Abstract
Open-field behavioral scoring is widely used to assess spinal cord injury (SCI) outcomes, but has limited usefulness in describing subtle changes important for posture and locomotion. Additional quantitative methods are needed to increase the resolution of locomotor outcome assessment. This study used gait analysis at multiple speeds (GAMS) across a range of mild-to-severe intensities of thoracic SCI in the rat. Overall, Basso, Beattie, and Bresnahan (BBB) scores and subscores were assessed, and detailed automated gait analysis was performed at three fixed walking speeds (3.5, 6.0, and 8.5 cm/sec). Variability in hindpaw brake, propel, and stance times were analyzed further by integrating across the stance phase of stepping cycles. Myelin staining of spinal cord sections was used to quantify white matter loss at the injury site. Varied SCI intensity produced graded deficits in BBB score, BBB subscores, and spinal cord white matter and total volume loss. GAMS measures of posture revealed decreased paw area, increased limb extension, altered stance width, and decreased values for integrated brake, propel, and stance. Measures of coordination revealed increased stride frequency concomitant with decreased stride length, resulting in deviation from consistent forelimb/hindlimb coordination. Alterations in posture and coordination were correlated to impact severity. GAMS results correlated highly with functional and histological measures and revealed differential relationships between sets of GAMS dynamics and cord total volume loss versus epicenter myelin loss. Automated gait analysis at multiple speeds is therefore a useful tool for quantifying nuanced changes in gait as an extension of histological and observational methods in assessing SCI outcomes.
Key words: : BBB score, DigiGait™, footprint, myelin, rat
Introduction
Contusion injury in the rat is a widely used experimental model for investigating spinal cord injury (SCI). This approach has been used with variations in injury location and intensity to study the sensorimotor impairments that result from SCI, and it provides models in which to assess new therapeutic interventions. Perhaps the most well-characterized instrument for evaluating functional deficits in gait and therapeutic efficacy after SCI is the Basso, Beattie, and Bresnahan (BBB) scale for assessing open-field locomotion.1 The BBB scale has been validated, is widely used, and is highly standardized,1 but only addresses a subset of readily observable attributes of gait. BBB scoring includes observation of basic movements limited to ankle, knee, and hip range of motion and plantar placement of the hindlimb with or without weight support. Additionally, observation of coordination, hindpaw position during locomotion, toe clearance, tail position, and trunk stability are scored. However, limitations in the BBB scale emerge from the categorical nature by which attributes are scored. Certain milestone movements, such as coordination, must be observed before additional criteria, such as toe clearance, can be scored.2 These limitations are addressed, in part, through the utilization of BBB subscores,2 which elucidate gains of function not otherwise scorable on the standard BBB scale in the absence of milestone indicators. BBB subscores represent measures of paw position, toe clearance, trunk control, and tail position made independent of all other observable traits. Notably, other attributes of SCI recovery, such as speed of locomotion, paw area, stance width, and step cycle dynamics, are not addressed by the BBB scale or subscale. Importantly, the BBB scale is not designed to measure the dynamics of forelimb movement.
Footprint analysis was developed to quantitate specific gait attributes, some of which are not captured by BBB scoring. Data gathering for footprint analysis by inking paws is both imprecise and labor intensive and does not restrict locomotion to specific speeds. Locomotion speed represents a source of variability in gait analysis that is not controlled experimentally in BBB scoring or in footprint analysis. By analyzing gait at several fixed speeds across a range of walking speeds, the contribution of gait speed can be experimentally controlled and analyzed as a source or error in gait analysis or as a contributor to alterations in abnormal gait dynamics. BBB-scored behaviors, by definition, are observed only at the self-determined ambulatory speed of each subject. Inherent differences in speed of movement across different injury intensities can also lead to confounded data; for example, more severely injured rats locomote at slower speeds. Accordingly, there is a need to address gait in SCI at known, fixed speeds.
Automated gait analysis reveals aspects of recovery from spinal injury that may be too rapid, complex, or subtle for visual observation. Further, automated measurement of gait dynamics removes the observer as a source of variability. It greatly increases the number and complexity of experimental outcomes that can be simultaneously gathered and potentially minimizes investigator time and effort while increasing throughput in data gathering. Many approaches, including the use of contact electrodes,3 robotic gait analysis devices,4 and digital footprint analysis,5 have been used to automate gait analysis to avoid potential observer bias or limitations. Video-based approaches, such as three-dimensional video analysis8 and CatWalk™,9–12 can improve the scope and details of data gathered, but as with BBB scoring, are limited to the gait speed at which the animal chooses to locomote. Hamers and colleagues identified this factor as a specific limitation of the CatWalk method, noting that much more information would be apparent using multiple fixed locomotion speeds.10
Video analysis of locomotion at variable speeds provides additional information. The DigiGait™ (Mouse Specifics, Inc., Quincy, MA) and TreadScan systems consist of a camera mounted beneath a variable speed transparent treadmill and were developed to expand the parameters of footprint analysis that can be assessed. The TreadScan 6,7 has been used for multi-speed analysis of functional outcomes in a mouse spinal transection model6 and mouse contusion model.17 The DigiGait system has been used to evaluate gait disturbances in rodent models of sciatic nerve injury,13 paw inflammation,14 and SCI.15,16 A previous study used DigiGait analysis at a single running-gait speed to complement BBB,16 but the researchers concluded that analysis at additional treadmill speeds would be beneficial. Gait analysis at multiple speeds (GAMS) using the DigiGait apparatus allows automated quantitation of numerous detailed attributes of gait; importantly, data can be gathered at specific user-selected treadmill speeds.
This study was undertaken to establish predictive measurements of SCI outcomes in rats with a range of mild to very severe contusion injuries. In light of limitations of previous approaches, we hypothesized that GAMS would produce highly correlated associations among injury intensity, histological outcomes, and locomotion and would complement and augment the BBB scoring system. Previous studies have typically selected a subset of gait measures that have not assessed the complexities of step cycle, posture, and coordination that comprehensive GAMS has the potential to address. Here, comprehensive, automated gait analysis was used at multiple walking speeds to characterize subtle changes in a broad range of complex step cycle, posture, and coordination dynamics. The GAMS results complemented BBB scores and correlated highly with injury impact intensity and spinal cord white matter loss at the injury epicenter. The results of this study demonstrate that varying intensity of SCI produces significant, graded changes in gait that can be resolved by GAMS, which complements and extends the usefulness of the BBB scale.
Methods
Subjects
Fifty-two Fisher 344 male rats housed in an Association for Assessment and Accredidation of Laboratory Animal Care–accredited facility were used in the study. All procedures were approved by the University of Kansas Medical Center Animal Care and Use Committee (Kansas City, KS) and complied with all federal and state regulations. Animals were randomly assigned to five different groups, four of which were injured with different forces: 125 kDyn (“mild”); 175 kDyn (“moderate”); 200 kDyn (“severe”); or 225 kDyn (“very severe”); and a laminectomy with no impact (sham). All sham and SCI subjects were included in the study and had a presurgical baseline BBB of 21. Eight subjects were identified as statistical outliers and were removed from the study on the basis of postsurgical BBB scores that varied more than two standard deviations from their group mean.
Surgery/care
Surgeries were performed on 100- to 110-day-old rats under ketamine (80 mg/kg)/xylazine (7 mg/kg) anesthesia under aseptic conditions. Sham animals received an eighth thoracic segment (T8) laminectomy alone, whereas injured animals underwent T8 laminectomy and contusion injury using an Infinite Horizon spinal cord impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA) with 125, 175, 200, or 225 kDyn impact. Displacement distance reported by the impactor software for each contusion was recorded at the time of surgery. At the conclusion of surgery, 0.25% bupivacaine HCl was applied locally to the incision site. Buprenex (0.01 mg/kg, subcutaneously [s.c.]) was injected immediately after surgery and 1 day later. All animals were monitored daily until the end of the experiment. On the first week after surgery, the rats received a daily s.c. injection of 30,000 U of penicillin (Combi-Pen 48) in 5 mL of saline to prevent infections and dehydration. Rats' bladders were expressed twice-daily until animals recovered urinary reflexes. From the second week onward, animals were supplemented with vitamin C pellets (BioServ, Frenchtown, NJ) to avert urinary tract infection.18
Behavioral assessments
BBB scoring was performed as previously described1 1–3 days before SCI and at 1, 7, 14, 21, and 28 days postimpact; the latest time point corresponds to when SCI outcomes are known to plateau.19–21 For observational purposes, a straight alley with a darkened goal box at the end was utilized as described by Wong and colleagues.22 For 1 week preceding testing, animals were habituated to the straight-alley apparatus. Scores and subscores2 were recorded for the left and right sides of each subject. Sided scores showed the greatest variation at the first week; statistical analysis revealed that the sided scores did not vary significantly within subjects (p=0.46). Therefore, left- and right-side scores were averaged and rounded to the nearest integer.
