Abstract
Locomotor training (LT) after spinal cord injury (SCI) is a rehabilitative therapy used to enhance locomotor recovery. There is evidence, primarily anecdotal, also associating LT with improvements in bladder function and reduction in some types of SCI-related pain. In the present study, we determined if a step training paradigm could improve outcome measures of locomotion, bladder function, and pain/allodynia. After a T10 contusive SCI trained animals (adult male Wistar rats), trained animals began quadrupedal step training beginning 2 weeks post-SCI for 1 h/day. End of study experiments (3 months of training) revealed significant changes in limb kinematics, gait, and hindlimb flexor-extensor bursting patterns relative to non-trained controls. Importantly, micturition function, evaluated with terminal transvesical cystometry, was significantly improved in the step trained group (increased voiding efficiency, intercontraction interval, and contraction amplitude). Because both SCI and LT affect neurotrophin signaling, and neurotrophins are involved with post-SCI plasticity in micturition pathways, we measured bladder neurotrophin mRNA. Training regulated the expression of nerve growth factor (NGF) but not BDNF or NT3. Bladder NGF mRNA levels were inversely related to bladder function in the trained group. Monitoring of overground locomotion and neuropathic pain throughout the study revealed significant improvements, beginning after 3 weeks of training, which in both cases remained consistent for the study duration. These novel findings, improving non-locomotor in addition to locomotor functions, demonstrate that step training post-SCI could contribute to multiple quality of life gains, targeting patient-centered high priority deficits.
Key words: : allodynia, bladder cystometry, exercise, locomotion, nerve growth factor
Introduction
Spinal cord injury (SCI) results in motor, sensory, and autonomic deficits. Historically, renal failure from urinary tract complications was the leading cause of death for persons with SCI.1 Although this has changed, today urological complications are responsible for most clinical conditions and hospital readmissions for patients with SCI,2 who are about 11 times more likely to die from diseases of the urinary system than non-injured persons.3 Daily, frequent bladder management is needed to avoid and control incontinence, bladder overdistention, vesicoureteral reflux, pyelonephritis, lower urinary tract infections, cystitis, high intravesical pressures, and autonomic dysreflexia.4 Bladder dysfunction ranks as a top disorder affecting quality of life post-SCI.5,6
Post-SCI pain is also a major concern of persons with SCI. Pain prevalence is estimated at around 81% of persons with SCI, 41% of whom are categorized as having at-level neuropathic pain according to the International Association for the Study of Pain.7 Persons with SCI with at-level neuropathic pain and incomplete SCI more frequently experience allodynia, pain evoked by a non-noxious stimulus.8,9 Pain affects patients' ability to sleep, work, and participate in daily activities.10 Many treatments of post-SCI pain involve medication, but lasting effectiveness remains elusive.11 Physical therapy strategies and/or regular activity/exercise are regularly used for treatment of pain and are consistently perceived by patients with SCI as one of the most helpful and effective treatments.11,12
Locomotor training (LT) is emerging as a safe and effective therapy for post-SCI motor deficits. LT was first developed based on animal studies of task specific stepping after spinal transection indicating that the mammalian spinal cord can perform a motor task based on interpretation of afferent inputs in the absence of supraspinal control.13–18 To date, LT studies have focused on motor performance outcomes, such as muscle electromyography, gait analysis, kinematics, balance, and distances walked.
Case studies have documented bladder and sensory function recovery because of LT strategies,19,20 but potential effects of LT on changes in non-locomotor functions have yet to be systematically studied. Our goal was to determine if step training could improve not only locomotor functions, but also non-locomotor functions (bladder function and at-level allodynia) using a severe contusion model of SCI in the male rat.
Methods
All animal procedures were performed according to the National Institutes of Health guidelines and the protocols reviewed and approved by the Institutional Animal Use and Care Committee at the University of Louisville, School of Medicine. Sixteen male Wistar rats (Harlan Sprague Dawley, Inc, Indianapolis, IN) approximately 60 to 70 days old weighing 160 to 180 g were individually housed in an animal room with a 12-hour light and dark cycle. Animals were randomly divided into two equal groups pre-training. One group (n=8) was quadrupedally trained 1 h daily for 12 weeks and a second group (n=8) served as non-trained controls. Observers were kept blind to the group assignment, and baseline behavioral and functional assessment measurements were obtained for both groups for comparison with measurements during the 13-week post-SCI experiment period (see Fig. 1 timeline).
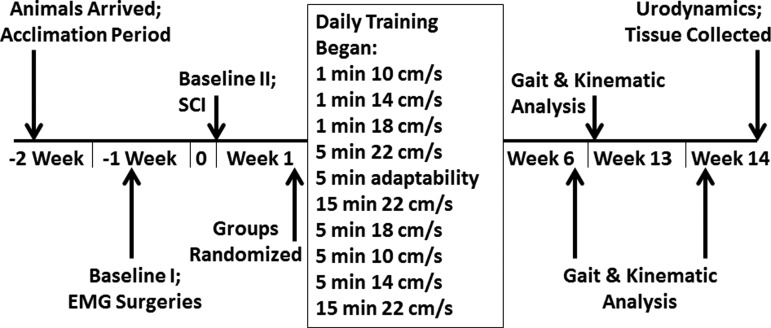
FIG. 1.
Timeline of experimental design. Locomotor step training began 2 weeks after injury following the protocol shown here with varying speeds. Basso-Beattie-Bresnahan (BBB), at-level allodynia, and below-level thermal testing occurred weekly or biweekly. Kinematics, gait analysis, and electromyography (EMG) were collected periodically. Urodynamic evaluation was performed at the terminal experiment. Trained and non-trained rats had the same testing at all time points. SCI, spinal cord injury.
Surgical procedures
Placement of electrodes for hindlimb electromyography
After a 1 week acclimation period and gentling, animals were equipped with electromyography (EMG) head plugs and EMG electrodes 1 week before SCI. Briefly, animals were anesthetized with a mixture of ketamine (80 mg/kg body weightt; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (10 mg/kg body weightt; Akorn Inc, Decatur, IL) injected intraperitoneally. The area covering the skull, left hindlimb, and back were shaved and cleaned with antiseptic solution. Incisions were made in the skin over the skull between the ears and the hindlimb regions above the target muscles. After exposure of the skull surface by removing fascia and connective tissue, the skull was gently scored with a scalpel blade, and holes were carefully drilled to allow for the placement of three small screws (Small Parts, Inc., Logansport, IN). Dental cement (Teets “cold cure” methyl methacrylate, Diamond Springs, CA) was used to secure the head plug with 9 wires (12 inches of Cooner wire on each channel) to the skull surface. The electrode wires from the head plug were subcutaneously tunneled from the skull to the left hindlimb. Warm sterile saline was continuously applied to each muscle throughout the surgery to prevent drying.
Approximately 1 mm of insulation was stripped from the ends of the wires before insertion into the muscle bellies of the left vastus lateralis (VL), semitendinosus (ST), soleus (SOL), and tibialis anterior (TA) muscles using a 23-gauge, 1 inch needle. Each muscle was back stimulated with an Isolated Pulse Stimulator (A-M Systems, Model 2100) to ensure that it contracted. Connective tissue was sutured with 5-0 chromic gut, and the skin closed with 4-0 Ethilon (Butler Schein, Dublin, OH).
Spinal cord injuries
One week after EMG implants, animals were re-anesthetized with a ketamine/xylazine mixture. Body temperatures were maintained at 36–37°C on a heating pad until the animal recovered from the anesthetic. The spinal cord was exposed by a laminectomy of the T9 vertebra, which overlies the T10 portion of the spinal cord.21 The Infinite Horizon impactor device (Precision Systems and Instrumentation, LLC; Fairfax Station, VA) was used to make a 210 kilodyne contusion injury.22 The rostral and caudal sections of vertebrae (T8 and T10) were secured during impaction using stabilizing forceps. The musculature and subcutaneous tissue was sutured closed (Ethicon 4-0 non-absorbable surgical suture), and Michel clips were used to close the skin. Animals were hydrated with 5% dextrose lactated Ringer (5 mL/100 g, subcutaneously). All animals were cared for three times a day to express bladders, inspect wounds, note changes in an animal's general condition, and clean the animals.
Post-surgical care
Animals were given subcutaneous injections of an analgesic, buprenorphine (Buprenex, 0.5 mg/kg, Butler Schein, Dublin, OH) for analgesia twice a day for 2 days. To prevent possible infections, animals were also given 0.5 mL of dual penicillin (PenJect,® The Butler Company, Columbus, OH) single dose perioperatively as a general prophylactic, 5 mg/kg gentamicin (GentaFuse,® Butler Schein; Dublin, OH) once per day for 5 days to prevent bladder infections.
Transvesical catheter
After the last training session, a transvesical bladder catheter was implanted under urethane anesthesia (1 g/kg).23 Animal temperatures were maintained with a circulating water-heating pad. The bladder was exposed via a midline abdominal incision through skin and musculature. A purse-string suture (4-0 Ethilon) was placed in the urothelium of the bladder dome. PE-60 tubing (the tip previously heated to form a collar ∼2 mm from the end) was inserted through the bladder dome within the suture limits and secured. The catheter was then connected to an infusion pump and pressure transducer.24
Behavioral procedures
Training paradigm
Training interventions initiated acutely post-SCI may be detrimental to recovery efforts by exacerbating secondary injury cascades25; therefore we initiated training at 2weeks post-SCI. Because we know the spinal cord interprets velocity and load,26 our training paradigm used multiple speeds within each session, and trainers adjusted the body weight support as needed. In addition, animals were trained quadrupedally, which may facilitate locomotor recovery via bidirectional interlimb coordination through neural coupling, much as arm swing is coupled in bipedal human locomotion.27,28 The quadrupedal position is distinctly different from many previous studies in which rats are trained in an upright/bipedal position. The upright position itself can improve stepping.29 Training was also accomplished with manual assistance, which may differ from robotic assist strategies and is discussed elsewhere.30–33 Lastly, more repetition and practice facilitates training efficacy34; thus, we opted for hour-long sessions.
Step training was performed on the Rodent Robotic Motor Performance System (Robomedica, Inc., Irvine, CA), which consisted of a computer-controlled, spring-actuated body weight support (BWS) mechanism. At the start of every session, the Robomedica device was initialized and the BWS set to zero. Each individual animal's body weight was entered into the computer so that the BWS arm adjusted the load appropriately to zero. The animals wore Lycra® vests (Robomedica, Inc.) that were harnessed to the BWS mechanism arm with hook-and-loop material.
