Abstract
Progesterone is an anti-inflammatory and promyelinating agent after spinal cord injury, but its effectiveness on functional recovery is still controversial. In the current study, we tested the effects of chronic progesterone administration on tissue preservation and functional recovery in a clinically relevant model of spinal cord lesion (thoracic contusion). Using magnetic resonance imaging, we observed that progesterone reduced both volume and rostrocaudal extension of the lesion at 60 days post-injury. In addition, progesterone increased the number of total mature oligodendrocytes, myelin basic protein immunoreactivity, and the number of axonal profiles at the epicenter of the lesion. Further, progesterone treatment significantly improved motor outcome as assessed using the Basso-Bresnahan-Beattie scale for locomotion and CatWalk gait analysis. These data suggest that progesterone could be considered a promising therapeutical candidate for spinal cord injury.
Key words: : CatWalk, oligodendrocytes, progesterone, spare white matter, spinal cord injury
Introduction
After spinal cord injury (sci), primary mechanical insult is followed by the activation of a secondary cascade of events that ultimately causes progressive degeneration of the neural tissue. These secondary events include vascular abnormalities, ischemia-reperfusion, glutamate excitotoxicity, neuronal and oligodendrocyte death, oxidative cell injury, glial reactivity, axonal loss, and a robust inflammatory response.1–3
Effective treatments should ideally act on part or all of those events to finally spare tissue and preserve axonal tracts, oligodendrocytes, and myelin sheaths. No gold standard therapy for SCI has been established,4–6 although clinical trials with methylprednisolone (NASCIS II and III) have demonstrated modest therapeutic benefits.7,8
Progesterone (PROG) could represent a good candidate for therapy, because it is neuroprotective, promyelinating, and anti-inflammatory in pathologies of peripheral and central nervous systems.9–12
Specifically, in experimental brain trauma, PROG reduces edema and inflammatory cytokines, prevents neuronal loss and mitochondrial dysfunction, and improves functional outcomes.13–15 This has permitted the development of two Phase II clinical trials that have recently shown significant improvements in patients with traumatic brain injury receiving PROG.16–18
In our previous studies, we demonstrated beneficial effects of PROG after complete section of spinal cord: it restores the expression levels of several molecular markers, subsides chromatolysis in motoneurons,19,20 enhances the differentiation of oligodendrocyte precursor cells by increasing the expression of pro-oligodendrogenic factors such as Olig2 and Nkx2.2,21 and decreases the activation and proliferation of astrocytes and microglial cells.22
In this study, we have assessed histological and functional effect of PROG with multiple approaches, including magnetic resonance imaging (MRI), stereological cell counting, open field based Basso-Bresnahan-Beattie (BBB) scale for locomotion, CatWalk gait analysis, Hargreaves plantar test, and dynamic Von Frey.
Our investigation provided compelling evidence that PROG significantly improved tissue preservation and functional outcome after spinal cord contusion. These results showed that PROG could be considered a strong candidate for the therapy of SCI, beause it has been considered for traumatic brain injury.
Methods
Animals
Young adult male Wistar rats (300–335 g, 12 weeks old) obtained from Harlan-interfauna Ibérica (Barcelona, Spain) were maintained in a 12:12 h light:dark cycle, and received food and water ad libitum. Rats were handled in accordance with the guidelines published in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, the principles laid out in the Guidelines for the Use of Animals in Neuroscience Research published by the Society for Neuroscience, and European Union guidelines (Council Directive 86/609/EEC). Experimental procedures were approved by the Ethical Committee for Animal Welfare at the National Paraplegics Hospital (CEBA). Special care was taken to use the minimum number of animals needed for statistical accuracy.
SCI
Male Wistar rats were submitted to a moderate-severe contusive spinal cord injury as described previously.23,24 Briefly, animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (45 mg/kg, Normon Veterinary Division, Madrid, Spain) and Xilagesic (2% xylazine, 10 mg/kg, Calier, Barcelona, Spain). Once the absence of reflexes had been checked, the rats were injected with a low dose of atropine (50 μg/kg body weight; Brown Medical, Barcelona, Spain) to reduce salivary and bronchial secretions and to avoid the induction of bradycardia and possible cardiac arrest by the surgery or xylazine. Artificial tears were applied to the eyes to prevent corneal abrasion and infection.
We performed a laminectomy of T8 vertebra, and the vertebral column was stabilized by clamping spiny processes of T7 and T9 vertebrae. Spinal cord contusion was performed with the Infinite Horizon™ device (Precision Systems and Instrumentation, Lexington, KY), applying a force of 200 Kdyn without any additional dwell time.25 Force and displacement curves generated by the Infinite Horizon™ were checked to confirm that injuries were performed with similar intensities and profiles, showing no artifacts indicative of an erroneous/abnormal lesion, therefore ensuring consistency and reproducibility of the injury. This method enabled us to reduce the number of lesioned animals to a minimum, avoiding animal suffering without compromising the statistical accuracy. The injury produced by this method is equivalent to that described as moderate-severe by other groups.26,27 Sham operated animals received the same protocol of laminectomy but without contusion.
Postoperative care included a subcutaneous injection of Buprex (buprenorphine, 0.05 mg/kg; Schering Plough, Madrid, Spain) and a prophylactic subcutaneous antibiotic injection 1 h after the lesion and on the following day (Baytril, Enrofloxacine, 1 mg/kg; Bayer, Kiel, Germany). The animals were fed with wet extruded rodent food, and manual bladder expression was used until they were self-voiding (within 10 days). The animals were monitored for hydration and eventual infections until the end of the experiment.
PROG treatment
Injured animals received daily subcutaneous injections of natural PROG (16 mg/kg/day, Sigma Aldrich, n=8, SCI+PROG group) or vehicle (castor oil, Sigma Aldrich, n=8, SCI group) for 60 days until sacrifice. PROG was given to awake animals 1 h after injury. This dose of PROG has been shown to prevent edema and neuronal loss and to improve cognitive responses after brain-contusion injury,28 to induce oligodendrogenesis and remyelination after spinal cord injury,21,29 and to decrease reactive gliosis.22 Sham-operated rats (n=8, CTL) received a laminectomy but not SCI.
Magnetic resonance data acquisition and analysis
Rats were anesthetized and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer 60 days after injury. The spinal cord was dissected out and post-fixed for 4 h in the same solution at 4°C. Spinal cords were placed in Fluorinert (Sigma, Madrid, Spain), and MRI was performed on a 4.7-T Bruker BioSpec system (Bruker, Karlsruhe, Germany) with a H1 surface coil. Three dimensional T2-weighted Fast Spin Echo images (T2W-3D) were acquired with the following parameters: repetition time (TR) 1650 msec, echo time (TE) 70 msec, number of averages (NA)=1, slice thickness 0.5 mm, 16 sagittal slices per sample, field of view (FOV) 2.56×1.28×0.8 cm2, and data matrix 256×128×16. T2W-3D hyperintense lesions in the spinal cord were identified and the volumes and lengths were measured with Image J software (NIH, Bethesda, MD).23,30
Tissue processing for immunohistochemistry and Luxol Fast Blue (LFB) staining
After MRI studies, spinal cords were immersed in a solution of 30% sucrose in 0.1 M phosphate buffer during 72 h at 4°C. Tissue blocks of 5 mm including the epicenter of the spinal cord lesion in the middle of the block were then embedded in optimum cutting temperature compound (Tissue-Tek, Sakura Finetek, Zoeterwoude, NL), frozen on dry ice, cut into 50-mm thick serial coronal sections on a cryostat, and sequentially collected on slides. After immunohistochemistry and LFB staining, cross-sections were examined under a light microscope, at 40× or 60× magnification, equipped with a digital camera Panasonic GPKR2 22 connected to an Olympus BH2 microscope. Image analysis was performed using Bioscan Optimas II software or Image J, NIH image analysis software. All slides were assessed blindly with respect to treatment.
LFB staining
Sections were treated with 95% ethanol and left in LFB solution (0.1 mg% LFB in 95% ethanol with 10% acetic acid) at 60°C for 18 h. After several washes, sections were immersed in lithium carbonate and then 70% ethanol, rinsed in distilled water, dried, and mounted with Permount.
Immunohistochemistry
Immunostaining was performed as described previously21 using primary antibodies against myelin basic protein (MBP 1:100, Abcam, Cambridge, UK), neurofilament H (NF-H 1:1000, Abcam, Cambridge, UK), or adenomatus polyposis coli (APC) clone (clone CC1 1:100, Calbiochem, Darmstadt, Germany), and secondary biotinylated anti-rabbit immunoglobulin G (IgG) (1/200 Vector Laboratories, Burlingame, CA) or biotinylated anti-mouse IgG (1/200 Vector Laboratories). After the incubation with secondary antibodies, sections were incubated with avidin-biotin complex (ABC) for 30 min (ABC kit, Vector Laboratories) and finally revealed with diaminobenzidine tetrachloride (0.50 mg/mL, Sigma, St. Louis, MO) in the presence of 0.01% H2O2 for 7 min in the dark. The sections were given a final rinse in phosphate buffered saline, dehydrated in graded ethanols and xylene, and mounted with Permount. CTL experiments were performed to rule out interference of non-specific staining. These experiments were run in parallel and involved the incubation of tissue without primary antibodies.
