Abstract
The high levels of preexisting immunity against Adenovirus type 5 (Ad5) have deemed Ad5 unusable for translation as a human vaccine vector. Low seroprevalent alternative viral vectors may be less impacted by preexisting immunity, but they may also have significantly different phenotypes from that of Ad5. In this study we compare species D Ads (26, 28, and 48) to the species C Ad5. In vitro transduction studies show striking differences between the species C and D viruses. Most notably, Ad26 transduced human dendritic cells much more effectively than Ad5. In vivo imaging studies showed strikingly different transgene expression profiles. The Ad5 virus was superior to the species D viruses in BALB/c mice when delivered intramuscularly. However, the inverse was true when the viruses were delivered mucosally via the intranasal epithelia. Intramuscular transduction was restored in mice that ubiquitously expressed human CD46, the primary receptor for species D viruses. We analyzed both species C and D Ads for their ability to induce prophylactic immunity against influenza in the CD46 transgenic mouse model. Surprisingly, the species D vaccines again failed to induce greater levels of protective immunity as compared with the species C Ad5 when delivered intramuscularly. However, the species D Ad vaccine vector, Ad48, induced significantly greater protection as compared with Ad5 when delivered mucosally via the intranasal route in CD46 transgenic mice. These data shed light on the complexities between the species and types of Ad. Our findings indicate that more research will be required to identify the mechanisms that play a key role in the induction of protective immunity induced by species D Ad vaccines.
Introduction
The need for improved influenza vaccines has been recently highlighted. With the pandemic swine flu of 2009 and the most recent early and widespread high levels of influenza activity in the United States, people are becoming more educated to the fact that our current influenza vaccine program has significant limitations (Velan, 2011). Even in a normal season, 5–15% of the world's population is affected by epidemics and has upper respiratory tract infections annually, 3–5 million have severe illness, and 250,000–500,000 cases result in death (WHO, 2014). In the United States, seasonal influenza affects up to 20% of the population and results in 200,000 hospitalizations and approximately 37,000 deaths each year. The World Health Organization (WHO) states, “Influenza rapidly spreads around the world in seasonal epidemics and imposes a considerable economic burden in the form of hospital and other health care costs and lost productivity. In the United States of America, it is estimated that influenza epidemics cost up to $167 billion per year” (Molinari et al., 2007). Our society is becoming less and less trusting of our traditional influenza vaccine (Borjesson and Enander, 2013). A vaccine technology that the majority of people now know is only effective 59% of the time (Osterholm et al., 2012). Although recent studies have shown that high-dose influenza vaccine formulations have been shown to improve the vaccine efficacy in the elderly and immunocompromised, there is still a need to improve the current vaccine technology (Falsey et al., 2009; McKittrick et al., 2013).
Ad type 5 (Ad5) has been shown to be a very good viral vector for use as an influenza vaccine. It has been proven to be an effective influenza vaccine in many animal models and against many strains of influenza, including highly pathogenic avian influenza (HPAI) (Lo et al., 2008; Park et al., 2009; Toro and Tang, 2009; Zhou et al., 2010). However, Ad5 seroprevalence is an undeniable fundamental problem to its use as an influenza vaccine vector in humans. There are very high levels of preexisting immunity to Ad5 in all populations (Abbink et al., 2007; Mast et al., 2010; Barouch et al., 2011). In many populations the preexisting immunity is 100% by the age of 2 (Abbink et al., 2007). The failure of the HIV vaccine STEP trial emphasized the limitations of using this virus as a vaccine vector (Sekaly, 2008; McMichael and Haynes, 2012; Cohen, 2013). Our solution is the creation of new vaccine vectors that are based on less common types of adenovirus (Ad) and study them as viral vectored influenza vaccines.
Ads are naked, icosahedral viruses with double-stranded DNA genomes of ∼36 kb. There are approximately 60 different types of Ad divided into 7 species (A–G) (Wy Ip and Qasim, 2013). Researchers, including ourselves, have begun to look into the use of low-seroprevalent Ads as alternative viral vectored vaccines (Abbink et al., 2007; Waddington et al., 2008; Schuldt et al., 2012; Teigler et al., 2012; Zahn et al., 2012; Baden et al., 2013; Weaver, 2013; Weaver and Barry, 2013). While these alternative viral vectors may have a lower seroprevalence, the differences in their biology as compared with Ad5 have started to come into question. It is a risky assumption to assume that all Ads share the same phenotype (Coughlan et al., 2012). In this study we show that there is a significant degree of difference in phenotypes even within the same species of Ad. Using a transgenic human CD46-expressing mouse model, we were able to restore the intramuscular (i.m.) species D transduction efficiency. However, i.m. vaccination was not improved. Vaccine efficacy was improved only when the species D Ad, Ad48 in particular, was delivered by the intranasal (i.n.) route in a human CD46 transgenic mouse model. As species D Ads are generally associated with the gastrointestinal and ocular mucosal tissues, they may be optimal vectors for the induction of mucosal immunity (Fields et al., 2013). These results indicate the importance of evaluating these new alternative serotypes as vaccine vectors for potential translation into human clinical trials.
Materials and Methods
Viruses
The species C and D Ad constructs used in this study were cloned and modified as previously described (Weaver and Barry, 2013). The modified viral genomes were digested from their plasmid backbones using PacI and transfected into 293 cells. The rescued virus was amplified and purified on two sequential CsCl gradients. Each Ad serotype was modified to express an eGFP-luciferase (GFPLuc) fusion protein in place of the adenoviral E3 genes in order to make replication-competent (RC) vectors. Since replication-defective (RD) viral vectors are considered to be safer alternatives, we created an RD E1-deleted Ad28 expressing GFPLuc (Ad28-GFPLuc) for comparison of the viral vector platforms. RD species D Ads were rescued as previously described with the exception that 293-E4-pIX cells (Microbix) were used to amplify the virus. Ad28 was chosen because it was neither the best nor the worst at transduction in vitro and in vivo and we felt that it was a good middle representative of the three species D Ads. This also reduced the number of animals needed for the studies. Since there was no significant difference in the in vivo transduction levels by either RD or RC platform, we chose to test only RD viruses for use as influenza vaccines. These vaccines were constructed by deleting the E1 genes and replacing them with a CMV expression cassette expressing a centralized H1 hemagglutinin (HA) gene. We chose to use a centralized H1 antigen (HA1-con) since, in the case of vaccine mismatch, it has been shown to induce broader levels of anti-influenza immunity as compared with wild-type HA genes (Weaver et al., 2011).
