 and Panx3 have higher sequence homology than Panx2 [80]. In addition, Panx3 channels have been reported to release ATP, placing these channels as possible candidates to replace Panx1 function [72]. While these observations are provocative and may point towards coordinated regulation of Panxs it remains unclear whether these two families of proteins share regulatory elements at the transcriptional and post-transcriptional levels.
Figure 1. Panx3 is upregulated in the arterial wall of a global Panx1 knockout animal.
Immunocytochemical analysis of Panx1 and Panx3 expression in isolated mouse thoracodorsal arteries (TDA). In wild type C57Bl/6 animals (top panels) Panx1 is abundantly expressed in the endothelial and smooth muscle cells, with minimal Panx3 expression detected. Parallel analysis of Panx3 expression in isolated TDA from a global Panx1 knockout mouse (Panx1−/−) revealed a dramatic upregulation of Panx3 when Panx1 was genetically ablated (lower panels). Asterisks indicate the blood vessel lumen. Nuclei are stained with DAPI (blue) and autofluorescence of the internal elastic lamina is in green. Scale bar = 10 µm
4. Coupling Panx channels to receptor activation
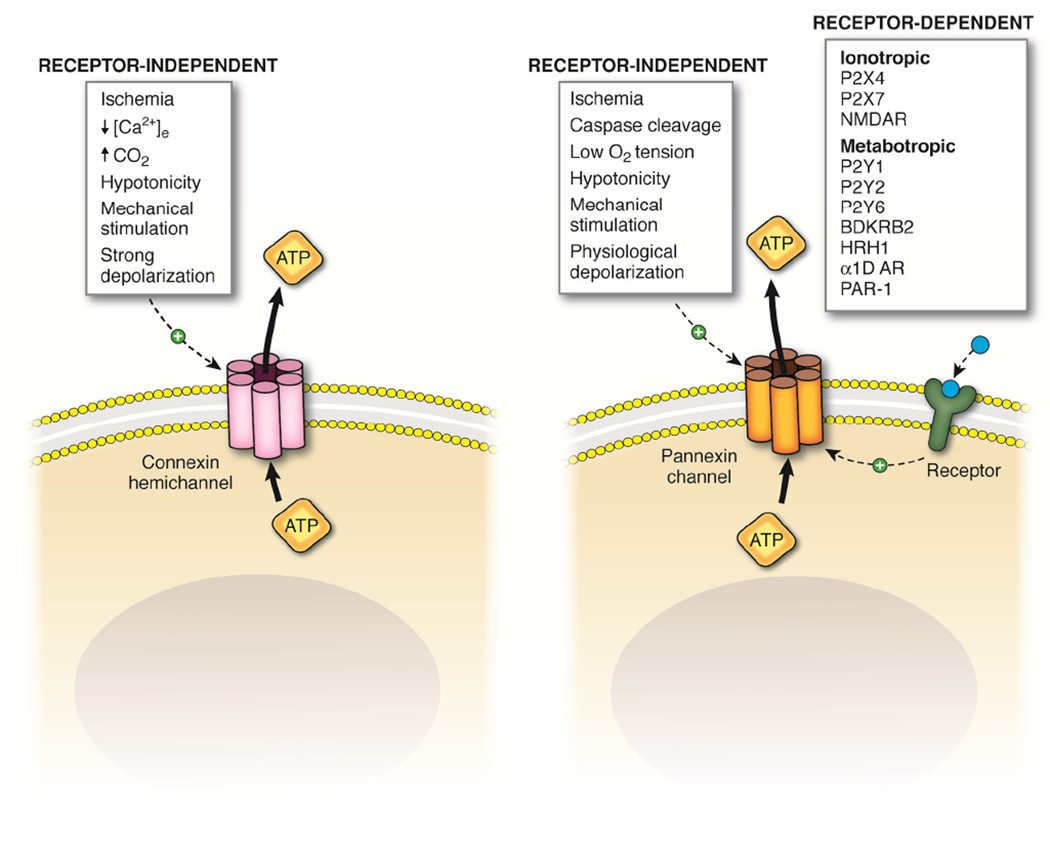
Panx channels and Cx hemichannels have emerged as prominent regulators of cellular ATP release, as described above, and much effort has focused on determining the molecular basis for how each of these channels can be activated. There are a number of biological cues that have shed light on the particular circumstances in which Cx hemichannels and Panx channels open. Cx hemichannels have been reported to open in response to ischemia and subsequent decreases in [Ca2+]e and pH (increased CO2), as well as in response to hypotonic stress, mechanical stimulation and strong membrane depolarizations. Panx1 channel activation has been implicated in response to a number of the same factors including ischemia, low O2 tension, hypotonic stress and mechanical stimulation; however, their activation has also been reported in response to caspase-cleavage of the Panx1 C-tail and depolarizations in the physiological range. The comparison of Cx hemichannel vs. Panx channel mediated ATP release over the past 10 years has brought about intriguing evidence that Panx channel activation (Panx1 in particular) may be tightly coupled to plasma membrane receptors in a number of cell types and biological processes. To date, however, knowledge on similar regulation of Cx hemichannels by receptor activation has not been reported. The current evidence for Cx hemichannel-mediated ATP release points toward a role for these channels during pathological conditions such as ischemia and inflammation, pathologies that are often associated with decreases in [Ca2+]e and large fluctuations in membrane potential. While Panx channels have also been implicated in these pathological states, there is an accumulation of evidence that they release ATP under physiological conditions in response to the activation of several receptor types. Among these receptors are purinergic P2X(4/7) [85,91,128] and P2Y(1/2/6) [129,130] receptors, bradykinin B2 receptors (BDKRB2) [131], histamine H1 receptors (HRH1) [102], α1D-adrenergic receptors (α1D-AR) [7,132], protease activated receptor 1 (PAR-1) [88] and N-methyl-D-aspartate receptors (NMDA) [90] (Fig. 2).
