Abstract
Mesenchymal stem cells (MSCs), as well as osteoblastic cells derived from these MSCs, have been shown to be key components of the hematopoietic stem cell (HSC) niche. In this study, we wished to examine whether other cell types that are known to differentiate from MSCs similarly regulate the stem cell niche, namely cells of the adipocyte lineage. Recent studies have examined the role that adipocytes play in the biology of the HSCs in different bone locations and in transplantation settings; however, none have examined their role under homeostatic conditions. We compared the ability of adipocytic and nonadipocytic cell lines to support primitive hematopoietic cells in vitro. Preadipocytic cell lines demonstrated enhanced support of hematopoietic cells. Similarly, primary bone marrow (BM) cells treated with troglitazone, a drug that enhances adipogenesis, also demonstrated augmented support over control-treated stromal cells. We further examined the effects of increased adipocyte number in vivo under homeostatic conditions using troglitazone treatment and found that these alterations had no effect on HSC frequency. Taken together, we demonstrate that cells of the adipocyte lineage promote the ability of stromal cells to support primitive hematopoietic cells in vitro, yet alterations of adipocyte number and volume in vivo have no effect. These data suggest that adipocytes are not a component of the adult BM HSC niche under homeostatic conditions.
Introduction
Hematopoietic stem cells (HSCs) in the adult mouse bone marrow (BM) are known to be localized near the endosteal surface of bone or associated with sinusoidal endothelium. The stromal cells surrounding the HSCs create a nonrandom microenvironmental niche that regulates HSC function and is composed of a number of cell types, including mesenchymal stem cells (MSCs), osteoblasts, and endothelial cells [1–6]. These cells act upon the HSCs through secretion of soluble factors or by direct cell-to-cell contact mechanisms.
While the role of the MSCs, and the osteoblastic cells derived from these stem cells, appears to have a clearly defined role in regulating HSC physiology, the role of another cell type derived from the MSCs, the adipocyte, is less clear. This is predominantly due to opposing findings of multiple studies. It was originally assumed that adipocytes were simply passive space fillers in the BM upon observing the turnover of red marrow to yellow marrow attributable to age [7]. Naveiras et al., however, identified a reduction in HSC number after comparing adipocyte-rich tail vertebrae marrow with that of adipocyte-free thoracic vertebrae and noted an accelerated recovery after BM ablation of genetically modified fatless mice [8]. More recently, peroxisome proliferator-activated receptor gamma (PPARγ) inhibitor treatment of mice following chemotherapy led to a reduced number of adipocytes, which correlated with an increased rate of recovery of the hematopoietic system [9]. These data implied that adipocytes are predominantly negative regulators of the BM microenvironment in vivo. Chitteti et al. further supported this by showing the inefficient proliferation of Lin−c-Kit+Sca-1+ (LKS) cells and colony-forming unit-culture production with GZL stromal cell line high in adipocyte content. The negative regulative affect being accredited to the increased expression of adiponectin and neuropilin-1 [10].
Conversely, adipocytes have also been found to support HSCs, reappearing at day 7 after irradiation injury, corresponding to the initiation of hematopoietic proliferation [11]. In vitro adipocytes have been shown to be able to support myelopoiesis and lymphopoiesis and suppress human HSC differentiation, thus prolonging cell survival [12–14]. Additionally, adipocytes have been found to secret adipokines, cytokine family growth factors that play a role in hematopoiesis. Leptin-deficient obese ob/ob mice have impairments in hematopoiesis, which could be restored following the treatment with exogenous Leptin [15]. This factor also causes a proliferative effect in HSCs, showing increases in lymphopoiesis, myelomonocytic progenitor cells, and synergizes with stem cell factor (SCF) in the proliferation of primitive hematopoietic progenitors [16,17]. Interleukin-6 (IL-6) and IL-8 are growth factors derived from adipocytes that have roles in the proliferation and differentiation of hematopoietic cells [18]. Adiponectin enhances HSC proliferation in vitro and when expanded can more efficiently reconstitute lethally irradiated hosts through AdipoR1-mediated signaling [19]. CXCL12-abundant reticular cells are adipo-osteogenic progenitors that produce large amount of CXCL12 and SCF, which are required for the proliferation and maintenance of HSCs [20].
In this study, we investigated the role of adipocytes in the HSC microenvironment under homeostatic conditions. Using troglitazone, an antidiabetic drug known to be a PPAR-γ agonist [21], we increased adipocyte number both in vitro and in vivo and observed whether these changes in adipocyte numbers produced significant changes in primitive hematopoietic cell populations. We provide evidence that while increased adipocyte number in stromal cells led to augmented support of primitive hematopoietic cells in vitro, increased adipocyte number in vivo had no effect. These results argue against a role for adipocytes in the stem cell niche under homeostatic conditions.
