Abstract
Background: The hyperinsulinemic euglycemic clamp is the gold standard for assessment of insulin resistance and requires frequent, accurate measurements of blood glucose concentrations, typically utilizing the YSI 2300 STAT Plus™ glucose analyzer (YSI, Inc., Yellow Springs, OH). Despite its accuracy, the YSI has several limitations, including its cost, lengthy run time, need for trained personnel, frequent maintenance, and large blood volumes. Simpler hospital-grade hand-held glucose meters are now available but have not been validated for use in pediatric clamp settings. Our objective was to evaluate the accuracy, precision, and reliability of the StatStrip® (SS) hospital glucose monitoring system (Nova Biomedical, Waltham, MA) relative to the YSI 2300 STAT glucose analyzer in pediatric hyperinsulinemic euglycemic clamps.
Subjects and Methods: Four hundred sixty blood specimens drawn from 11 pediatric patients undergoing hyperinsulinemic euglycemic clamps were simultaneously analyzed by SS and YSI. Outcome measures included SS bias relative to YSI and glucose measurement precision on SS and YSI.
Results: The SS showed a slight positive bias of 0.75±2.83 mg/dL versus the YSI. Percentage coefficients of variance for SS and YSI were 9.53% and 9.25%, respectively. Using a Bland–Altman plot, the limits of agreement were ±5.7 mg/dL. The coefficient of repeatability for SS was 6.63; the coefficient of individual agreement between the YSI and SS was 0.995.
Conclusions: The SS is a suitable replacement for the YSI in pediatric hyperinsulinemic euglycemic clamp studies, is easier to use, more cost-effective, and faster, and requires less blood. Future euglycemic clamp studies can consider utilizing this methodology.
Introduction
Insulin resistance is thought to increase risk for the development of several comorbidities, including cardiovascular disease, type 2 diabetes mellitus (T2DM), hypertension, polycystic ovarian syndrome (PCOS), sleep apnea, and nonalcoholic fatty liver disease. Insulin resistance is also increasingly recognized as being important in type 1 diabetes mellitus (T1DM).1 Youth with T2DM and T1DM are more insulin resistant than youth without diabetes of similar body mass index.2,3 Obesity, an increasingly common problem in pediatrics, also increases insulin resistance. Finally, insulin resistance rises during puberty in all adolescents, secondary to increases in levels of growth hormone and sex steroids characteristic of puberty.4 Thus, insulin resistance assessments are increasingly required in pediatric research studies.
The most rigorous method used to assess insulin resistance in a research setting is the hyperinsulinemic euglycemic clamp. The euglycemic clamp typically requires glucose readings every 5–10 min for several hours. Blood glucose concentrations during the euglycemic clamp are most commonly measured with the YSI 2300 STAT Plus™ glucose and lactate analyzer (YSI, Inc., Yellow Springs, OH), considered to be a gold standard method. The YSI analyzer utilizes a steady-state measurement methodology, based on the glucose oxidase technique (YSI STAT 2300 Plus laboratory manual). However, the YSI requires relatively large sample volumes (0.5 mL per sample), which over the length of a clamp can create blood volume problems, especially in pediatric studies. In addition, the YSI requires extensive training to use and maintain, significant set-up time to allow the machine to warm-up and calibrate, often requires centrifugation of each blood sample prior to analysis, and takes 3–4 min to obtain results. These limitations decrease efficiency and increase cost.
A point-of-care glucose meter, using modified glucose oxidase–based amperometric test strip technology to measure whole blood glucose, has recently been introduced (StatStrip® [SS] hospital glucose monitoring system; Nova Biomedical, Waltham, MA). The SS measures whole blood glucose concentrations in 6 s from a 1.2-μL sample of venous, arterial, or capillary whole blood. Previous studies have demonstrated acceptable accuracy and reliability of the SS for the point-of-care measurement of glucose in adult5,6 and neonatal7,8 settings. In addition, one study assessed the performance during adult combined hyperinsulinemic euglycemic and hyperglycemic clamps9 and concluded that the SS demonstrated acceptable performance. However, the accuracy of the SS meter compared with the YSI analyzer has not previously been reported in a pediatric population outside of the setting of neonatal hypoglycemia. Our goal was to assess the accuracy, precision, and reliability of the SS relative to the YSI, in youth with and without obesity and diabetes during a three-stage inpatient hyperinsulinemic euglycemic clamp, in order to determine the suitability of widespread adoption in pediatric clamp studies.