Figure 2. Mechanisms of activation of connexins hemichannels and pannexin channels.
Schematic illustrating the receptor-dependent and independent activation of Cx hemichannels and Panx channels. Cx hemichannels activate in response to a number of receptor-independent cues including ischemic stress, decreased extracellular calcium concentrations, mechanical stimulation, hypotonicity and strong membrane depolarizations. Panx channels also activate in response to a number of receptor-independent stimuli, including ischemic stress, caspase cleavage of their intracellular C-tails, lowered oxygen tension and physiological depolarizations. In addition, and unique from connexins hemichannels, Panx channels open in response to activation of a number of membrane receptors including the ionotropic P2X4 and P2X7 purinergic receptors and N-methyl-D-aspartate receptors (NMDAR) as well as metabotropic P2Y1, P2Y2 and P2Y6 receptors, bradykinin B2 receptors (BDKRB2), histamine H1 receptors (HRH1), α1D-adrenergic receptors (α1D-AR) and protease activated receptor-1 (PAR-1).
The involvement of purinergic receptors in the promotion of Panx1-mediated ATP release has been most evident during inflammation and inflammasome activation. The inflammasome is a multiprotein complex containing caspase enzymes whose activation signals processing and secretion of the inflammatory cytokine IL-1β [133]. Recent studies have suggested that activation of the P2X7 receptor orchestrates inflammasome activation through the induction of a large pore formed by Panx1 channels. [91,134–136]. This signaling axis involves coupling of P2X7 receptors to Panx1 channels that when activated may release ATP and allow the influx of other small ions which is required for caspase-1 processing and IL-1β release in macrophages, neurons and other immune cells [85,91]. Though Panx1 channels were originally thought to form the sole large pore permeation pathway during prolonged P2X7 receptor activation, several studies have emerged indicating the existence of a separate conductance pathway independent of Panx1 channels [137,138]. Another recently identified regulator or this complex is the P2X4 receptor. The P2X4 receptors has been shown to forming a complex with P2X7 and Panx1, functioning to promote reactive oxygen species generation and enhance inflammasome activation [128]. It is currently unknown whether ATP released from Panx1 channels in this complex serves to amplify signaling through P2X7 and P2X4 receptors, or whether the primary function of the Panx1 channels resides as an influx pathway for extracellular ions. Additionally, the Panx1 channel has been shown to be negatively regulated by extracellular ATP, providing a feedback mechanism for tight control of channel activity, which may suggest that the ATP liberated from Panx1 channels during inflammasome activation serves to protect the cell from chronic ATP release and apoptosis [139].