Materials and Methods
Cell lines
The OP9, M2-10B4, and MC3T3-E1 cell lines were all obtained from the American Type Culture Collection. The ST2 cell line was a kind gift of Dr. Baruch Frenkel, and the 3T3-L1 cell line was a kind gift of Dr. Steven Mittelman. The OP9 cell line was maintained in minimum essential medium (α-MEM) without ribonucleosides, supplemented with 20% fetal bovine serum (FBS) and penicillin/streptomycin (P/S; all from Mediatech, Inc.). The MC3T3-E1 cell line was grown in α-MEM with ribonucleosides, supplemented with 10% FBS and P/S. The 3T3-L1 cell line was maintained in Dulbecco's modified Eagle medium supplemented with 10% FBS and P/S. Both the ST2 and M2-10B4 cells lines were maintained in RPMI1640 supplemented with 10% FBS and P/S. All cell lines were grown in a humidified atmosphere at 37°C/5% CO2. To induce adipogenesis of the cell lines, confluent cultures were maintained in the regular growth medium supplemented with 10 μM troglitazone and 1 μM dexamethasone for 2 weeks. To induce osteogenesis of the cell lines, confluent cultures were maintained in the regular growth medium supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerolphosphate for 2 weeks.
Animals
Six- to 8-week-old male C57Bl/6 and B6.SJL mice (Taconic Farms, Inc.) were obtained and used in accordance with the University of Southern California Institutional Animal Care and Use Committee guidelines. Mice were housed in sterilized microisolator cages and received autoclaved food and water ad libitum.
Primary cell culture
BM mononuclear cells (MNCs) were obtained from the hind limbs of C57Bl/6 mice and maintained in M5300 long-term culture media (StemCell Technologies) until confluent. To induce adipogenesis of primary stromal cells, BM MNCs were maintained in the long-term culture medium with 10 μM troglitazone (Sigma-Aldrich).
Cobblestone area-forming cell assay
The cell lines or primary BM stromal cells were plated in a 96-well plate at 2×105 cells per well as the feeder layer and irradiated (35 Gy, cell lines; 15 Gy, primary BM stromal cells). Whole BM MNCs isolated from C57Bl/6 mice were seeded in serial dilution atop the confluent stromal layer. The cells were maintained in α-MEM (Mediatech, Inc.) supplemented with 10% FBS, P/S, and 1 μM hydrocortisone in a humidified atmosphere at 33°C/5% CO2. The presence of cobblestone areas was scored on week 5, and the frequency of cobblestone area-forming cells (CAFCs) were calculated using L-Calc software (StemCell Technologies). To examine the role of contact versus noncontact support, BM MNCs were plated at the same limiting dilutions into transwells (0.2 μm Anopore Membrane; Nunc International). Media were replaced weekly from both the 96-well plate and the transwells. For the addition of signaling inhibitors to the CAFC assay, inhibitors were added when BM MNCs were plated onto the feeder layer and continually added weekly along with media changes. Concentrations of the inhibitors were as follows: γ-Secretase Inhibitor II (30 μM; CalBioChem), Dickkopf-1 (100 ng/mL; R&D Systems), and Cyclopamine (5 μM; CalBioChem). To examine the role of adiponectin, a neutralizing anti-adiponectin antibody (1 μg/mL; Abcam) was added to the culture medium and continually added weekly along with media changes.
Oil Red-O assay
Confluent cell lines or primary stromal cells were fixed with 10% formalin and stained with Oil Red-O solution (Sigma-Aldrich) for 10 min. Images were acquired under an inverted Olympus phase-contrast microscope. To quantify the staining, Oil Red-O dye was eluted with 100% isopropanol for 10 min. Absorbance was measured using the Molecular Devices SpectraMax M5 at 490 nm using 100% isopropanol as the blank.
In vivo injections
C57/Bl6 mice were injected intraperitoneally with troglitazone (5 mg/kg in 0.1 mL saline solution) three times a week for 4 weeks. The weight of each mouse was weighed before each injection.
Histological analysis
Tibias from the troglitazone- or control-treated mice were dissected, fixed in formalin, decalcified in Immunocal, processed for embedding in paraffin, and sectioned at 5 μm (Thermo Scientific; Shandon Finesse ME+). Sections were stained with hematoxylin and eosin (Fischer Scientific) or for tartarate-resistant acid phosphatase-positive (TRAP+) cells using TRAP and alkaline phosphatase double-stain kit (Takara Bio, Inc.) according to the manufacturer's instructions. Pictures of stained sections were taken at 4× magnification (Carl Zeiss Axio Imager Z1 Microscope). Images were aligned at the growth plate starting from the left-hand side. Visible adipocytes, >30 μm, were quantified from the growth plate to the end of the image on the right-hand side. Quantification of the adipocyte volume as a percentage of the total BM volume was obtained following tracing of the adipocytes using AxioVision software (Zeiss). TRAP+ cells were quantified across the whole-bone section in the trabecular bone region immediately below the growth plate.