GAMS was performed with the Mouse Specifics DigiGait System. Computerized digital footprint images were generated by high-speed video recording from the ventral aspect of the animal. Baseline GAMS imaging was performed 1–3 days before SCI and repeated 28 days postimpact at three predetermined speeds of 3.5, 6.0, and 8.5 cm/sec. Actual treadmill speeds were directly determined by timing several full revolutions of the treadmill belt (of known length) across the range of speeds to accurately calibrate the testing speed. Based on observation and previously published findings,17 rats were not pretrained on the treadmill because generous pretraining inhibited the desired normal walking behavior. Data were evaluated across five impact groups, and over 40 metrics were generated by the Mouse Specifics analysis software. Gait analysis was performed with animals exhibiting a minimum BBB score of 10, representing weight bearing with occasional weight-supported steps. All gait dynamic indices were calculated with version 12.1 of the Mouse Specifics analysis software. Left- and right-limb values were averaged to generate one hindlimb (HL) and one forelimb (FL) value for each subject at each walking speed. To refine and expand the DigiGait system analysis of step-cycle dynamics, the stance phase of step cycles was integrated across time in an additional data analysis. Stance is the portion of the step cycle when the paw is in contact with the surface and can be divided into brake (from the first contact to peak stance) and propel (from peak until end of contact) phases. The Basler camera utilized with the DigiGait system captures images at a constant frame rate; accordingly, an area under the curve was calculated by adding the paw area measurements for each video frame captured during a complete stance. All complete HL stances that were observed for a given animal were averaged to generate one value per subject, resulting in a measure termed “integrated stance.” Additionally, integrated values were divided into brake and propel phases, which were temporally separated at the moment of peak stance, as defined by the video frame with the highest paw contact area.
Histology
At the end of 4 weeks, animals were euthanized with Beuthanasia-D and transcardially perfused with 0.1 M of phosphate-buffered saline (PBS), containing 10 U/mL of heparin, followed by 4% formaldehyde in PBS. Thoracic spinal cord segments were collected, starting from one vertebral segment rostral to two segments caudal from the laminectomy site (approximately 1.2 cm).
Transverse 20-μm frozen sections were collected at 100-μm intervals, resulting in approximately 120 sections per subject for analysis. Sections were stained for myelin using eriochrome cyanin (EC).23 All sections were imaged with 4×objective and 10×ocular magnification using a Nikon 80i microscope and Elements imaging software (Nikon Instruments, Melville, NY). Imaged sections were analyzed using MetaMorph image analysis software (Molecular Devices, LLC, Sunnyvale, CA). Images of EC-stained serial sections were processed using thresholding techniques; subsequently, borders of spinal cord sections were circumscribed and myelin-stained areas calculated by the image analysis software. Total volume of the injured spinal cord was calculated as a sum of total section areas multiplied by distances between sections starting from the most rostral section showing any damage through the most caudal section showing damage. Cord atrophy is reported as the differences in total volume of injured cords compared to total volumes of uninjured cords of identical length. For each injured cord, the injury epicenter was defined as the section within the injury with the least amount of myelin staining; white matter loss was assessed by measuring the area of myelin staining in this section, as compared to uninjured cords at a comparable level.
Statistical analysis
Statistical analyses were performed using SigmaPlot 11.0 (Systat Software, Inc., Chicago, IL). Group differences were evaluated by analysis of variance (ANOVA) or repeated-measures two-way ANOVA, as appropriate, with Bonferroni's or Student-Newman-Keuls' post-hoc tests when significance of p<0.05 was obtained. Data are presented as average±standard error of the mean. Spearman's rank-order correlation analysis was utilized to measure the strength of correlation among BBB scores, GAMS data parameters, and histological findings. Correlation values are reported as rs, the Spearman rank-order correlation coefficient.
Results
Tissue damage after injury
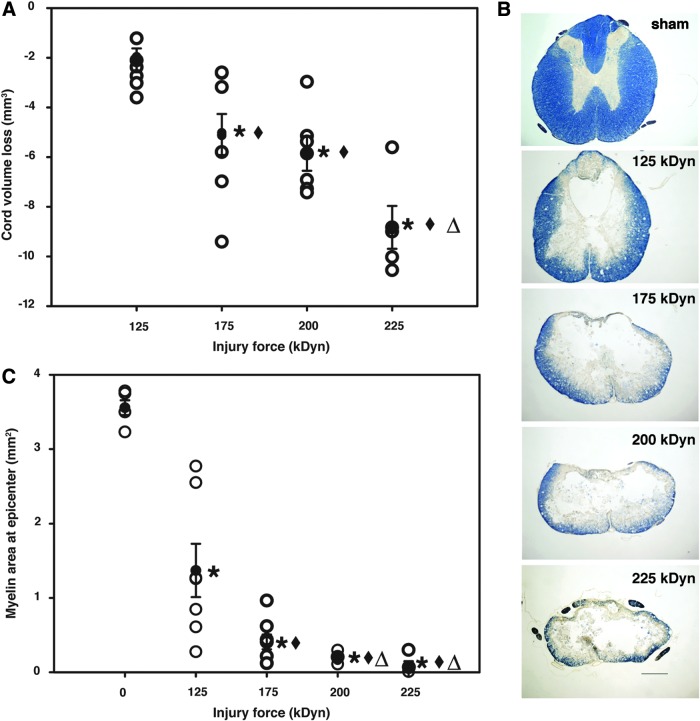
Tissue damage caused by T8 contusion was evaluated 4 weeks after injury. All contused spinal cords showed morphological changes consistent with damage; however, cord total volume loss was not significant in rats with the mild (125 kDyn) injury (Fig. 1A). All other injury groups showed cord total volume loss, compared to sham or mild injury groups, and rats with very severe (225 kDyn) injury were also significantly different from rats with moderate (175 kDyn) injury. Cord atrophy was significantly and linearly correlated with cord displacements at injury (ρ=0.658; p<0.035).
FIG. 1.
Assessment of the magnitude of spinal cord injuries. Open circles represent individual data points; closed circles represent group averages with standard error of the mean. (A) Cord volume loss, expressed as total cord volume relative to sham-operated controls. Note that cord total volume loss gradually increased with increasing injury severity (125 kDyn/mild, 175 kDyn/moderate, 200 kDyn/severe, and 225 kDyn/very severe; n=5, 7, 8, 6, and 5 from sham to very severe injury groups, respectively). *p<0.05, compared to sham-treated control spinal cords; ♦p<0.05, compared to mild injury; Δp<0.05, compared to moderate injury. (B) Representative images of cord injury epicenters stained with eriochrome cyanin 4 weeks after injury (scale bar, 500 μm). (C) White matter loss at epicenter of injury, measured as the amount of myelin staining at the injury epicenter. *p<0.05, compared to sham; ♦p<0.05, compared to mild injury. Color image is available online at www.liebertpub.com/neu
Representative images of transverse sections from sham spinal cord and injury epicenters from injury groups after EC myelin staining are shown in Figure 1B. There was a significant and gradually increasing loss of myelination in all injury groups, relative to sham animals; all groups were significantly different from each other, except severe (200 kDyn) and very severe (Fig. 1C). Calculated myelin loss in epicenter areas was significantly correlated with cord displacement values at the time of injury (ρ=0.922; p<0.0001).