Subjects and Methods
Eleven three-stage hyperinsulinemic euglycemic clamps (10, 16, and 80 mU of insulin/m2/min) were performed as previously described3 in adolescents with normal weight, obesity, T1DM, T2DM, and PCOS. Informed consent and/or assent as appropriate was obtained in all participants as per local Institutional Review Board guidelines. Subjects were screened with a complete blood count and excluded if anemia was present. Participants with diabetes were taking insulin and/or metformin, and obese and PCOS participants were taking no medications. Regular insulin was administered intravenously to assess the insulin resistance in adipose, hepatic, and skeletal muscle tissue. Intravenous glucose concentrations were measured every 5 min for approximately 4.5 h to titrate a 20% dextrose infusion, with a euglycemic goal of 95 mg/dL throughout each clamp. At each time point, 0.5 mL of blood was drawn into a syringe, and approximately 0.3–0.4 mL was placed in a 1-mL heparinized microcentrifuge tube and inverted several times. The microcentrifuge tube was centrifuged for at least 30 s, and the plasma glucose concentration was measured in duplicate by the YSI using the two electrodes. The remaining blood in the syringe was immediately placed on a glucose monitoring test strip, and the whole blood glucose concentration was measured by the SS in triplicate. During one clamp, additional whole blood glucose concentrations were measured by the YSI in duplicate. Both devices were properly maintained and calibrated according to the manufacturer's instructions.
The YSI was used as the reference method to determine the accuracy of the SS. The values of the SS and YSI were averaged and paired based on time point. Time points lacking a complete measurement set (n=2 for YSI and n=3 for SS) were excluded so that a valid comparison could be made between the SS and YSI. At each time point the mean YSI result and the mean SS result were calculated. The mean, SD, coefficient of variation, median, 25th percentile, and 75th percentile were calculated for the mean SS result and the mean YSI result across the entire dataset. To visually inspect the concordance between SS and YSI, mean results for each were plotted on an x-y scatter plot with a superimposed line of identity. Bland–Altman plots were constructed to assess the bias between SS and YSI. In addition, a modified Bland–Altman plot was constructed to graphically assess the performance of SS relative to YSI within a ±5 mg/dL error limit. The coefficient of repeatability was calculated as described by Bland and Altman.10 The coefficient of individual agreement (ΨNMSD) was calculated as described by Haber and Barnhart.11 Statistical analyses were performed using SAS software (version 9.2 of the SAS System for Windows; SAS Institue, Cary, NC). Data are presented as mean±SD values unless otherwise specified.
Results
In total, 460 paired glucose values from matched time points were obtained from 11 participants undergoing hyperinsulinemic euglycemic clamps. Descriptive characteristics of the participants appear in Table 1. All 11 participants were female with an average age of 16.0±1.6 years and an average body mass index of 33.2±6.6 kg/m2. Of the 11 patients, seven had PCOS, two had T2DM, one had T1DM, and one patient was an obese control.
Table 1.
Characteristics of 11 Female Participants Undergoing Hyperinsulinemic Euglycemic Clamp Studies
| Disease category | Number of participants | Age (years) | BMI (kg/m2) | Hct (%) |
|---|---|---|---|---|
| Obese control | 1 | 17 | 35.2 | 43.5 |
| T1DM | 1 | 18 | 29.6 | 39.6 |
| T2DM | 2 | 17 | 33.2 | 39.3 |
| PCOS | 7 | 15.3 | 33.5 | 42.5 |
| Overall | 11 | 16.0±1.6 | 33.2±6.6 | 41.5 |
BMI, body mass index; Hct, hematocrit; PCOS, polycystic ovarian syndrome; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Descriptive statistics of blood glucose measurements made by the SS and YSI appear in Table 2. The SS showed a slight positive mean bias of 0.75±2.83 mg/dL (range, −6.50 to +14.9 mg/dL). Coefficients of variation were also determined for the SS and YSI, calculated at 9.53 mg/dL and 9.25 mg/dL, respectively. In addition, the coefficient of repeatability and ΨNMSD of the SS were calculated for precision analysis. As the average difference for paired measurements on the SS (0.18±3.31) was not significantly different from zero, a coefficient of repeatability for the SS was calculable and found to be 6.63. The estimated value of the ΨNMSD for the SS and YSI methods was 0.995 (95% confidence interval, 0.978–1.012). This indicates that disagreement between the two methods will not exceed the disagreement between replicated observations using the same method by more than 1%.