Trained animals engaged in quadrupedal step training, beginning 2 weeks after SCI surgery, 7 days a week, 58 min per day, for a total of 81 sessions using a three-tiered training approach. The “warm-up period,” which began at a speed of 10 cm/sec, allowed rats to acclimate to the robotic system. Gradually, speeds were increased at various intervals (14 cm/sec, 18 cm/sec, and 22 cm/sec) for 8 min. Next, the animal trained at an “adaptability speed” for 5 min, which was the best pace that each animal achieved proper plantar placement—e.g. complete toe extension, no ankle rotation, and incorporation of forelimb-hindlimb coordination with minimal assistance. Finally, during a “retraining period,” the animals cycled through all speeds again, with the majority of the time (30 min) spent at 22 cm/sec (Fig. 1).
Quadrupedal training was chosen over bipedal training to engage interlimb coordination through any residual fibers to accomplish gains in overground locomotion. All BWS was provided as needed using manual assistance at the hip flexor region by the trainers (no BWS was provided by the Robomedica arm). If an animal could achieve quality steps with minimal weight support assistance, this support was provided by the trainer at the hip flexor region or by holding the tail. Rats were encouraged to step independently as they began to gain consistent stepping and more stability without collapsing and dragging their hindlimbs. It was noted that occasional flicking of the tail occurred during training, and some animals tried to hold their tail up or to the side during training, but their tail was down in the home cage. Tail position is accounted for during Basso-Beattie-Bresnahan (BBB) subscoring.
Rats were not required to complete the 58 min session during the first week of training if they exhibited signs of stress—i.e., porphyrin staining at eyes and nose, irregular breathing, or excessive diarrhea. All trained animals were able to complete the 58 min by 10 sessions (four rats did not complete the full 58 min during the first week). The non-trained group was not exposed to the treadmill system except during EMG and kinematic evaluation sessions. To minimize differences in stress and handling, the non-trained group was handled at least four times per week (sometimes more). Animals were never stimulated to step by perineum or tail pinching. Noxious stimuli was avoided during training sessions—e.g., if an animal had skin abrasion on a paw, animals ceased from training until the issue was resolved (one rat for 3 days), because potentially noxious input may inhibit spinal learning.35
At-level allodynia
Behavioral testing of SCI rats for sensitivity to normally innocuous stimuli (touch and gentle squeeze/pressure) was performed using our published grading scale for the scoring of pain-like behavior to trunk stimulation in the rat.36 Levels of noise and other distractions were kept to a minimum. All test sessions were started at approximately the same time of day (9 AM before the start of training). The dorsolateral trunk (T7–T9 dermatomal level) just above the T10 spinal injury level was tested bilaterally for at-level mechanical hypersensitivity to touch and gentle touch/squeeze. Two baseline measurements (at least 3 days apart) were performed before injury for all rats.
The top of the cage was removed, and the animal was allowed to acclimate to the environment for 2 min. Each animal was stroked at the dorsolateral trunk five times bilaterally with an interstimulus interval of 1 min between sides (while in its cage) with a No. 5 paintbrush (1.5×0.5 cm bristles; average pressure, 15 g) in an alternating rostral/caudal plane.36 After each stroking stimulus, the presence/absence of any evoked responses that are indicative of pain was noted. The three typical evoked pain-like responses, as observed previously with both noxious stimulation in uninjured rats and touch stimuli in SCI rats based on previous studies,21,37 were (1) a freezing response (stopping of normal activity and staying still in response to the stimulus), (2) escape (any movement of the animal away from the stimulus probe), and (3) grabbing at or pushing away the stimulus probe with their forepaws. It has been shown that these evoked responses are not present in decerebrate rats, indicating higher-order processing.38 Therefore, the perception of pain is likely involved with these responses.38
In addition, head orientation, which is reflexive in nature (present in decerebrate rats), was not counted as an evoked pain-like behavioral response.38 An animal must show an evoked pain-like behavioral response at least 60% of the time in a given session to be considered responsive to the testing stimulus (i.e., an animal responded to at least three of five stimuli/strokes per side).37 Responses to brush, if present, were further assessed for threshold values using a set of Semmes-Weinstein monofilaments (20 filament set, 15 of which are in the range of 0.008 g to 15 g; Stoelting Co, Wood Dale, IL). Animals designated as responders to brush (15 g stimulus) were then given a numeric score based on their associated responses to filament testing, receiving a minimum of 4 (4=freeze, 5=escape, 6=grab/push—as the aggressiveness of the behavioral response increases, so does the score) to a maximum of 10 (see Hall and associates36 for scoring scale).
The Semmes-Weinstein monofilaments were chosen over our Electro Von Frey anesthesiometer (IITC Model 2390), which was not very sensitive in detecting the lower range of forces. Depending on the behavioral response of the animal to brush, the experimenter chose an appropriate Semmes-Weinstein monofilament (usually mid-range) so as not to overstimulate and sensitize the animal. For example, to assist in this procedure, the monofilaments are grouped into sensitivity ranges based on the manufacturer's specifications, where green coded filaments (0.008 g–0.07 g) are more sensitive than the next size blue coded (0.16 g–2.0 g) filaments. An initial filament stimulus was applied by pressing the tip of the filament into the dorsolateral trunk (T7–T9) until it bent. If the animal responded, a lower gram filament was applied to test the animal's sensitivity. In between filament probing, the animal is left alone for a 1 min interstimulus interval. The process is repeated until the lowest gram filament that the animal responds to 60% of the time is determined. If the animal was not responsive to the initial probing stimulus, the next greatest gram filament (and repeated if needed) was used to determine the threshold of the animal's sensitivity.
For those animals not responsive to brush stroke (i.e., evoked pain-like behavioral response to less than 60% of the stimuli—less than three of five strokes), a gentle squeeze/pressure test was conducted to determine if the animal had increased sensitivity to a stronger mechanical stimulus over a wider surface area (which also normally does not provoke avoidance behaviors and is thus considered innocuous). In this instance, the animal's skin is gently squeezed with a pair of modified Adson tissue forceps (2.0-mm–wide tips), which is equivalent to the 60 g Semmes-Weinstein monofilament. Gentle squeeze/pressure was applied to the dorsolateral trunk (T7-T9) five times bilaterally, with an interstimulus squeeze interval of 1 min. As with touch-evoked agitation, any evoked pain-like responses were observed and documented (0=no response, 1=freeze, 2=escape, 3=grab/push).
After the testing session, animals were scored for their degree of at-level allodynia based on a 10-point scale, with 10 being the maximum score an animal can receive.36 Scores from each weekly testing were documented for each animal and averaged together to obtain a mean weekly allodynia score for each group.
Thermal hyperalgesia
Hindlimb withdrawal latency to an applied heat stimulus was measured using a modified Hargreaves' method as described previously.39 Briefly, animals were placed in individual acrylic enclosures on a glass plate, which was situated over a light box. Animals were habituated to the apparatus 1 week before the start of the study. Animals were allowed to acclimate to their environment as the heat source warmed to a constant testing temperature of 32°C (20–30 min). A radiant heat stimulus was directed onto the glabrous surface of the paw (IITC Life Sciences Inc., Model 400, Woodland Hills, CA). The tester, positioned in front of the unit, recorded the time to paw withdrawal in response to the heat. To prevent tissue damage, the device was pre-set for a cutoff time (20 sec), where the light source automatically shut off if the animal did not respond. Each hindpaw was tested five times. Five min (minimum) were allowed between each trial, and these measurements were then averaged for each limb and compared with pre-injury baseline values.
Locomotor assessment
The BBB scale, an open-field locomotor assessment, was used to evaluate hindlimb function in the rats.40 Once per week, each animal was placed in an open field and tested for 4 min by the same two scorers, who were presented with the trained and non-trained animals in random order. Locomotor behavior was calculated using a 21-point scale, which rates parameters such as individual joint movements (0–7), weight support (8–13) and paw placement (14–21). Intact animals demonstrate a locomotor score of 21, whereas animals that exhibit complete paralysis of the hindlimbs are scored as 0. Animals were placed in the open field for 4 min before baseline for acclimation. A baseline measurement was collected before surgically implanted EMG electrodes. A second baseline was collected post-EMG surgery to ensure no damaging effects (all animals scored 21). Weekly testing was assessed after SCI.
Kinematic and EMG data acquisition
All trained and non-trained animals were weighed and hindquarters shaved. The bony landmarks on the lateral side of the left hindlimb were marked with permanent marker: iliac crest, greater trochanter, lateral malleolus, and metatarsophalangeal joint (ilium, hip, ankle, toe) for the recording of locomotion.41 The original marks were placed by one person to avoid interperson variability and darkened periodically to maintain accurate recording throughout the study.
Before the testing session, the Robomedica, Labview, and Kinematic systems were initialized and the BWS set to zero. Animals were then put in the harness and attached to the robotic arm using Velcro straps. A flexible cable was connected to the head plug for EMG recording. The test was completely unassisted—i.e. each animal stepped without any help from the trainer, not even tail holding. Kinematic and EMG data were collected while the animal took 10 unassisted steps at 22 cm/sec. If an animal failed to step, a maximum recording session of 2 min was captured. Electromyograms of all muscles were recorded simultaneously.
Hindlimb kinematic recordings were acquired at 30, 60, and 80 days post-SCI using the MaxTraq motion analysis system version 2.4 (Innovision Systems, INC; Columbiaville, MI). Two cameras, positioned on the left side of the animal, were used to capture the angles. The iliac crest, hip, ankle (lateral malleolus), and toe were digitized, and the hip-ankle-toe (HAT) and iliac crest-hip-ankle (IHA) angles were marked. Naïve rats' locomotions were recorded, but were not suitable for comparative analysis because they refused to walk while harnessed on the treadmill or even within a Plexiglas chamber surrounding the treadmill with a sugared cereal reward.
Kinematic and gait data analysis
Using the MaxTraq software, a three-dimensional (3D) compilation was made from the original Audio Video Interleave files, and the IHA (lateral malleolus), and toe (metatarsophalangeal joint) were digitized.42 From the 3D files, stick figures and angle-angle plots were generated and further analyzed in Excel. As with all of our analysis, assigned file names kept the experimenter blind (trained vs. non-trained). HAT and IHA angles were analyzed by comparing peak flexion, extension, and excursions (i.e., the minimum, maximum, and the difference between those angles) between trained and non-trained group means.
Stance (the time between contact and liftoff of the left foot), swing (the time between liftoff and contact), step duration (the time between two successive left foot contacts), and steps/sec were analyzed for both the trained and non-trained groups. For stance, if an animal did not lift the paw to take a step, the maximum score allowed was 5 sec. Swing was calculated as the difference between step cycle duration and stance. These parameters are represented as box plots showing the 75th and 25th percentiles (±95th and 5th percentiles) at 22 cm/sec where the dashed line is the mean and the solid line is the median for each group. Outliers are identified with a closed circle.