In addition to P2X receptors, a number of P2Y receptor isoforms have been reported to induce Panx1 channel activation. Specifically, the P2Y1 and P2Y2 isoforms, when expressed together with Panx1 channels in oocytes, activate Panx1 channel currents in response to ATP [129]. It was also reported that increases in cytoplasmic [Ca2+] positively regulate Panx1 channel activity in these cells and the downstream signaling through P2Y receptors may induce Panx1 activation through a calcium-dependent mechanism. More recently, Panx1 activation downstream of P2Y6 receptor activation was shown to promote ATP release from urothelial cells which promotes bladder overreactivity [130]. Given the strong correlation between purinergic receptor activation and Panx1-dependent ATP release, certain cell types may utilize an ATP-induced ATP release pathway for cellular communication.
Along with the P2Y receptors mentioned above, several GPCRs have been coupled to activation of Panx1 channels. Activation of the BDKRB2 with bradykinin causes an increase in intracellular calcium that has been reported to be downstream of ATP release from Cx hemichannels and Panx1 channels in human subcutaneous fibroblasts [131]. In this study the authors report that blocking Cx hemichannel activity with 2-octanol, reduced a portion of the bradykinin-induced intracellular calcium event, but did not measure the effect of Cx hemichannel blockers on ATP release in response to bradykinin. In support of a role for Panx1 channel-mediated ATP release in these cells, blockade of Panx1 channels with 10panx1 significantly attenuated ATP release. In addition, removal of extracellular calcium in these studies did not affect the magnitude of ATP release. Since Cx hemichannels open in response to decreases in [Ca2+]e, ATP release should increase under these conditions, unless all Cx hemichannels in these cells were already open, which would be extremely detrimental to cell viability. A separate study from the same group has reported that in addition to activation of BDKRB2, Panx1 channels can also be activated in human subcutaneous fibroblasts by histamine acting through the HRH1 [102].
In the vasculature, receptor coupling to Panx1 channels have been observed in both smooth muscle and endothelial cells. In primary human umbilical vein endothelial cells, cell activation with the protease thrombin causes significant release of ATP into the extracellular milieu [88]. The effects of thrombin on this process were subsequently shown to be mediated by direct activation of PAR-1 expressed on these endothelial cells, leading to a rise in intracellular calcium and downstream activation Panx1 channels liberating ATP. At the level of vascular smooth muscle, Panx1 channels have been linked to vasoconstriction in response to α1D-AR activation [7]. α1D-ARs receive sympathetic input from perivascular nerves that release the AR agonist norepinephrine (NE). Binding of NE to the α1D-ARs promotes Panx1 channel activation releasing ATP that then binds to purinergic receptors located on the same or neighboring VSMCs ultimately causing vasoconstriction in resistance arteries [7,132]. In these studies, overexpression (utilizing a Panx1 plasmid) or knockdown of Panx1 with targeted siRNAs strengthened and reduced α1D-AR mediated vasoconstriction, respectively.