Quantification of tissue sparing
Serial sections were taken every 0.5 mm from 2.5 rostral up to 2.5 mm caudal to the epicenter and were assayed for LFB staining. The spared area of white and gray matter in each section was quantified using NIH image analysis software Image J. The white matter was determined to be spared if LFB staining was grossly normal in appearance and density (lacking cysts and degeneration) and spared gray matter estimation was based on comparison with normal gray matter cytoarchitecture observed in naïve animals.25,31 We calculated the volume of spared white matter (SWM) and spared gray matter (SGM) by multiplication the spared area of each section by section thickness. The volume of spared tissue was normalized as a percentage of the volume of white and gray matter measured at the same spinal levels in intact rats.
Optical density measurement of MBP
Optical density of MBP staining was measured as described previously.21 Briefly, inmmunostaining intensity was determined in areas (50,000 μm2) covering three white matter regions: dorsal funiculus, lateral funiculus, and ventral funiculus, anatomically delimited by the method described in Labombarda and associates.21 Computer-assisted image analysis (Bioscan Optimas II) was used to transform differences in color intensity of immunopositive areas into gray differences, and results were expressed as the mean±standard error of the mean inverse logarithm of gray intensity per unit area (mm2) (ILIGV/area=LIGV).20 Sections were processed simultaneously under identical light beam, wavelength, and gray-scale threshold throughout the experiment and quantification analysis. LIGV was averaged for each section and expressed as a percentage of CTL intact animals.
Stereological APC (CC1) counting
Serial sections were taken every 0.5 mm from 2.5 rostral up to 2.5 mm caudal to the epicenter and were assayed for APC immunostaining. The number of total APC+cells was estimated using the optical dissector method with total section thickness as the dissector height and a counting frame of 60 mm×50 mm.32 Section thickness was estimated using the fluorescent properties of hematoxylin, so counterstained sections were examined under a Nikon Eclipse E 800 confocal scanning laser microscope. A total of 240 counting frames (24 per section covering dorsal, lateral, and ventral white matter funiculus) were assessed per animal. Knowing the volume of the dissector in each section, we calculated the number of APC+cells/mm3. To obtain the total number of APC+cells, we multiplied the number of cells/mm3 per the total volume of spinal cord (the summation of cross-sectional areas×thickness section×distance between slices).
Quantification of axonal profiles
We obtained micrographs of ventral, dorsal, and lateral white matter with a bright field microscope using a 60×objective. The final area of the picture was 25,000 mm2, with a final pixel size of 0.239 microns×0.179 microns. We performed a semi-automated quantification of axonal profiles using NIH image analysis software Image J. Pictures were transformed in 8-bit grayscale images, then converted to binary images using manual threshold. Dilate, Watershed and Erode filters were applied to the images, and finally the automated counting tool included in Image J was used. To obtain total axonal profile in white matter, we multiplied axonal profile density (axonal profile/mm2) per white matter area of dorsal, ventral, and lateral funiculus. Finally, the number of axonal profiles per funiculus was added, and axonal preservation was expressed as percentage of total axonal profile in white matter with respect to CTL animals.
Open-field locomotion
Open-field locomotion was evaluated by using the BBB locomotor scale according to the instructions published in the original articles.26,27 Briefly, animals were gently placed to the open field during several sessions. Then, two trained observers (blind to hormone treatment) scored rats for 4 min at the same time of the day at 3, 7, 14, 30, and 60 days after SCI. One researcher recorded all the data on a score sheet, and the other one kept the animal moving in the open field. Movements elicited by the touch of an examiner were not scored. A score of 0 points defines no movement of the hindlimbs (HL), and the maximum of 21 points defines normal locomotion as observed in unlesioned rats. Score includes criteria such as joint movements, weight support, forelimb-hindlimb (FL-HL) coordination, and tail position.
Because interlimb coordination can be quite difficult to assess in the open field, we corrected open field BBB scoring using an objective assessments of coordination with the CatWalk system (CatWalk-based BBB score) according to Koopmans and colleagues.33 We acquired and studied five uninterrupted runs with the CatWalk system on the day of open field testing, considering “consistent coordination” when Regularity Index (RI) (Table 1) scored 100% in 5/5 runs; “frequent coordination” when RI scored 100% in 3/5 or 4/5 runs; “occasional coordination” when RI scored 100% in 1/5 or 2/5, and “no coordination” when RI scored 100% in 0/5 runs. We also used the BBB subscoring scale to improved the sensitivity of the BBB by scoring the higher motor functions (i.e., toe clearance, predominant paw position, trunk stability, and tail position) regardless of the other BBB parameters, adding them together to yield a single score (maximum score=13).34
Table 1.
CatWalk Parameters Analyzed
| Explanation | |
|---|---|
| Base-of-support (BOS) | Distance between the two hindpaws, as measured perpendicular to the walking direction. |
| Stride length | Distance between the placement of a paw and the subsequent placement of the same paw. |
| Print area | The total surface area of the complete print. |
| Max area | Maximum area of a paw that comes into contact with the glass plate; it is the print area at maximum contact. |
| Paw angle | Estimate of the angle (in degrees) of the paw axis relative to the horizontal plane. |
| Print intensity | The mean brightness of all pixels of the print at Max contact. Intensity ranges from 0 to 255. The intensity of a signal depends on the degree of contact between a paw and the glass plate and increases with increasing pressure. Therefore, intensity is an indirect measure of weight support of the different paws. |
| Stance duration | Time of contact of the hindpaw with the glass floor. |
| Swing duration | Time that the hindpaw is not in contact with the glass floor. |
| Duty cycle | Expresses stance duration as a percentage of the duration of the step cycle. It is calculated as follows: Duty cycle: stand/stand+swing * 100 %. |
| Regularity index (RI) | An index for the degree of interlimb coordination during gait, as measured by the number of normal step sequence patterns (NSSP), multiplied by four (number of paws), divided by the number of paw placements, and multiplied by 100%; RI=(NSSP×4)/number of paw placements×100%, With respect to RI, six NSSP have been described previously36 and involve cruciate, alternate, and rotary step patterns. In healthy (fully coordinated) animals, its value is 100%; loss of interlimb coordination obviously leads to a decrease in this parameter. |
| Phase dispersions (PD) | Measure for interlimb coordination based on time-relationships between footfalls. The moment of initial contact of one paw (the Target) is related (expressed as a percentage) to the stride cycle of another paw (the Anchor). PD can be calculated between limbs on the same grindle (forepaws or hindpaws - grindle pair), between limbs on the same side (ipsilateral left or ipsilateral right - lateral pair) and between diagonal paws (opposite front/hindpaws - diagonal pair). Within diagonal pairs (RF-LH, LF-RH) and ipsilateral pairs (RF-RH, LF-LH), the Anchor is always one of the front paws. Within girdle pairs (LF-RF, LH-RH), the Anchor paw is always one of the left paws. |
| Phase dispersions variability | The standard deviation of the mean of PD values for each pair of paws. Coordinated locomotor activity is characterized by minor PD variability during uninterrupted locomotion. It is a measure of accurancy in the inter-limb coordination |
RF, right forelimb; RH, right hindlimb; LF, left forelimb; LH, left hindlimb.
CatWalk-automated quantitative gait analysis
While the general locomotor performance was evaluated using the BBB scale, a more detailed analysis of locomotion was performed using the CatWalk gait analysis system in rats that were able to maintain weight support with their HL (BBB score above 9). CatWalk measures a large number of both static and dynamic gait parameters and enables quantitative assessment of locomotion.33,35–39
Briefly, the CatWalk system consists of a glass runway that contains light from a fluorescent tube. Internal reflection causes the light to be restricted to the glass surface plate under normal circumstances. When an object touches the glass surface, however, the light exits the glass, thereby only illuminating the contact area. Hence, placement of the rat paws on the glass floor lights up the corresponding areas on the glass floor. The run of the animal across the glass runway is detected by a video camera (Pulnix TM 765E, Pulnix Inc., U.K.) positioned underneath the glass plate. The signal is digitized (50 frames/sec) by a Picolo Diligent frame grabber board. The data are acquired, compressed, stored, and eventually analyzed by the CatWalk 7.1 software program.35
One week before the surgery, animals were trained to walk across the CatWalk, baseline scores were recorded, and rats were then tested on days 14, 30, and 60 post-surgery (when animals regained at least frequent weight supported stepping). For locomotor analysis, the following criteria concerning walkway crossing had to be met: (1) the rat crossed the walkway without any interruption or hesitation with a consistent pace, (2) a minimum of five correct crossings with about four step cycles (i.e., each paws positioned four times) were acquired and analyzed per animal, and (3) to avoid a high variability in gait velocities, only runs with a stable crossing time of the runway between 3 and 4 sec were included.
Because no left-right differences were present in any of the gait parameters investigated, corresponding left and right gait parameters were averaged before analysis. The analyzed parameters are described in Table 1. For a full description of the locomotor parameters, see Hamers and coworkers.36
Sensory function
Sixty days after injury, rats were brought into the behavior room 1 h before the test session to allow them to habituate to the environment. Animals were placed alone in each behavioral apparatus for 15 min before testing, and trials of a particular sensory test were separated by at least 1 hr. They received sugared cereal rewards throughout testing to keep them from attending to the movements and procedures of the examiner. The average value of three readings was used as the tolerance threshold for each hindpaw.