The species C Ad5 RC vector contained a CMV-GFPLuc expression cassette in between the E1A and B genes. This vector also had a partial E3 deletion that resulted in overexpression of the ADP gene as previously described (Doronin et al., 2000). Ad5-RD was created using the Stratagene Ad-Easy system and had a CMV expression cassette in place of the E1 genes and had a complete E3 deletion.
Cells
Peripheral blood mononuclear cells (PBMCs) were collected under a Mayo Clinic institutional review board-approved protocol and informed consent was obtained from all donors. PBMCs were used to generate human monocyte-derived immature dendritic cells by the “adherence method” or by CD14 isolation as previously described (Gottschalk et al., 2003; Leen et al., 2004). Adherent or CD14-selected monocytes were then cultured in Cell Genix/GlutaMAX-I medium with 800 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sargramostim Leukine; Immunex) and 1000 U/ml interleukin-4 (IL-4; R&D Systems) for 5 days, with IL-4 and GM-CSF replenishment on days 2 and 4. On day 5, they were cryopreserved. A549, RAW 264.7, and Jurkat cells were obtained from ATCC. A549 cells were cultured in complete Dulbecco's modified Eagle's medium (cDMEM) that contained 10% heat-inactivated fetal calf serum (Gibco) and penicillin/streptomycin at 100 U/ml (Gibco). RAW 264.7 and Jurkat cells were cultured in complete RPMI medium (cRPMI) that contained 10% heat-inactivated fetal calf serum and penicillin/streptomycin at 100 U/ml.
In vitro transduction
Six-well plates were seeded with cells 24 hr before infection. The cells were infected with 1×104 vp/cell of each virus. Images of GFP fluorescence were collected and the luciferase activity was determined at 24 hr postinfection using the reporter passive lysis 5× buffer and luciferase assay reagent (LAR; Promega) as described by the manufacturer protocol. Briefly, the medium was removed and 800 μl of DPBS was added. About 200 μl of 5× passive lysis buffer was added to each well and mixed. The plate was incubated at room temperature for 5 min and 200 μl of LAR was added. The plate was shaken on an orbital shaker and luciferase activity was measured using a Beckman Coulter DTX 880.
Ethics statement
Female BALB/c mice (6–8 weeks old) were purchased from Charles River Laboratories. The human CD46+ transgenic mice were established on a C57/BL7× C3H hybrid genetic background and supplied by the Mayo Clinic Toxicology Animal Core (Mrkic et al., 1998). CD46+ transgenic mice (6–10 weeks old) were generated under the breeding protocol IACUC A61312. The mice were housed in the Mayo Clinic Animal Facility under the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines with animal use protocols approved by the Mayo Clinic Animal Use and Care Committee (protocol A64612). All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Mayo Clinic.
In vivo luciferase imaging
Mice were anesthetized by intraperitoneal (i.p.) injection with ketamine (140 mg/kg)/xylazine (5.55 mg/kg) and were immunized by i.m. or i.n. routes. Mice immunized by the i.m. route received 1×1010 vp/mouse in two 25 μl injections into each quadricep muscle (50 μl total volume). Mice immunized by the i.n. route received 1×1010 vp/mouse of Ad in two 10 μl instillations into each nare (20 μl total volume). The luciferase-expressing viruses were administered as indicated and the mice were imaged 24 hr after administration on a Lumazone Imaging System (Roper Scientific) as described by Hofherr et al. (2008). Mice were anesthetized with ketamine/xylazine and injected i.p. with d-luciferin at a concentration of 20 mg/ml in PBS in a volume of 200 μl, and the mice were immediately placed into the Lumazone and images were captured. All images were taken with a 10 min exposure and 4×4 binning. Data analysis was performed on each image using background-subtracted sum intensities detected by the Lumazone Imaging Software and graphed using Prism Graphing Software.
Immunizations and influenza virus challenge
Mice were anesthetized by i.p. injection with ketamine (140 mg/kg)/xylazine (5.55 mg/kg). Mice immunized by the i.m. route received the specified Ad virus in two 25 μl injections into each quadricep muscle (50 μl total volume). Mice immunized by the i.n. route received the Ad vaccine in two 10 μl instillations into each nare (20 μl total volume). We chose to test Ad26 and 48 because they represented the high and low levels of in vitro and in vivo transduction and would represent the extremes of the 3 species D Ads. Additionally, fewer animals were required for the vaccine studies. Three weeks after immunization, the mice were challenged i.n. with mouse-adapted influenza A/PR/8/34. The mice were anesthetized with ketamine/xylazine as described for animal immunizations. The anesthetized mice were placed on their back and challenged with 100 MLD50 (50% mouse lethal doses) of influenza virus A/PR/8/34 using two 10 μl instillations into each nare (20 μl total volume). The mice were observed for morbidity and weight loss over subsequent days and were euthanized if weight loss reached 25% of initial body weight. Since the mean weight loss is no longer representative once an animal is removed from a group, weight loss data were censored when survival dropped below 100%.
Statistical analyses
All data were analyzed using GraphPad Prism 4 software. Statistical significance was determined using Unpaired, two-tailed T-tests, ANOVA with Bonferroni posttest, and Log-rank (Mantel–Cox). p-Values ≤0.05 were considered statistically significant.