Flow cytometric analysis
BM MNCs were stained with biotinylated lineage antibodies (anti-CD3, anti-CD4, anti-CD8, anti-Ter119, anti-Gr-1, anti-Mac-1, and anti-B220), anti-Sca-1, anti-Flk-2, and anti-c-Kit (Pharmingen) and then labeled with a secondary fluorescent-conjugated streptavidin. The cells were then analyzed on a BD LSR II cytometer (BD Biosciences) and gated using FlowJo flow cytometry analysis software.
Competitive repopulation assay
250,000 BM MNCs from troglitazone- or control-treated mice (CD45.2) were mixed with 250,000 BM MNCs from a B6.SJL mouse (CD45.1). These cells were then injected into the tail vein of B6.SJL mice that were lethally irradiated at 9 Gy ∼24 h prior transplantation. Engraftment levels and multilineage reconstitution were measured in peripheral blood samples obtained from the tail of recipients starting week 4. PE anti-mouse CD45.1, FITC anti-mouse CD45.2, APC anti-mouse CD3e, PE-Cy7 anti-mouse CD11b, and biotin anti-mouse B220 antibodies (all from eBioscience) were used to stain the peripheral blood samples.
Statistical analysis
Comparison of experimental groups was performed using the unpaired two-tailed Student's t-test as appropriate for the data set. P<0.05 was considered significant.
Results
Preadipocytic cell lines demonstrate superior support of primitive hematopoietic cells
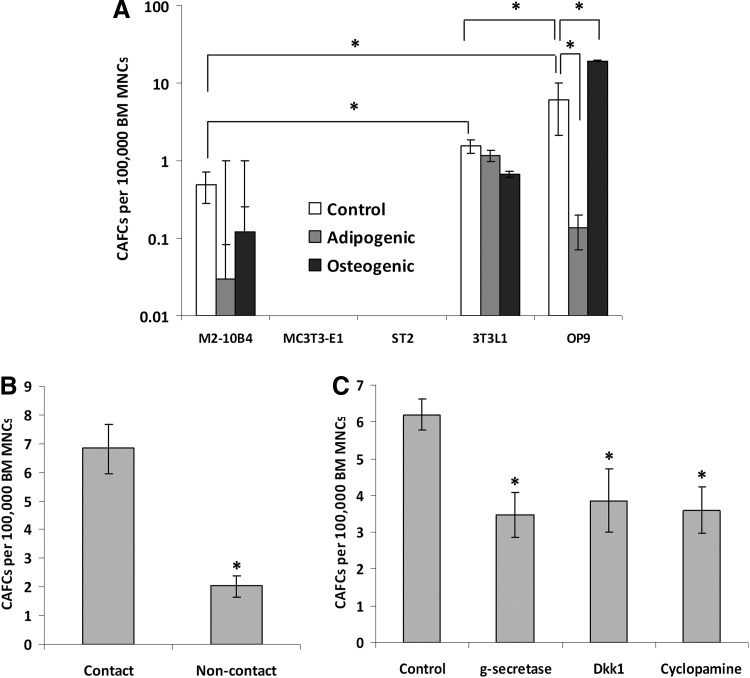
We initially wished to test whether cells of the adipocytic lineage would support hematopoietic cell growth in vitro. To achieve this, we performed CAFC assays at 5 weeks with five different cell lines. These included M2-10B4 (a mouse BM stromal cell line derived from a C57BL/6J×C3H/HeJ F1 mouse [22]), MC3T3-E1 (a preosteoblast cell line derived from a C57Bl/6 mouse [23]), ST2 (a BM stromal cell line derived from BC8 mice that can differentiate into osteoblasts and adipocytes [24–26]), 3T3-L1 (a preadipocyte substrain of the embryonic fibroblast cell line 3T3 [27]), and OP9 (a newborn calvaria BM stromal cell line derived from a C57BL/6×C3H F2–op/op mouse [28]). As shown in Fig. 1A, the M2-10B4, 3T3-L1, and OP9 cell lines were able to support primitive hematopoietic cells for extended periods in culture, whereas the MC3T3-E1 and ST2 cell lines were not. The significantly enhanced supportive capacity of the preadipocyte OP9 and 3T3-L1 cell lines over the other cell lines was further examined through differentiating the cell lines toward an adipogenic lineage using troglitazone (a PPAR-γ agonist that promotes the differentiation of adipocytes from MSCs), or an osteogenic lineage, and investigating any alterations in the function of the stromal cell layer. Differentiation of the cells was confirmed through Oil Red-O staining for adipogenic cells and Q-PCR of Runx2 expression for osteogenic cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). These data demonstrated that the MC3T3-E1 cells were unable to be differentiated, whereas the ST2 cells were able to be differentiated, yet continued to be unable to support primitive hematopoietic cells. This is presumably due to a lack of supportive factors released from this cell line since ST2 cells grown under normal conditions were unable to support hematopoietic cells, suggesting that differentiation toward an adipogenic or osteogenic lineage could not overcome the deficiency in the supportive ability. There were no significant alterations in the ability of the M2-10B4 or 3T3-L1 cell lines to support week 5 CAFCs (Fig. 1A) upon differentiation to the different lineages. The significant decrease in the ability of the OP9 cell line upon adipogenic differentiation to support primitive hematopoietic cells was presumably due to the close to 100% conversion of the cell line to mature adipocytes (Supplementary Fig. S1).