Mechanical sensitivity
The sensitivity to touch stimuli was assessed by using the dynamic plantar aesthesiometer (Ugo Basile, Varese, Italy), an automated apparatus based on the Von Frey filament principle. Rats were placed in a raised cage with a wire mesh floor over the stimulator unit. The metal filament was applied to the center of the palmar surface of the hindpaw, and upward force was increased from 1 to 50 g over 15 sec. Force at withdrawal was recorded for both hindpaws.40,41
Thermal sensitivity
The Plantar test (Ugo Basile, Varese, Italy) was used to evaluate thermal hyperalgesia according to the Hargreaves method.42 Rats were placed in a cage with a glass floor, over a movable infrared light source. The light source was positioned under the center of the palmar hindpaw, and it was turned off automatically when the rat lifted the paw, allowing the measurement of time between the beginning of the light beam and the elevation of the foot. To prevent tissue damage, a 20-sec cutoff time was set. Thermal withdrawal latency was recorded for both hindpaws.40,41
Statistical analysis
All the statistical analysis and graphs were performed with Prism 5.0 software (www.graphpad.com/support). All the data are presented as means±standard errors. For BBB and BBB subscoring analysis, the nonparametric Mann-Whitney U test was used. A two-way analysis of variance of repeated measures followed by the Bonferroni post-test was used to determine statistical differences between treatments in the different rostrocaudal regions from the epicenter or at different time points after injury
One-way analysis of variance followed by the Newman Keuls post-test or t test was used to determine statistical differences between three or two treatments, respectively, at 60 days. The number of rats was used as the n number (n=8 for all treatments). A p<0.05 was considered to be significant.
Results
SCI
The real force at the impact site and the mean amount of spinal cord displacement, two key parameters in defining the severity of injury, did not show significant differences between SCI and SCI+PROG groups, ensuring consistency and reproducibility of the injury (force: SCI 204.3±1.42 Kdyn vs. SCI+PROG 204.9±1.66 Kydn; displacement: SCI 1188±53.47 mm vs. SCI+PROG 1212±33.56 mm). After surgery, animals showed an initial drop in body weight during the first postoperative days, increasing afterward until the end of the study. There were no differences between the groups (data not shown).
PROG reduced secondary damage and increased spared tissue
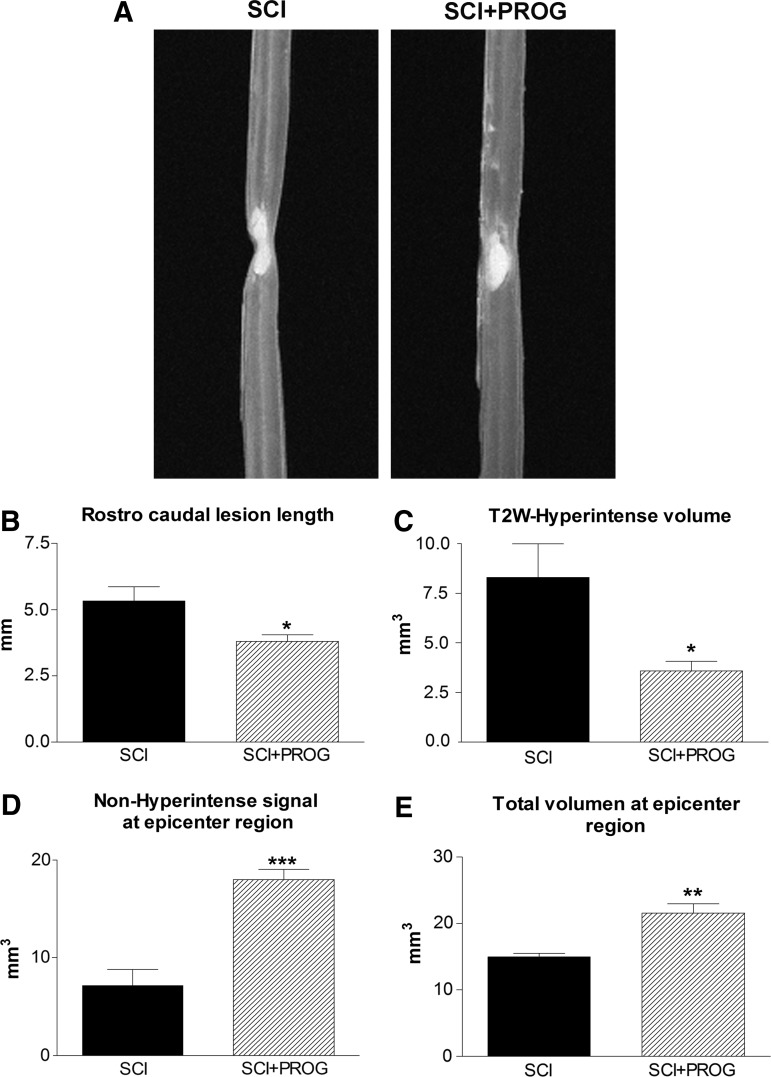
We determined the effect of PROG on the extension of the lesion 60 days after injury by quantifying ex vivo the edema-related hyperintense signaling in T2W-3D MRI, which mainly reflected the cyst already formed.43–45 PROG reduced volume and length of T2 hyperintense signals when compared with SCI rats (Fig. 1A, B, C). In addition, the non-hyperintense region and the total volume of spinal cord at the epicenter region were significantly increased in PROG+SCI rats 60 days after the injury (Fig. 1D, E).
FIG. 1.
Representative T2-weighted three-dimensional magnetic resonance imaging (T2W-3D MRI) showing smaller hyperintense region in spinal cord injury plus progesterone (SCI+PROG) rats (A). Effects of PROG treatment on reducing lesion extension (B), cyst formation (C), preservation of non-hyperintense tissue (D), and total spinal cord volume (E) at the epicenter region after 60 days of injury. All values are expressed as mean±standard error of the mean, *p<0.05 vs. SCI, **p<0.01 vs. SCI, ***p<0.001 vs. SCI, t test.
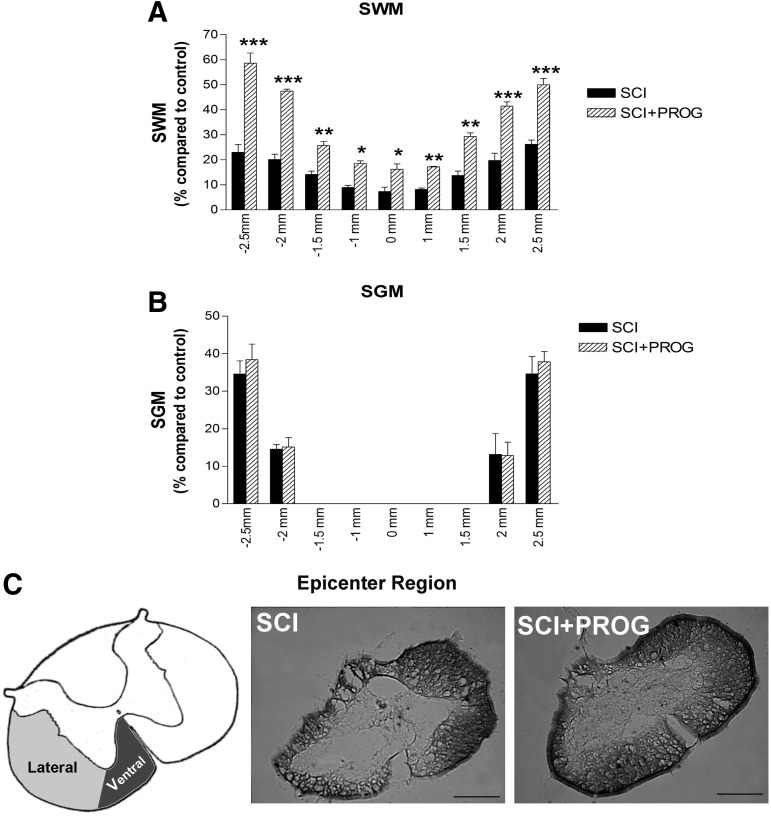
Next, we studied the effect of PROG administration on the extension of the lesion and tissue sparing at the histological level. We performed LFB staining on serial coronal sections taken between 2.5 mm rostrally and 2.5 mm caudally from the epicenter, and we quantified SWM and SGM (Fig. 2).
FIG. 2.
Effect of progesterone (PROG) on white and grey matter damage measured at 60 days after lesion. An increase in spred white matter (SWM) preservation was observed in spinal cord injury (SCI)+PROG rats at all distances measured from the epicenter (A). PROG did not modify the volume of spared grey matter (SGM) along the segment (B). Micrographs showing coronal section of spinal cord stained with Luxol Fast Blue from the epicenter region. Spinal cord contusion produced a cavitation at the epicenter, leaving a greater sparse region of intact white matter in the ventral-lateral margins (lateral and ventral funiculus) in SCI+PROG group (C). All values are expressed as mean±standard error of the mean. For A and B: ***p<0.001 vs. SCI, **p<0.01 vs. SCI, *p<0.05 vs. SCI, two-way analysis of variance of repeated measures followed by Bonferroni post-test. For C: scale bar=200 μm.
After 60 days, injured animals lost approximately 77% of white matter in segments located 2.5 mm rostral and caudal to the epicenter, while PROG treatment reduced white matter decrease to about 42% (i.e., 2.5 mm rostral: SCI: 22.99±3.03%; SCI+PROG: 58.60±4.06, p<0.001, Fig .2A). The amount of white matter preservation at the epicenter itself was approximately 7% in SCI rats, while in SCI+PROG animals, it was about 16% (SCI: 7.29±1.74%; SCI+PROG: 16.19±2.17%, p<0.05, Fig. 2A). The preservation of SWM was observed between SCI and SCI+PROG groups at all distances measured from the epicenter (Fig. 2A).
On the other hand, no statistical differences were observed between the SCI and SCI+PROG rats in SGM (SCI vs. SCI+PROG, p>0.05, Fig. 2B).