Finally, Panx1 channel activation has been observed downstream of NMDAR activation in pyramidal neurons in response to ischemia [89,90]. During conditions of cerebral ischemia, a period of anoxic depolarization ensues in hippocampal neurons that is promoted by the opening of Panx1 channels leading to excitotoxicity and cell death [89,140]. These initial observations by Thompson et al supported a functional role for Panx1 channels in anoxic depolarization in isolated hippocampal neurons, but have since been debated in an in situ brain slice model [141]. This latter study was unable to identify a discernable role for Panx1 channels during ischemic insult in isolated hippocampal brain slices using a pharmacological approach to block Panx channel activity. However, more recent evidence from Weilinger et al. utilizing a conditional Panx1 knockout mouse model demonstrated a significant role for Panx1-mediated currents in ischemia-induced anoxic depolarizations [90]. This event is mediated by activation of NMDARs expressed at the neuronal synapse which promotes activation of Src Family Kinases (SFKs) and Panx1 channels. Using a novel competing peptide designed against a region of the Panx1 C-tail that contains a potential tyrosine phosphorylation site, as well as a pharmacological inhibitor of SFKs called PP2, Weilinger et al. identified a specific role for SFKs in promoting Panx1 channel activity during ischemia [90]. In sum, the growing evidence for activation of a number of receptors imparting activation of Panx1 channels may suggest that this is a defining difference between Cx hemichannel and Panx1 channels.
5. Conclusions
Defining particular roles for Cx hemichannels and Panx channels in cellular ATP release has been an enduring problem in the field of purinergic signaling. These two families of channel forming proteins share a number of similarities, despite lacking sequence homology, which have hindered the development of specific inhibitors to test their function, respectively. While there are apparent similarities, such as membrane topology, plasma membrane localization and functional overlap as channels capable of releasing ATP, Cxs and Panxs appear to harbor unique characteristics affecting channel formation, gating and activation. Importantly, the Panxs have emerged as physiological ATP release channels while the role of Cx hemichannels under physiological settings in not clear. Ascribing a particular function to Panxs vs Cxs in health and disease remains a difficult problem and is associates with factors including the pharmacological selectivity between channels, methodological differences in assessing channel function and genetic alterations associated with knockout animal models. Great strides have been made in many of these areas, particularly at the genetic level with the advent of inducible, cell-type specific knockout animals. In addition, more rigorous pharmacological approaches are being employed in combination with genetic models to increase the accumulation of evidence for the involvement of Cx hemichannels and Panx1 in many physiological and pathological contexts. As we continue to assign new roles for Panxs and Cxs in ATP release, it will remain important to not rely on a single technique for ascribe function to one channel or the other, but to employ a rigorous examination of channel function through multiple assays. Finally, as research on Panx channels has continued to grow, it is becoming increasingly evident that these channels may be intimately linked to receptor activation in many cell types and tissues, providing a unique segregation from Cx hemichannels. Future studies will be important to elucidate the potential for Cx hemichannel to release ATP under physiological conditions in response to specific receptor activation. In conclusion, despite the current limitations, there is still much to learn about the unique properties between Cx hemichannels and Panx channels and their respective roles in human physiology and disease.