FIG. 1.
Stromal cell line support of primitive hematopoietic cells. (A) Cobblestone area-forming cell (CAFC) frequency of bone marrow (BM) mononuclear cells (MNCs) grown on stromal layer cell lines derived from five different cell lines representing adipogenic, osteogenic, and fibroblastic cells. (B) Long-term culture frequency of BM MNCs that had been cultured in noncontact conditions using the OP9 cell line as the stromal cell layer. (C) CAFC frequency of BM MNCs cultured in contact conditions in the presence of inhibitors of the Notch pathway (γ-secretase inhibitor), the Wnt pathway (Dickkopf-1), or the hedgehog pathway (Cyclopamine). All graphs represent the mean of three independent experiments, error bars represent SEM. *P<0.05.
Enhanced cell support of the OP9 cell line is mediated through contact-dependent mechanisms
To ascertain if the enhanced support by the OP9s was mediated by cell contact mechanisms, we performed long-term culture assays under normal contact conditions or by separating the hematopoietic cells from the stromal layer using a transwell insert (noncontact conditions). As shown in Fig. 1B, noncontact conditions resulted in a significant marked decrease in the ability of the stromal cells to support primitive hematopoietic cells for extended periods of time in culture suggesting that cell contact-dependent mechanisms are important for the enhanced support shown by the OP9 cell line. This does not discern what known pathways may be involved, therefore we performed CAFC assays in the presence of inhibitors of the Notch pathway (γ-secretase inhibitor), the Wnt pathway (Dickkopf-1), or the Hedgehog pathway (Cyclopamine), all of which have been implicated as regulators of HSC physiology [29–31]. As shown in Fig. 1C, all three inhibitors resulted in significant marked decreases in the ability of the stromal cells to support week 5 CAFCs, implying that multiple pathways are active in the support of HSCs. Adipocytic cells have a positive augmentative effect on the ability of stromal cells to support primitive hematopoietic cells that seems to be driven by multiple cell contact-dependent mechanisms.
Stimulation of adipocyte differentiation augments primary stromal cell support of primitive hematopoietic cells
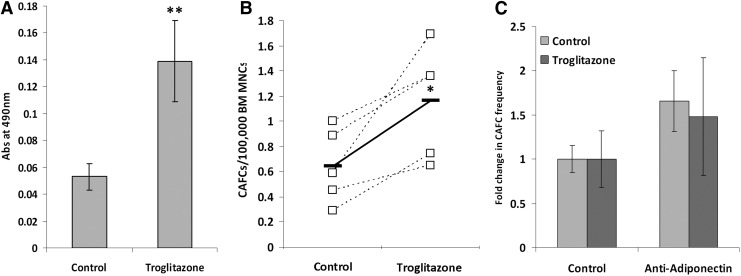
Considering the enhanced support of primitive hematopoietic cells by the preadipocytic cell lines, OP9 and 3T3-L1, we subsequently tested whether increased adipocytic cell numbers in primary stromal cell cultures led to a similar enhanced support of primitive hematopoietic cells in vitro. Primary adult murine BM stromal cell cultures were grown in the presence or absence of 10 μM troglitazone and then assessed for their supportive ability in CAFC assays. Troglitazone treatment led to a marked increase in the number of adipocytes in primary stromal cell cultures confirming the biased differentiation toward the adipocytic lineage (Fig. 2A; Supplementary Fig. 2). It has been reported that troglitazone can have an effect on the differentiation of cells of the myeloid lineage as well, although there are conflicting reports as to whether it stimulates or inhibits osteoclast function [32–34]. Therefore, we assessed the fraction of myeloid cells in the stromal cell layers and found them to be comparable (96.8% for control-treated cells vs. 94.9% for troglitazone-treated cells). Analysis of the number of TRAP+ osteoclastic cells in the stromal cell layer demonstrated an almost undetectable level in the control cultures, which was not increased in the troglitazone-treated stromal cell layer (data not shown). Assessing the ability of these troglitazone-treated stromal cells to support primitive hematopoietic cells, these conditions demonstrated a significant enhancement in their ability to support week 5 CAFCs (Fig. 2B). Since adiponectin has been shown to increase HSC proliferation in vitro, we examined whether this played a role in the enhanced support of the troglitazone-treated stromal layers. Analysis of the fold change in the CAFC frequency in the presence of a neutralizing anti-adiponectin antibody did not result in any changes (Fig. 2C), further suggesting that the effects were not due to a secreted factor, rather cell contact-dependent mechanisms. These in vitro experiments, though, confirm that cells of the adipocytic lineage promote the proliferation and/or survival of primitive hematopoietic cells.