Additional quantitative assessment of the area of white matter at lateral and ventral funiculus was performed at the epicenter itself, because this parameter is related to supraspinal control.46,47 This area was significantly larger in contusioned animals treated with PROG than in the vehicle group (Fig. 2C, SCI+PROG: 0.38 mm2±0.034 vs. SCI: 0.27 mm2±0.067, p<0.05).
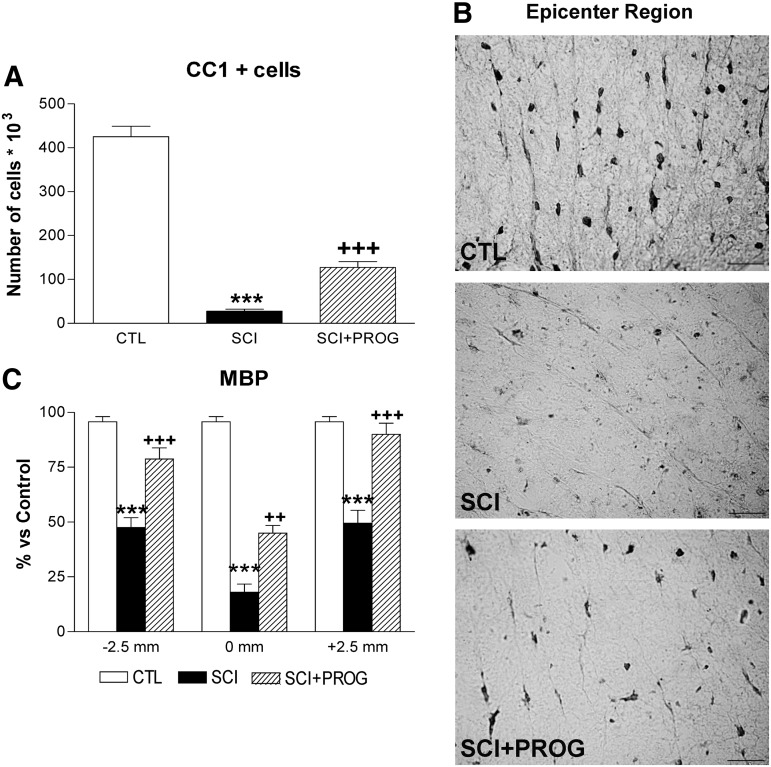
PROG administration increased oligodendrocyte numbers and reduced myelin damage after SCI
We studied the effect of PROG administration on oligodendrocyte preservation. We stereologically quantified the number of CC1+cells in SWM within a 5 mm segment of the lesion epicenter.
After 60 days of spinal cord contusion, lesioned animals showed a 93.46% reduction in the total number of CC1+cells throughout the white matter, while PROG-treated rats showed a higher number of cells, losing about 65% of oligodendrocytes compared with sham-operated rats (CTL: 425 103±13.18 103, SCI: 27.83 103±4.30 103, SCI+PROG: 127.4 103±13.18 103; p<0.001 SCI vs. CTL; p<0.001 SCI vs. SCI+PROG; Fig. 3A, B).
FIG. 3.
Progesterone (PROG) increased the oligodendrocytes number and reduced myelin damage 60 days after lesion. Spinal cord injury (SCI) animals showed a reduction in the total number of CC1+cells throughout the white matter compared with control (CTL) rats, while the in SCI+PROG group, the reduction was lower (A). Micrographs showing CC1 immunohistochemistry in ventrolateral white matter at the epicenter region in the different groups of animals (B). Myelin basic protein (MBP) immunoreactivity increased in SCI+PROG rats compared with SCI rats at 2.5 mm rostral, 2.5 mm caudal, and the midpoint−0mm- from the epicenter (C). All values are expressed as mean±standard error of the mean. For A: ***p<0.001 vs. CTL, +++ p<0.001 vs. SCI, one-way analysis of variance (ANOVA) followed by Newman Keuls post-test. For C: ***p<0.001 vs. CTL, ++ p<0.01 vs. SCI, +++ p<0.001 vs. SCI, two-way ANOVA of repeated measures followed by Bonferroni post-test. For B: scale bar=20 μm.
Next, we measured MBP immunoreactivity in the SWM of 2.5 mm rostral, 2.5 mm caudal, and the midpoint of the epicenter region (Fig. 3C). MBP immunoreactivity diminished dramatically in SCI rats at all studied regions but was partially preserved in SCI animals treated with PROG (SCI vs. SCI+PROG, p<0.01 at all regions, Fig. 3C). Therefore, increased oligodendrocyte density in the PROG-treated group was accompanied by better myelin preservation.
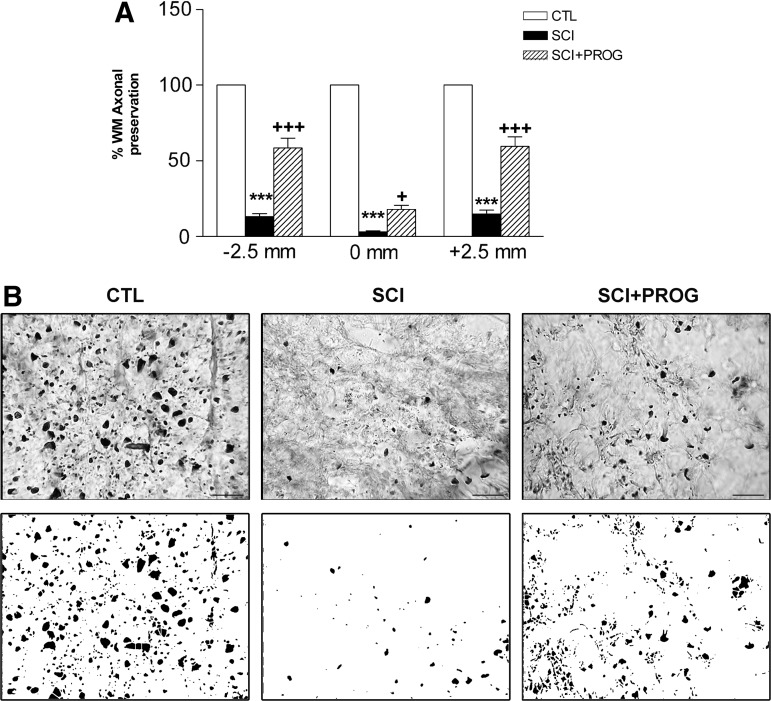
PROG improved axonal preservation after spinal cord contusion
We also evaluated the number of axonal profiles on sections immunostained against NF-H. Quantification revealed a higher axonal preservation in PROG-treated animals compared with vehicle-treated ones (SCI+PROG vs. SCI, p<0.05 or less at all regions, Fig. 4A, B). This axonal protection was verified along the whole 5-mm segment.
FIG. 4.
Progesterone (PROG) improved axonal preservation 60 days after injury at 2.5 mm rostral, 2.5 mm caudal, and at the midpoint−0mm- of the epicenter (A). Micrographs show neurofilament (NF-H) immohistochemistry in ventrolateral white matter at the epicenter region in the different groups of animals. Spinal cord injury (SCI) group showed less axonal profiles than control (CTL), while in SCI+PROG rats, the number was recovered (B upper panel). Examples of processed images used for quantification are shown (B lower panel). All values are expressed as mean±standard error of the mean. For A: ***p<0.001 vs. CTL, +++ p<0.001 vs. SCI, two-way analysis of variance of repeated measures followed by Bonferroni post-test. For B: scale bar=20 μm.
PROG improved locomotor outcome after SCI
Open-field locomotion
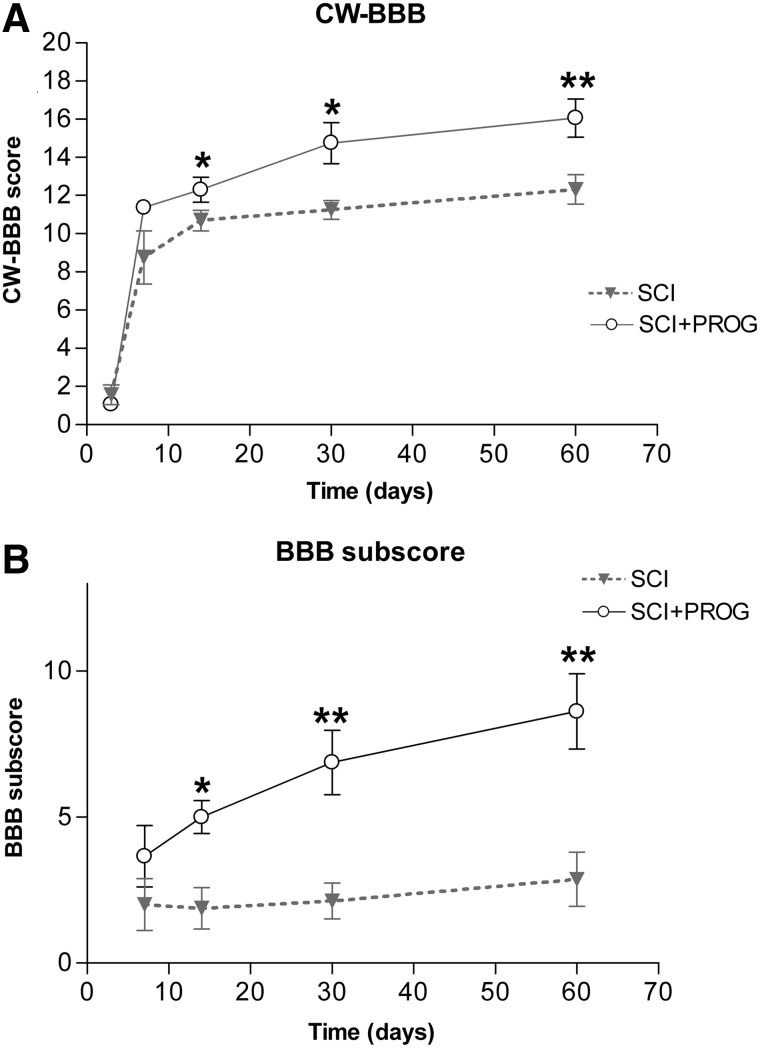
The general locomotor performance was evaluated using the BBB scale, but interlimb coordination was corrected with the RI value obtained in the CatWalk gait analysis (CW-BBB) (see Methods, Table 1).