Acknowledgments
This work was supported by National Institutes of Health grants HL088554 (B.E.I.), HL107963 (B.E.I.) and an American Heart Association predoctoral fellowship (A.W.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hassan AB, El-Gendi AY. Effect of adenosine triphosphate (ATP) on arterial blood pressure and renal blood flow in normal and bled dogs. Zentralbl Veterinarmed A. 1981;28:152–158. doi: 10.1111/j.1439-0442.1981.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 2.Tarasova OS, Golubinskaya VO, Kosiakov AN, Borovik AS, Timin EN, Rodionov IM. The role of purinergic and adrenergic transmitters of the sympathetic system in the control of arterial blood pressure variability. J Auton Nerv Syst. 1998;70:66–70. doi: 10.1016/s0165-1838(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 3.Wolf MM, Berne RM. Coronary vasodilator properties of purine and pyrimidine derivatives. Circ Res. 1956;4:343–348. doi: 10.1161/01.res.4.3.343. [DOI] [PubMed] [Google Scholar]
- 4.Winbury MM, Papierski DH, Hemmer ML, Hambourger WE. Coronary dilator action of the adenine-ATP series. J Pharmacol Exp Ther. 1953;109:255–260. [PubMed] [Google Scholar]
- 5.Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- 6.Hopwood AM, Burnstock G. ATP mediates coronary vasoconstriction via P2x- purinoceptors and coronary vasodilatation via P2y-purinoceptors in the isolated perfused rat heart. Eur J Pharmacol. 1987;136:49–54. doi: 10.1016/0014-2999(87)90777-1. [DOI] [PubMed] [Google Scholar]
- 7.Billaud M, et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G, Brouns I, Adriaensen D, Timmermans JP. Purinergic Signaling in the Airways. Pharmacol Rev. 2012 doi: 10.1124/pr.111.005389. [DOI] [PubMed] [Google Scholar]
- 9.Thomas T, Ralevic V, Bardini M, Burnstock G, Spyer KM. Evidence for the involvement of purinergic signalling in the control of respiration. Neuroscience. 2001;107:481–490. doi: 10.1016/s0306-4522(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 10.Funk GD. Neuromodulation: purinergic signaling in respiratory control. Compr Physiol. 2013;3:331–363. doi: 10.1002/cphy.c120004. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 12.Sueta T, Paki B, Everett AW, Robertson D. Purinergic receptors in auditory neurotransmission. Hear Res. 2003;183:97–108. doi: 10.1016/s0378-5955(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 13.Sneddon P, Westfall TD, Todorov LD, Mihaylova-Todorova S, Westfall DP, Kennedy C. Modulation of purinergic neurotransmission. Prog Brain Res. 1999;120:11–20. doi: 10.1016/s0079-6123(08)63542-6. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 15.Koeppen M, Di Virgilio F, Clambey ET, Eltzschig HK. Purinergic regulation of airway inflammation. Subcell Biochem. 2011;55:159–193. doi: 10.1007/978-94-007-1217-1_7. [DOI] [PubMed] [Google Scholar]
- 16.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G401–G410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2013;368:1260. doi: 10.1056/NEJMc1300259. [DOI] [PubMed] [Google Scholar]
- 19.Li D, et al. Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:e81–e89. doi: 10.1161/ATVBAHA.111.239095. [DOI] [PubMed] [Google Scholar]
- 20.Ragazzi E, Chinellato A. Purinergic receptors, prostacyclin and atherosclerosis. Pharmacol Res. 1992;26:123–129. doi: 10.1016/s1043-6618(05)80125-2. [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013 doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72:5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- 23.Buxton IL, Yokdang N, Matz RM. Purinergic mechanisms in breast cancer support intravasation, extravasation and angiogenesis. Cancer Lett. 2010;291:131–141. doi: 10.1016/j.canlet.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deli T, Csernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res. 2008;14:219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- 25.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 26.Matute C, Cavaliere F. Neuroglial interactions mediated by purinergic signalling in the pathophysiology of CNS disorders. Semin Cell Dev Biol. 2011;22:252–259. doi: 10.1016/j.semcdb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 28.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970;45:272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahl G, Levine E, Rabadan-Diehl C, Werner R. Cell/cell channel formation involves disulfide exchange. Eur J Biochem. 1991;197:141–144. doi: 10.1111/j.1432-1033.1991.tb15892.x. [DOI] [PubMed] [Google Scholar]
- 34.Foote CI, Zhou L, Zhu X, Nicholson BJ. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. J Cell Biol. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–322. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97(1985):1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 38.Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- 39.Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg GS, Lampe PD, Sheedy D, Stewart CC, Nicholson BJ, Naus CC. Direct isolation and analysis of endogenous transjunctional ADP from Cx43 transfected C6 glioma cells. Exp Cell Res. 1998;239:82–92. doi: 10.1006/excr.1997.3872. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 42.Nualart-Marti A, et al. Role of connexin 32 hemichannels in the release of ATP from peripheral nerves. Glia. 2013;61:1976–1989. doi: 10.1002/glia.22568. [DOI] [PubMed] [Google Scholar]