EMG data analysis
EMG data were collected from the SOL, TA, ST, and VL muscles using two 8-channel hardwired systems (Neuralynx Lynx-8, Bozeman, MT). Data were digitally sampled by an on-line analog to digital (A–D) conversion system (National Instrument, Austin, TX) at 2000 Hz and processed by custom-written acquisition software. Burst duration (sec) and mean burst-to-step ratio were calculated for each animal.43,44 To calculate muscle activation amplitude, integrated mean amplitude (μV.s) (which is less sensitive to movement artifacts) was used. Mean values were compared between treatment groups.
Urodynamic analysis via non-stop transvesical cystometry
The bladder was emptied, and the initial bladder volume (mL) was recorded before the start of the experiment. Physiologic saline was infused into the bladder at a rate of 0.25 mL/min to evoke voiding contractions.23 Urodynamic data (voiding and non-voiding events, bladder pressure) and experimenter notes were recorded on video for offline playback and analysis with Datawave software (www.dwavetech.com). Voiding efficiency was calculated as the percent volume voided per volume saline infused. Residual volume was measured directly by withdrawal of saline through the catheter and bladder manipulation.
Cystometrogram (CMG) parameters are the mean of five consecutive cycles (which were sampled approximately 15 min after the start of saline infusion). CMGs were analyzed for resting pressure (mm Hg), maximal amplitude of contraction (mm Hg; peak pressure minus resting pressure), contraction time (sec), and intercontraction interval (sec). Cystometry data were collected for all eight non-trained and six of the seven trained rats (one rat stopped breathing before the CMG recording and was immediately perfused for tissue retrieval).
Analysis of nerve growth factor (NGF), neurotrophin factor 3 (NT3), and brain-derived neurotrophic factor (BDNF) in the bladder
RNA extraction and real-time reverse transcription polymerase chain reaction (qRT-PCR)
The whole bladder was dissected from the prostate, blotted dry, weighed, flash frozen in liquid nitrogen, and stored at −80°C. Samples were coded until results were obtained. The following procedures were performed according to the manufacturer's instructions and similar to our previous studies.45,46 RNA was extracted with TRIZOL (Invitrogen). Quantity and quality was evaluated via a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies). RNA samples with 260 nm/280 nm ratios above 1.9 and 260 nm/230 nm ratios above 1.8 met quality control standards. RNA samples were DNase treated with TURBO DNA-free kit (ABI) to eliminate genomic DNA contamination followed by cDNA synthesis from 2 μg RNA with the QuantiTect Reverse Transcription Kit (Qiagen).
Four potential genes (ACTb, GAPDH, 18S, and Rpl7a) were screened using cDNA from the bladders of SCI trained, SCI non-trained, and naïve animals to identify an appropriate control that was stable throughout each condition, and ACTb was chosen as the best normalizer (least variation between condition) because it was not affected by SCI or training. Quantitative real time PCR was performed in triplicates with 20 ng cDNA with primers for NGF, BDNF, NT3, and β-actin (ACTb, reference gene) using SYBR-Green (Applied Biosystems) on the Rotorgene real time cycler (Qiagen/Corbett Research). The following sequences were used: NGF F, 5′-CAA CAG GAC TCA CAG GAG CA 3′; NGF R, 5′-CAC ACA CGC AGG CTG TAT CT 3′; BDNF F, 5′-GCC ACA ATG TTC CAC CA 3′; BDNF R, 5′ CGT TTG CTT CTT TCA TGG G 3′; NT3 F, 5′ TTC TGC CAC GAT CTT ACA GG 3′; NT3 R, 5′ ACA TCT ACC ATC TGC TTG GAG 3′; ACTb F, 5′-TGA CCC AGA TCA TGT TTG AG 3′; ACTb R, 5′- CTC TTT AAT GTC ACG CAC GA 3′.
The following cycle parameters were used: incubation at 95°C for 4 min followed by 40 cycles of 95°C for 12 sec and 60°C for 30 sec. A melt curve was performed at the end of the run (ramp from 50 degrees to 99 degrees) to confirm specificity of amplification products. Reaction efficiency between primer sets were accounted for using standard curve quantification. Positive control cDNA was prepared from embryonic day 17 whole rat head. No reverse transcriptase and no template served as negative controls.
Histology of lesion epicenter
Immediately after completion of the urodynamic study, animals were perfused transcardially with a solution of physiologic saline and heparin. Spinal cord tissue containing the lesion area was then removed and immersed in 4% paraformaldehyde for at least 48 h, followed by 30% sucrose/phosphate buffer solution with 1% sodium azide for at least 24 h and until the tissue was cut transversely on the cryostat (Leica CM 1850). The tissue was sectioned at 18-μm thickness and stained with both Luxol fast blue and cresyl violet (Kluver-Barrera method).
Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI) and the Nikon E400 microscope were used to obtain measurements to quantify the lesion epicenter (based on total lesion area) as previously described.36,47 Briefly, white matter was divided into four regions (dorsal columns, dorsolateral funiculus, ventrolateral funiculus, and the ventromedial funiculus) as well as each area subdivided into left and right sides. The central canal, medial edges of the dorsal horn, and the tips of the ventral horn were used as landmarks for the divisions. The percent of white matter sparing (WMS) was determined by dividing the white matter remaining at the epicenter by the average area of white matter present in more intact sections. The intact area of white matter for a given region was estimated by averaging together measurements from two sections 2 mm rostral and two sections 2 mm caudal to the epicenter.
Statistics
One animal was excluded from the trained group because of (1) poor health (inadequate weight gain and poor grooming/appearance throughout the study) and (2) during terminal dissection, a black granular substance (presumably dried blood) was found throughout the abdominal cavity. None of the other animals (trained or non-trained) displayed those signs.
Analysis was performed using SigmaStat, IBM SPSS, and Microsoft Excel. The Levene test for inequality of variance was performed to test equality of variance. Repeated behavioral tests, such as BBB, at-level allodynia, Hargreaves, gait analysis, were compared between the treatment groups using repeated measures analysis of variance (ANOVA) (fixed effects) for tests of within subject and between subject effects, followed by Bonferroni post-hoc t tests with a significance level of p<0.05 (n=7 trained vs. n=8 non-trained).
Terminal or post-training tests, such as mean angular amplitudes (n=7 trained vs. n=8 non-trained), electromyography (n=3 trained vs. n=5 non-trained; all animals with chronic implants that were intact for the study's 4 month duration), cystometry (n=6 trained vs. n=8 non-trained; one animal expired before CMG recording), and qRT-PCR (n=7 trained vs. n=8 non-trained) results were compared with unpaired two tailed t tests, trained vs non-trained, significance level p<0.05. The Pearson correlation and multiple regression analyses (linear regression, enter method, two cystometry measures with NGF or voiding efficiency per analysis) were performed on select cystometry and qRT-PCR variables (significant or approached significance). All values reported in the manuscript are mean±standard deviation.
Results
Training leads to improved bladder function
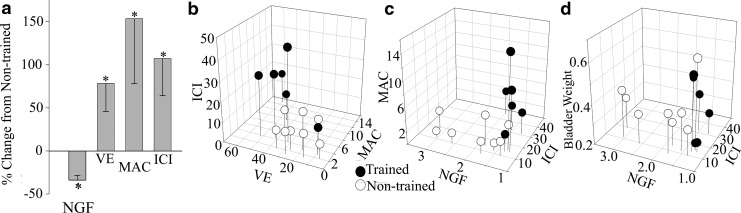
A 1-h daily quadrupedal step training regimen using manual trainer assistance was initiated at 2 weeks post-contusion injury and continued for 3 months (Fig. 1). Post-training, non-stop transvesical urodynamics was used to evaluate voiding parameters in all rats with SCI. The mean voiding efficiency (percent volume voided/volume infused) of the trained group was significantly greater than non-trained (Fig. 2a, trained 39.78±17.72; non-trained 22.29±11.02, p=0.042). This was accompanied by a significant increase in the maximum amplitude of bladder contraction (MAC, pressure in mm Hg) and a significantly increased intercontraction interval (ICI, time in seconds) (Fig. 2, MAC p=0.043, trained 6.54±4.75, non-trained 2.56±1.39; ICI p=0.018, trained 25.40±12.98, non-trained 12.25±3.77). Contraction time (trained 25.23±15.35, non-trained 16.03±3.67), resting pressure (trained 16.10±5.52, non-trained 17.80±2.46), and bladder weight (trained 0.36±0.11, non-trained 0.40±0.10 [for reference, naïve age-matched controls 0.22±0.03]) demonstrated no differences between groups.
FIG. 2.
Bladder nerve growth factor (NGF) mRNA and cystometry. (a) Percent change from the non-trained mean for NGF (fold change), VE (voiding efficiency, percent volume voided per volume saline infused), MAC (maximal amplitude of contraction, pressure in mm Hg), and ICI (intercontraction interval, seconds). Statistics were performed on the means of each parameter. Mean NGF significantly decreased and mean VE, ICI, significantly increased with training. (b) Three-dimensonal (3D) relationship between cystometry measures. (c, d) 3D relationship between NGF and cystometry measures. Significant group differences were detected by group using multiple regression analysis.
Bladder weight (g), however, significantly correlated (Pearson correlation) with voiding efficiency in only the trained group (p=0.004, r=− 0.946, r2=0.895, n=6). Bladder hypertrophy post-SCI can result from detrusor sphincter dyssynergia in a manner similar to bladder outlet obstruction, which is reversible with outlet relief.48,49 The sphincter must be relaxed during a bladder contraction to allow emptying. Based on our findings, we would infer that the sphincter of the trained rats was in partial coordination to allow for the flow of urine, an increase in voiding efficiency, and the significant relationship between bladder weight and voiding efficiency for trained rats.
Training affected neurotrophin mRNA in the bladder
The afferents responsible for transducing bladder stimuli undergo anatomical, functional, and neurochemical plasticity in response to SCI and SCI-related tissue pathologies such as cystitis (bladder inflammation).48,50–52 These plasticity phenomena have been linked to increased production of NGF in bladder tissues.49,53–56 Given that step training affected bladder function and that training affects neurotrophin production in other tissues (muscle, spinal cord),57,58 we assessed neurotrophin mRNA in the bladders of both trained and non-trained rats by qPCR. The expression level of each transcript was determined relative to β-actin. NGF mRNA was significantly decreased in the bladders of trained versus non-trained rats (Fig. 2, trained 1.37±0.31; non-trained 2.08±0.75, p=0.038; naïve [n=5] 1.33±0.56). Levels of BDNF and NT3 mRNA were not different between groups. NGF plays a large role in the excitability and activity of the bladder. NGF administration alone can induce bladder overactivity.56,59 Our finding that SCI animals that had LT also had decreased bladder NGF mRNA and increased micturition function (longer ICI, which is a goal in the context of SCI) suggests that training was able to ameliorate bladder overactivity after SCI through changes in NGF.