All rats manifested significant bilateral HL paralysis immediately after injury. After 14 days, SCI rats improved their scores, showing consistent weight-supported HL steps but no FL-HL coordination (CW-BBB score=11), while PROG-treated rats demonstrated consistent coordinated FL-HL movements (CW-BBB score=14) (p<0.05 SCI vs. SCI+PROG, Fig. 5A). This pattern was maintained for the duration of the assessment period. Behavioral improvements seen in PROG-treated animals were present at each time point after 14 days (p<0.05 at all time points, Fig 5A).
FIG. 5.
Integration of the CatWalk(CW)-based coordination into the Basso-Bresnahan-Beattie (BBB) locomotor scale results in the CatWalk-based BBB, which showed that spinal cord injury plus progesterone (SCI+PROG) animals regained better locomotor function when compared with SCI rats (A). BBB subscore also showed marked differences between SCI and SCI+PROG rats (B). All values are expressed as mean±standard error of the mean, *p<0.05 vs. SCI, **p<0.01 vs. SCI, ***p<0.001 vs. SCI, nonparametric Mann-Whitney U test.
As indicated in Methods, the BBB subscore evaluates aspects of fine motor control regardless of the other BBB parameters.34,48 After 14 days, the BBB subscore of SCI animals became stable at a score of 2, whereas PROG-treated animals eventually reached a higher score between 5 and 8 (Fig. 5B). At 60 days post-injury, PROG rats presented better subscores than the SCI group: 75% of PROG rats had the tail consistently up, 50% had consistent trunk stability and parallel paw position of HL during the stance, and 62% showed consistent toe clearance. In the SCI group, only 37% of rats had the tail up, none of the rats reached trunk stability and parallel paw position, and 12% showed consistent toe clearance.
CatWalk-automated quantitative gait analysis
Fourteen days post-injury, all rats in both groups reached a BBB score higher than 9, allowing us to test their locomotor performance also by CatWalk gait analysis. Importantly, gait velocity, which strongly affects the variations in gait parameters, is controlled using the Cat-Walk36 by training the animals to make runs with a stable crossing time. During this study, no significant differences were found between animal groups with respect to crossing time or average body weight, another parameter that affects gait (data not shown). There were no significant differences in any studied parameter for the functions of the FL between SCI and SCI+PROG rats.
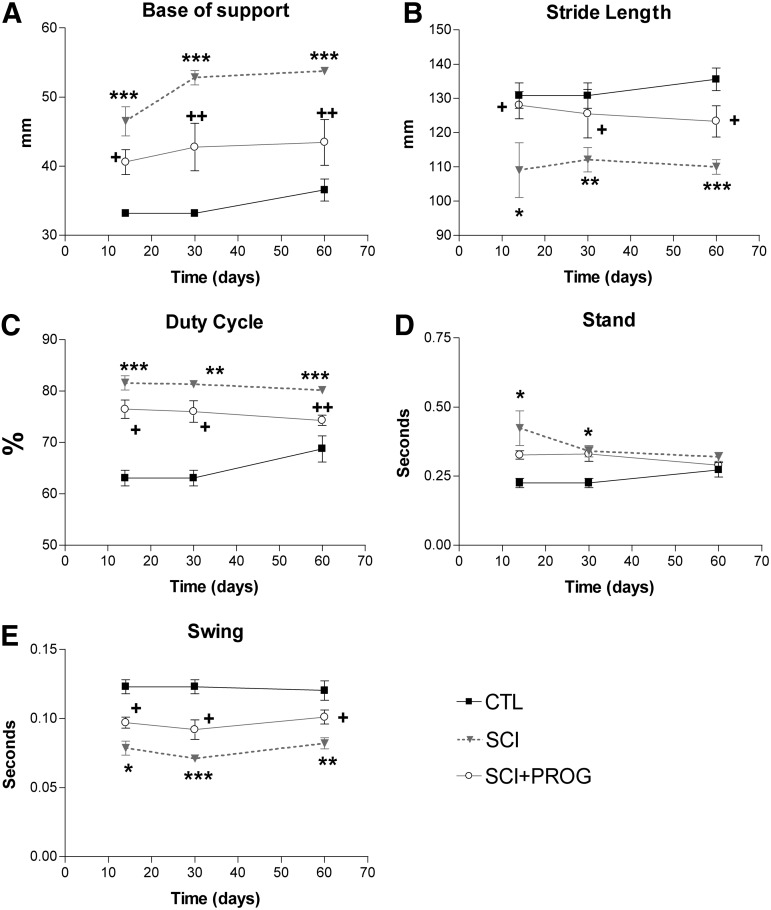
Base of support (BOS) of the hindpaws
The distance between the hindpaws during locomotion is an indicator of trunk stability of the animal. BOS was higher in SCI rats throughout the test period compared with sham control animals (p<0.001 at all time points, SCI vs. CTL, Fig. 6A). PROG-treated rats showed a decrease in BOS with respect to SCI rats (p<0.05 or less at all time points, SCI vs. SCI+PROG, Fig. 6A). During the study, BOS increased slightly over time in all groups, probably because of the normal increases in body weight (CTL, SCI, SCI+PROG; Time: F (2, 42)=5.24, p<0.05, Fig. 6A).
FIG. 6.
CatWalk gait parameters analysis showed that progesterone (PROG) improved overground locomotion. Base of support (A), Stride length (B), Duty cycle (C), Stand phase (D), and Swing phase (E). All values are expressed as mean±standard error of the mean, *p<0.05 vs. contgrol (CTL), **p<0.01 vs. CTL, ***p<0.001 vs. CTL, + p<0.05 vs. spinal cord injury (SCI), ++ p<0.01 vs. SCI, two-way analysis of variance of repeated measures followed by Bonferroni post-test.
Stride length
Stride length decreased significantly in injured rats compared with sham-operated animals (p<0.05 or less at all time points, SCI vs. CTL, Fig. 6B). PROG-treated rats, however, showed an increased stride length compared with SCI rats (p<0.05, SCI vs. SCI+PROG, Fig. 6B) and remained closer to sham-operated values (ns, SCI+PROG vs. CTL, Fig. 6B)
Duty cycle, Stance, and Swing
The Duty cycle is the percent of the total step cycle during which a paw is on the ground (Table 1). The Duty cycle of the hindpaws increased in injured rats (p<0.05 or less at all points, SCI and SCI+PROG vs. CTL, Fig. 6C), but this increase was smaller in rats treated with PROG (p<0.05 or less at all time points, SCI vs. SCI+PROG, Fig. 6C).
The time that the hindpaw was not in contact with the glass floor (Swing phase) decreased in all injured animals, but to a lesser extent in PROG-treated animals throughout the study (p<0.05 at all time points SCI vs. SCI+PROG, Fig. 6E). The time that the hindpaws were in contact with the floor (Stance phase), however, was significantly increased in both groups of lesioned animals only after 14 and 30 days (p<0.05 SCI and SCI+PROG vs. CTL, Fig. 6D).
Print area, Max area, and Paw angle
Even though the duration of the Stance phase did not change among groups, we evaluated additional characteristics of the Stance by studying Print area, Max area, and Paw angle (see Table 1). After 14 and 30 days, both injured groups showed statistically significant increases in Print area and Max area compared with sham, but without significant differences between them (data not shown). After 60 days, however, the Max Area and Print area values remained similar in SCI rats while in SCI+PROG animals, both parameters decreased, reaching values similar to those of sham-operated rats (Table 2). Paw angle increased throughout the study in injured animals, but to a lesser extent in those treated with PROG (data not shown). Data at 60 days are shown in Table 2.
Table 2.
Max Area, Print Area and Print Intensity after 60 days of Spinal Cord Injury
| Treatments | Max area (mm2) | Print area (mm2) | Paw angle (degrees) | Print intensity (a.u) |
|---|---|---|---|---|
| CTL | 42.35±2.38 | 56.94±3.86 | 5,26±1.17 | 84.79±2.96 |
| SCI | 67.35±4.33** | 110.80±8.83*** | 12.57±3.00* | 88.22±2.67 |
| SCI+PROG | 51.27±5.57 # | 85.27±8.87 # | 4.73±0.87# | 81.90±4.32 |
CTL, control; SCI, spinal cord injury; PROG, progesterone.
p<0.05 vs. CTL; ***p<0.001 vs. CTL; # p<0.05 vs. SCI. One-way analysis of and Student Newman Keuls post-test.