Table 2.
Overall Mean StatStrip and YSI Analyzer Blood Glucose Readings
| Mean | SD | Median | 25th percentile | 75th percentile | CV | ΨNMSD | CoR | |
|---|---|---|---|---|---|---|---|---|
| Mean SS (mg/dL) | 96.5 | 9.20 | 95.7 | 91.0 | 101.0 | 9.53 | 0.995 | 6.63 |
| Mean YSI (mg/dL) | 95.7 | 8.85 | 94.7 | 90.7 | 99.9 | 9.25 | — | — |
| Bias (mean SS – mean YSI) | 0.75 | 2.83 | 0.5 | −1.20 | 2.50 | — | — |
A dash indicates not applicable.
ΨNMSD, coefficient of individual agreement; CoR, coefficient of repeatability; CV, coefficient of variation; SS, StatStrip.
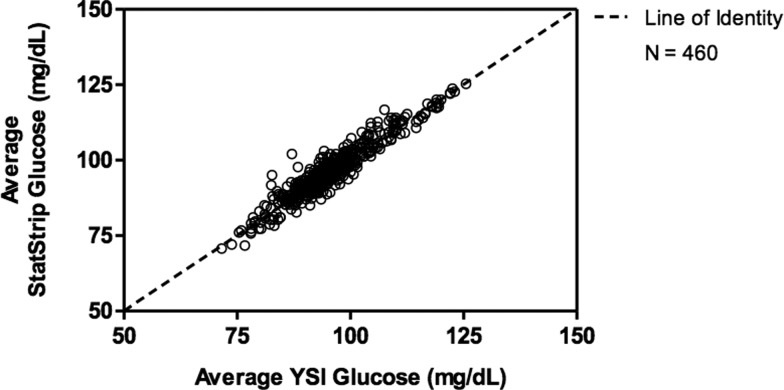
Figure 1 is a scatter plot of the SS triplicate average against the average of the YSI electrode measurements. There is a 45° line of identity superimposed on the plot, showing where the points would fall if the YSI and the SS measures were always in agreement. More of the samples fell above the line of identity, indicating that the SS value tends to be higher than the YSI value.
FIG. 1.
Scatter plot of the average StatStrip versus YSI glucose results.
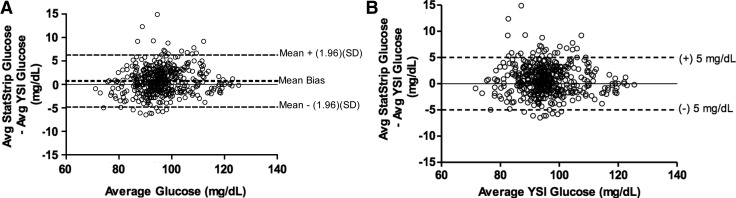
A Bland–Altman plot demonstrating the bias observed between the average SS glucose result and the average YSI result relative to the average glucose concentration across both SS and YSI appears in Figure 2A. The horizontal lines on the plot represent the 95% limits of agreement (mean±1.96 [SD]) for the mean difference between the YSI and SS measures. As seen in Figure 2A, the 95% limits of agreement were between −4.8 mg/dL and 6.3 mg/dL. To assess how many data points fell within ±5 mg/dL of the mean difference between the YSI and SS, a difference that could lead to dextrose infusion rate changes during a hyperinsuliemic euglycemic clamp, a modified Bland–Altman plot showing the bias between the average SS results and the average YSI results relative to the average YSI results appears in Figure 2B with modified horizontal lines corresponding to ±5 mg/dL. Ninety-four percent of SS values fell within a ±5 mg/dL error limit of the average YSI result.