Multiple regression analysis revealed that MAC (p<0.05, Beta weight 0=0.44), and training group (p<0.05, Beta weight=0.51) were good predictors of voiding efficiency (R2=0.870, p<0.001, N=14). Therefore, training modified the relationship of voiding efficiency and MAC. Multiple regression analysis of cystometry parameters with NGF was significant (R2=0.69, p<0.01, N=14), although only training was a significant predictor of NGF (p<0.005, Beta weight=0.88). Examination of the relationships (Pearson correlations) between NGF and voiding efficiency with ICI and bladder weight revealed that ICI was strongly correlated with NGF in the training group only (r=0.84, p<0.05, n=6), and voiding efficiency and bladder weight were highly correlated only in the training group (r=0.95, p<0.005, n=6). Therefore, training modified the relationship of NGF to MAC and ICI, as well as the relationship between voiding efficiency and bladder weight. These relationships are demonstrated with descriptive 3D graphs in Figure 2b–d.
Training decreased at-level allodynia but had no effect on below-level thermal sensitivity
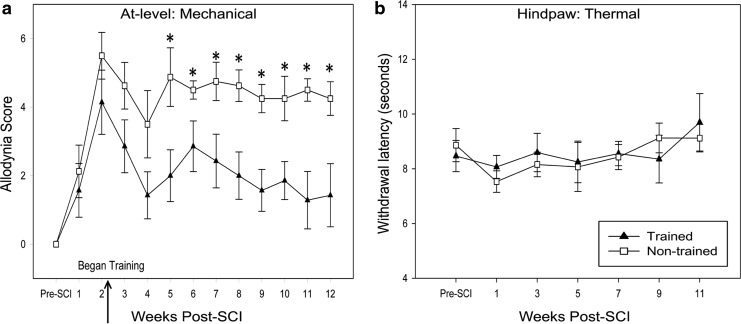
In this study, we applied non-noxious mechanical stimuli to the dermatomes immediately rostral to the injury (at-level). Based on several categories of pain-related behavioral responses indicative of higher order processing, animals were scored for sensitivity.36 After contusion, at-level allodynia developed in all animals by 14 days post-SCI (all animals scored greater than zero). After 3 weeks of training (i.e., 5 weeks after injury), at-level allodynia scores for the trained group became significantly lower than non-trained for the duration of training (Fig. 3a). The contusion injury did not induce significant thermal sensitivity relative to pre-injury withdrawal thresholds. Step training did not alter hindpaw thermal sensitivity compared with non-trained controls (p>0.05; Fig. 3b). It has been suggested that the rhythmicity and loading that occur during step training have more pain relieving efficacy than swim or stand training.60 These results support step training's efficacy in relieving post-SCI pain—in this case, at-level allodynia.
FIG. 3.
Mechanical and thermal sensory testing. (a) Mechanical allodynia testing demonstrated that training initiated at 2 weeks (W) post-spinal cord injury (SCI) leads to a significant decrease of allodynia scores in at-level dermatomes compared with non-trained controls (*p=0.027 W5, p=0.046 W6, p=0.029 W7, p=0.006 W8, p=0.003 W9, p=0.016 W10, p=0.002 W11, p=0.015 W12). In agreement with Basso-Beattie-Bresnahan scoring, it took approximately 3 weeks to see a significant training effect. (b) The Hargreaves test for thermal hyperalgesia of the hindpaws did not reveal significant differences between trained and non-trained groups. The mean of 10 trials (5 on right and 5 on left hindpaws) is shown. Importantly, training did not exacerabate thermal hyperalgesia.
Training leads to improved overground and treadmill locomotion
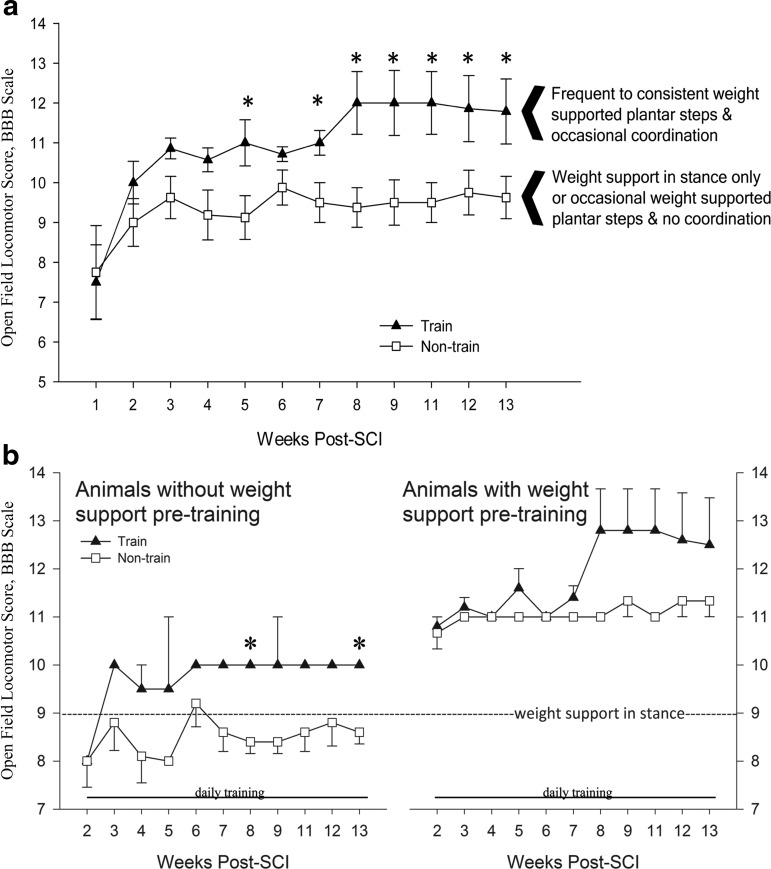
Pre-training BBB means agreed with previous functional outcomes using the Infinite Horizon Impactor.22,61 The trained group as a whole scored significantly higher BBBs beginning after 3 weeks of training (5 weeks post-SCI). During post-hoc analysis of individual animal trends, we discovered that the effect of step training on BBB scores was dependent on intrinsic weight support (Fig. 4). Animals that could achieve weight support before training went on to gain coordinated forelimb-hindlimb stepping. It is very important to note that non-trained animals that could also achieve weight support at 2 weeks post-SCI never gained coordinated forelimb-hindlimb stepping. LT still leads to significant improvements (compared with non-trained) even without weight support. Note that mean BBB scores (trained vs. non-trained) were equal pre-training (without weight support: n=2 trained 8.0±0.0, n=5 non-trained 8.0±1.22; with weight support: n=5 trained 10.8±0.45, n=3 non-trained 10.3±1.15).
FIG. 4.
Basso-Beattie-Bresnahan (BBB) scoring. (a) Trained group means were significantly higher than non-trained at several weeks (W) (n=7 trained vs. n=8 non-trained; *p=0.035 W5, p=0.029 W7, p=0.013 W8, p=0.023 W9, p=0.016 W11, p=0.05 W12, p=0.041 W13). (b) Post-hoc analysis revealed that the effect of step training on BBB scores was dependent on intrinsic weight support. In animals that could not weight support before training commenced, significant differences were detected (n=2 trained vs. n=5 non-trained; *p=0.011 W8; p=0.019 W13). With this training paradigm, it took approximately 6 weeks of training to see improvements in locomotion in animals that could weight support before training commenced. Functional differences between trained and non-trained occurred in consistency of weight supported plantar stepping and forelimb to hindlimb coordination.
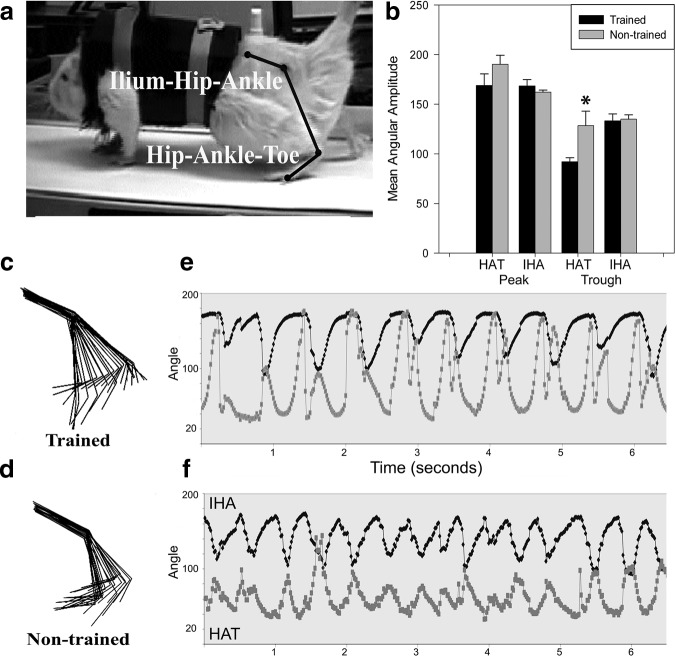
Kinematic analysis of maximum (joint extension), minimum (joint flexion), and excursion (joint range of motion) angles formed by the HAT and IHA during stepping at 22 cm/sec was compared between groups post-training. Trained animals demonstrated significantly greater ankle flexion than non-trained controls (Fig. 5b, trained HAT 92.03±10.64; non-trained HAT 128.46±41.34, p=0.041). Hip and ankle angular excursions, stride length, phase relationship, toe-hip distance, and toe velocity were not different between groups (data not shown).
FIG. 5.
Hindlimb kinematics. Trainers did not touch or assist the animals during locomotor assessments (except to position the paws in a plantar position before the treadmill belt started). (a) Maxtraq diagram showing the ilium-hip-ankle (IHA) and hip-ankle-toe (HAT) angles. (b) Maximum and minimum (peaks and troughs) mean angular amplitudes of the hip and ankle joints at the completion of the study. Trained animals demonstrate significantly greater mean ankle flexion (trained HAT 92.03±10.64; non-trained HAT 128.46±41.34, p=0.041). (c, d) Stick figure representation of two animals with comparable Basso-Beattie-Bresnahan (BBB) scores from the trained (BBB=12) and non-trained (BBB=11) groups at the completion of the study. Trained animals had larger mean angular excursions at both the hip and ankle (trained excursions: HAT 76.95±25.64, IHA 35.25±9.98; non-trained excursions: HAT 61.73±28.06, IHA 27.20±9.90). (e, f) Phase relationship of stepping via the hip and ankle angular pattern of peaks and troughs (or extension and flexion). The trained animal demonstrates a greater range of motion in the ankle (e vs. f, light gray trace).