FIG. 2.
Effect of increased adipocyte number on primary stromal cell support of primitive hematopoietic cells. BM MNCs were grown as stromal layers in the presence of 10 μM troglitazone or control dimethyl sulfoxide (DMSO). (A) Quantification of the total adipocyte concentration following solubilization of the Oil Red-O dye. (B) CAFC frequency of BM MNCs co-cultured on stromal cell layers grown in the presence of troglitazone or control conditions. Five independent experiments are shown, with the mean represented as the solid line. (C) CAFC frequency of BM MNCs co-cultured on stromal cell layers grown in the presence of troglitazone or control conditions with added neutralizing anti-adiponectin antibody. Two independent experiments are shown. *P<0.05; **P<0.001.
Troglitazone treatment leads to increased BM adipocyte frequency
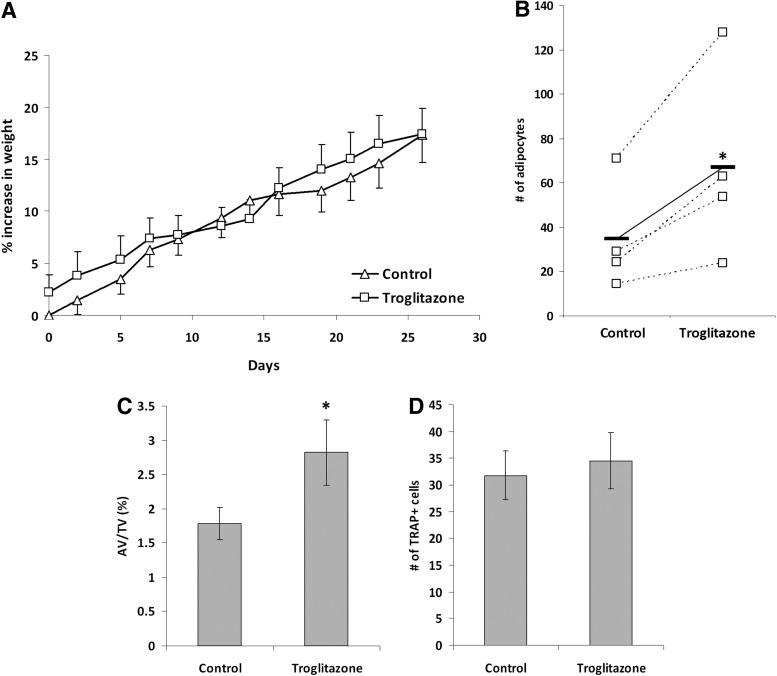
To enable us to examine the role of adipocytes in the HSC niche in vivo, we first wished to establish a model where cells of the adipocyte lineage are specifically modulated. Troglitazone has previously been used to increase adipocyte volume in the BM of mice through addition of it to food [35]. In our study, we treated wild-type C57Bl/6 mice with 5 mg/kg of troglitazone three times per week for 4 weeks. This treatment did not lead to any observable differences in the weight of the treated mice compared with controls (Fig. 3A), suggesting that subcutaneous or visceral fat was not significantly affected. However, the examination of the BM through histological analysis confirmed a specific significant increase in the number of adipocytes in the BM (Fig. 3B; Supplementary Fig. S3), as well as an increase in the total adipocyte volume relative to total BM volume (Fig. 3C). As discussed earlier, troglitazone may affect the differentiation of the osteoclasts, which may in turn affect the HSCs [36,37]. Therefore, we quantified the number of TRAP+ cells in the BM. No significant differences were observed (Fig. 3D), suggesting that the effects of Troglitzone were limited to cells of the adipogenic lineage. This model therefore provided us with a method in which to examine the specific effects of increased adipocytic cells in vivo on the primitive hematopoietic cells under homeostatic conditions.
FIG. 3.