Results
In vitro comparison of species C and species D Ads
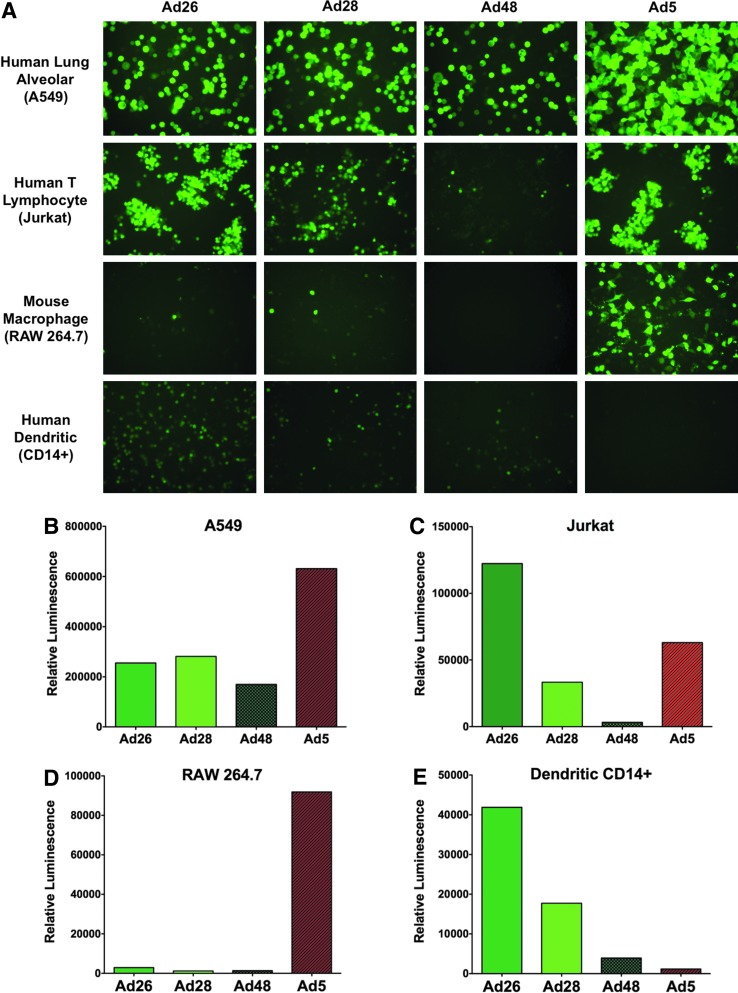
While Ad5 induces a robust immune response as a vaccine carrier, it suffers from Ad-specific preexisting immunity in most humans. To circumvent this problem, we cloned the genomes of three low-seroprevalent species D Ads, Ad26, Ad28, and Ad48, and modified them to express GFPLuc in place of E1 or E3 genes (Weaver and Barry, 2013). As an initial test of vector functions, several cell lines were infected with recombinant species D Ad26, Ad28, and Ad48 viruses and compared with cells infected with a species C Ad5 virus (Fig. 1). The species C Ad5 transduced the A549 human lung cells at least 2-fold more efficiently than the species D Ads (Fig. 1A and B). Ad26 was more efficient than Ad5 for infection of Jurkat human T lymphocyte cells (Fig. 1A and C). In contrast, Ad5 virus was more than 30-fold more efficient in transducing RAW264.7 mouse monocyte/macrophages (Fig. 1A and D). Most interestingly, the species D viruses were much more efficient at transducing human dendritic cells in vitro (Fig. 1A and E). Of the species D viruses, Ad26 mediated 36-fold higher transduction of human dendritic cells than Ad5 (Fig. 1E). These data suggested that the species D Ads have different cell tropism than Ad5 and that this may have utility when vaccinating against influenza.
FIG. 1.
Species C and D Ad tissue tropism. Species C and D viruses expressing GFPLuc were used to infect human lung carcinoma cells (A549) (B), human T lymphocyte (Jurkat) (C), mouse macrophages (RAW 264.7) (D), and human dendritic cells (CD14+) (E). Representative images are shown at 400× magnification (A). The cells were lysed at 24 hr postinfection using 5× passive lysis buffer. Luciferase assay reagent was added and luminescence was measured using a Beckman Coulter DTX 880. Luminescence values represent the luciferase activity of the entire well of a 6-well plate. Ad, adenovirus. Color images available online at www.liebertpub.com/hum
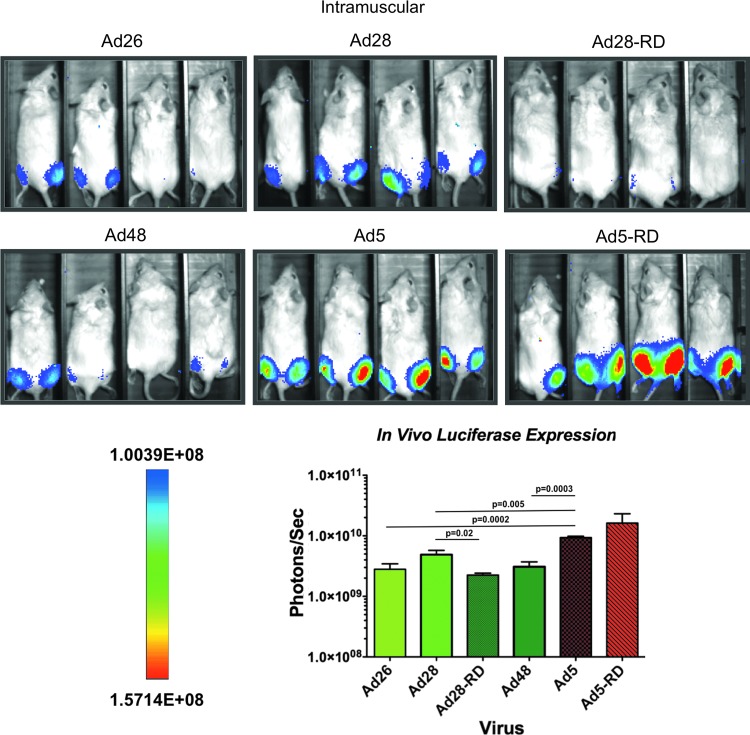
In vivo transduction by Ads after i.m. injection
Mice were immunized i.m. with 1×1010 vp/mouse of species D and species C Ads and luciferase expression was imaged 24 hr later (Fig. 2). By this route, Ad26, Ad28, and Ad48 produced significantly lower levels of luciferase activity as compared with the Ad5 vector (p≤0.0002, p<0.005, and p<0.0003, respectively). When RD E1-deleted Ad28-RD was tested, it also induced significantly lower luciferase expression than Ad5 and this expression was statistically lower than that mediated by its RC Ad28 counterpart (p=0.02). In contrast, Ad5-RD had higher luciferase expression than RC Ad5, although these differences did not reach statistical significance.