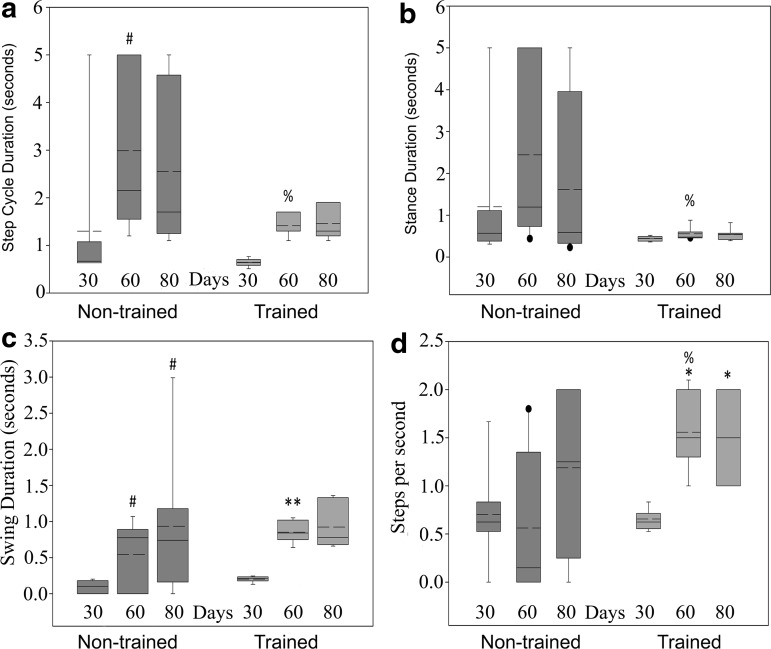
Gait analysis was performed at 30, 60, and 80 days post-training. Improvements were detected in step cycle duration, stance, swing, and steps per second. Step cycle duration (the time between two successive left foot contacts) of the non-trained group increased significantly from 30 to 60 days and remained stable at 80 days (Fig. 6a, non-trained 30: 1.76±2.01, 60: 2.99±1.70, 80: 2.55±1.67, p=0.037). Compared with non-trained controls, the trained group had significantly shorter step cycle duration at the 60 day time point and remained stable at 80 days (Fig. 6a, trained 30: 0.64±0.08, 60:1.41±0.23, 80: 1.46±0.34, p=0.031). The trained group also demonstrated less variability than the non-trained for each time point.
FIG. 6.
Gait analysis. Animals were recorded stepping at 22 cm/sec for gait analysis, completely unassisted. (a) Step cycle duration is the time between two successive left foot contacts. The mean step cycle duration of the non-trained group became significantly longer (30 vs. 60 days, #p=0.037; 30: 1.76±2.0, 60: 2.99±1.7). Trained animals had a significantly shorter mean step cycle duration than non-trained animals at the 60 day time point (trained 1.41±0.23, non-trained 2.99±1.7, %p=0.031). (b) Stance duration is the time between contact and liftoff of the left foot. Trained animals had significantly shorter mean stance duration compared with non-trained animals at the 60 day time point (trained 0.56±0.16, non-trained 2.45±2.13, %p=0.037). (c) Swing duration is the difference between step cycle and stance durations. No significant differences were detected between groups. All animals initially had short swing durations, which improved with time during the study; non-trained 30: 0.10±0.08, 60: 0.54±0.46, 80: 0.93±0.94; trained 30: 0.20±0.04, 60: 0.85±0.14, 80: 0.92±0.30 (#non-trained 30 vs. 60, p=0.011 and 80, p=0.019; **trained 30 vs. 60, p=0.001). (d) In terms of the number of steps per second, trained and non-trained groups demonstrated similar function at 30 days (0.66±0.11 vs. 0.70±0.50), but by the 60 day time point, trained animals took significantly more steps per second than non-trained animals (trained 1.56±0.38, non-trained 0.56±0.74, %p=0.007). Within the trained group, animals took significantly more steps per second at 60 and 80 days compared with 30 days (*30 vs. 60 p=0.008 and 80: 1.50±0.41, p=0.026), indicative of the positive effects of training on the ability of these animals to take successful steps. In addition, animals in the trained group showed less variability than animals in the non-trained group for each measure.
Further group differences were found when comparing the stance and swing phases of the step cycle (Fig. 6b). Stance duration increased in the non-trained group with time from SCI, but remained stable in the trained group. Stance duration was significantly shorter in the trained group at the 60 day time point compared with non-trained controls and remained stable at 80 days (Fig. 6b, non-trained 60: 2.45±2.13, trained 60: 0.56±0.16, 80: 0.53±0.14, p=0.037). The swing phase significantly increased with time for both groups (Fig. 6c, non-trained 30: 0.10±0.08, 60: 0.54±0.46, p=0.011, 80: 0.93±0.94, p=0.019; trained 30: 0.20±0.04, 60: 0.85±0.14, p=0.001, 80: 0.92±0.30). These findings suggest that, at the 30 day time point, both trained and non-trained animals took quick, short steps or no steps (hindpaw dragging), which resulted in a short swing phase followed by recovery. The trained group took significantly more steps per second at 60 and 80 days than 30 (Fig. 6d trained 30: 0.66±0.11, 60: 1.56±0.38, p=0.008, 80: 1.50±0.41, p=0.026). The trained group also took significantly more steps per second compared with non-trained controls at 60 days (Fig. 6d, non-trained 60: 0.56±0.74, p=0.007).
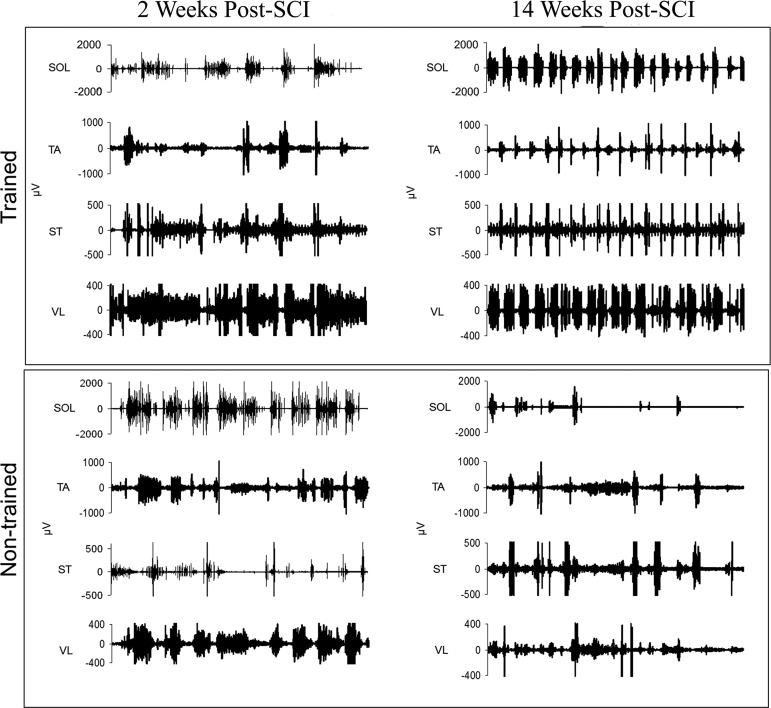
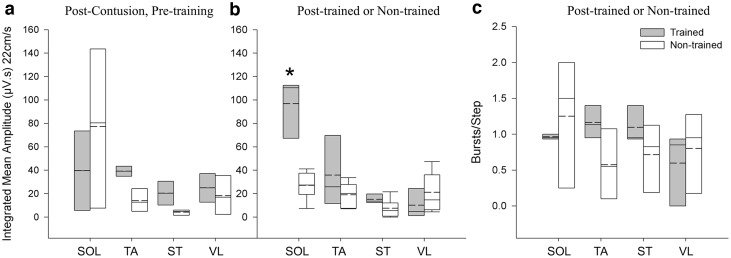
EMG data from hindlimb muscles during unassisted treadmill stepping (22 cm/sec) were compared at 2 weeks post-SCI (pre-training) and after 80 days of training (or non-training). The burst pattern became more organized with training, whereas non-trained animals displayed irregular bursting, coactivation, and decreased activity (Fig. 7). Decreased muscle activity and EMG amplitudes have been previously reported for chronic spinalized rats.62 Trained animals' mean EMG amplitudes significantly increased in the SOL muscle, an ankle extensor and plantar flexor, compared with non-trained controls (Fig. 8b, trained 96.72±25.48; non-trained 27.10±13.06, p=0.003). Although not significant, trained animals approximated a 1:1 burst-to-step ratio for SOL, TA, and ST (Fig. 8c), while non-trained animals had greater variability (SOL: trained 0.96±0.03, non-trained 1.25±0.96).
FIG. 7.
EMG traces from hindlimb muscles during unassisted treadmill stepping (22 cm/sec) were compared at 2 weeks post-spinal cord injury (SCI) (pre-training) and after 80 days of training (or non-training). “Before and after” EMG activity of four muscles soleus (SOL), tibialis anterior (TA), semitendinosus (ST), and vastus lateralis (VL) are shown for the left hindlimb of one trained and one non-trained animal at matched time points. Post-training, the trained animal exhibits a more coordinated burst pattern while the non-trained animal's EMG activity has uncoordinated bursting activity.
FIG. 8.
Electromyography (EMG) quantification. (a) EMG integrated mean amplitudes from four muscle of the left hindlimb of animals before and after training. Pre-training, there were no significant differences between trained and non-trained mean amplitudes. (b) Post-training, the trained group demonstrated a significant increase in the soleus (SOL) muscle mean amplitude compared with non-trained controls (p=0.003). (c) Burst-to-step ratios for each recorded muscle. Although no significant difference was detected, trained animals display ratios closer to 1:1 and have less variability than non-trained controls, especially noticeable in the SOL muscle (SOL: trained 0.96±0.03, non-trained 1.25±0.96). TA, tibialis anterior; ST, semitendinosus; VL, vastus lateralis.
No differences in WMS at epicenter or ventrolateral funiculus
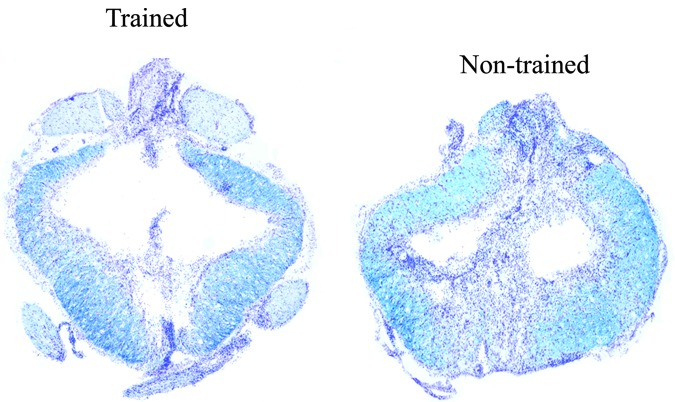
Severe contusion resulted in complete loss of grey matter at the epicenter of the lesion and a rim of spared white matter. There was no significant difference in the initial injury parameters of force or displacement (force (kdyn) trained 216.50±6.7; non-trained 217.25±8.1; displacement (μm) trained 1218.75±109.0; non-trained 1300.5±81.6). There was no significant difference overall in the percent WMS at the epicenter (trained 18.84±6.1; non-trained 13.62±4.2). An example showing WMS from a trained and non-trained rat is provided in Figure 9. In addition, no significant difference was found in degree of asymmetry of percent ventrolateral funiculus WMS (trained: 20.29±17.3; non-trained: 19.34±12.1), which we have shown to be related to the presence of at-level allodynia,36 suggesting that the lesion alone cannot explain the observed behavioral differences between the two groups. A correlation analysis between ventrolateral funiculus (VLF) WMS and BBB score revealed, using the non-parametric Spearman Rank correlation, no significant correlation for both the trained (rS=0.59, p>0.05, n=7) and non-trained (rS=0.22, p>0.05, n=8) groups of rats.