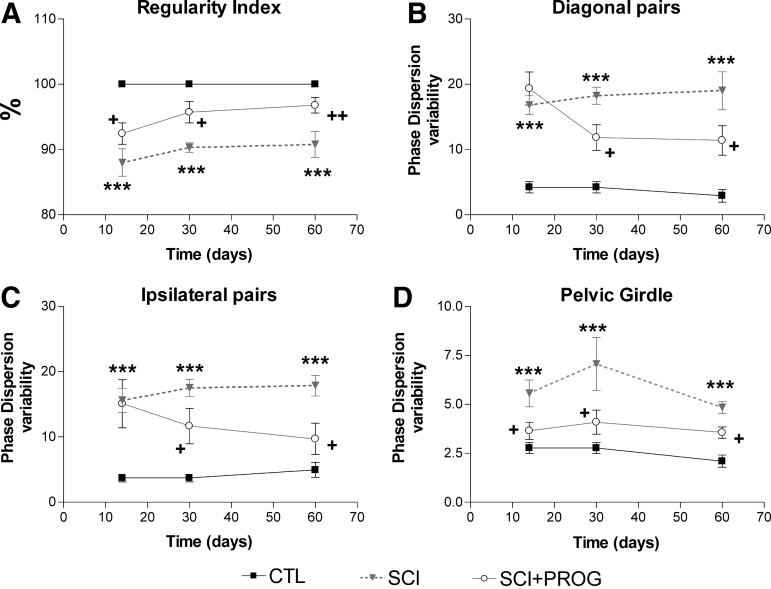
RI
This parameter reflects FL-HL coordination, correlating the order of paw placements of the animal with the normal step sequence patterns.35,36 In sham-operated animals, all runs reached a RI of 100%, showing perfect interlimb coordination. In injured animals, mean RI was reduced in both groups from 2 weeks after injury (p<0.05 vs. CTL, Fig. 7), but the decrease was smaller in PROG-treated animals at all time points (p<0.05 SCI vs. SCI+PROG, Fig. 7). These PROG-treated animals recovered interlimb coordination reaching RI values similar to control rats at 30 days (95.72±1.64, ns SCI+PROG vs. CTL) and 60 days (96.76±1.19, ns SCI+PROG vs. CTL, Fig. 7).
FIG. 7.
PROG treatment improved interlimb coordination after injury. Spinal cord contusion decreased RI in SCI rats compared to CTL animals, but in a lesser extent in SCI+PROG (A). PD variability was increased in SCI rats compared to CTL in all the studied pairs of paw. However, in SCI+PROG rats, PD variability was lower compared to SCI in diagonal pair (B), ipsilateral pair (C) a pelvic girdle (D). All values are expressed as mean±SEM, ***p<0.001 vs CTL, +p<0.05 vs SCI. A two-way ANOVA of repeated measures followed by Bonferroni post-text.
Phase dispersions (PD)
Another parameter used to estimate coordination is the measurement of PD, which, in contrast to the RI, determine the paw placements with respect to timing (Table 1). Changes in PD variability represent a measure of interlimb coordination: during uninterrupted locomotion, coordinated limb pairs show very small phase dispersion variability, whereas limbs that move largely autonomously display a high variability.33,49
We averaged the values of complementary pairs to perform statistical analysis: PD diagonal pairs is the average between left forward-right hindlimb (LF-RH) and right forward-left hindlimb (RF-LH) and PD ipsilateral pairs is the average between right forward-right hindlimb (RF-RH) and left forward-left hindlimb (LF-LH). We also estimated the PD variability of pectoral (LF-RF) and pelvic (LH-RH) girdles.
Injury severely increased PD variability in all pairs of paws compared with sham animals (ipsilateral pairs: F2,42=42.26, p<0.001, Fig. 7B; diagonal pairs: F2,42=38.27, p<0.001, Fig. 7C; pelvic girdle: F2,42=31.37, p<0.001, Fig. 7D). Significant effects of PROG treatment became apparent after 14 days, however. The PD variability was significantly lower in SCI+PROG than in SCI rats in the ipsilateral and diagonal pairs and in the pelvic girdle (p<0.05 SCI vs. SCI+PROG Fig. 7B, C, D). The value of PD variability was stable inside each group over time, not existing statistically significant differences from 14 onward (ipsilateral pairs: F2,42=2.43, ns, Fig. 7B; diagonal pairs: F2,42=1.02, ns, Fig. 7C, pelvic girdle: F2,42=3.50, ns, Fig. 7D).
On the other hand, changes in PD mean value represent changes in the step patterns during gait.49 Lesioned animals showed changes in the mean PD for ipsilateral and diagonal pairs vs sham-operated controls, but no difference was observed between SCI and SCI+PROG rats (data not shown)
Sensory function
Animals were tested for mechanical and thermal sensitivity to explore sensory functions after 60 days of spinal cord contusion.
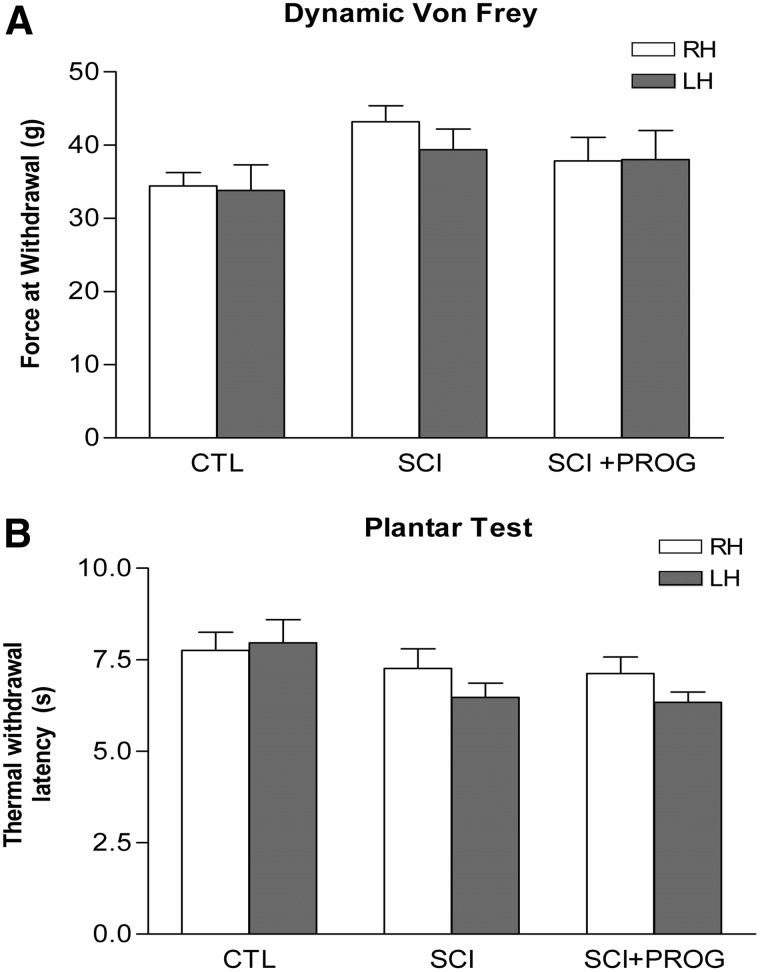
Mechanical sensitivity
There was no significant difference in the filament force necessary to elicit a hindpaw withdrawal between the SCI rats and sham-operated animals, indicating no significant mechanical allodynia in our model of contusion (Fig. 8A). In addition, chronic PROG administration did not change the threshold of response to mechanical stimulation (Fig. 8A). This was supported also by the absence of differences in some CatWalk parameters such as the duration of Stance phase (Fig. 6D) and the pressure applied during Stance (Print intensity) and by the increase in the area contacted by the paw during the stand (Print area) and the increase of the area contacted at the moment of maximum paw-floor contact (Max area) (Table 2). Decreases in those parameters have been previously related to mechanical allodynia.50,51
FIG. 8.
Neither PROG nor spinal cord injury induced mechanical or thermal allodynia after 60 days of spinal cord contusion. Dynamic Von Frey (A). Plantar test (B). All values are expressed as mean ±SEM and no statistical differences were found by two-way ANOVA. RH, right hindlimb; LH, left hindlimb.
Thermal sensitivity
As for mechanical stimulation, no statistically significant differences were observed between groups, showing that our model did not produce thermal hyperalgesia and that PROG administration did not induce changes in the nociceptive behavior (Fig. 8B).
Discussion
The current study demonstrated that PROG treatment after spinal cord contusion was beneficial at the anatomical, histological, and functional levels. Because at present there are no universally accepted treatments for this neurological disorder, our present investigation identifies PROG as a promising candidate for clinical trials.
PROG reduced secondary injury and white matter pathology
By MRI techniques, we observed that the volume of hyperintense signal was smaller in rats treated with PROG, suggesting that the steroid effectively reduced edema formation after spinal trauma. This is in agreement with previous reports showing that PROG reduces edema formation after traumatic brain injury16 by decreasing the expression of aquaporin-4 at the zone of injury52 and by stabilizing the blood–brain barrier with its antioxidant effects.53 The improvement in tissue preservation and total spinal cord volume may imply a secondary damage reduction, an effect expected given the already described effects of PROG in decreasing inflammation and reactive gliosis13,22,54,55 reducing oxidative stress53 and decreasing excitotoxicity via indirect conversion into allopregnenalone.9,56–58
Further, PROG notably reduced the extension of the hyperintense signal in a rostrocaudal direction. This would imply that PROG may be beneficial for injuries located near the lumbar or cervical enlargements, where limiting the expansion of the primary damage would preserve levels containing, for example, central pattern generators for locomotion or breathing control.