FIG. 2.
In vivo analysis of transgene expression and biodistribution by species C and D Ad vectors by systemic immunization. Groups of four BALB/c mice were administered 1×1010 viral particles of the indicated vectors intramuscularly. Twenty-four hours later the animals were anesthetized, injected with luciferin, and imaged for luciferase activity. All images were taken with a 10 min exposure and 4×4 binning. Color images available online at www.liebertpub.com/hum
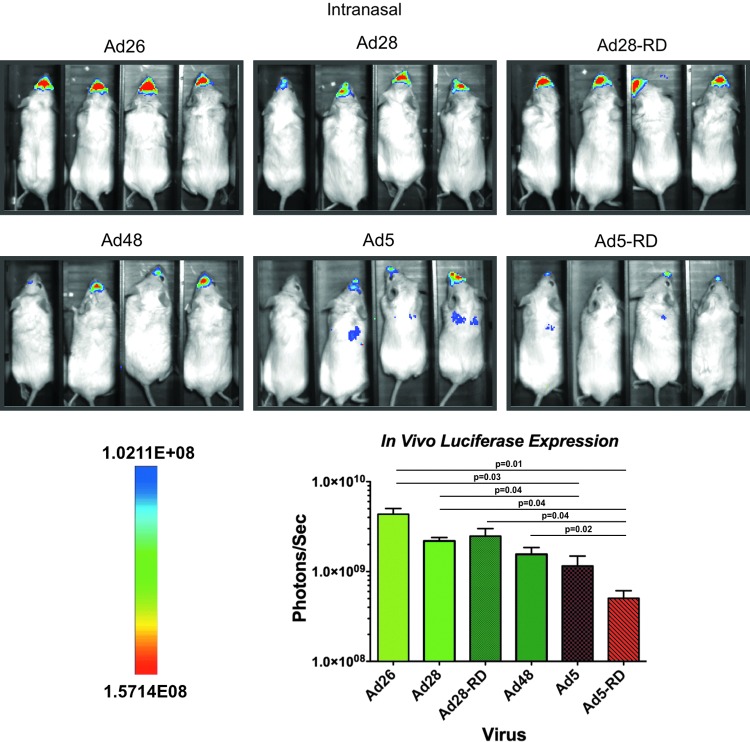
In vivo transduction by Ads after i.n. administration
The Ad vectors were administered i.n. at a dose of 1×1010 vp/mouse to compare mucosal transgene expression levels. Imaging at 24 hr demonstrated that the species D Ads mediated higher luciferase expression in the nasal mucosa than the species C Ad5 viruses (Fig. 3). In particular, Ad26 produced 3- and 7-fold higher expression than Ad5 and Ad5-RD, respectively (p=0.03 and 0.01, respectively). All species D Ad viruses induced statistically higher levels of luciferase expression as compared with the species C Ad5-RD virus (p≤0.05). Ad28 and Ad28-RD produced equivalent levels of luciferase activity when delivered intranasally.
FIG. 3.
In vivo analysis of transgene expression and biodistribution by species C and D Ad vectors by mucosal immunization. Groups of four BALB/c mice were administered 1×1010 viral particles of the indicated vectors intranasally. Twenty-four hours later the animals were anesthetized, injected with luciferin, and imaged for luciferase activity. All images were taken with a 10 min exposure and 4×4 binning. Color images available online at www.liebertpub.com/hum
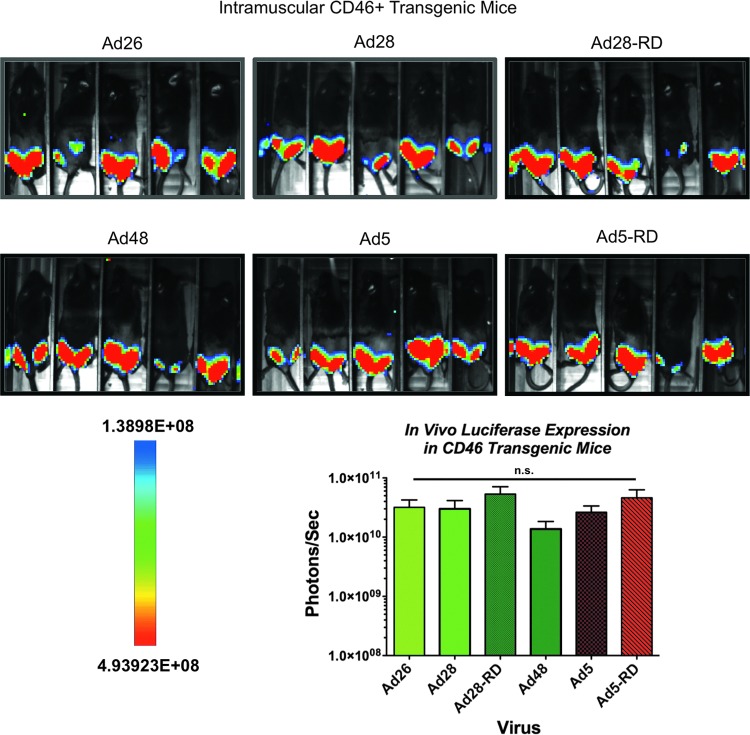
In vivo transduction in CD46 transgenic mice
Several studies indicate that CD46 is the main receptor for species D Ad binding (Wu et al., 2004; Wang et al., 2007; Li et al., 2012; Teigler et al., 2012). Since mice have limited expression of CD46 and mouse CD46 may not be sufficiently homologous for use as a receptor, we decided to investigate the use of CD46 transgenic mice for future studies (Tsujimura et al., 1998). Individual mice were immunized intramuscularly with 1×1010 vp of Ad26, 28, and 48 and compared with Ad5 (Fig. 4). Because of interference, the hair covering the quadriceps was removed by shaving. As expected, there were no statistically significant differences in virus transduction and luciferase expression levels of the species C and D Ads (Fig. 4). Overall, the species D Ad virus transduction levels were restored to a level equivalent to that of species C Ad5. We attempted to evaluate the in vivo expression levels of i.n. immunized CD46 mice. However, we were unable to reach detectable levels. This problem can be attributed to the signal being blocked by the dark skin and facial hair over the nasal area (data not shown).