FIG. 9.
Histology. Example shows the lesion epicenter from a trained and non-trained male rat with spinal cord injury. A bilateral rim of tissue sparing with staining of both Luxol fast blue and cresyl violet (Kluver-Barrera method) can be seen. Total percent white matter sparing measured (relative to rostral/caudal intact sections from the same animal, not shown) and Basso-Beattie-Bresnahan (BBB) scores assessed during the final week of testing for the animals shown was 19.9%/BBB=16 (trained) and 18.7%/BBB=12 (non-trained). Color image is available online at www.liebertpub.com/neu
Discussion
Step training post-SCI resulted in significant multi-system functional gains. Beneficial outcomes after 3 months of daily 1-h quadrupedal step training sessions with manual assistance included improved bladder function, reduced at-level allodynia, improved overground walking (BBB), hindlimb EMG, and kinematics. It is important to note that for this study, only male rats were used because the SCI population is 80% male. Sex differences (not examined in the present study) do exist in response to SCI, training, micturition, and development of pain as noted in a variety of human and animal studies.63–71
Step training
A 1 h per day training paradigm with weight bearing stepping was controlled using a treadmill system and incorporated specific aspects of locomotion aimed at facilitating recovery. Repetitive practice of weight bearing steps likely induces plasticity within the lumbosacral spinal cord, leading to functional improvements not only for locomotion but for other circuitries as well (see below), because this spinal cord region contains single neurons having multiple somato-visceral convergent inputs.72,73 A similar training strategy is currently being used in human studies with benefits for multiple functional systems as well.19
As with the human studies, manual assistance is being provided. Unlike robotic systems,33,74 manual assistance allows for variable stepping trajectories and challenges the rats to weight support and control their trunk on their own. While studies aimed at limiting movements (hindlimb immobilization) have a negative impact on locomotor recovery,75 strategies aimed at increasing animal activity through environmental enrichment have also shown improvements for locomotor skills.76,77 Male rats being examined in current studies with control groups exposed to the same environments for equal amounts of time do not exhibit significant improvements in BBB scores and kinematic analysis (Program No. 85.17. Neuroscience Meeting Planner. Society for Neuroscience, 2012).
Step training and bladder function
Detrusor sphincter dyssynergia (bladder and external urethral sphincter [EUS] contracting simultaneously) is a major impediment to efficient voiding post-SCI. This results in large residual volumes predisposing persons with SCI to recurrent urinary tract infections and cystitis. The mechanisms contributing to improved bladder function with step training post-SCI in the current study are unknown, but may include interactions through increased extensor activity and/or somatosensory feedback during stepping. Lower limb extensor/flexor activation, for example, can alter EUS and detrusor muscle activity. Post-SCI, flexion reflexes are overactive78 and may exacerbate detrusor sphincter dyssynergia.4
In the present study, step training was found to significantly increase SOL EMG amplitude. Greater extensor activity, which has been shown to inhibit EUS activity,79,80 may contribute to the measured increase in voiding efficiency by reducing the severity of detrusor sphincter dyssynergia and decreasing urethral resistance. Also, electrical stimulation of the foot or posterior tibial nerve can increase bladder capacity and decrease bladder overactivity in both humans and animals.81–85 Somatosensory feedback during stepping may be similar to foot electrostimulation leading to the measured increase in ICIs. It will be important for subsequent studies to directly explore the impact of training on sphincter and detrusor activity.
Step training and other forms of exercise also regulate neurotrophin levels and may contribute to locomotor and sensory recovery.57,58,60 Step training may improve bladder function through the regulation of NGF or other molecules. Our results demonstrated reduced bladder NGF mRNA in the step trained group (vs. non-trained) while BDNF and NT3 remained unchanged. NGF has long been implicated in bladder dysfunction disorders86 and has been proposed as a potential biomarker screening tool for clinical bladder disorders.87 NGF administration into the normal bladder induces hyperactivity and increases firing rate of dissociated bladder afferents.50 NGF production increases in the bladder after SCI88 and has been proposed to underlie detrusor hyperreflexia and detrusor sphincter dyssynergia.89 A reduction of NGF (or return to baseline levels) via step training would facilitate return of normal bladder function.
Step training and neuropathic pain
Attempts to manage chronic pain and dysesthesia often use exercise therapies.11,12 Greater asymmetrical lesion damage/sparing is associated with abnormal inputs to higher centers, altered responsiveness, and nociceptive-like behaviors.36,37,90–92 Despite similar degrees of VLF damage asymmetry relative to non-trained controls, step trained animals demonstrated a normalized response to mechanical stimulation of at-level dermatomes, indicative of a reduction in hypersensitivity.
Multiple potential underlying mechanisms exist that may explain the step training induced allodynia changes in the present study. For example, immediate and long-term exercise induced analgesia may involve intrinsic mechanisms such as increased endogenous opioid production.93,94 Administration of naloxone, an opioid receptor antagonist, was seen to reverse treadmill exercise associated reductions of mechanical hyperalgesia in a rodent model of chronic muscle pain.95 Pre-treatment with naloxone, ρ-chlorophenylalanine methyl ester (a serotonin synthesis inhibitor), or a bilateral adrenalectomy (adrenals are a source of endogenous opioids in addition to the pituitary) in a model of visceral nociception blocked the exercise induced hyponociception in mice, implicating endogenous opioids and serotonergic mechanisms.96
There appears to be a threshold of intensity and duration of exercise to induce analgesic responses and increase plasma beta-endorphin levels.97 Our results support the hypothesis that long-term exercise (60 min daily for 12 weeks) decreases post-SCI pain. In addition, extended training duration is associated with improvements in both gait and increased patient quality of life and physical function.98
Decreases in at-level allodynia may also result from somatosensory stimulation during step training. Afferent input achieved from loading and rhythmicity of stepping has been shown to successfully influence locomotion and modulate efferent output.99 Treadmill stepping for 60 min has also led to increases of serotonin (5-HT) in laminae III–V, possibly from activation of the nucleus raphe magnus during motor training.100 Serotonergic neurons in certain brainstem nuclei project to the dorsal horn,101 and decreases in serotonin-positive fibers below the level of SCI are associated with neuropathic pain; intrathecal delivery of 5HT can attenuate allodynia.102
Engesser-Cessar and colleagues103 showed that wheel running in SCI mice led to an improvement in locomotion as well as an increase in serotonin-positive fibers below the level of the injury. Serotonin can also inhibit primary afferent evoked inputs in laminae IV–VII104 that project to supraspinal centers through ventral and lateral white matter tracts and may represent a mechanism through which step training may activate descending serotonergic pathways and suppress incoming noxious inputs in an asymmetrical VLF lesion.
Step training and locomotion
For a recent review of LT for the recovery of locomotion, see Battistuzzo and coworkers.105 In this study, step trained contused male rats moderately improved overground walking (BBB) associated with consistent weight bearing steps and degree of forelimb-hindlimb coordination. The non-trained control group maximized their recovery by 6 weeks post-SCI but never reached the levels of the LT group, suggesting that the daily stepping had a significant effect beyond spontaneous recovery attained from WMS.75,106,107 Similar WMS between groups suggests that recovery occurred through mechanisms that facilitated or enhanced remaining pathways. This does not rule out regeneration (short distance or collateral sprouting) or remyelination, however. In addition, it should be considered that LT may have prevented a functional decline of or modified the spinal circuitry below the lesion.108–112
Continued task specific stepping practice is essential to maintaining gait improvements in both humans and animals.113–117 Therefore, other gains achieved from LT (bladder or sensory function) might also diminish once training ceases. Thus, assessing function well beyond the end of training is an important future experiment.
Conclusions
Activity based training can influence urologic and pain outcomes that are important to persons with SCI irrespective of the influence on locomotion that is moderate and dependent on intrinsic weight support capacity. Our results support observations in several recent patient case studies suggesting that LT, such as body weight supported step training, may be beneficial for the alleviation of post-SCI bladder dysfunction and pain and thus justifies further investigation. These novel findings demonstrating multiple improvements in non-locomotor as well as locomotor systems suggest that step training after SCI could translate to significant quality of life gains for persons living with a wide range of functional deficits post-SCI.