Our histological measurements support and extend our MRI findings: SWM values were higher at all distances measured in PROG-treated rats in accordance with the MRI data. A remarkable agreement across studies indicates that only 5–10% tissue sparing is necessary for the recovery of plantar stepping (BBB score=10–11).27,59–61 Accordingly, SWM was about 7% at the epicenter of lesioned rats that showed a consistent stepping (mean BBB score=11) 60 days after the lesion. Interestingly, PROG treatment preserved 16% of SWM at the epicenter, and rats demonstrated consistent plantar stepping with FL-HL coordination (mean BBB score=14). This confirms that a small increase in SWM may result in a substantial recovery of locomotor function, probably by preserving more supraspinal and propiospinal inputs.27,49,61
In this regard, after PROG treatment, the areas of dorsolateral and ventrolateral funiculus were significantly larger at the epicenter site. White matter tracts that occupy the outer rim of the cord such as the rubro-, raphe-, reticulo- and vestibulospinal tracts are part of the descending brainstem system that appear to provide important, if not essential, input to the central pattern generator (CPG) to initiate locomotion and produce complex and precise locomotor patterns.46,47,62 Thus, axon sparing of these tracts by PROG treatment may underline the described locomotor recovery.
PROG and its reduced metabolite, allopregnenalone, are well recognized promyelinating factors in the peripheral and central nervous systems in several diseases.9–12,63–67 We have demonstrated in previous works that chronic PROG administration increases the expression of transcription factors that define oligodendrocyte linage, such as Olig2 and Nkx2.2 after spinal cord transection.21,29 In the present work, we report that PROG treatment reduced the loss of oligodendrocyte numbers and MBP immunoreactivity produced by SCI. This effect could be because of preservation of the myelin-producing cells, but also to an increase in oligodendrocyte numbers by differentiation of oligodendrocyte precursors into mature myelinating oligodendrocytes, promoting the remyelination process. Either way, the effects on oligodendrocyte number could contribute to the reported PROG actions in locomotor outcome, because therapeutical strategies that limit white matter damage and oligodendrocyte death have been shown to improve recovery after SCI,68,69 and spontaneous functional recovery correlates well with mature oligodendrocyte number in white matter after spinal cord contusion.70 PROG could have acted directly on oligodendrocytes, or indirectly by modulating the inflammatory environment, because PROG treatment decreases gliosis and cytokine production after spinal cord transection22 (author unpublished results). Further studies should be performed to answer this question.
We completed the evaluation of SWM with the study of axonal profile preservation at the epicenter. Quantification revealed a higher axonal preservation in PROG-treated animals along the whole 5-mm segment compared with SCI rats. PROG could protect axons by reducing inflammation, oxidative stress, reactive gliosis, edema, and excitotoxicity, but also preserving the integrity of the myelin sheath.71–73 Otherwise, PROG could have promoted axonal regeneration of supraspinal or propiospinal neurons.
Finally, PROG produced no significant changes in SGM. This is in accordance with previous reports, which described that functional deficits after midthoracic injuries are mainly attributed to white matter damage.74–76
PROG improved locomotor outcome after spinal cord injury
Behavioral recovery is the highest goal in the search for therapeutically relevant repair strategies for SCI. An important aspect of the observed locomotor outcome is the recovery of coordination. As it was demonstrated by others,27,35,49,77 rats after thoracic spinal cord trauma exhibited no interlimb coordination (CW-BBB score=11, high PD variability of ipsilateral and diagonal pairs of HL). The group treated with PROG, however, regained better locomotor function presenting consistent FL-HL coordination (CW-BBB score≥14, and values close to control animals in coupling between FL-HL, RI, and PD variability of the ipsilateral and diagonal pairs). It is generally accepted that coordinated gait can only be generated when there is a functional connection between the CPGs of the individual limbs located in the lumbar and cervical spinal cord.78–81
The recovery of FL-HL coordination in the PROG-treated rats indicated that communication between FL and HL CPGs has improved. Possible mechanisms underlying the recovery may be: (1) the preservation of descending brainstem systems, which are important in activation of CPG (rubro-, vestibulo-, reticuloraphe- and propiospinal tracts)46,47,62,78; (2) the formation of new intraspinal circuits that might contact long propiospinal neurons bridging the lesion and arborizing on lumbar neurons46,82; (3) neuroprotection and CPG plasticity. In this regard, we have published that PROG increases the synthesis of BDNF after spinal cord transection,19 which, in turn, like IGF-1, may induce synaptogenesis and CPG plasticity.33,83 Additional tract-tracing experiments to verify theses hypothesis would be needed, however.
The greater trunk stability in PROG-treated rats could produce the improvement of the following gait parameters: BOS, Swing phase, Print- and Max area, and Stride length. Particularly, an increased BOS is a common feature of SCI36,84,85 and has been explained as an adaptation for an instable gait.35 Thus, recovery of a normal hindlimb BOS, 60 days post-injury in PROG-treated rats, could be related to improvements in trunk stability. These data agrees with the BBB subscore, which indicated that in SCI+PROG group, 50% of the rats showed trunk stability, while none of the rats showed trunk stability in the SCI group. Alternatively, pronounced external hindpaw rotation might increase BOS measurements.36 In this regard, PROG also decreased the hindpaw rotation after injury, possibly reducing also the BOS. Indeed, the BBB-subscore showed that 50% of the animals in the SCI+PROG group reached a normal parallel paw position, while none of the rats reached the appropriate position in the SCI group.
In accordance with previous reports, Duty cycle increased after SCI,35,84 because of the reduction of the Swing phase. Conversely, after PROG treatment Duty cycle almost reached control values, because Swing phase was increased. That means that the rat paw falls to the ground faster in vehicle-treated rats than in PROG-treated rats. Even though the duration of the Stance phase did not change among groups, we evaluated additional characteristics of the Stance by studying Print and Max areas. Both parameters increased after SCI as reported.35 In PROG-receiving rats, however, Print and Max areas reached values similar to those of sham-operated rats. The decreasing of the Print area, as suggested by Hamers and coworkers,36 may be because of the consistent toe clearence that showed in 50% of the SCI+PROG rats.
Stride length normally decreases after injury36,38,86; however, PROG treatment raised this parameter, and rats tended to recover the ability to step a normal distance. These results might be the consequence of an improvement in BOS, the increase of Swing phase, and greater trunk stability.
Treatments that produce beneficial effects after SCI also may present undesired effects. In our current study, we checked whether PROG effects on locomotion were accompanied by the development of mechanical allodynia or thermal hyperalgesia. In the spinal contusion model, it has been described that normal sensory function is supported by as little as 10% of white matter sparing.49 Accordingly, we did not find mechanical allodynia or thermal hyperalgesia in rats with SCI, and we assessed that PROG did not induce such behaviors either, neither measured by classical tests, such as hot and cold plate and the Hargreaves method, nor with CatWalk parameters normally decreased in animals in which allodynia is developing (Stance, Print Intensity, Print and Max area).50,51
Data obtained in the present study demonstrate that PROG improved locomotor outcome after thoracic contusion. There is no general consensus about the functional effects of PROG after SCI, however. While improvement of motor and histological outcome has been reported in one study,87 no recovery is observed in another.88 The discrepancies between our present investigation and the report by Fee and colleagues88 may be mainly because of the duration of PROG treatment. They performed an acute treatment of 5 days, while we treated the rats with PROG during all the experiment (60 days), suggesting that a long-term treatment is needed to observe the behavior outcome.
Conclusion
The current study adds further evidence to support PROG as a potential effective treatment after SCI, as it has been used for traumatic brain injury.
We suggest that beneficial actions of PROG on locomotor outcome could be related to the reduction of secondary damage and the preservation or regeneration of axons and myelin of the descending pathways, and with the increase of oligodendrocytes number, either by inhibiting their apoptosis or promoting their differentiation. Most of the clinical trials for SCI are directed to limiting secondary injury to prevent neurological function loss and to provide the anatomical substrate for further reparation.89 In this line of evidence, PROG treatment holds promise for success. In fact, multi-potential drugs such as PROG, which have pleiotrophic sites of action and modulate multiple injury mechanisms, could be the focus of future clinical trials in spinal cord trauma.