FIG. 2.
(A) Bland–Altman plot demonstrating the bias observed between the average (Avg) StatStrip and YSI results relative to the average glucose concentration across both StatStrip and YSI datasets (n=460). The mean bias observed was 0.73 mg/dL, and the 95% limits of agreement defined as the mean ±1.96 SD were between −4.8 and 6.3 mg/dL. (B) Modified Bland–Altman plot showing the bias between the Avg StatStrip and YSI results relative to the Avg YSI results (n=460). Note that 94% of the results fell within a ±5 mg/dL error limit.
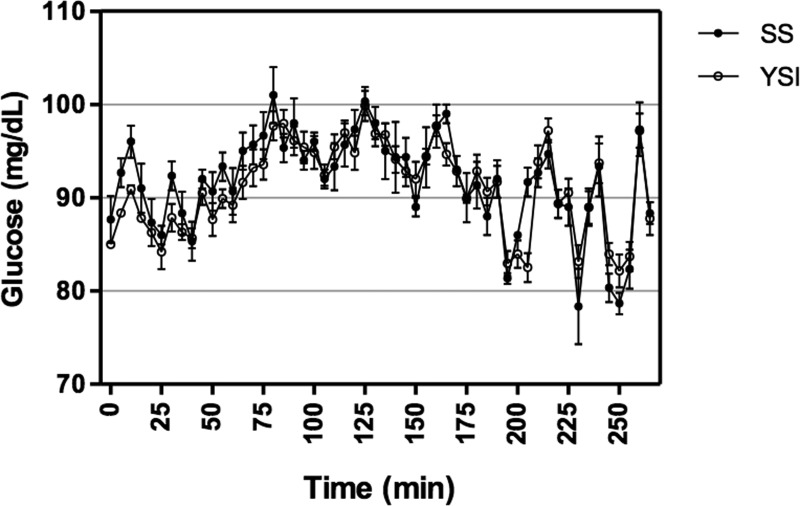
A representative plot of YSI and SS glucose readings from a participant undergoing a hyperinsulinemic euglycemic clamp is shown in Figure 3. Initial discrepancies between the SS and YSI at the beginning of the euglycemic clamp minimize with time. Despite the small positive bias of the SS, the overall peaks and troughs of the SS and YSI data coincide.
FIG. 3.
Representative participant plot showing the mean±SD blood glucose measurements for the StatStrip (SS) and YSI over the course of a hyperinsulinemic euglycemic clamp.
Discussion
Blood glucose measurements that are rapid, accurate, and reliable are required in a hyperinsulinemic euglycemic clamp setting in order to assess tissue sensitivity to insulin and to maintain patient safety. The YSI analyzer has been considered the gold standard for blood glucose concentration measurements in a research setting, but its limitations—including its requirement of dedicated personnel, frequent calibrations, a 3–4-min run-time, and cost—have led investigators to explore point-of-care glucose meters as a replacement for the YSI analyzer, but older versions were not felt to be adequate. In our hands, the recently introduced hospital-grade SS hand-held device performed well but demonstrated a slight positive bias relative to the YSI in the setting of a euglycemic clamp. The ΨNMSD also indicated that the agreement between the YSI and SS is better than acceptable. Previous glucose clamp studies (combination hyperinsulinemic euglycemic and hyperglycemic clamps) in adults by Rabiee et al.9 similarly reported a positive bias, although their bias was greater at 8.5 mg/dL.