Open-field locomotor assessment
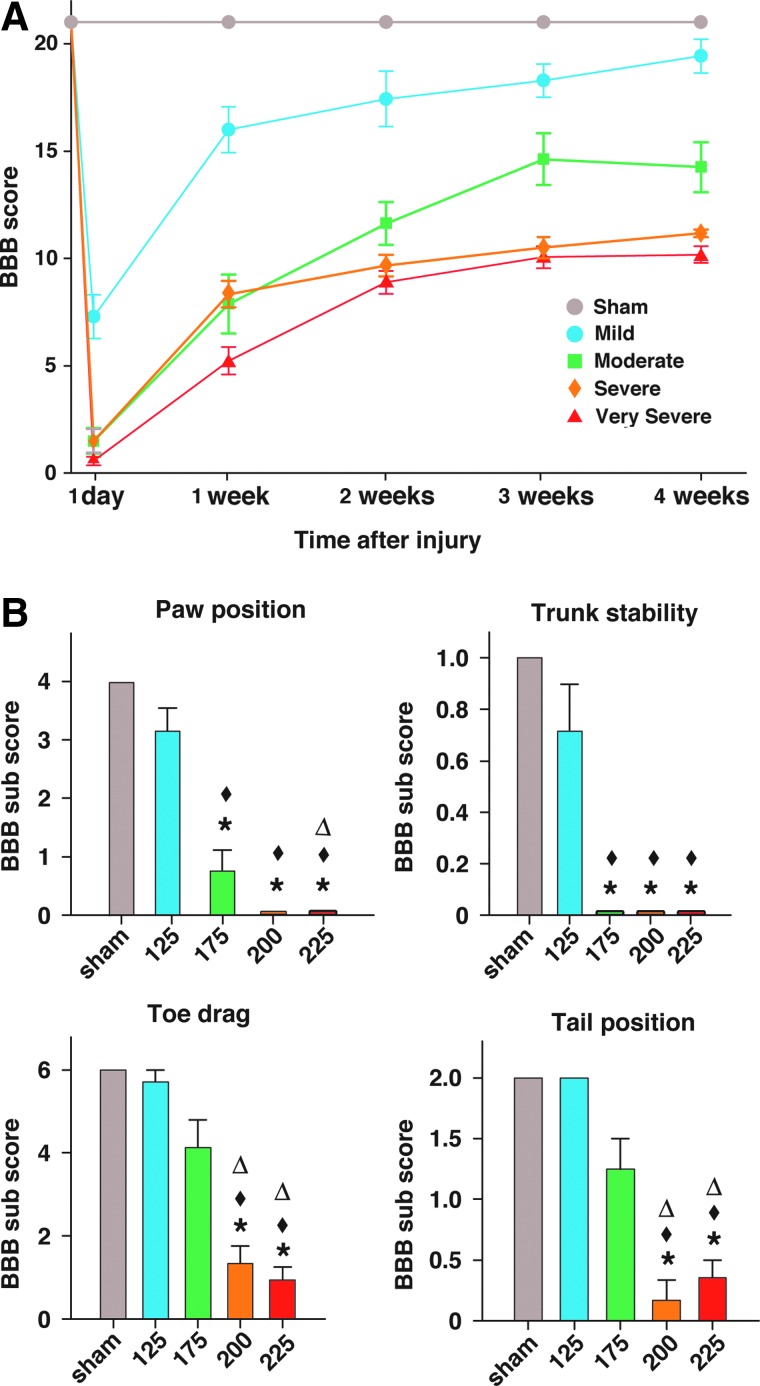
Subjects' locomotion was scored weekly by using the BBB open-field locomotor test (Fig. 2A). Each of the four different injury groups had distinct locomotor recovery outcomes; all groups were significantly different from sham. Analysis of the overall BBB scores at 4 weeks post-SCI showed that the group with the mild injury had trunk instability and was significantly different from all other injury groups. Animals with moderate injury stepped with consistent coordination, but had deficits in paw placement, toe clearance, and stability, and were significantly different from rats with very severe injury. Animals in the severe injury group had frequent-to-consistent weight-supported stepping, but no coordination. The very severely injured rats, on average, had only occasional weight-supported plantar steps; however, the severe and very severe injury groups were not significantly different from each other. These characteristics correspond to group average BBB scores at 4 weeks of 19, 14, 11, and 10, respectively (Fig. 2A). BBB scores measured at 4 weeks after injury were correlated to spinal cord displacement values measured at injury (ρ=0.808; p<0.0001).
FIG. 2.
BBB scores and subscores of spinal-injured rats. (A) BBB scores (n=7, 8, 6, and 17 from mild-to-very-severe injury groups, respectively). (B) BBB subscores, analyzed according to subcategories. *p<0.05, compared to sham; ♦p<0.05, compared to mild injury; Δp<0.05, compared to moderate injury. BBB, Basso, Beattie, and Bresnahan. Color image is available online at www.liebertpub.com/neu
Cord total volume loss predicted BBB outcomes (linear correlation, ρ=0.842; p<0.0001), whereas epicenter white matter sparing had a curvilinear relationship with BBB scores (fourth-order polynomial regression, ρ=0.953; p<0.0001).
Analysis of BBB subscores at 4 weeks postinjury are shown in Figure 2B. BBB subscore totals at 4 weeks were significantly different from sham for the moderate—very severe injuries, but not the mild injury, and injury groups differed significantly from each other, except for the severe versus very severe injuries. More important, BBB subscores (Figure 2B) showed that moderately injured rats had significant deficits in paw position and trunk stability, compared to mildly injured rats. Additionally, moderately injured rats showed smaller deficits in toe drag and tail position, compared to severely injured rats.
Refinement of posture and movement analyses after contusion injury using gait analysis at multiple speeds
Posture
Postural data were analyzed at peak stance, and data shown in Figures 3–5 were collected from subjects walking on the treadmill at 6 cm/sec, which was the optimal speed for detecting a large dynamic range of postural differences among injury groups. Data from all treatment groups were compared to preinjury values, and experimental groups were compared to each other at 4 weeks after injury. Gait analysis data were gathered from animals exhibiting a minimum BBB score of 10. Subjects with lower BBB scores were less able to locomote at increased testing speeds; accordingly, the number of walking subjects from which data could be gathered was reduced at more-severe injury intensities.
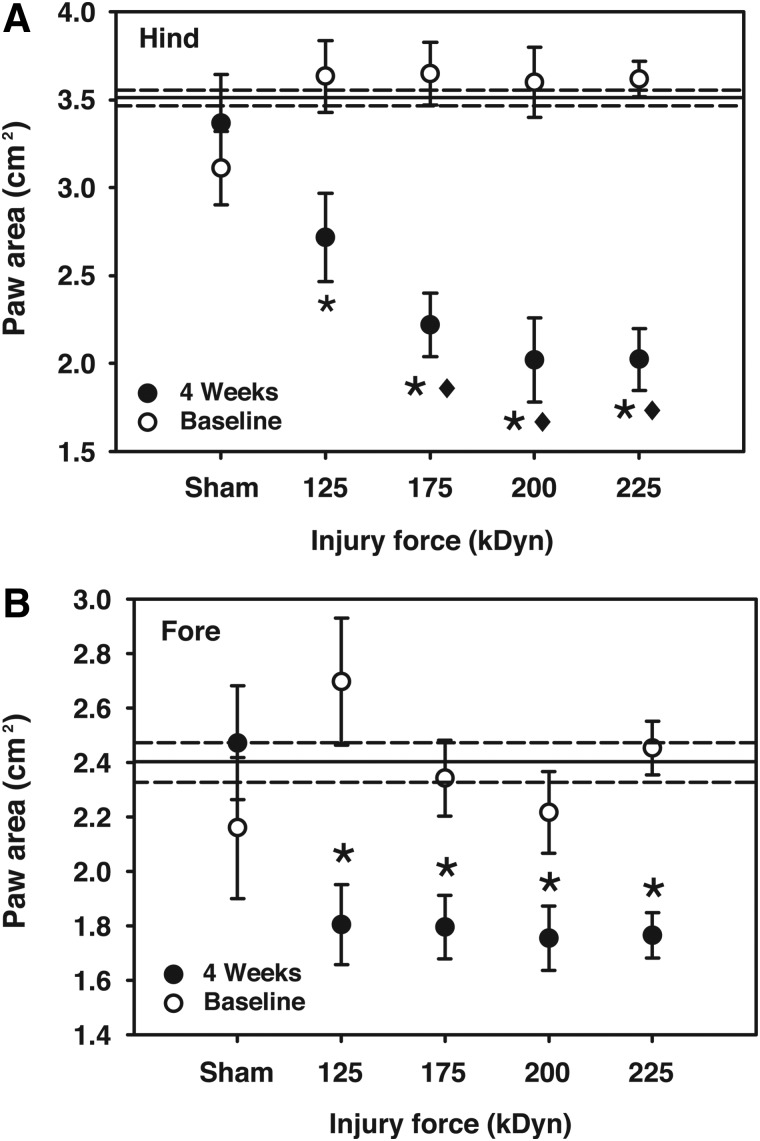
FIG. 3.