FIG. 4.
Restoration of species D Ad transduction in CD46 transgenic mice. Groups of five human CD46+ transgenic mice were administered 1×1010 viral particles of the indicated vectors intramuscularly. Twenty-four hours later the animals were anesthetized, injected with luciferin, and imaged for luciferase activity. All images were taken with a 10 min exposure and 4×4 binning. Color images available online at www.liebertpub.com/hum
Protection of CD46+ mice by systemic i.m. immunization
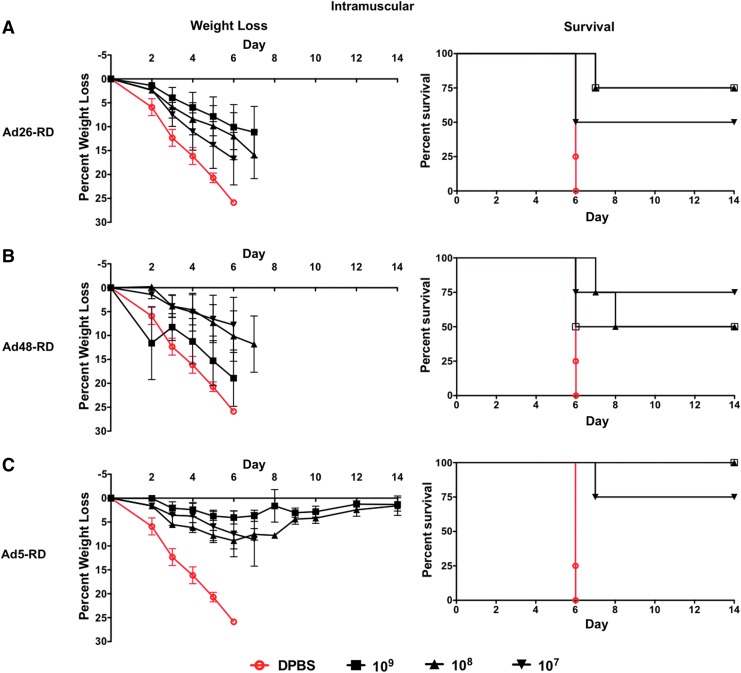
In order to determine if the restoration of i.m. transduction by species D Ads in CD46+ mice would improve vaccine efficacy, we immunized CD46+ transgenic mice i.m. with serial dilutions of Ad26-RD, Ad48-RD, and Ad5-RD viruses expressing the centralized hemagglutinin (HA1-con). The mice were challenged i.n. 3 weeks postimmunization with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Unfortunately, restoring the transduction levels of the species D Ad viruses did not result in the restoration of vaccine efficacy, at least not by the i.m. route. Weight loss, disease, and death was observed in all mice immunized with Ad26-RD and Ad48-RD vaccines (Fig. 5A and B). However, the species C Ad5-RD vaccine was capable of protecting mice from severe disease at all doses (Fig. 5C). In the case of i.m. immunization in CD46 mice, the species D Ad5-RD was a superior vaccine as compared with the species D Ads 26-RD and Ad48-RD.
FIG. 5.
Systemic intramuscular immunization weight loss and survival. The vaccine efficacy of Ad26 (A), Ad48 (B), and Ad5 (C) vaccines on weight loss, disease, and death in intramuscularly immunized human CD46+ transgenic mice was determined by vaccinating with serial dilutions of Ad-vectored viruses (1×109 to 1×107 vp/mouse). Three weeks postvaccination the mice were challenged intranasally with 100 MLD50 of mouse-adapted influenza virus A/PR/8/34. The mice were monitored for weight loss and disease. Mice that lost ≥25% of their body weight were removed from the study and humanely euthanized. Color images available online at www.liebertpub.com/hum
Protection of CD46+ mice by mucosal i.n. immunization
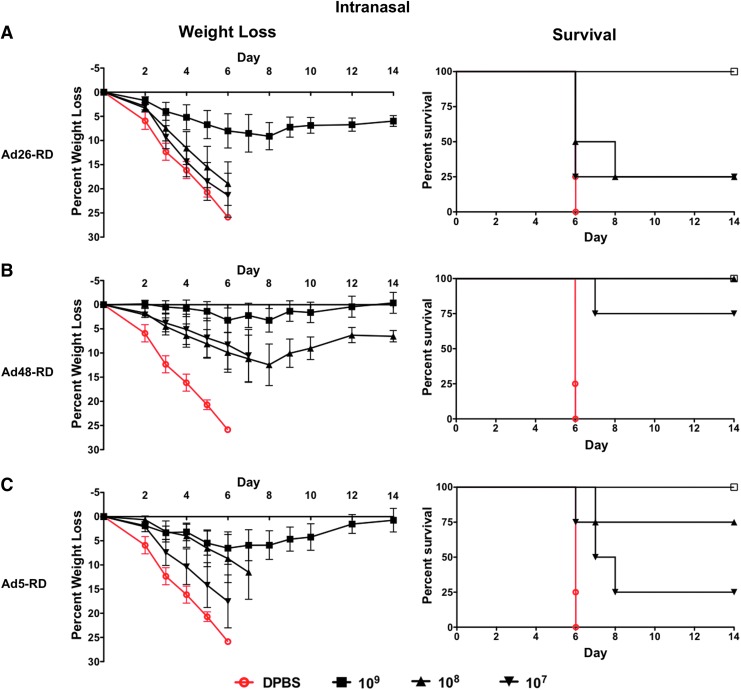
Previous studies have shown that species D Ads could induce protective anti-influenza immunity in BALB/c mice when immunized intranasally (Weaver and Barry, 2013). While we were unable to obtain in vivo imaging of i.n. mice, our i.m. data indicate that species D Ad transduction is restored in the CD46+ transgenic mice. Since CD46 expression is constitutive and ubiquitous in our mouse model, we can only assume that transduction via the CD46 receptor is also occurring in the intranasally immunized mice. In vivo transduction data indicate that species D Ads are transducing BALB/c mice via an alternative receptor (Fig. 3) (Weaver and Barry, 2013). Here we determine if the restoration of the CD46 receptor would result in a synergistic improvement of vaccine efficacy in i.n. immunized mice. We immunized groups of CD46+ mice intranasally with serial dilutions of Ad26-RD, Ad48-RD, and Ad5-RD vaccines. The mice were then challenged 3 weeks after immunization with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Based on previous studies, the species D Ad vaccines were better at protecting mice when delivered i.n. as compared with i.m. (Weaver and Barry, 2013). As expected, both Ad26-RD and Ad48-RD induced greater levels of protection when delivered intranasally. Ad26-RD and Ad5-RD vaccines induced similar levels of protection against the influenza virus challenge (Fig. 6A and C). However, the Ad48-RD vaccine induced the greatest level of protection and was the only vaccine to induce 100% survival at a dose of 1×108 vp (Fig. 6B). Interestingly, Ad48-RD delivered i.n. was as good at inducing protective immunity as Ad5-RD delivered intramuscularly. This is in contrast to Ad5-RD, where the greatest levels of protection are induced by i.m. immunization.