One concern of using point-of-care glucose meters arises from error introduced by variations in hematocrit.12 Both the YSI and SS utilize biosensors that are a function of the molality of glucose; however, blood glucose measurements are normally reported in concentration units of molarity. In plasma, the discrepancy between molality and molarity, due to proteins and lipids displacing water within the plasma, is negligible. However, in whole blood, hemoglobin within erythrocytes displaces a large volume of water, increasing the difference between molality and molarity values.13 Thus, there is a discrepancy between glucose measurements made in plasma and whole blood that has been previously reported as approximately 10–15%.14,15 To address this concern, the International Federation of Clinical Chemistry and Laboratory Medicine recommended in 2001 that point-of-care glucose devices report plasma-equivalent concentrations of glucose using a conversion factor of 1.11 to convert whole blood glucose concentrations to plasma-equivalent concentrations.13 To evaluate the accuracy of this correction factor in our data, blood glucose readings obtained from the YSI analyzer using whole blood were multiplied by 1.11 and compared with plasma YSI readings from the same samples. The corrected results from the whole blood YSI reading were highly correlated with the plasma YSI values (Pearson coefficient of 0.998, P≤0.05 [data not shown]). The manufacturer states SS measures and corrects for hematocrit interference by measuring hematocrit in one well and correcting for hematocrit levels in its blood glucose reading.16 Numerous studies have supported this claim, including a recent study by Hopf et al.12 demonstrating that SS is >98% accurate compared with the YSI when used over a hematocrit range of 0.19–0.43 in an intensive care unit setting. Acceptable accuracy was defined in Hopf et al.12 using the ISO15197:2003 criteria; of all the point-of-care devices measured, SS showed the best absolute accuracy.
Findings by previous researchers have reported less precision in the SS measurement compared with the YSI measurement of glucose during adult clamp studies.9 Indeed, the coefficient of variance for data obtained by SS was greater than that for the data collected by the YSI. However, the calculated coefficient of repeatability for the SS was acceptable—indicating that 95% of repeated blood glucose readings on SS will not vary by more than 6.63 mg/dL. Although the manufacturer designed the SS to accept blood samples both from skin punctures and from syringes, our observations suggest that some of the variability seen with the SS may be due to the rate and the angle at which the blood is absorbed by the test strip from the syringe. We also observed instances where blood clotted or was not appropriately absorbed into the test strip well. Despite these observations, the larger variability does not limit the use of the SS because it is possible to perform triplicate readings if two readings disagree by more than 5 mg/dL in far less time than it takes to obtain one measurement from the YSI.
Clamp studies require clinical judgment of glucose trends across sequential time points. To assess what impact using the SS has on clinical decision making in a clamp setting, a modified Bland–Altman plot was analyzed to see what percentage of data points fell within a clinically acceptable range, using the YSI as the reference. A range of ±5 mg/dL was chosen as clinically acceptable, as a change of 5 mg/dL above or below the last glucose reading would likely influence the outcome by prompting a change in the dextrose infusion rate. Ninety-four percent of average SS results were within ±5 mg/dL of the YSI average, indicating that SS shows good agreement with YSI values. It should be noted that if the bias of the SS relative to the YSI is known by an investigator, then the investigator can take this factor into account in deciding target glucose concentrations. A limitation, however, is that the glucose infusion rates were adjusted based on the YSI, and not directly from an equivalent clamp using the SS.
Although our study validated the use of the SS in a pediatric population at normal blood sugar levels, we cannot comment on the accuracy and reliability of the SS relative to the YSI at hypoglycemic or hyperglycemic ranges. Our future studies will include assessment in the hyperglycemic range in the setting of hyperglycemic clamps. An additional limitation is that our participants were without anemia and potential interfering medications, limiting the ability to generalize to a more abnormal population.
In summary, compared with the YSI, the SS meter offers the advantages of glucose readings in less time, user-friendly and portable operation, lower cost, and, of particular interest in pediatric populations, lower blood volume samples. Approximately 30 mL of blood per participant was required to perform our reported YSI measurements, as opposed to approximately 6 mL per participant to perform the SS measurements. Additionally, using the YSI costs at our institution the SS saved approximately $240 per clamp. Our study is unique in that it assesses the accuracy, precision, and reliability of the SS in hyperinsulinemic euglycemic clamps in youth and provides data in a wide variety of pediatric patients—including patients with T1D, T2D, and PCOS and obese control participants. Based on its successful performance, the SS is an acceptable substitute for the YSI in pediatric hyperinsulinemic euglycemic clamp settings.