Hind- or forepaw surface area utilized at peak stance while walking at a treadmill speeds of 6.0 cm/sec. Reference lines for baseline grand mean±one standard error of the mean are included. Note that both fore- and hindpaw surface areas were decreased at peak stance at all impact levels (125 kDyn/mild, 175 kDyn/moderate, 200 kDyn/severe, and 225 kDyn/very severe; *p<0.05, compared to baseline; ♦p<0.05, compared to 4-week 125 kDyn impact; repeated-measures two-way analysis of variance with Student-Newman-Keuls' post-hoc test; n=4–14).
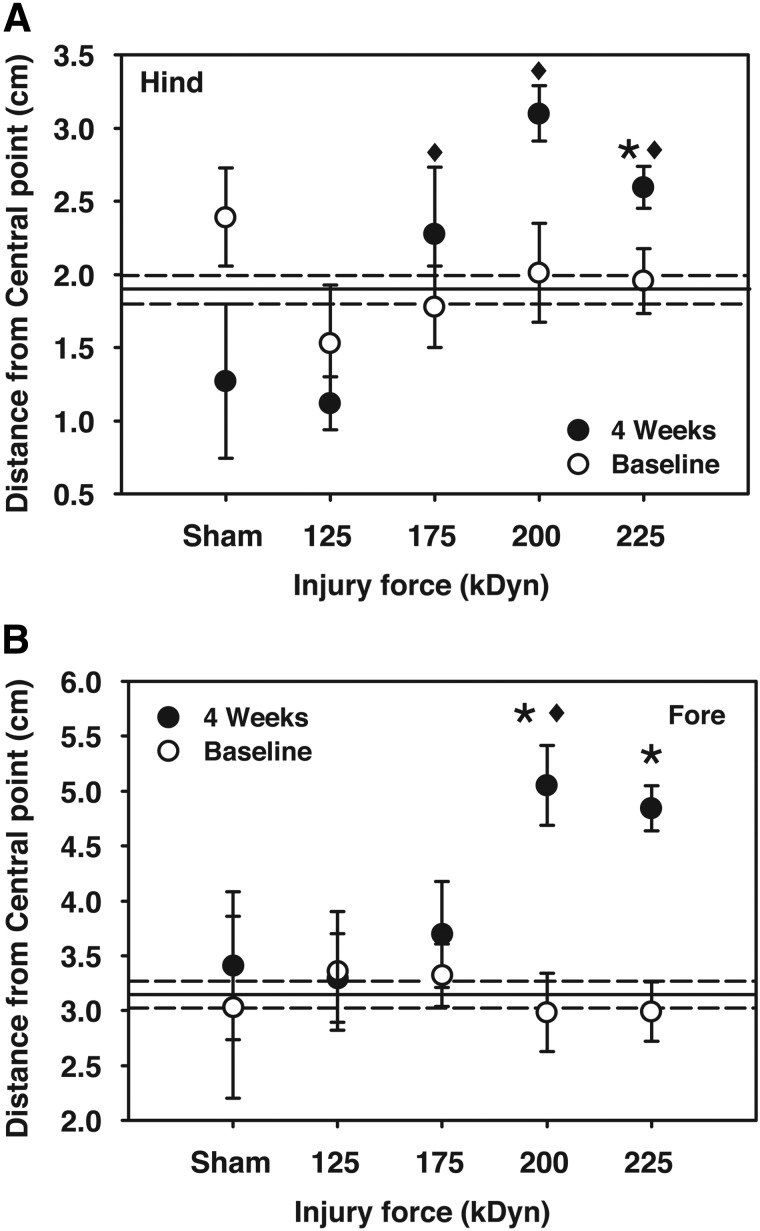
FIG. 4.
Hind- or forepaw distance from the center of body on the rostrocaudal axis while walking at a treadmill speed of 6.0 cm/sec. Reference lines for baseline (grand mean±one standard error of the mean) are included. Note that both hind- and forepaw distances were increased at the severe and very severe impact levels (125 kDyn/mild, 175 kDyn/moderate, 200 kDyn/severe, and 225 kDyn/very severe; *p<0.05, compared to baseline; ♦p<0.05, compared to 4-week mild injury; repeated-measures two-way analysis of variance with Student-Newman-Keuls' post-hoc test; n=4–14).
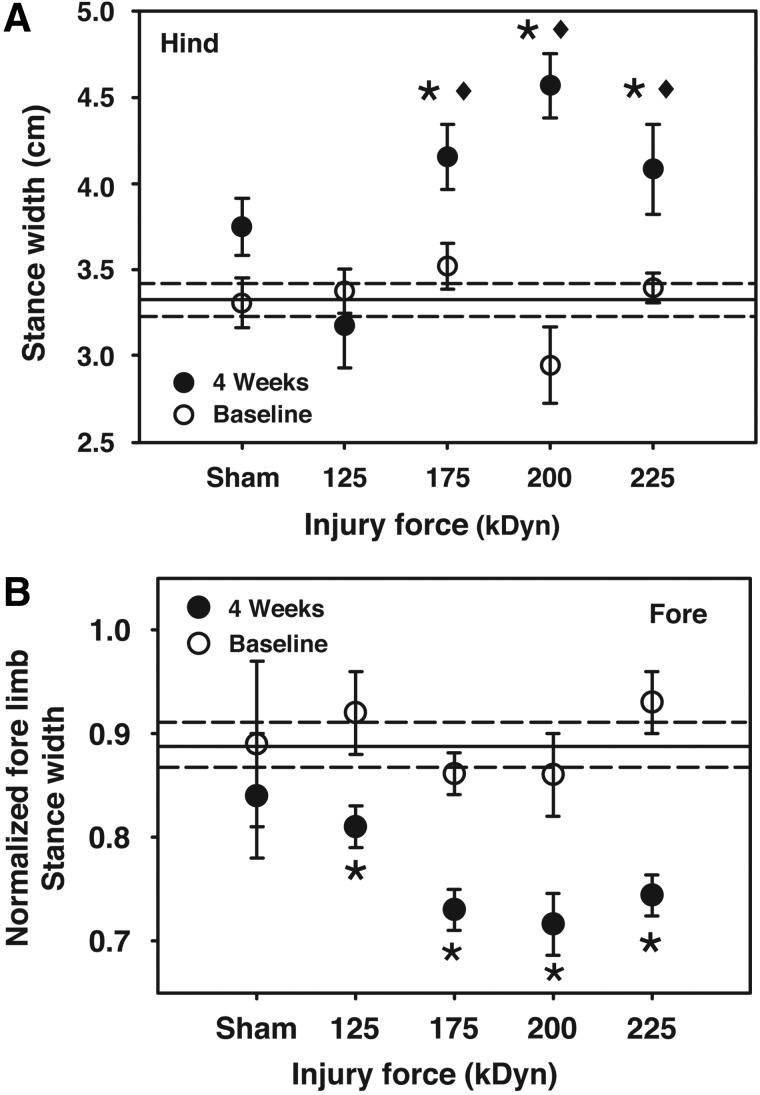
FIG. 5.
Hindpaw stance width and forepaw normalized stance width while walking at a treadmill speed of 6.0 cm/sec. Reference lines for baseline (grand mean±one standard error of the mean) are included. Note that hindpaw stance width was increased at moderate, severe, and very severe impacts. Additionally, moderate, severe, and very severe impacts were all increased above mild impact. Normalized forepaw stance widths were decreased at all impact intensities (125 kDyn/mild, 175 kDyn/moderate, 200 kDyn/severe, and 225 kDyn/very severe; *p<0.05, compared to baseline; ♦p<0.05, compared to 4 week mild injury; repeated-measures two-way analysis of variance with Student-Newman-Keuls' post-hoc test; n=4–14).