Troglitazone treatment increases the number of adipocytes in the BM. Mice were treated with troglitazone (5 mg/kg, three times per week) or DMSO control for 4 weeks. (A) Percent increase in total body weight of the mice over the treatment period. These data represent the mean of three independent experiments, with five mice in each group for each experiment. (B) Quantification of the number of adipocytes in each section. The dotted lines represent an individual experiment where five mice were examined in each group. The solid line represents the mean of all experiments. (C) Quantification of the volume of the adipocytes expressed as a percentage of the total BM volume of the same sections. These data represent the mean of three independent experiments, with five sections in each group for each experiment. (D) Quantification of the number of tartarate-resistant acid phosphatase-positive (TRAP+) cells in the metaphysis region of each section. These data represent the mean of three independent experiments, with five sections in each group for each experiment. *P<0.05.
Increased adipocyte frequency does not alter HSC frequency in vivo
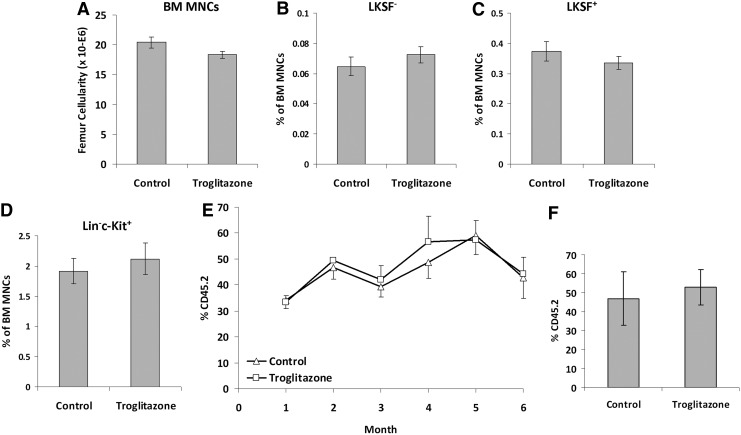
To assess whether the increase in adipocyte frequency and volume in the BM resulted in alterations of the primitive hematopoietic cells, we examined the frequency of these cells in the BM following troglitazone treatment of C57Bl/6 mice. As shown in Fig. 4A, troglitazone treatment did not result in any significant alteration in the cellularity of the BM. Analysis of the primitive hematopoietic cell subsets similarly did not show any significant differences in the frequency of the more primitive Lin−c-Kit+Sca-1+Flk-2− (LKSF−) or the more differentiated LKSF+ or lin−c-Kit+ cells (Fig. 4B–D). Since no alterations in the frequency of the cells do not necessarily rule out a functional change in the HSCs, we next performed a competitive repopulation analysis of HSC function. Transplanting BM MNCs derived from troglitazone- or control-treated mice, we did not observe any significant difference in the contribution to hematopoiesis at early or late time points (Fig. 4E), again suggesting that the increase in adipocyte frequency in the BM did not lead to functional differences in the primitive hematopoietic cells. Therefore, from these data, we can conclude that an increase in adipocyte number and volume in the BM does not influence the biology of the HSCs and thus are not a key component of the HSC niche.
FIG. 4.
Effect of in vivo troglitazone treatment on the frequency and function of primitive hematopoietic cells. Mice were treated with troglitazone (5 mg/kg, three times per week) or DMSO control for 4 weeks and the BM MNCs were obtained. (A) Cellularity, (B) Lin−c-Kit+Sca-1+Flk-2− (LKSF−) cell frequency, (C) LKSF+ cell frequency, and (D) lin-c-Kit+ cells frequency is shown. (E) Competitive transplant of BM MNCs from troglitazone- or control-treated mice following 4 weeks of treatment. (F) Engraftment in the BM at the 6-month time point. These data represent the mean of three independent experiments, with five mice in each group for each experiment, error bars represent SEM.
Discussion
While the role of the MSCs, and the MSC derived osteoblastic cells, in the HSC niche have been extensively described [1–3], the role of other MSC derived cells, specifically the adipocyte, remains controversial. Initial reports suggested that adipocytes may be positive regulators of HSC function; however, this was based on studies examining the effects of adiponectin on the expansion of a purified HSC population ex vivo, rather than the effects of the adipocytes themselves [19]. More recently, a key study, which suggested that adipocytes are negative regulators of the stem cell niche, made these conclusions from examining the frequency of the HSCs in adipocyte-rich tail vertebrae marrow to that of adipocyte-free thoracic vertebrae [8]. An important caveat here is that it is not clear if the two different locations also differ in the composition of other stromal supportive cells, not just cells of the adipocytic lineage, and thus may have an influence on the primitive hematopoietic cells.