Acknowledgments
The authors would like to acknowledge Hui Zhong (UCLA) for her assistance with the EMG electrode surgeries; Darlene Burke for statistical guidance; Chandresh Shah, Krish Gopathi, and Neil Bodduluri for animal training; James Armstrong, Jason Fell, Menka Sanghvi, Yangsheng Chen, Johnny Morehouse, and Matt Nitzken for technical assistance. Supported by grants from the NIH NCRR Grant RR015576 (CHH, JCP, SJH), NRSA Grant F31NS070329 (PJW), Department of Defense (CHH), Owsley B. Frazier Endowment (SJH), and the Kentucky Head and Spinal Cord Injury Research Trust (JCP, SJH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tribe C.R. (1963). Causes of death in the early and late stages of paraplegia. Paraplegia 1, 19–47 [DOI] [PubMed] [Google Scholar]
- 2.Pagliacci M.C., Franceschini M., Di Clemente B., Agosti M., and Spizzichino L. (2007). A multicentre follow-up of clinical aspects of traumatic spinal cord injury. Spinal Cord 45, 404–410 [DOI] [PubMed] [Google Scholar]
- 3.DeVivo M., and Stover S. (1995). Long-term survival and causes of death, in: Spinal cord injury: Clinical outcomes from the model systems. Stover S.L., Delisa J.A., and Whiteneck G.G. (eds). Aspen Publishers, Inc.: Gaithersburg, MD, pps. 289–316 [Google Scholar]
- 4.Tai C., Roppolo J.R., and de Groat W.C. (2006). Spinal reflex control of micturition after spinal cord injury. Restor. Neurol. Neurosci. 24, 69–78 [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 6.Ditunno P.L., Patrick M., Stineman M., and Ditunno J.F. (2008). Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 46, 500–506 [DOI] [PubMed] [Google Scholar]
- 7.Siddall P.J., McClelland J.M., Rutkowski S.B., and Cousins M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 [DOI] [PubMed] [Google Scholar]
- 8.Siddall P.J., and Loeser J.D. (2001). Pain following spinal cord injury. Spinal Cord 39, 63–73 [DOI] [PubMed] [Google Scholar]
- 9.Siddall P.J., Taylor D.A., McClelland J.M., Rutkowski S.B., and Cousins M.J. (1999). Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 81, 187–197 [DOI] [PubMed] [Google Scholar]
- 10.Rintala D.H., Loubser P.G., Castro J., Hart K.A., and Fuhrer M.J. (1998). Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity, and relationship with impairment, disability, handicap, and subjective well-being. Arch. Phys. Med. Rehabil. 79, 604–614 [DOI] [PubMed] [Google Scholar]
- 11.Warms C.A., Turner J.A., Marshall H.M., and Cardenas D.D. (2002). Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin. J. Pain 18, 154–163 [DOI] [PubMed] [Google Scholar]
- 12.Heutink M., Post M.W., Wollaars M.M., and van Asbeck F.W. (2011). Chronic spinal cord injury pain: pharmacological and non-pharmacological treatments and treatment effectiveness. Disabil. Rehabil. 33, 433–440 [DOI] [PubMed] [Google Scholar]
- 13.Barbeau H., and Rossignol S. (1987). Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95 [DOI] [PubMed] [Google Scholar]
- 14.Giuliani C.A., and Smith J.L. (1987). Stepping behaviors in chronic spinal cats with one hindlimb deafferented. J. Neurosci. 7, 2537–2546 [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgson J.A., Roy R.R., de Leon R., Dobkin B., and Edgerton V.R. (1994). Can the mammalian lumbar spinal cord learn a motor task? Med. Sci. Sports Exerc. 26, 1491–1497 [PubMed] [Google Scholar]
- 16.Howland D.R., Bregman B.S., Tessler A., and Goldberger M.E. (1995). Development of locomotor behavior in the spinal kitten. Exp. Neurol. 135, 108–122 [DOI] [PubMed] [Google Scholar]
- 17.Lovely R.G., Gregor R.J., Roy R.R., and Edgerton V.R. (1986). Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 92, 421–435 [DOI] [PubMed] [Google Scholar]
- 18.Michele Basso D., Murray M., and Goldberger M.E. (1994). Differential recovery of bipedal and overground locomotion following complete spinal cord hemisection in cats. Restor. Neurol. Neurosci 7, 95–110 [DOI] [PubMed] [Google Scholar]
- 19.Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G., and Edgerton V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schalow G. (2010). Cure of urinary bladder functions in severe (95%) motoric complete cervical spinal cord injury in human. Electromyogr. Clin. Neurophysiol. 50, 155–179 [PubMed] [Google Scholar]
- 21.Hubscher C.H., and Johnson R.D. (1999). Changes in neuronal receptive field characteristics in caudal brain stem following chronic spinal cord injury. J. Neurotrauma 16, 533–541 [DOI] [PubMed] [Google Scholar]
- 22.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 23.Maggi C.A., Santicioli P., and Meli A. (1986). The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J. Pharmacol. Methods 15, 157–167 [DOI] [PubMed] [Google Scholar]
- 24.Harkema S.J., Hurley S.L., Patel U.K., Requejo P.S., Dobkin B.H., and Edgerton V.R. (1997). Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 77, 797–811 [DOI] [PubMed] [Google Scholar]
- 25.Smith R.R., Brown E.H., Shum-Siu A., Whelan A., Burke D.A., Benton R.L., and Magnuson D.S. (2009). Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J. Neurotrauma 26, 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beres-Jones J.A., and Harkema S.J. (2004). The human spinal cord interprets velocity-dependent afferent input during stepping. Brain 127, 2232–2246 [DOI] [PubMed] [Google Scholar]
- 27.Dietz V. (2011). Quadrupedal coordination of bipedal gait: implications for movement disorders. J. Neurol. 258, 1406–1412 [DOI] [PubMed] [Google Scholar]
- 28.Huang H.J., and Ferris D.P. (2009). Upper and lower limb muscle activation is bidirectionally and ipsilaterally coupled. Med. Sci. Sports Exerc. 41, 1778–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slawinska U., Majczynski H., Dai Y., and Jordan L.M. (2012). The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J. Physiol. 590, 1721–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai L.L., Fong A.J., Otoshi C.K., Liang Y., Burdick J.W., Roy R.R., and Edgerton V.R. (2006). Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J. Neurosci. 26, 10564–10568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidler J.M., and Wall A.E. (2005). Alterations in muscle activation patterns during robotic-assisted walking. Clin. Biomech. (Bristol, Avon) 20, 184–193 [DOI] [PubMed] [Google Scholar]
- 32.Israel J.F., Campbell D.D., Kahn J.H., and Hornby T.G. (2006). Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys. Ther. 86, 1466–1478 [DOI] [PubMed] [Google Scholar]
- 33.Ziegler M.D., Zhong H., Roy R.R., and Edgerton V.R. (2010). Why variability facilitates spinal learning. J. Neurosci. 30, 10720–10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha J., Heng C., Reinkensmeyer D.J., Roy R.R., Edgerton V.R., and De Leon R.D. (2007). Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrauma 24, 1000–1012 [DOI] [PubMed] [Google Scholar]
- 35.Grau J.W., Washburn S.N., Hook M.A., Ferguson A.R., Crown E.D., Garcia G., Bolding K.A., and Miranda R.C. (2004). Uncontrollable stimulation undermines recovery after spinal cord injury. J. Neurotrauma 21, 1795–1817 [DOI] [PubMed] [Google Scholar]
- 36.Hall B.J., Lally J.E., Vukmanic E.V., Armstrong J.E., Fell J.D., Gupta D.S., and Hubscher C.H. (2010). Spinal cord injuries containing asymmetrical damage in the ventrolateral funiculus is associated with a higher incidence of at-level allodynia. J. Pain 11, 864–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubscher C.H., and Johnson R.D. (2006). Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp. Neurol. 197, 177–188 [DOI] [PubMed] [Google Scholar]
- 38.Woolf C.J. (1984). Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain 18, 325–343 [DOI] [PubMed] [Google Scholar]
- 39.Hargreaves K., Dubner R., Brown F., Flores C., and Joris J. (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 [DOI] [PubMed] [Google Scholar]
- 40.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 41.Kuerzi J., Brown E.H., Shum-Siu A., Siu A., Burke D., Morehouse J., Smith R.R., and Magnuson D.S. (2010). Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp. Neurol 224, 178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beare J.E., Morehouse J.R., DeVries W.H., Enzmann G.U., Burke D.A., Magnuson D.S., and Whittemore S.R. (2009). Gait analysis in normal and spinal contused mice using the TreadScan system. J. Neurotrauma 26, 2045–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy R.R., Hutchison D.L., Pierotti D.J., Hodgson J.A., and Edgerton V.R. (1991). EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol. 70, 2522–2529 [DOI] [PubMed] [Google Scholar]
- 44.de Leon R., Hodgson J.A., Roy R.R., and Edgerton V.R. (1994). Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 654, 241–250 [DOI] [PubMed] [Google Scholar]
- 45.Harrison B.J., Flight R.M., Gomes C., Venkat G., Ellis S.R., Sankar U., Twiss J.L., Rouchka E.C., and Petruska J.C. (2014). IB4-binding sensory neurons in the adult rat express a novel 3′ UTR-extended isoform of CaMK4 that is associated with its localization to axons. J. Comp Neurol. 522, 308–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hougland M.T., Harrison B.J., Magnuson D.S., Rouchka E.C., and Petruska J.C. (2013). The transcriptional response of neurotrophins and their tyrosine kinase receptors in lumbar sensorimotor circuits to spinal cord contusion is affected by injury severity and survival time. Front. Physiol 3, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward P.J., and Hubscher C.H. (2012). Persistent polyuria in a rat spinal contusion model. J. Neurotrauma 29, 2490–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kruse M.N., Bray L.A., and de Groat W.C. (1995). Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J. Auton. Nerv. Syst. 54, 215–224 [DOI] [PubMed] [Google Scholar]
- 49.Steers W.D., Kolbeck S., Creedon D., and Tuttle J.B. (1991). Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J. Clin. Invest. 88, 1709–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimura N., and de Groat W.C. (1999). Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J. Neurosci. 19, 4644–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimura N., Erdman S.L., Snider M.W., and de Groat W.C. (1998). Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience 83, 633–643 [DOI] [PubMed] [Google Scholar]
- 52.Zinck N.D., Rafuse V.F., and Downie J.W. (2007). Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 204, 777–790 [DOI] [PubMed] [Google Scholar]
- 53.Dmitrieva N., Shelton D., Rice A.S., and McMahon S.B. (1997). The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78, 449–459 [DOI] [PubMed] [Google Scholar]
- 54.Elliott J., MacLellan A., Saini J.K., Chan J., Scott S., and Kawaja M.D. (2009). Transgenic mice expressing nerve growth factor in smooth muscle cells. Neuroreport 20, 223–227 [DOI] [PubMed] [Google Scholar]
- 55.Guerios S.D., Wang Z.Y., Boldon K., Bushman W., and Bjorling D.E. (2008). Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R111–R122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamb K., Gebhart G.F., and Bielefeldt K. (2004). Increased nerve growth factor expression triggers bladder overactivity. J. Pain 5, 150–156 [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Pinilla F., Ying Z., Opazo P., Roy R.R., and Edgerton V.R. (2001). Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 13, 1078–1084 [DOI] [PubMed] [Google Scholar]
- 58.Ying Z., Roy R.R., Edgerton V.R., and Gomez-Pinilla F. (2005). Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 193, 411–419 [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura N., Bennett N.E., Hayashi Y., Ogawa T., Nishizawa O., Chancellor M.B., de Groat W.C., and Seki S. (2006). Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J. Neurosci. 26, 10847–10855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutchinson K.J., Gomez-Pinilla F., Crowe M.J., Ying Z., and Basso D.M. (2004). Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127, 1403–1414 [DOI] [PubMed] [Google Scholar]