- 43.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eltzschig HK, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 45.Schock SC, Leblanc D, Hakim AM, Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 46.Davidson JO, Green CR, Nicholson LF, O'Carroll SJ, Fraser M, Bennet L, Gunn AJ. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol. 2012;71:121–132. doi: 10.1002/ana.22654. [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran S, Xie LH, John SA, Subramaniam S, Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS One. 2007;2:e712. doi: 10.1371/journal.pone.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren Q, Riquelme MA, Xu J, Yan X, Nicholson BJ, Gu S, Jiang JX. Cataract- causing mutation of human connexin 46 impairs gap junction, but increases hemichannel function and cell death. PLoS One. 2013;8:e74732. doi: 10.1371/journal.pone.0074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klaassen LJ, Fahrenfort I, Kamermans M. Connexin hemichannel mediated ephaptic inhibition in the retina. Brain Res. 2012;1487:25–38. doi: 10.1016/j.brainres.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 50.Lee JR, Derosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J Invest Dermatol. 2009;129:870–878. doi: 10.1038/jid.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 52.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong CW, et al. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 54.Lu D, Soleymani S, Madakshire R, Insel PA. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 2012 doi: 10.1096/fj.12-204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci U S A. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fasciani I, et al. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology. 2013;75C:479–490. doi: 10.1016/j.neuropharm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 58.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008;132:315–327. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci U S A. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflugers Arch. 1999;437:345–353. doi: 10.1007/s004240050788. [DOI] [PubMed] [Google Scholar]
- 61.Orellana JA, et al. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60:53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Figueroa V, Saez PJ, Salas JD, Salas D, Jara O, Martinez AD, Saez JC, Retamal MA. Linoleic acid induces opening of connexin26 hemichannels through a PI3K/Akt/Ca(2+)-dependent pathway. Biochim Biophys Acta. 2013;1828:1169–1179. doi: 10.1016/j.bbamem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Batra N, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponsaerts R, D'Hondt C, Hertens F, Parys JB, Leybaert L, Vereecke J, Himpens B, Bultynck G. RhoA GTPase switch controls Cx43-hemichannel activity through the contractile system. PLoS One. 2012;7:e42074. doi: 10.1371/journal.pone.0042074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiori MC, Figueroa V, Zoghbi ME, Saez JC, Reuss L, Altenberg GA. Permeation of calcium through purified connexin 26 hemichannels. J Biol Chem. 2012;287:40826–40834. doi: 10.1074/jbc.M112.383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 67.Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebihara L, Tong JJ, Vertel B, White TW, Chen TL. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest Ophthalmol Vis Sci. 2011;52:882–889. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem J. 2010;432:133–143. doi: 10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- 70.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 72.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193:1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swayne LA, Sorbara CD, Bennett SA. Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J Biol Chem. 2010;285:24977–24986. doi: 10.1074/jbc.M110.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cowan KN, Langlois S, Penuela S, Cowan BJ, Laird DW. Pannexin1 and Pannexin3 exhibit distinct localization patterns in human skin appendages and are regulated during keratinocyte differentiation and carcinogenesis. Cell Commun Adhes. 2012;19:45–53. doi: 10.3109/15419061.2012.712575. [DOI] [PubMed] [Google Scholar]
- 76.Ambrosi C, et al. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 78.Sosinsky GE, et al. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penuela S, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 81.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2011;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schenk U, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 84.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 85.Gulbransen BD, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chekeni FB, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Godecke S, Roderigo C, Rose CR, Rauch BH, Godecke A, Schrader J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol. 2012;302:C915–C923. doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- 89.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 90.Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012;32:12579–12588. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spray DC, Ye ZC, Ransom BR. Functional connexin "hemichannels": a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 95.D'Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 96.Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- 97.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- 99.Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- 100.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 101.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinheiro AR, et al. Histamine induces ATP release from human subcutaneous fibroblasts, via pannexin-1 hemichannels, leading to Ca2+ mobilization and cell proliferation. J Biol Chem. 2013;288:27571–27583. doi: 10.1074/jbc.M113.460865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol. 1995;488(Pt 3):721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L623–L630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- 105.Martin PE, Wall C, Griffith TM. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br J Pharmacol. 2005;144:617–627. doi: 10.1038/sj.bjp.0706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol. 1997;503(Pt 1):99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang N, et al. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–552. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 110.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 112.Wang N, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ponsaerts R, et al. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010;24:4378–4395. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 114.Seminario-Vidal L, et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin- induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 116.Manohar M, Hirsh MI, Chen Y, Woehrle T, Karande AA, Junger WG. ATP release and autocrine signaling through P2X4 receptors regulate gammadelta T cell activation. J Leukoc Biol. 2012;92:787–794. doi: 10.1189/jlb.0312121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Contreras JE, et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orellana JA, Diaz E, Schalper KA, Vargas AA, Bennett MV, Saez JC. Cation permeation through connexin 43 hemichannels is cooperative, competitive and saturable with parameters depending on the permeant species. Biochem Biophys Res Commun. 2011;409:603–609. doi: 10.1016/j.bbrc.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lohman AW, et al. S-nitrosylation inhibits pannexin 1 channel function. J Biol Chem. 2012 doi: 10.1074/jbc.M112.397976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bargiotas P, et al. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:20772–20777. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dvoriantchikova G, Ivanov D, Barakat D, Grinberg A, Wen R, Slepak VZ, Shestopalov VI. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS One. 2012;7:e31991. doi: 10.1371/journal.pone.0031991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 127.Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 128.Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz O, Ojcius DM. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Timoteo MA, Carneiro I, Silva I, Noronha-Matos JB, Ferreirinha F, Silva-Ramos M, Correia-de-Sa P. ATP released via pannexin-1 hemichannels mediates bladder overactivity triggered by urothelial P2Y receptors. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 131.Pinheiro AR, Paramos-de-Carvalho D, Certal M, Costa C, Magalhaes-Cardoso MT, Ferreirinha F, Costa MA, Correia-de-Sa P. Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun Signal. 2013;11:70. doi: 10.1186/1478-811X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sumi Y, Woehrle T, Chen Y, Yao Y, Li A, Junger WG. Adrenergic receptor activation involves ATP release and feedback through purinergic receptors. Am J Physiol Cell Physiol. 2010;299:C1118–C1126. doi: 10.1152/ajpcell.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanjanamekanant K, Luckprom P, Pavasant P. P2X7 receptor-Pannexin1 interaction mediates stress-induced interleukin-1 beta expression in human periodontal ligament cells. J Periodontal Res. 2013 doi: 10.1111/jre.12139. [DOI] [PubMed] [Google Scholar]
- 137.Marques-da-Silva C, Chaves MM, Rodrigues JC, Corte-Real S, Coutinho-Silva R, Persechini PM. Differential modulation of ATP-induced P2X7-associated permeabilities to cations and anions of macrophages by infection with Leishmania amazonensis. PLoS One. 2011;6:e25356. doi: 10.1371/journal.pone.0025356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alberto AV, Faria RX, Couto CG, Ferreira LG, Souza CA, Teixeira PC, Froes MM, Alves LA. Is pannexin the pore associated with the P2X7 receptor? Naunyn Schmiedebergs Arch Pharmacol. 2013;386:775–787. doi: 10.1007/s00210-013-0868-x. [DOI] [PubMed] [Google Scholar]
- 139.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–C255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 141.Madry C, Haglerod C, Attwell D. The role of pannexin hemichannels in the anoxic depolarization of hippocampal pyramidal cells. Brain. 2010;133:3755–3763. doi: 10.1093/brain/awq284. [DOI] [PMC free article] [PubMed] [Google Scholar]