A recent study has provided evidence for the existence of two distinct niches in the BM, a reconstituting niche and a homeostatic niche [38]. Previous studies have shown an accelerated recovery of the hematopoietic system after BM ablation of genetically modified fatless mice, thus adipocytes presumably play a negative role in the proposed reconstituting niche. Here, we wished to examine the role of the adipocyte in the adult murine BM homeostatic niche. We first examined the supportive capacity of different cell lines and found the OP9 and 3T3-L1 preadipocytic cell lines to be superior. The OP9 cells line is a commonly used cell line to support primitive cells, including those of the hematopoietic and embryonic lineages [39,40]. While this demonstrated the crucial role of cell contact for the augmented support, we further wished to analyze mature adipocytes in a primary setting. Therefore, we chose a model system where we could specifically modulate cells of the mesenchymal lineage to preferentially differentiate toward adipocytes. Troglitazone is an antidiabetic drug that acts as an agonist for PPAR-γ [21]. Culture of primary BM stromal cells with troglitazone resulted in a significant increase in the frequency of adipocytes, which actually led to an increased support of primitive hematopoietic cells. Why these results are in contrast to those previously published in vitro reports [10] are not clear, but it may be due to our use of primary BM cells.
We further examined the effects of troglitazone in vivo and demonstrated that we could specifically increase the frequency of adipocytes in the BM following 4 weeks of treatment, with no other increases in body weight. This suggested that subcutaneous or visceral fat was not significantly affected and that other observations would not be significantly affected by other side effects induced by the treatment including alterations in hormone levels. In contrast to the augmented support of primitive hematopoietic cells in vitro, in vivo increases in adipocytes had no effect on the hematopoietic stem or progenitor cells. This is in contrast to previous in vivo studies but may reflect key differences between the reconstituting and homeostatic niche. Other studies by us have shown that the manipulation of the stromal compartment over a 4-week period led to significant changes in the primitive hematopoietic cell frequency [2]. The reasons for the lack of an effect on the primitive hematopoietic cells in this model system are many; however, one may relate to the fact that the adipocytes are not co-localized with the HSCs. In an in vitro setting, cell-to-cell contact mechanisms are inherently being altered by forcing HSCs to contact cells in ways that normally may not occur in vivo. These forced interactions may demonstrate an effect on the support of HSCs, yet may not correctly reflect in vivo physiology. It is thus important to validate these in vitro observations in a more natural homeostatic environment. Troglitazone treatment allowed for the selective increase in adipocyte volume and number, while seemingly not affecting any other component of hematopoiesis. As suggested in Supplementary Fig. S3, the increase in adipocytes was not localized to one region of the BM cavity, rather they appear to be in a random distribution. However, to formally rule out adipocytes as being active in the hematopoietic niche under homesostatic conditions, direct visualization of the HSCs in real time would have to be performed. Unfortunately, this has only been achieved with moderate success recently [41,42]. Our functional data thus argue against a role of adipocytes in the HSC niche under homeostatic conditions.
Supplementary Material
Acknowledgments
The authors would like to thank Dilani Rosa for technical assistance. We thank the University of Southern California Flow Cytometry Core Facility (supported by P30 CA014089 from the National Cancer Institute) for assistance with the flow cytometry analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN. and Frenette PS. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846 [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, et al. (2003). Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841 [DOI] [PubMed] [Google Scholar]
- 4.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J. and Aguila HL. (2004). Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103:3258–3264 [DOI] [PubMed] [Google Scholar]
- 5.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C. and Morrison SJ. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121 [DOI] [PubMed] [Google Scholar]
- 6.Ding L, Saunders TL, Enikolopov G. and Morrison SJ. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corre J, Barreau C, Cousin B, Chavoin JP, Caton D, Fournial G, Penicaud L, Casteilla L. and Laharrague P. (2006). Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol 208:282–288 [DOI] [PubMed] [Google Scholar]
- 8.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F. and Daley GQ. (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460:259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu R-J, Wu M-Q, Li Z-J, Zhang Y. and Liu K-Y. (2013). Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int J Hematol 97:58–72 [DOI] [PubMed] [Google Scholar]
- 10.Chitteti BR, Cheng Y-H, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, Kacena MA. and Srour EF. (2010). Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood 115:3239–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki K. and Allen TD. (1991). Ultrastructural and morphometric alterations in bone marrow stromal tissue after 7 Gy irradiation. Blood Cells 17:527–549 [PubMed] [Google Scholar]