- 61.Cao Q., Zhang Y.P., Iannotti C., DeVries W.H., Xu X.M., Shields C.B., and Whittemore S.R. (2005). Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp. Neurol. 191, Suppl 1, S3–S16 [DOI] [PubMed] [Google Scholar]
- 62.Roy R.R., Zhong H., Khalili N., Kim S.J., Higuchi N., Monti R.J., Grossman E., Hodgson J.A., and Edgerton V.R. (2007). Is spinal cord isolation a good model of muscle disuse? Muscle Nerve 35, 312–321 [DOI] [PubMed] [Google Scholar]
- 63.Cruz Y., and Downie J.W. (2005). Sexually dimorphic micturition in rats: relationship of perineal muscle activity to voiding pattern. Am. J. Physiol. Regul. Integ. Comp. Physiol. 289, R1307–R1318 [DOI] [PubMed] [Google Scholar]
- 64.Farooque M., Suo Z., Arnold P.M., Wulser M.J., Chou C.T., Vancura R.W., Fowler S., and Festoff B.W. (2006). Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord 44, 182–187 [DOI] [PubMed] [Google Scholar]
- 65.Hubscher C.H., Fell J.D., and Gupta D.S. (2010). Sex and hormonal variations in the development of at-level allodynia in a rat chronic spinal cord injury model. Neurosci. Lett. 477, 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang J., Chaloupka E.C., Mastrangelo M.A., and Hoffman J.R. (2002). Physiological and biomechanical analysis of treadmill walking up various gradients in men and women. Eur. J. Appl. Physiol. 86, 503–508 [DOI] [PubMed] [Google Scholar]
- 67.Lacroix-Fralish M.L. (2008). Sex-specific pain modulation: the growth factor, neuregulin-1, as a pro-nociceptive cytokine. Neurosci. Lett. 437, 184–187 [DOI] [PubMed] [Google Scholar]
- 68.Norrbrink Budh C., Lund I., Hultling C., Levi R., Werhagen L., Ertzgaard P., and Lundeberg T. (2003). Gender related differences in pain in spinal cord injured individuals. Spinal Cord 41, 122–128 [DOI] [PubMed] [Google Scholar]
- 69.Sipski M.L., Jackson A.B., Gomez-Marin O., Estores I., and Stein A. (2004). Effects of gender on neurologic and functional recovery after spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1826–1836 [DOI] [PubMed] [Google Scholar]
- 70.Streng T., Santti R., and Talo A. (2002). Similarities and differences in female and male rat voiding. Neurourol. Urodyn. 21, 136–141 [DOI] [PubMed] [Google Scholar]
- 71.Wood K., Wilhelm J.C., Sabatier M.J., Liu K., Gu J., and English A.W. (2012). Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev. Neurobiol. 72, 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berkley K.J., Hubscher C.H., and Wall P.D. (1993). Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J. Neurophysiol. 69, 545–556 [DOI] [PubMed] [Google Scholar]
- 73.Honda C.N. (1985). Visceral and somatic afferent convergence onto neurons near the central canal in the sacral spinal cord of the cat. J. Neurophysiol. 53, 1059–1078 [DOI] [PubMed] [Google Scholar]
- 74.Dominici N., Keller U., Vallery H., Friedli L., van den Brand R., Starkey M.L., Musienko P., Riener R., and Courtine G. (2012). Versatile robotic interface to evaluate, enable and train locomotion and balance after neuromotor disorders. Nat. Med. 18, 1142–1147 [DOI] [PubMed] [Google Scholar]
- 75.Caudle K.L., Brown E.H., Shum-Siu A., Burke D.A., Magnuson T.S.G., Voor M.J., and Magnuson D.S. (2011). Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabil. Neural Repair 25, 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Meeteren N.L., Eggers R., Lankhorst A.J., Gispen W.H., and Hamers F.P. (2003). Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J. Neurotrauma 20, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 77.Lankhorst A.J., ter Laak M.P., van Laar T.J., van Meeteren N.L., de Groot J.C., Schrama L.H., Hamers F.P., and Gispen W.H. (2001). Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J. Neurotrauma 18, 203–215 [DOI] [PubMed] [Google Scholar]
- 78.Hornby T.G., Rymer W.Z., Benz E.N., and Schmit B.D. (2003). Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J. Neurophysiol. 89, 416–426 [DOI] [PubMed] [Google Scholar]
- 79.Jolesz F.A., Ruenzel P.W., and Henneman E. (1988). Reflex inhibition of urethral sphincters to permit voiding in paraplegia. Arch. Neurol. 45, 38–40 [DOI] [PubMed] [Google Scholar]
- 80.Jolesz F.A., Cheng-Tao X., Ruenzel P.W., and Henneman E. (1982). Flexor reflex control of the external sphincter of the urethra in paraplegia. Science 216, 1243–1245 [DOI] [PubMed] [Google Scholar]
- 81.Chen G., Larson J.A., Ogagan P.D., Shen B., Wang J., Roppolo J.R., de Groat W.C., and Tai C. (2012). Post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves in the foot. J. Urol. 187, 338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McGuire E.J., Zhang S.C., Horwinski E.R., and Lytton B. (1983). Treatment of motor and sensory detrusor instability by electrical stimulation. J. Urol. 129, 78–79 [DOI] [PubMed] [Google Scholar]
- 83.Peters K.M., Macdiarmid S.A., Wooldridge L.S., Leong F.C., Shobeiri S.A., Rovner E.S., Siegel S.W., Tate S.B., Jarnagin B.K., Rosenblatt P.L., and Feagins B.A. (2009). Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J. Urol. 182, 1055–1061 [DOI] [PubMed] [Google Scholar]
- 84.Tai C., Chen M., Shen B., Wang J., Roppolo J.R., and de Groat W.C. (2011). Irritation induced bladder overactivity is suppressed by tibial nerve stimulation in cats. J. Urol. 186, 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tai C., Shen B., Chen M., Wang J., Liu H., Roppolo J.R., and de Groat W.C. (2011). Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int. 107, 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steers W.D., and Tuttle J.B. (2006). Mechanisms of Disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat. Clin. Pract. Urol. 3, 101–110 [DOI] [PubMed] [Google Scholar]
- 87.Liu H.T., and Kuo H.C. (2009). Urinary nerve growth factor levels are elevated in patients with overactive bladder and do not significantly increase with bladder distention. Neurourol. Urodyn. 28, 78–81 [DOI] [PubMed] [Google Scholar]
- 88.Vizzard M.A. (2000). Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 161, 273–284 [DOI] [PubMed] [Google Scholar]
- 89.de Groat W.C., and Yoshimura N. (2006). Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 152, 59–84 [DOI] [PubMed] [Google Scholar]
- 90.Gerke M.B., Duggan A.W., Xu L., and Siddall P.J. (2003). Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience 117, 715–722 [DOI] [PubMed] [Google Scholar]
- 91.Wang G., and Thompson S.M. (2008). Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J. Neurosci. 28, 11959–11969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao P., Waxman S.G., and Hains B.C. (2007). Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J. Neurosci. 27, 8893–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koltyn K.F. (2000). Analgesia following exercise: a review. Sports Med. 29, 85–98 [DOI] [PubMed] [Google Scholar]
- 94.Stagg N.J., Mata H.P., Ibrahim M.M., Henriksen E.J., Porreca F., Vanderah T.W., and Philip Malan T., Jr. (2011). Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology 114, 940–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bement M.K., and Sluka K.A. (2005). Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch. Phys. Med. Rehabil. 86, 1736–1740 [DOI] [PubMed] [Google Scholar]
- 96.Mazzardo-Martins L., Martins D.F., Marcon R., Dos Santos U.D., Speckhann B., Gadotti V.M., Sigwalt A.R., Guglielmo L.G. and Santos A.R. (2010). High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J. Pain 11, 1384–1393 [DOI] [PubMed] [Google Scholar]
- 97.Schwarz L., and Kindermann W. (1992). Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 13, 25–36 [DOI] [PubMed] [Google Scholar]
- 98.Hicks A.L., Adams M.M., Martin Ginis K., Giangregorio L., Latimer A., Phillips S.M., and McCartney N. (2005). Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 43, 291–298 [DOI] [PubMed] [Google Scholar]
- 99.Dietz V., and Harkema S.J. (2004). Locomotor activity in spinal cord-injured persons. J. Appl. Physiol. 96, 1954–1960 [DOI] [PubMed] [Google Scholar]
- 100.Gerin C., Teilhac J.R., Smith K., and Privat A. (2008). Motor activity induces release of serotonin in the dorsal horn of the rat lumbar spinal cord. Neurosci. Lett. 436, 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Basbaum A.I., and Fields H.L. (1984). Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann. Rev. Neurosci. 7, 309–338 [DOI] [PubMed] [Google Scholar]
- 102.Hains B.C., Everhart A.W., Fullwood S.D., and Hulsebosch C.E. (2002). Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp. Neurol. 175, 347–362 [DOI] [PubMed] [Google Scholar]
- 103.Engesser-Cesar C., Ichiyama R.M., Nefas A.L., Hill M.A., Edgerton V.R., Cotman C.W., and Anderson A.J. (2007). Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 25, 1931–1939 [DOI] [PubMed] [Google Scholar]
- 104.Shay B.L., and Hochman S. (2002). Serotonin alters multi-segmental convergence patterns in spinal cord deep dorsal horn and intermediate laminae neurons in an in vitro young rat preparation. Pain 95, 7–14 [DOI] [PubMed] [Google Scholar]
- 105.Battistuzzo C.R., Callister R.J., Callister R., and Galea M.P. (2012). A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J. Neurotrauma 29, 1600–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Leon R.D., Hodgson J.A., Roy R.R., and Edgerton V.R. (1998). Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 79, 1329–1340 [DOI] [PubMed] [Google Scholar]
- 107.Fouad K., Metz G.A., Merkler D., Dietz V., and Schwab M.E. (2000). Treadmill training in incomplete spinal cord injured rats. Behav. Brain Res. 115, 107–113 [DOI] [PubMed] [Google Scholar]
- 108.Cote M.P., and Gossard J.P. (2004). Step training-dependent plasticity in spinal cutaneous pathways. J. Neurosci. 24, 11317–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cote M.P., Menard A., and Gossard J.P. (2003). Spinal cats on the treadmill: changes in load pathways. J. Neurosci. 23, 2789–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ichiyama R.M., Courtine G., Gerasimenko Y.P., Yang G.J., van den Brand R., Lavrov I.A., Zhong H., Roy R.R. and Edgerton V.R. (2008). Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 28, 7370–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petruska J.C., Ichiyama R.M., Jindrich D.L., Crown E.D., Tansey K.E., Roy R.R., Edgerton V.R., and Mendell L.M. (2007). Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 27, 4460–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dietz V. (2012). Neuronal plasticity after a human spinal cord injury: positive and negative effects. Exp. Neurol. 235, 110–115 [DOI] [PubMed] [Google Scholar]
- 113.De Leon R.D., Hodgson J.A., Roy R.R., and Edgerton V.R. (1999). Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 81, 85–94 [DOI] [PubMed] [Google Scholar]
- 114.Magnuson D.S., Smith R.R., Brown E.H., Enzmann G., Angeli C., Quesada P.M., and Burke D. (2009). Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil. Neural Repair 23, 535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singh A., Balasubramanian S., Murray M., Lemay M., and Houle J. (2011). Role of spared pathways in locomotor recovery after body-weight-supported treadmill training in contused rats. J. Neurotrauma 28, 2405–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wernig A., Nanassy A., and Müller S. (1998). Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord 36, 744–749 [DOI] [PubMed] [Google Scholar]
- 117.Wirz M., Colombo G., and Dietz V. (2001). Long term effects of locomotor training in spinal humans. J. Neurol. Neurosurg. Psychiatry 71, 93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]