FIG. 6.
Mucosal intranasal immunization weight loss and survival. The vaccine efficacy of Ad26 (A), Ad48 (B), and Ad5 (C) vaccines on weight loss, disease, and death in the intranasally immunized in human CD46+ transgenic mice was determined by vaccinating with serial dilutions of Ad-vectored viruses (1×109 to 1×107 vp/mouse). Three weeks postvaccination the mice were challenged intranasally with 100 MLD50 of mouse-adapted influenza virus A/PR/8/34. The mice were monitored for weight loss and disease. Mice that lost ≥25% of their body weight were removed from the study and humanely euthanized. Color images available online at www.liebertpub.com/hum
Discussion
As an alternative to the highly seroprevalent species C Ad5 vector, we have characterized three human species D Ad vaccine vectors. An initial characterization of cell tropism showed that species D viruses had lower levels of luciferase expression in human lung epithelial cells as compared with the species C Ad5. This could be explained by the fact that Ad5 is a respiratory pathogen and is able to replicate efficiently in human lung A549 cells, whereas Ad26, Ad28, and Ad48 were all isolated from rectal swabs from a 9-month-old male, 30-month-old male, and an immunocompromised adult, respectively (Hierholzer et al., 1991; Schnurr and Dondero, 1993). Both species C and D viruses were capable of infecting human T lymphocytes; however, there was a high degree of variation in the species D viruses. Again, this emphasizes the differences in Ad phenotypes, even within the same species. Species C viruses were also much more capable of infecting macrophages as compared with all of the species D viruses. This observation has been observed in other studies in which Ad was used to eliminate Kupffer cells that ultimately allowed for greater transgene expression (Shashkova et al., 2008; Khare et al., 2011). Most interestingly, the species D Ads were much more capable of transducing the professional antigen presenting human dendritic cells. Again, we see a high level of variation in the 3 species D Ads; however, the lowest level of Ad48 is still >2-fold higher than the species C Ad5.
The primary receptor for Ad5 virus entry is the coxsackie and Ad receptor (CAR). Unfortunately, CAR is sequestered to the basolateral membrane of the mucosal epithelia, making Ad5 less effective at transducing the nasal and lung epithelial cells (Grubb et al., 1994; Zabner et al., 1997). In addition, CAR is not expressed on dendritic cells, making them refractory to Ad5 transduction (Mercier et al., 2004). Previous studies by our group have modified Ad5 to express a chimeric fiber protein that utilizes the reovirus sigma protein for transduction into dendritic cells via the junctional adhesion molecule 1 (JAM1) as the primary receptor (Mercier et al., 2004). While these modified viruses show lower levels of transduction by the i.n. route, they induced equivalent T cell immunity (Weaver et al., 2012). The primary receptor for species D Ads is CD46, which is expressed on all human nucleated cells, including dendritic cells, and therefore more readily transduced (Ni et al., 2005). This is of interest since dendritic cells play a key role in the development and maintenance of immune responses and a vaccine vector that is capable of transducing these cells may be a more effective vaccine.