Acknowledgments
The authors would like to thank Nova Biomedical Corporation for providing the glucose testing equipment and supplies for this research. Additionally, we thank the research volunteers for their participation, the CTRC nursing staff, and CHC laboratory staff for making these studies possible. K.J.N. is supported by grants 7-11-CD-08 from the American Diabetes Association, R56DK088971 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, JDRF5-2008-291 from the JDRF, Adult CTRC MO1-RR00051 from the National Institutes of Health, and Pediatric CTRC MO1 RR00069 from the National Institutes of Health. M.C.-G. is supported by grant CTSI TL1 RR02577 from the University of Colorado, a mentored grant from the Thrasher Pediatric Research Foundation, and grant T32 DK063687 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. K.C. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Summer Medical Student Research Program. K.A.L. was supported by the University of Colorado Pediatric Summer Student Research Program.
Author Disclosure Statement
K.A.L., K.C., A.W., L.P., M.C.-G., and K.J.N. declare no competing interests exist. T.S.I. was previously employed by Nova Biomedical (Waltham, MA).
K.A.L. and K.J.N. researched data, wrote and reviewed/edited the manuscript, and contributed to the discussion. K.C. researched data, wrote the manuscript, and contributed to the discussion. A.W. and L.P. researched data, reviewed/edited the manuscript, and contributed to the discussion. T.S.I. and M.C.-G. reviewed/edited the manuscript and contributed to the discussion.
References
- 1.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diel C: Report of the National Heart, Lung, and Blood Institute–National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 2005;111:3489–3493 [DOI] [PubMed] [Google Scholar]
- 2.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE: Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG: Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab 2009;94:3687–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV: Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215–219 [DOI] [PubMed] [Google Scholar]
- 5.Chan PC, Rozmanc M, Seidon-Long I, Kwan J: Evaluation of a point-of-care glucose meter for general use in complex tertiary care facilities. Clin Biochem 2009;42:1104–1112 [DOI] [PubMed] [Google Scholar]
- 6.Karon BS, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, Presti S, Santrach PJ: Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther 2008;10:111–120 [DOI] [PubMed] [Google Scholar]
- 7.Kitsommart T, Ngerncham S, Wongsiridej P, Kolatat T, Jirapaet KS, Paes B: Accuracy of the StatStrip versus SureStep Flexx glucose meter in neonates at risk of hypoglycemia. Eur J Pediatr 2013;172:1181–1186 [DOI] [PubMed] [Google Scholar]
- 8.Ngerncham S, Piriyanimit S, Kolatat T, Wongsiridej P, Inchgarm L, Kitosommart R, Vutrapongwatana P, Jeerapaet K: Validity of two point of care glucometers in the diagnosis of neonatal hypoglycemia. Indian Pediatr 2012;49:621–625 [DOI] [PubMed] [Google Scholar]
- 9.Rabiee A, Magruder JT, Grant C, Salas-Carrillo R, Gillette A, DuBois J, Shannon RP, Anderson DK, Elahi D: Accuracy and reliability of the Nova StatStrip® glucose meter for real-time blood glucose determinations during glucose clamp studies. J Diabetes Sci Technol 2010;4:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 11.Haber M, Barnhart HX: A general approach to evaluating agreement between two observers or methods of measurement from quantitative data with replicated measurements. Stat Methods Med Res 2008;17:151–169 [DOI] [PubMed] [Google Scholar]
- 12.Hopf S, Graf B, Grubner M: Comparison of point-of-care testing glucose results from intensive care patients measured with network-ready devices. Diabetes Technol Ther 2011;13:1047–1056 [DOI] [PubMed] [Google Scholar]
- 13.Lyon ME, Lyon AW: Predicted discrepancies between direct reading whole blood biosensors and central lab plasma methods: predicting and avoiding medical error. Presented at the 23rd International AACC CPOCT Symposium, Boston, MA, September22–25, 2010 [Google Scholar]
- 14.Caraway WT, Watts NB: Carbohydrates. In: Tietz E, ed.Textbook of Clinical Chemistry. Philadelphia: W.B. Saunders Co., 1986: 784–789 [Google Scholar]
- 15.Burrin JM, Alberti KG: What is blood glucose: can it be measured? Diabet Med 1990;7:199–206 [DOI] [PubMed] [Google Scholar]
- 16.Kost GJ, Tran NK, Louie RF, Gentile NL, Abad VJ: Assessing the performance of handheld glucose testing for critical care. Diabetes Technol Ther 2008;10:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]