The areas of the fore- and hindpaws in contact with the treadmill surface were recorded at the time of peak stance (“paw area”). This measure was decreased at 4 weeks postsurgery for all impact intensities, compared to baseline values (Fig. 3, both panels). In the hindpaw, paw area of animals in the moderate-to-very-severe impact groups was significantly less than animals receiving mild injury (Fig. 3A). Similar decrements in fore- and hindpaw area were observed at 3.5 and 8.5 cm/sec (data not shown).
Distances of the fore- and hindpaws from the center of body on the rostrocaudal axis (“central point”) at peak stance represent the magnitude of FL or HL extension. Both measures showed incremental increases after moderate-to-very-severe injury. HL distances significantly increased after very severe injury. FL distances were significantly greater after both severe and very severe injuries (Fig. 4B). The HL extension of animals receiving moderate-to-very-severe injuries was significantly greater than sham treatment or mild injury (Fig. 4A). A similar pattern of altered reaching and extension was observed at 3.5 and 8.5 cm/sec walking speeds (data not shown).
Compared to baseline, stance width of the HL significantly increased after moderate-to-very-severe injuries (Fig. 5B). Additionally, these animals had significantly larger stance widths, compared to animals with mild injury. Changes in HL stance width were similar at 3.5- and 8.5-cm/sec walking speeds. Repeated-measures two-way ANOVA analysis revealed differences in FL stance width between the global baseline and values at 4 weeks postinjury (p=0.015), but did not show within-group differences (data not shown). However, normalization of FL stance width to body and paw width (the Mouse Specifics “FL weight support” index) revealed injury-related changes in this metric at all injury levels (Fig. 5B).
Step-cycle dynamics
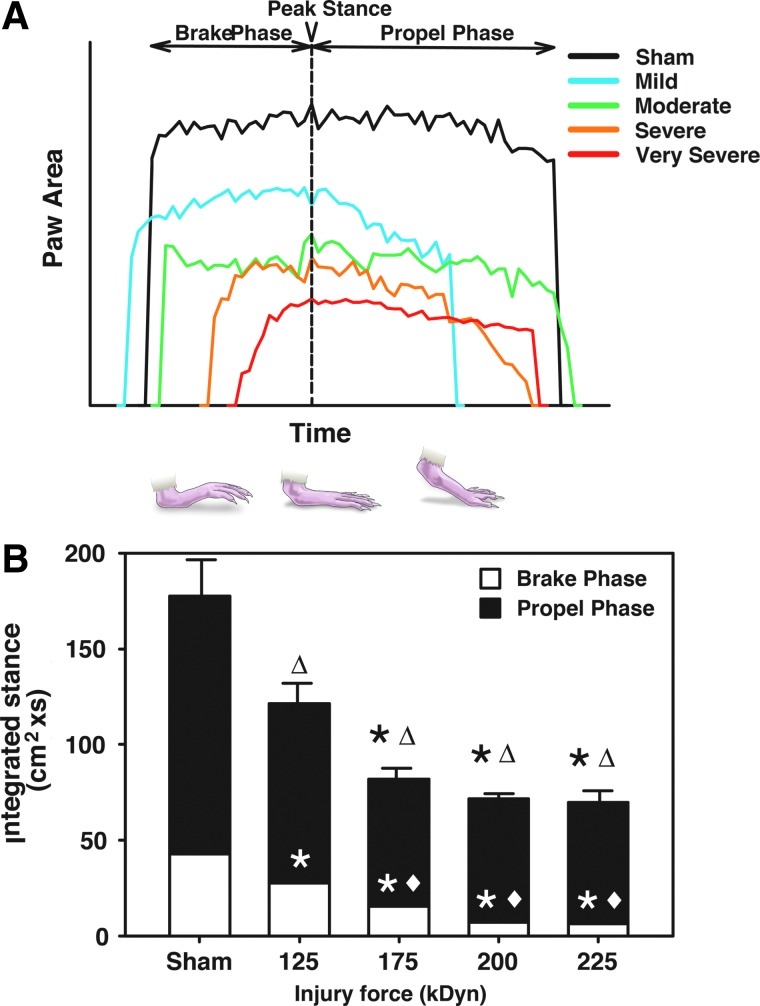
Step-cycle dynamics were analyzed at 6 cm/sec, corresponding to a walking speed achieved by even the most injured groups, and these data were less variable than those gathered at higher treadmill speeds. Braking time (defined as the amount of time from first contact with the treadmill to time of peak stance) and propel time (the moment of peak stance through full lifting off the belt) were dependent on walking speed. Analysis of these metrics across impact intensities yielded an inconsistent pattern of alteration (data not shown). To clarify differences, an area under the paw utilization/time curve was calculated for each step observed (Fig. 6A). Expanding the analysis of the relationship between brake, propel, and stance by integrating paw area values across the entire stance results in an “integrated stance” that revealed consistent, significant, injury-intensity–dependent decrements in gait (Fig. 6B). Significant decrements in the brake phase of the integrated stance were observed at all impact levels. Decrements in the propel phase of the integrated stance were noted at moderate-to-very-severe injuries. Integrated stance was significantly decreased at all levels of impact intensity. Additionally, significant differences between the brake phase of the integrated stance of mild injury animals and those receiving moderate-to-severe injuries were observed (Fig. 6B).
FIG. 6.
Stance Integration. (A) Representative hindpaw stances measured at a treadmill speed of 6.0 cm/sec at 4 weeks post-SCI. The stance includes brake and propel phases, which were temporally divided by the point of peak stance. The curves shown are representative single steps aligned at peak stance, which may not fully convey the breadth of changes happening within a treatment group. (B) Integrated stance results across injury intensity. Note that the integrated propel phase was significantly decreased at moderate-to-very-severe injury levels, whereas integrated brake phase and integrated stance were decreased at all injury levels (125 kDyn/mild, 175 kDyn/moderate, 200 kDyn/severe, and 225 kDyn/very severe; *p<0.05, compared to sham [brake or propel phase]; Δtotal integrated stance, p<0.05, compared to sham, ♦p<0.05, compared to mild injury; analysis of variance with Student-Newman-Keuls' post-hoc test; n=3–7). Color image is available online at www.liebertpub.com/neu
Coordination
Measures of coordination generated by the analysis software were analyzed in tandem to generate a pattern of fluid gait. In general, the most robust alterations in coordinated movement were observed at a walking speed of 8.5 cm/sec.
Stride frequency, as measured in steps per second, was unchanged in HL at 4 weeks post-SCI, but all animals receiving an impact displayed significantly increased stride frequency in the FL (Fig. 7A). Correlation of FL stride frequency to BBB was stronger than any other correlations between GAMS metrics and BBB in either FL or HL (Table 1). Increased FL stride frequency was also observed at the lower walking speed of 6.0 cm/sec, but was absent at 3.5 cm/sec (data not shown).