When we looked at the in vivo tropism of the viral vectors, we found that the species C Ad5 virus was much more capable of transducing muscle in BALB/c mice. The species C Ad5 virus had statistically higher levels of transgene expression levels as compared with all of the species D viruses. This was a disappointing discovery, but not unexpected when we consider the differences in cell tropism observed in vitro. In contrast, when the viruses were delivered to BALB/c mice mucosally by i.n. immunization, we found the opposite result. In this case, the species D Ad viruses had significantly higher transgene expression level than the species C viruses. An interesting note was the inversion of transgene expression by the RC and RD versions of the viruses. In the case of i.m. immunization, Ad5-RD was greater than Ad5, whereas Ad28 was greater than Ad28-RD. The opposite was observed when the viruses were delivered mucosally by i.n. immunization. Mucosally, Ad5 was greater than Ad5-RD, whereas Ad28 and Ad28-RD were almost equivalent. Even though these differences are modest, they may still be important. This indicates that the RD form of the species D Ad is equivalent to the RC form. This is important since the defective form has a limited capacity to express viral proteins without the E1 gene. Therefore, the defective form is a safer vaccine platform while at the same time remaining as potent as an RC form. Of note, we found that the species C Ad5 intranasally immunized mice had a small amount of detectable luciferase expression in the lower respiratory tract, whereas no luciferase signal was detected in the lower respiratory tract of species D Ad immunized mice (Fig. 3). We hypothesize that since mouse macrophages are more susceptible to Ad5 virus it may be that this expression is because of alveolar macrophage transduction. Additionally, species C Ads cause human respiratory disease and may be more apt to induce disease in a broader range of lung tissue in mice as compared with species D Ads.
In general, BALB/c mice do not express CD46, the primary receptor for species D Ads (Wu et al., 2004). Mice express CD46 only in the testis, and mouse and human CD46 are ∼50% divergent at the amino acid level (Inoue et al., 2003). Our data clearly show species D Ad transduction of mouse mucosal tissue and luciferase expression in vivo. Therefore, either mouse CD46 is also expressed in the mucosal epithelium or the species D viruses are transducing through a secondary receptor. One explanation for the high levels of species D Ad transduction in the nasal mucosa of the BALB/c mice is that some studies have suggested sialic acid as an alternative receptor (Arnberg et al., 2000a,b, 2002). The use of transgenic mice expressing human CD46 allowed us to explore the use of the vaccines in the context of the primary receptor. We were able to restore the i.m. transduction of the species D Ads to a level equal to the Ad5 virus. We assumed that this restoration of transgene expression would lead to increased levels of vaccine efficacy. However, we were surprised to find out that the species D vaccines were no better when delivered i.m. regardless of the presence of human CD46. We can only speculate as to why the vaccine efficacy was not restored in the i.m. immunized CD46 transgenic mice. Since human CD46 is a complement regulatory protein that functions to control innate immune responses, it may also act to quench the vaccine-induced immunity (Oglesby et al., 1992; Hartman et al., 2008). Perhaps while serving as the primary receptors for species D Ads, CD46 may also induce immune dysfunction in the vaccinated mice as indicated by the lack of improved vaccine efficacy. The Ad5 vaccine does not interact with CD46 and there was no difference in vaccine efficacy in either BALB/c or CD46 transgenic mice (BALB/c data not shown). When mice were immunized mucosally by the i.n. route, we did see an improvement of vaccine efficacy by the species D vaccine but not the Ad5 species C vaccine. Again, the availability of a secondary receptor as well as the primary CD46 receptor may explain this difference. In addition, there may be different immune responses to the vaccine in the mucosal compartment. It is very possible that the interaction between the Ad vaccine and CD46 in the systemic and mucosal immune compartments results in significantly different vaccine-induced immune responses. Future studies that investigate this interaction may reveal the mechanisms for the regulation of adaptive immunity in these two different compartments.
A previous study that investigated the use of low-seroprevalent Ads as vaccines for influenza also showed enhanced vaccine efficacy by the mucosal route in BALB/c mice (Weaver and Barry, 2013). In the absence of CD46 it was shown that species D Ad vaccines were equally effective at inducing anti-influenza immunity when the vaccines were delivered mucosally. The previous studies were performed using E3-deleted RC species D Ad vaccines, whereas this study used E1-deleted RD vaccines. It is possible that since the RC vaccines do not possess the immunoevasive E3 genes they are more immunogenic or it could be that transcription of viral genes is also playing a role in the induced immune responses. Although there are many possibilities to explain the observed differences in vaccine efficacy, only future studies on the viral vaccine and host interactions will elucidate these mechanisms. Hopefully, these studies will shed light on and improve our ability to drive antipathogen immune responses.
Acknowledgments
We thank the Biodefense and Emerging Infectious Diseases Repository for reagents used in this study. This work was supported by NIH NIAID Grant AI097241.
Author Disclosure Statement
All authors acknowledge that there are no competing financial interests.