- 12.Gimble JM, Dorheim MA, Cheng Q, Medina K, Wang CS, Jones R, Koren E, Pietrangeli C. and Kincade PW. (1990). Adipogenesis in a murine bone marrow stromal cell line capable of supporting B lineage lymphocyte growth and proliferation: biochemical and molecular characterization. Eur J Immunol 20:379–387 [DOI] [PubMed] [Google Scholar]
- 13.Gimble JM, Robinson CE, Wu X. and Kelly KA. (1996). The function of adipocytes in the bone marrow stroma: an update. Bone 19:421–428 [DOI] [PubMed] [Google Scholar]
- 14.Glettig DL. and Kaplan DL. (2013). Extending human hematopoietic stem cell survival in vitro with adipocytes. Biores Open Access 2:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claycombe K, King LE. and Fraker PJ. (2008). A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA 105:2017–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR. and Matthews W. (1996). A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6:1170–1180 [DOI] [PubMed] [Google Scholar]
- 17.Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S. and Nakahata T. (1997). Leptin stimulate the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood 90:3438–3443 [PubMed] [Google Scholar]
- 18.Hoch M, Eberle AN, Peterli R, Peters T, Seboek D, Keller U, Muller B. and Linscheid P. (2008). LPS induces interleukin-6 and interleukin-8 but not tumor necrosis factor-alpha in human adipocytes. Cytokine 41:29–37 [DOI] [PubMed] [Google Scholar]
- 19.DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U. and Reya T. (2007). Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol 178:3511–3520 [DOI] [PubMed] [Google Scholar]
- 20.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K. and Nagasawa T. (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33:387–399 [DOI] [PubMed] [Google Scholar]
- 21.Grossman SL. and Lessem J. (1997). Mechanisms and clinical effects of thiazolidinediones. Expert Opin Investig Drugs 6:1025–1040 [DOI] [PubMed] [Google Scholar]
- 22.Sutherland HJ, Eaves CJ, Lansdorp PM, Thacker JD. and Hogge DE. (1991). Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood 78:666–672 [PubMed] [Google Scholar]
- 23.Sudo H, Kodama HA, Amagai Y, Yamamoto S. and Kasai S. (1983). In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 96:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K. and Nishikawa S. (1988). B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J 7:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka E, Yamaguchi A, Hirose S. and Hagiwara H. (1999). Characterization of osteoblastic differentiation of stromal cell line ST2 that is induced by ascorbic acid. Am J Physiol 277:C132–C138 [DOI] [PubMed] [Google Scholar]
- 26.Ding J, Nagai K. and Woo JT. (2003). Insulin-dependent adipogenesis in stromal ST2 cells derived from murine bone marrow. Biosci Biotechnol Biochem 67:314–321 [DOI] [PubMed] [Google Scholar]
- 27.Green H. and Meuth M. (1974). An established pre-adipose cell line and its differentiation in culture. Cell 3:127–133 [DOI] [PubMed] [Google Scholar]
- 28.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K. and Bickel PE. (2006). OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res 47:450–460 [DOI] [PubMed] [Google Scholar]
- 29.Stier S, Cheng T, Dombkowski D, Carlesso N. and Scadden DT. (2002). Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99:2369–2378 [DOI] [PubMed] [Google Scholar]
- 30.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R. and Weissman IL. (2003). A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423:409–414 [DOI] [PubMed] [Google Scholar]
- 31.Trowbridge JJ, Scott MP. and Bhatia M. (2006). Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci U S A 103:14134–14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okazaki R, Toriumi M, Fukumoto S, Miyamoto M, Fujita T, Tanaka K. and Takeuchi Y. (1999). Thiazolidinediones inhibit osteoclast-like cell formation and bone resorption in vitro. Endocrinology 140:5060–5065 [DOI] [PubMed] [Google Scholar]
- 33.Schwab AM, Granholm S, Persson E, Wilkes B, Lerner UH. and Conaway HH. (2005). Stimulation of resorption in cultured mouse calvarial bones by thiazolidinediones. Endocrinology 146:4349–4361 [DOI] [PubMed] [Google Scholar]
- 34.Wan Y. (2010). PPARγ in bone homeostasis. Trends Endocrinol Metab 21:722–728 [DOI] [PubMed] [Google Scholar]
- 35.Tornvig L, Mosekilde L, Justesen J, Falk E. and Kassem M. (2001). Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int 69:46–50 [DOI] [PubMed] [Google Scholar]
- 36.Lymperi S, Ersek A, Ferraro F, Dazzi F. and Horwood NJ. (2011). Inhibition of osteoclast function reduces hematopoietic stem cell activity in vivo. Blood 117:1540–1549 [DOI] [PubMed] [Google Scholar]
- 37.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A. and Blin-Wakkach C. (2012). Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med 209:537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lassailly F, Foster K, Lopez-Onieva L, Currie E. and Bonnet D. (2013). Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood 122:1730–1740 [DOI] [PubMed] [Google Scholar]
- 39.Nakano T, Kodama H. and Honjo T. (1994). Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265:1098–1101 [DOI] [PubMed] [Google Scholar]
- 40.Kodama H, Nose M, Niida S, Nishikawa S. and Nishikawa S. (1994). Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol 22:979–984 [PubMed] [Google Scholar]
- 41.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP. and Scadden DT. (2009). Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, et al. (2009). Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457:97–101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.