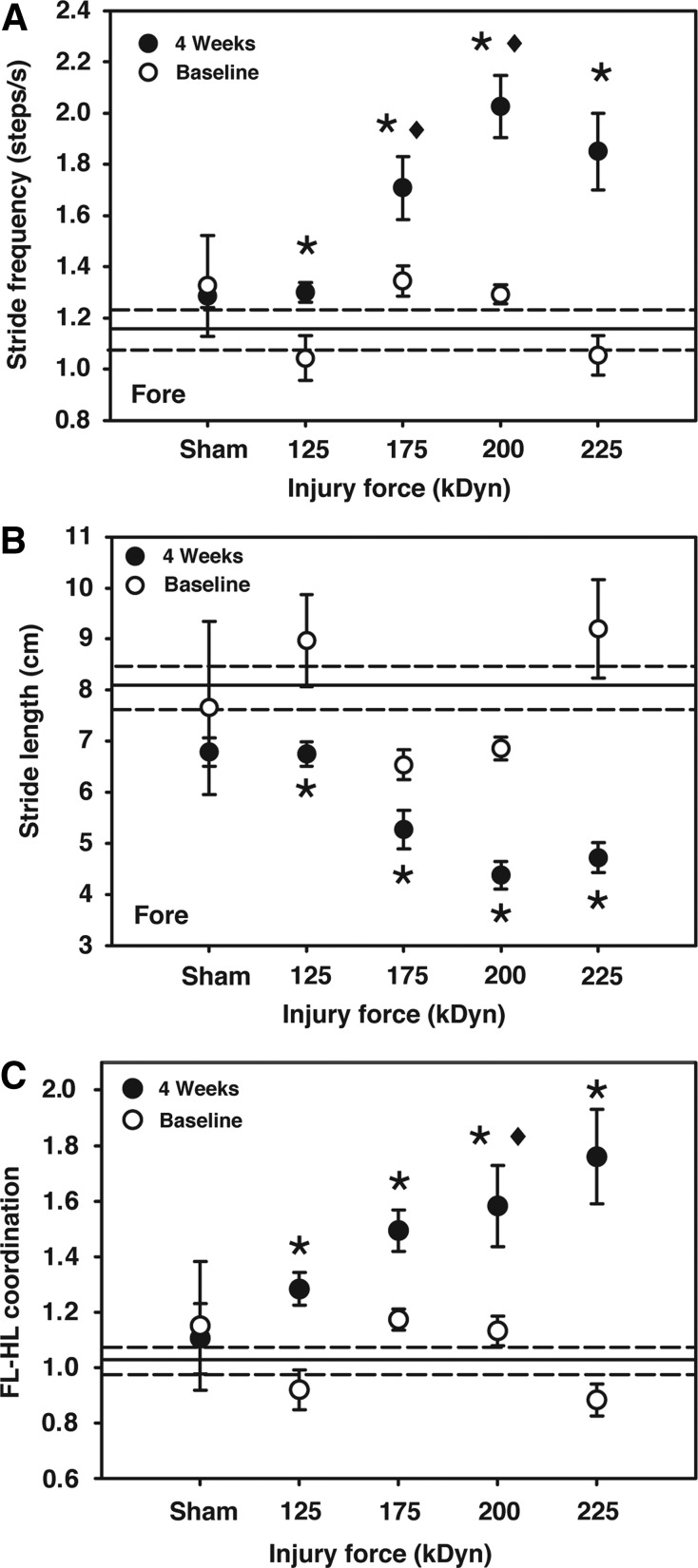
FIG. 7.
Stride dynamics. (A) Forepaw stride frequency while walking at a treadmill speed of 8.5 cm/sec. Hindpaw stride frequency was unchanged with SCI (data not shown), whereas forepaw stride frequency was significantly increased at all impact intensities. (B) Forepaw stride lengths while walking at a treadmill speed of 8.5 cm/sec. Hindpaw stride length was unchanged with SCI (data not shown), whereas forepaw stride length was significantly decreased at all impact intensities. (C) FL-HL coordination while walking at a treadmill speed of 8.5 cm/sec. Normal gait, by definition, has a coordination value of approximately 1.0. Note that coordination values significantly increased at all impact intensities. Reference lines for baseline (grand mean±one standard error of the mean) are included on each panel (125 kDyn/mild, 175 kDyn/moderate, 200 kDyn/severe, and 225 kDyn/very severe; *p<0.05, compared to baseline; ♦p<0.05, compared to mild injury; repeated-measures two-way analysis of variance with Student-Newman-Keuls' post-hoc test; n=2–15). FL-HL, forelimb/hindlimb.
Table 1.
Correlation Coefficients of Selected GAMS Parameters to BBB Scores and Histological Measures
| GAMS measurement | Limb | Correlation with BBB (Fig. 2A) (rs) | Correlation with myelin (Fig. 1C) (rs) | Correlation with volume loss (Fig. 1A) (rs) | |
|---|---|---|---|---|---|
| Body posture dynamics 6.0 cm/sec | Paw area (cm2) (Fig. 3) | Fore | 0.389 | 0.571 | −0.427 |
| Hind | 0.705 | 0.823 | −0.701 | ||
| Central distance (cm) (Fig. 4) | Fore | n.s. | n.s. | n.s. | |
| Hind | 0.743 | −0.693 | 0.637 | ||
| Normalized forelimb Stance width (cm) (Fig. 5) |
Fore | 0.465 | 0.550 | −0.486 | |
| Stance width (cm) (Fig. 5) | Hind | −0.453 | n.s. | n.s. | |
| Gait coordination dynamics 8.5 cm/sec | Stride length (cm) (Fig. 8) | Fore | 0.786 | 0.643 | −0.829 |
| Hind | n.s. | n.s. | n.s. | ||
| Stride frequency (steps/sec) (Fig. 7) | Fore | −0.804 | 0.660 | 0.852 | |
| Hind | n.s. | n.s. | n.s. | ||
| FL-HL coordination (Fig. 9) | −0.706 | −0.694 | 0.755 | ||
| Stance integration 6.0 cm/sec | Brake AUC (Fig. 6B) | Hind | 0.735 | 0.631 | −0.623 |
| Propel AUC (Fig. 6B) | Hind | 0.733 | 0.834 | −0.779 | |
| Stance total AUC (Fig. 6B) | Hind | 0.774 | 0.794 | −0.777 |
Spearman's correlation analysis of GAMS measurements to BBB scores, myelin content, and total cord volume loss was conducted on data obtained at 4 weeks post–spinal cord injury across all impact levels. Correlations shown are at the walking speeds indicated in Figures 3–10. Correlations are significant at p<0.05 (n=21–28), except where noted as nonsignificant (n.s.). Note that correlations with BBB score (function) are generally higher for hindpaws in body posture metrics and higher for forepaws in gait dynamics. Note that indices of body posture and integrated stance are more tightly associated with myelin content at the epicenter of the injury, whereas coordination dynamics are more closely associated with cord volume loss.
GAMS, gait analysis at multiple speeds; BBB, Basso, Beattie, and Bresnahan score; FL-HL, forelimb/hindlimb; AUC, area under the curve.
The increased FL stride frequency was accompanied by a decrease in stride length (Fig. 7B). In contrast, HL stride length and frequency were unchanged after contusion. Similar patterns were observed at lower walking speeds.
FL-HL coordination was calculated by the Mouse Specifics software as the sum of right and left FL step frequency divided by the sum of left and right HL step frequency; hence, the pattern of step frequency shown in Figure 7A reflects altered FL-HL coordination (Fig. 7C). Significant deviations from complete coordination, which is mathematically defined as 1.0, were noted at all impact levels. FL-HL coordination while walking at 8.5 cm/sec was linearly correlated to impact intensity with a Pearson's correlation value of 0.700 (p<0.0001).
Interlimb coordination was analyzed by phase dispersion analysis conducted with the analysis software. Mean phase dispersion values were calculated by the two respective limb-pair phase dispersions. Homologous phase dispersion (0.34±0.02) did not change after contusion. Similarly, ipsilateral phase dispersion (0.35±0.03) was unchanged at 4 weeks postinjury. Diagonal phase dispersion (0.19±0.02) significantly increased in animals with moderate-to-severe and very severe injuries (0.40±0.02, 0.43±0.10, and 0.38±0.03, respectively).
Discussion
SCI outcomes in humans are extremely variable,24 and the degree of sensory and motor losses may dictate the strategy and/or success of therapeutic interventions. Thus, there is a need for SCI models of varying severity. Because exercise-based, surgical, pharmacological, or stem-cell therapies may produce only incremental improvements in locomotor function, high-resolution, continuous measurement techniques are needed.
A novelty of the current study is its utilization of the entire package of GAMS metrics in a graded rat SCI, rather than selected individual measures, allowing assessment of interactions between speed and SCI severity. Testing SCI animals across a range of fixed treadmill speeds demonstrated that specific GAMS indices are optimally measured at specific walking speeds. This approach facilitated quantitation of subtle changes in a broad range of complex step-cycle, posture, and coordination dynamics that complemented BBB scoring. Tissue damage across impact groups in the current study was similar to that observed by others using the same contusion device19,21 and correlated with spinal cord displacement values at the time of injury. BBB scores at 4 weeks postinjury were highly correlated with spinal cord displacements.