References
- Abbink P., Lemckert A.A., Ewald B.A., et al. (2007). Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81, 4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N., Edlund K., Kidd A.H., and Wadell G. (2000a). Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74, 42–48 [PMC free article] [PubMed] [Google Scholar]
- Arnberg N., Kidd A.H., Edlund K., et al. (2000b). Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus alpha(v) integrins. J. Virol. 74, 7691–7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N., Pring-Akerblom P., and Wadell G. (2002). Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 76, 8834–8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L.R., Walsh S.R., Seaman M.S., et al. (2013). First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J. Infect. Dis. 207, 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D.H., Kik S.V., Weverling G.J., et al. (2011). International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29, 5203–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjesson M., and Enander A. (2013). Perceptions and sociodemographic factors influencing vaccination uptake and precautionary behaviours in response to the A/H1N1 influenza in Sweden. Scand. J. Public Health 42, 215–222 [DOI] [PubMed] [Google Scholar]
- Cohen J. (2013). AIDS research. More woes for struggling HIV vaccine field. Science 340, 667. [DOI] [PubMed] [Google Scholar]
- Coughlan L., Bradshaw A.C., Parker A.L., et al. (2012). Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol. Ther. 20, 2268–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K., Toth K., Kuppuswamy M., et al. (2000). Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J. Virol. 74, 6147–6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Treanor J.J., Tornieporth N., et al. (2009). Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J. Infect. Dis. 200, 172–180 [DOI] [PubMed] [Google Scholar]
- Fields B.N., Knipe D.M., and Howley P.M. (2013). Fields Virology. (Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia: ) [Google Scholar]
- Gottschalk S., Edwards O.L., Sili U., et al. (2003). Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood 101, 1905–1912 [DOI] [PubMed] [Google Scholar]
- Grubb B.R., Pickles R.J., Ye H., et al. (1994). Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature 371, 802–806 [DOI] [PubMed] [Google Scholar]
- Hartman Z.C., Appledorn D.M., Serra D., et al. (2008). Replication-attenuated human adenoviral type 4 vectors elicit capsid dependent enhanced innate immune responses that are partially dependent upon interactions with the complement system. Virology 374, 453–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J.C., Stone Y.O., and Broderson J.R. (1991). Antigenic relationships among the 47 human adenoviruses determined in reference horse antisera. Arch. Virol. 121, 179–197 [DOI] [PubMed] [Google Scholar]
- Hofherr S.E., Shashkova E.V., Weaver E.A., et al. (2008). Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 16, 1276–1282 [DOI] [PubMed] [Google Scholar]
- Inoue N., Ikawa M., Nakanishi T., et al. (2003). Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell Biol. 23, 2614–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare R., Chen C.Y., Weaver E.A., and Barry M.A. (2011). Advances and future challenges in adenoviral vector pharmacology and targeting. Curr. Gene Ther. 11, 241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen A.M., Sili U., Savoldo B., et al. (2004). Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood 103, 1011–1019 [DOI] [PubMed] [Google Scholar]
- Li H., Rhee E.G., Masek-Hammerman K., et al. (2012). Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J. Virol. 86, 10862–10865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.Y., Wu Z., Misplon J.A., et al. (2008). Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine 26, 2062–2072 [DOI] [PubMed] [Google Scholar]
- Mast T.C., Kierstead L., Gupta S.B., et al. (2010). International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28, 950–957 [DOI] [PubMed] [Google Scholar]
- McKittrick N., Frank I., Jacobson J.M., et al. (2013). Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann. Intern. Med. 158, 19–26 [DOI] [PubMed] [Google Scholar]
- McMichael A.J., and Haynes B.F. (2012). Lessons learned from HIV-1 vaccine trials: new priorities and directions. Nat. Immunol. 13, 423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier G.T., Campbell J.A., Chappell J.D., et al. (2004). A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc. Natl. Acad. Sci. USA 101, 6188–6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari N.A., Ortega-Sanchez I.R., Messonnier M.L., et al. (2007). The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25, 5086–5096 [DOI] [PubMed] [Google Scholar]
- Mrkic B., Pavlovic J., Rulicke T., et al. (1998). Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72, 7420–7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S., Bernt K., Gaggar A., et al. (2005). Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 16, 664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby T.J., Allen C.J., Liszewski M.K., et al. (1992). Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J. Exp. Med. 175, 1547–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm M.T., Kelley N.S., Sommer A., and Belongia E.A. (2012). Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12, 36–44 [DOI] [PubMed] [Google Scholar]
- Park K.S., Lee J., Ahn S.S., et al. (2009). Mucosal immunity induced by adenovirus-based H5N1 HPAI vaccine confers protection against a lethal H5N2 avian influenza virus challenge. Virology 395, 182–189 [DOI] [PubMed] [Google Scholar]
- Schnurr D., and Dondero M.E. (1993). Two new candidate adenovirus serotypes. Intervirology 36, 79–83 [DOI] [PubMed] [Google Scholar]
- Schuldt N.J., Aldhamen Y.A., Godbehere-Roosa S., et al. (2012). Immunogenicity when utilizing adenovirus serotype 4 and 5 vaccines expressing circumsporozoite protein in naive and adenovirus (Ad5) immune mice. Malar. J. 11, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaly R.P. (2008). The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205, 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova E.V., Doronin K., Senac J.S., and Barry M.A. (2008). Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 68, 5896–5904 [DOI] [PubMed] [Google Scholar]
- Teigler J.E., Iampietro M.J., and Barouch D.H. (2012). Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 86, 9590–9598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H., and Tang D.C. (2009). Protection of chickens against avian influenza with nonreplicating adenovirus-vectored vaccine. Poult. Sci. 88, 867–871 [DOI] [PubMed] [Google Scholar]
- Tsujimura A., Shida K., Kitamura M., et al. (1998). Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem. J. 330 (Pt 1), 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velan B. (2011). Acceptance on the move: public reaction to shifting vaccination realities. Hum. Vaccin. 7, 1261–1270 [DOI] [PubMed] [Google Scholar]
- Waddington S.N., Mcvey J.H., Bhella D., et al. (2008). Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132, 397–409 [DOI] [PubMed] [Google Scholar]
- Wang H., Liaw Y.C., Stone D., et al. (2007). Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J. Virol. 81, 12785–12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E.A. (2013). Vaccines within vaccines: the use of adenovirus types 4 and 7 as influenza vaccine vectors. Hum. Vaccin. Immunother. [Epub ahead of print] DOI: 10.4161/hv.27238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E.A., and Barry M.A. (2013). Low seroprevalent species d adenovirus vectors as influenza vaccines. PLoS One 8, e73313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E.A., Rubrum A.M., Webby R.J., and Barry M.A. (2011). Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS One 6, e18314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E.A., Mercier G.T., Gottschalk S., and Barry M.A. (2012). T-cell-biased immune responses generated by a mucosally targeted adenovirus-sigma1 vaccine. Mucosal. Immunol. 5, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2014). Influenza factsheet. www.who.int/mediacenter/factsheets/fszll/en/
- Wu E., Trauger S.A., Pache L., et al. (2004). Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78, 3897–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wy Ip W., and Qasim W. (2013). Management of adenovirus in children after allogeneic hematopoietic stem cell transplantation. Adv. Hematol. 2013, 176418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Freimuth P., Puga A., et al. (1997). Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J. Clin. Invest. 100, 1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., Gillisen G., Roos A., et al. (2012). Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PLoS One 7, e44115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Wu T.L., Lasaro M.O., et al. (2010). A universal influenza A vaccine based on adenovirus expressing matrix-2 ectodomain and nucleoprotein protects mice from lethal challenge. Mol. Ther. 18, 2182–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]