DigiGait indices of spinal cord injury outcomes
In BBB scoring, animals move freely during the testing period; they display a wide variety of locomotion speeds, but the test inherently only addresses spontaneously exhibited gait behaviors. Importantly, Basso points out that quantitative assay techniques need to appropriately match the nature of functional losses after SCI.2 Accordingly, speed of locomotion is a very important analysis parameter. Unlike BBB scoring or footprint analysis, where an animal ambulates at a self-directed, variable speed, GAMS uses fixed treadmill speeds. For example, Ek and colleagues15 assessed gait at a moderate-to-fast walking speed of 10 cm/sec. To add greater power, we employed three incrementally challenging speeds to measure locomotion, as suggested by previous studies in mouse SCI models.6,7
Recent studies have utilized multi-speed paradigms.6,17,25 However, speeds chosen in a rat study (10–30 cm/sec) were inappropriate for their SCI subjects, which displayed only occasional plantar stepping.25 That study also used a novel data analysis system (Virtual Dub software) developed by the researchers,25 which may have been limited by the software's inability to quantify sweeping-type limb motions or paw dragging. Similarly, Springer and colleagues16 used a speed of 35 cm/sec, which is not relevant for assessing locomotor function in animals without consistent gait. In contrast, our study used three moderately slow walking speeds, which was prudent given an average BBB score of 11.2 in severely injured animals at 4 weeks. This approach yielded different dynamic ranges of measurements, generating clear impact-related measures of gait deficit after SCI. This approach provides a powerful assessment tool for quantifying outcomes related to the intensity of injury.
Posture
Correct posture is required for normal ambulation, and its measurement is useful in assessing gait function. Paw area utilization, reaching/extension, and stance width metrics were considered simultaneously to describe the animal's posture while ambulating. In general, contused animals locomoted up on their toes in a manner that correlated to impact intensity. FLs reached farther distances to generate movement, whereas HLs exhibited decreased range of motion, as evidenced by the increased distance from the mid-line at the time of peak stance. This indicates that the animal is not able to retract the HL fully to place the paw under the hips at full stance while walking. HLs are splayed out, creating a wider base of support, as described previously.5,26 Normalization of FL stance width to body and paw width revealed changes at all injury levels. To compensate for the extended FL, FLs are placed closer together in order to generate movement with the same effort. Together, these data reveal a shift in the center of gravity of SCI animals, contributing to significant postural adjustments, relative to sham animals. In general, these metrics are altered at all walking speeds, correlate to BBB score and histological findings, and are injury intensity dependent. Posture measurements represent novel descriptors that are not included in the BBB scale.
Step-cycle integration
Measures of braking time and propulsion time are anecdotally related to limb strength and have been used previously as markers of SCI outcome.5,15,16,25,26 However, data collection for these parameters are heavily dependent on the speed of locomotion and the dynamic range available for measuring meaningful change. In our utilization of GAMS to describe these metrics, measurements of brake, propel, and stance time alone were speed dependent, variable between animals, and not meaningfully correlated with impact intensity. Therefore, the stance was integrated across time using the raw paw area per video frame output. Using this novel method, clear alterations in the brake and propel phases of the integrated stance were observed that were significantly correlated to SCI impact intensity. Both the brake and propel phases of the integrated stance significantly decreased in a manner correlated to impact intensity, white matter sparing, and displacement. These measures provide quantitative brake and propel phase data, which are more directly indicative of motor function of the large muscle groups that control the HL during walking than measures obtained by behavioral scoring.
Coordination
BBB scoring of the animals using our injury model revealed a loss of coordination for animals in the highest (200 and 225 kDyn) impact groups. However, GAMS revealed that the pattern of FL-HL stepping is altered for all impact groups after SCI. HL stride length and frequency are unchanged, but FL steps are shorter and thus occur more frequently (as observed previously)26 to maintain balance during gait while walking at a constant speed. The analysis software calculates a parameter termed “gait symmetry” that we have more descriptively renamed “FL-HL coordination.” This measurement mathematically compares FL and HL step frequency. This parameter is similar to the plantar stepping index reported by Kuerzi and colelagues.27 Using FL-HL coordination, we see a linear correlation between impact intensity and FL-HL coordination as well as significant deficits in coordination at all impact levels. Koopmans and colleagues similarly assessed coordination objectively using CatWalk analysis, but in the absence of other gait measures included in this study.9
The GAMS coordination metric is more sensitive than that observed in BBB scoring. Basso and colleagues2 acknowledge that scoring FL-HL coordination leads to a plateau in BBB scores in the central range of the scale. Whereas this plateau is partially addressed by BBB subscores (used in the current study), GAMS measures this coordination directly and continuously to allow assessment of subtle changes in this important functional range.
Phase dispersion measures of interlimb coordination are generally calculated at self-directed walking speeds and therefore may not be directly comparable to those obtained here at fixed walking speeds. The walking speeds chosen for this study allowed even the most severely injured animals to perform the task; thus, the lower speeds are below normal walking speed of an uninjured rat,28 resulting in high variability in the baseline data of animals preceding SCI. These caveats highlight the observation that moderate-to-severe injuries result in significant increases in ipsilateral phase dispersion.
Correlations between function and histology
In this study, spinal cord damage was intensity related, with only 39%, 15%, 9%, and 3% of spinal white matter spared at the injury epicenter in the mild-to-very-severe injuries, respectively. Major descending pathways producing muscle tone for weight support and lateral stability29 were affected in all injured rats. The corticospinal tract showed significant demyelination in all injuries, suggesting that damage to this tract may not determine gait deficits. SCI impact at the T8 level did not directly damage central pattern generators that contribute to recovery to the 9–11 range of the BBB scale. Reflex mechanisms in the spinal cord couple FL and HL activity.30 Myelin analysis also revealed damage to spinal cord tracts relevant to propriospinal projections,31,32 inputs to central pattern generators,33,34 and potential detour circuits.35
GAMS provides automated measures of FL-HL coordination that varied directly and continuously across graded SCI intensities. Because behavioral outcomes were also strongly correlated with white matter sparing at the injury epicenter, the FL-HL coordination measure provides a useful behavioral marker of FL-HL interconnections.
Importantly, the graded relationship between injury intensity and FL-HL coordination allows the assessment of small improvements in locomotor performance after moderate-to-severe SCI and was superior to BBB score in differentiating between severe and very severe injury. In a discussion of the complexities of BBB scoring, Basso2 emphasized FL-HL coordination as a crucial signpost of recovery in SCI outcomes. By measuring this directly and identifying graded gait changes across more severe injuries, GAMS provides a powerful tool for detailed assessment of SCI outcomes.
Comparing all of the sham and injured subjects facilitated detailed analyses of the correlations among impact intensity, spinal cord total volume loss, myelin loss, BBB scores, and GAMS measures of locomotion (Table 1). Overall, the relationships between GAMS measures and BBB scores, or between GAMS measures and epicenter myelin sparing or total volume loss, were highly significant with few exceptions (Table 1). Within these correlations, the magnitude of the Spearman rho values across these parameters revealed interesting and informative patterns. Table 1 shows that the relationship between body posture metrics (e.g., paw area and central distance) and BBB were stronger for HL than FL. Conversely, relationships between gait dynamics (e.g., stride length and frequency) were stronger for FL than HL (Table 1). These findings suggest that GAMS metrics may be useful in differentiating between HL function loss (i.e., HL paw area and central distance) and compensatory changes in FL use (i.e., FL stride length and frequency).
Similarly, comparison of the GAMS measures with the histological markers of SCI also revealed meaningful patterns. Even though most GAMS measures were significantly correlated with histological measures, body posture and integrated stance were more strongly related to myelin content, whereas coordination measures were more strongly related to total cord volume loss (Table 1). This suggests that GAMS measures of posture and stance provide better predictors of myelin loss at the focal point of the contusion, and that coordination measures better predict volume loss across the length of the cord.
Conclusions
SCI at increasing impact intensities produced graded BBB score deficits and cord damage. GAMS measurements showed that deficits in posture, step-cycle dynamics, and coordination also varied with SCI intensity. Data produced by GAMS correlated highly with BBB scores and histological measures. Posture and stance dynamics correlated better with myelin loss at the injury epicenter than with total cord volume loss. Conversely, gait coordination measures correlated more strongly with total cord volume loss.
The combination of GAMS with BBB and histology provides a robust means for quantifying nuances in SCI outcomes and extends the usefulness of observational scoring. Automated GAMS provides significant, differential predictors of myelin sparing and overall spinal cord total volume loss after SCI and can resolve a greater number of specific aspects of locomotion. These methods provide powerful tools for relating gait disturbances to SCI-induced histological damage and could be used to study subtle differences in SCI outcomes produced by potential therapeutic interventions, such as drugs, stem cell therapies, or brain-machine interface devices.
Acknowledgments
This study was generously supported by a gift from the Ronald D. Deffenbaugh Foundation. Core facility support was provided by the Kansas Intellectual and Developmental Disabilities Center (NICHD HD002528). The authors thank Paul Arnold, M.D. for editorial input, as well as the expert technical assistance provided by Eugene Gregory, Jing Huang, Phil Shafer, and Byunggil Yoo. The authors also thank the Center for Brain and Spinal Cord Repair, Ohio State University (Columbus, OH), for their invaluable service of providing a National Institutes of Health–sponsored Spinal Cord Injury Research Training Program.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 2.Basso D.M. (2004). Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma 21, 395–404 [DOI] [PubMed] [Google Scholar]
- 3.Majczyński H., Maleszak K., Górska T., and Slawińska U. (2007). Comparison of two methods for quantitative assessment of unrestrained locomotion in the rat. J. Neurosci. Methods 163, 197–207 [DOI] [PubMed] [Google Scholar]
- 4.Nessler J.A., De Leon R.D., Sharp K., Kwak E., Minakata K., and Reinkensmeyer D.J. (2006). Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: characterization of intrinsic recovery and comparison with BBB. J. Neurotrauma 23, 882–896 [DOI] [PubMed] [Google Scholar]
- 5.McEwen M.L. and Springer J.E. (2006). Quantification of locomotor recovery following spinal cord contusion in adult rats. J. Neurotrauma 23, 1632–1653 [DOI] [PubMed] [Google Scholar]
- 6.Leblond H., L'Esperance M., Orsal D., and Rossignol S. (2003). Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 23, 11411–11419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers S.A., DeVries W.H., Gruenthal M.J., Andres K.R., Hagg T., and Whittemore S.R. (2012). Sildenafil improves epicenter vascular perfusion but not hindlimb functional recovery after contusive spinal cord injury in mice. J. Neurotrauma 29, 528–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa L.M., Pereira J.E., Filipe V.M., Couto P.A., Magalhaes L.G., Bulas-Cruz J., Mauricio A.C., Geuna S., and Varejao A.S. (2010). The effect of gait speed on three-dimensional analysis of hindlimb kinematics during treadmill locomotion in rats. Rev. Neurosci. 21, 487–497 [DOI] [PubMed] [Google Scholar]
- 9.Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., and Joosten E.A. (2005). The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma 22, 214–225 [DOI] [PubMed] [Google Scholar]
- 10.Hamers F.P., Koopmans G.C., and Joosten E.A. (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma 23, 537–548 [DOI] [PubMed] [Google Scholar]
- 11.Hamers F.P., Lankhorst A.J., van Laar T.J., Veldhuis W.B. and Gispen W.H. (2001). Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma 18, 187–201 [DOI] [PubMed] [Google Scholar]
- 12.Jeong M.A., Plunet W., Streijger F., Lee J.H., Plemel J.R., Park S., Lam C.K., Liu J., and Tetzlaff W. (2011). Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J. Neurotrauma 28, 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piesla M.J., Leventhal L., Strassle B.W., Harrison J.E., Cummons T.A., Lu P. and Whiteside G.T. (2009). Abnormal gait, due to inflammation but not nerve injury, reflects enhanced nociception in preclinical pain models. Brain Res. 1295, 89–98 [DOI] [PubMed] [Google Scholar]
- 14.Berryman E.R., Harris R.L., Moalli M., and Bagi C.M. (2009). Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J. musculoskelet. Neuronal Interact. 9, 89–98 [PubMed] [Google Scholar]
- 15.Ek C.J., Habgood M.D., Callaway J.K., Dennis R., Dziegielewska K.M., Johansson P.A., Potter A., Wheaton B., and Saunders N.R. (2010). Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PloS One 5, e12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Springer J.E., Rao R.R., Lim H.R., Cho S.I., Moon G.J., Lee H.Y., Park E.J., Noh J.S., and Gwag B.J. (2010). The functional and neuroprotective actions of Neu2000, a dual-acting pharmacological agent, in the treatment of acute spinal cord injury. J. Neurotrauma 27, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beare J.E., Morehouse J.R., DeVries W.H., Enzmann G.U., Burke D.A., Magnuson D.S., and Whittemore S.R. (2009). Gait analysis in normal and spinal contused mice using the TreadScan system. J. Neurotrauma 26, 2045–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrmann D.L., Bresnahan J.C., Beattie M.S., and Shah B.R. (1992). Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma 9, 197–217 [DOI] [PubMed] [Google Scholar]
- 19.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A. and Lumpp J.E., Jr. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 20.Kloos A.D., Fisher L.C., Detloff M.R., Hassenzahl D.L., and Basso D.M. (2005). Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 191, 251–265 [DOI] [PubMed] [Google Scholar]
- 21.Cao Q., Zhang Y.P., Iannotti C., DeVries W.H., Xu X.M., Shields C.B., and Whittemore S.R. (2005). Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp. Neurol. 191, Suppl 1, S3–S16 [DOI] [PubMed] [Google Scholar]
- 22.Wong J.K., Sharp K., and Steward O. (2009). A straight alley version of the BBB locomotor scale. Exp. Neurol. 217, 417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabchevsky A.G., Fugaccia I., Sullivan P.G., and Scheff S.W. (2001). Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma 18, 513–522 [DOI] [PubMed] [Google Scholar]
- 24.Frigon A. (2011). Chapter 7 Interindividual variability and its implications for locomotor adaptation following peripheral nerve and/or spinal cord injury. Progr. Brain Res. 188, 101–118 [DOI] [PubMed] [Google Scholar]
- 25.Redondo-Castro E., Torres-Espin A., Garcia-Alias G., and Navarro X. (2013). Quantitative assessment of locomotion and interlimb coordination in rats after different spinal cord injuries. J. Neurosci. Methods 213, 165–178 [DOI] [PubMed] [Google Scholar]
- 26.García-Alías G., Torres-Espín A., Vallejo C., and Navarro X. (2010). Functional involvement of the lumbar spinal cord after contusion to T8 spinal segment of the rat. Restor. Neurol. Neurosci. 28, 781–792 [DOI] [PubMed] [Google Scholar]
- 27.Kuerzi J., Brown E.H., Shum-Siu A., Siu A., Burke D., Morehouse J., Smith R.R., and Magnuson D.S. (2010). Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp. Neurol. 224, 178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Górska T., Zmyslowski W., and Majczyński H. (1999). Overground locomotion in intact rats: interlimb coordination, support patterns and support phases duration. Acta Neurobiol. Exp. (Wars.) 59, 131–144 [DOI] [PubMed] [Google Scholar]
- 29.Fouad K., and Pearson K. (2004). Restoring walking after spinal cord injury. Progr. Neurobiol. 73, 107–126 [DOI] [PubMed] [Google Scholar]
- 30.Sherrington C.S. (1910). Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 40, 28–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed W.R., Shum-Siu A., Onifer S.M., and Magnuson D.S. (2006). Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience 142, 1195–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterling P., and Kuypers H.G. (1967). Anatomical organization of the brachial spinal cord of the cat. II. The motoneuron plexus. Brain Res. 4, 16–32 [DOI] [PubMed] [Google Scholar]
- 33.Cao Q., Xu X.M., Devries W.H., Enzmann G.U., Ping P., Tsoulfas P., Wood P.M., Bunge M.B., and Whittemore S.R. (2005). Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 25, 6947–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brockett E.G., Seenan P.G., Bannatyne B.A., and Maxwell D.J. (2013). Ascending and descending propriospinal pathways between lumbar and cervical segments in the rat: evidence for a substantial ascending excitatory pathway. Neuroscience 240, 83–97 [DOI] [PubMed] [Google Scholar]
- 35.Bareyre F.M., Kerschensteiner M., Misgeld T., and Sanes J.R. (2005). Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat. Med. 11, 1355–1360 [DOI] [PubMed] [Google Scholar]