Acknowledgments
We thank Dr. Angel Arevalo-Martin for his generous assistance with MRI interpretation and his contribution to the development of some quantification procedures. We also are grateful to Dr. Jorge Collazos-Castro for his selfless help and generosity in sharing his knowledge. We thank Javier Mazarío for his generous assistance with thermical and mechanical sensitivity tests. We thank Marién Fernandez, David Castejón, and Palmira Villa (CAI de Resonancia Magnética, Instituto Pluridisciplinar, Universidad Complutense de Madrid) for technical assistance with the MRI. This work was supported by the University of Buenos Aires (UBACyT 2011–2014: 20020100200053 - / UBACyT 2011–2014: 20020100100089). B. Paniagua was partially funded by Wings for Life Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ambrozaitis K.V., Kontautas E., Spakauskas B., and Vaitkaitis D. (2006). [Pathophysiology of acute spinal cord injury.] (Lith) Medicina (Kaunas) 42, 255–261 [PubMed] [Google Scholar]
- 2.Beattie M.S., Farooqui A.A., and Bresnahan J.C. (2000). Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma 17, 915–925 [DOI] [PubMed] [Google Scholar]
- 3.Borgens R.B., and Liu-Snyder P. (2012). Understanding secondary injury. Q. Rev. Biol. 87, 89–127 [DOI] [PubMed] [Google Scholar]
- 4.Kwon B.K., Okon E., Hillyer J., Mann C., Baptiste D., Weaver L.C., Fehlings M.G., and Tetzlaff W. (2011). A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 28, 1545–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammertse D.P. (2013). Clinical trials in spinal cord injury: lessons learned on the path to translation. The 2011 International Spinal Cord Society Sir Ludwig Guttmann Lecture. Spinal Cord51, 2–9 [DOI] [PubMed] [Google Scholar]
- 6.Rabchevsky A.G., Patel S.P., and Springer J.E. (2011). Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol. Ther. 132, 15–29 [DOI] [PubMed] [Google Scholar]
- 7.Bracken M.B. (1990). Methylprednisolone in the management of acute spinal cord injuries. Med. J. Aust. 153, 368. [PubMed] [Google Scholar]
- 8.Bracken M.B., Shepard M.J., Holford T.R., Leo-Summers L., Aldrich E.F., Fazl M., Fehlings M., Herr D.L., Hitchon P.W., Marshall L.F., Nockels R.P., Pascale V., Perot P.L., Jr., Piepmeier J., Sonntag V.K., Wagner F., Wilberger J.E., Winn H.R., and Young W. (1997). Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277, 1597–1604 [PubMed] [Google Scholar]
- 9.Ciriza I., Carrero P., Frye C.A. and Garcia-Segura L.M. (2006). Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J. Neurobiol. 66, 916–928 [DOI] [PubMed] [Google Scholar]
- 10.De Nicola A.F., Labombarda F., Deniselle M.C., Gonzalez S.L., Garay L., Meyer M., Gargiulo G., Guennoun R., and Schumacher M. (2009). Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front. Neuroendocrinol. 30, 173–187 [DOI] [PubMed] [Google Scholar]
- 11.Giatti S., Caruso D., Boraso M., Abbiati F., Ballarini E., Calabrese D., Pesaresi M., Rigolio R., Santos-Galindo M., Viviani B., Cavaletti G., Garcia-Segura L.M., and Melcangi R.C. (2012). Neuroprotective effects of progesterone in chronic experimental autoimmune encephalomyelitis. J. Neuroendocrinol. 24, 851–861 [DOI] [PubMed] [Google Scholar]
- 12.Schumacher M., Hussain R., Gago N., Oudinet J.P., Mattern C., and Ghoumari A.M. (2012). Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front. Neurosci. 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettus E.H., Wright D.W., Stein D.G., and Hoffman S.W. (2005). Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 1049, 112–119 [DOI] [PubMed] [Google Scholar]
- 14.Robertson C.L., Puskar A., Hoffman G.E., Murphy A.Z., Saraswati M., and Fiskum G. (2006). Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp. Neurol. 197, 235–243 [DOI] [PubMed] [Google Scholar]
- 15.Stein D.G. (2008). Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev. 57, 386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein D.G. (2011). Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience 191, 101–106 [DOI] [PubMed] [Google Scholar]
- 17.Wright D.W., Kellermann A.L., Hertzberg V.S., Clark P.L., Frankel M., Goldstein F.C., Salomone J.P., Dent L.L., Harris O.A., Ander D.S., Lowery D.W., Patel M.M., Denson D.D., Gordon A.B., Wald M.M., Gupta S., Hoffman S.W., and Stein D.G. (2007). ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 49, 391–402, 402 e1–2 [DOI] [PubMed] [Google Scholar]
- 18.Xiao G., Wei J., Yan W., Wang W., and Lu Z. (2008). Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit. Care 12, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez S.L., Labombarda F., Gonzalez Deniselle M.C., Guennoun R., Schumacher M., and De Nicola A.F. (2004). Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience 125, 605–614 [DOI] [PubMed] [Google Scholar]
- 20.Labombarda F., Gonzalez S.L., Gonzalez D.M., Guennoun R., Schumacher M., and de Nicola A.F. (2002). Cellular basis for progesterone neuroprotection in the injured spinal cord. J. Neurotrauma 19, 343–355 [DOI] [PubMed] [Google Scholar]
- 21.Labombarda F., Gonzalez S.L., Lima A., Roig P., Guennoun R., Schumacher M., and de Nicola A.F. (2009). Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia 57, 884–897 [DOI] [PubMed] [Google Scholar]
- 22.Labombarda F., Gonzalez S., Lima A., Roig P., Guennoun R., Schumacher M., and De Nicola A.F. (2011). Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp. Neurol. 231, 135–146 [DOI] [PubMed] [Google Scholar]
- 23.Arevalo-Martin A., Garcia-Ovejero D., Sierra-Palomares Y., Paniagua-Torija B., Gonzalez-Gil I., Ortega-Gutierrez S., and Molina-Holgado E. (2012). Early endogenous activation of CB1 and CB2 receptors after spinal cord injury is a protective response involved in spontaneous recovery. PLoS One 7, e49057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Ovejero D., Arevalo-Martin A., Paniagua-Torija B., Sierra-Palomares Y., and Molina-Holgado E. (2013). A cell population that strongly expresses the CB1 cannabinoid receptor in the ependyma of the rat spinal cord. J. Comp. Neurol. 521, 233–251 [DOI] [PubMed] [Google Scholar]
- 25.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 26.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 27.Basso D.M., Beattie M.S., and Bresnahan J.C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 [DOI] [PubMed] [Google Scholar]
- 28.Cutler S.M., Cekic M., Miller D.M., Wali B., VanLandingham J.W., and Stein D.G. (2007). Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma 24, 1475–1486 [DOI] [PubMed] [Google Scholar]
- 29.Labombarda F., Gonzalez S., Gonzalez Deniselle M.C., Garay L., Guennoun R., Schumacher M., and De Nicola A.F. (2006). Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J. Neurotrauma 23, 181–192 [DOI] [PubMed] [Google Scholar]
- 30.Arevalo-Martin A., Garcia-Ovejero D., and Molina-Holgado E. (2010). The endocannabinoid 2-arachidonoylglycerol reduces lesion expansion and white matter damage after spinal cord injury. Neurobiol. Dis. 38, 304–312 [DOI] [PubMed] [Google Scholar]
- 31.Ma M., Basso D.M., Walters P., Stokes B.T., and Jakeman L.B. (2001). Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol. 169, 239–254 [DOI] [PubMed] [Google Scholar]
- 32.Schmitz C., and Hof P.R. (2005). Design-based stereology in neuroscience. Neuroscience 130, 813–831 [DOI] [PubMed] [Google Scholar]
- 33.Koopmans G.C., Brans M., Gomez-Pinilla F., Duis S., Gispen W.H., Torres-Aleman I., Joosten E.A. and Hamers F.P. (2006). Circulating insulin-like growth factor I and functional recovery from spinal cord injury under enriched housing conditions. Eur. J. Neurosci. 23, 1035–1046 [DOI] [PubMed] [Google Scholar]
- 34.Basso D.M. (2004). Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma 21, 395–404 [DOI] [PubMed] [Google Scholar]
- 35.Hamers F.P., Koopmans G.C., and Joosten E.A. (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma 23, 537–548 [DOI] [PubMed] [Google Scholar]
- 36.Hamers F.P., Lankhorst A.J., van Laar T.J., Veldhuis W.B., and Gispen W.H. (2001). Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma 18, 187–201 [DOI] [PubMed] [Google Scholar]
- 37.Lankhorst A.J., ter Laak M.P., van Laar T.J., van Meeteren N.L., de Groot J.C., Schrama L.H., Hamers F.P., and Gispen W.H. (2001). Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J. Neurotrauma 18, 203–215 [DOI] [PubMed] [Google Scholar]
- 38.Singh A., Murray M., and Houle J.D. (2011). A training paradigm to enhance motor recovery in contused rats: effects of staircase training. Neurorehabil. Neural Repair 25, 24–34 [DOI] [PubMed] [Google Scholar]
- 39.Vlamings R., Visser-Vandewalle V., Koopmans G., Joosten E.A., Kozan R., Kaplan S., Steinbusch H.W., and Temel Y. (2007). High frequency stimulation of the subthalamic nucleus improves speed of locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience 148, 815–823 [DOI] [PubMed] [Google Scholar]
- 40.Dolan S., and Nolan A.M. (2007). Blockade of metabotropic glutamate receptor 5 activation inhibits mechanical hypersensitivity following abdominal surgery. Eur. J. Pain 11, 644–651 [DOI] [PubMed] [Google Scholar]
- 41.Obata K., Yamanaka H., Kobayashi K., Dai Y., Mizushima T., Katsura H., Fukuoka T., Tokunaga A., and Noguchi K. (2004). Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 24, 10211–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hargreaves K., Dubner R., Brown F., Flores C., and Joris J. (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 [DOI] [PubMed] [Google Scholar]
- 43.Bodley R. (2002). Imaging in chronic spinal cord injury—indications and benefits. Eur. J. Radiol. 42, 135–153 [DOI] [PubMed] [Google Scholar]
- 44.Goldberg A.L., Daffner R.H., and Schapiro R.L. (1990). Imaging of acute spinal trauma: an evolving multi-modality approach. Clin. Imaging 14, 11–16 [DOI] [PubMed] [Google Scholar]
- 45.Parashari U.C., Khanduri S., Bhadury S., Kohli N., Parihar A., Singh R., Srivastava R.N., and Upadhyay D. (2011). Diagnostic and prognostic role of MRI in spinal trauma, its comparison and correlation with clinical profile and neurological outcome, according to ASIA impairment scale. J. Craniovertebr. Junction Spine 2, 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballermann M., and Fouad K. (2006). Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci. 23, 1988–1996 [DOI] [PubMed] [Google Scholar]
- 47.Raineteau O., Fouad K., Bareyre F.M., and Schwab M.E. (2002). Reorganization of descending motor tracts in the rat spinal cord. Eur. J. Neurosci. 16, 1761–1771 [DOI] [PubMed] [Google Scholar]
- 48.Van Meeteren N.L., Eggers R., Lankhorst A.J., Gispen W.H., and Hamers F.P. (2003). Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J. Neurotrauma 20, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 49.Kloos A.D., Fisher L.C., Detloff M.R., Hassenzahl D.L., and Basso D.M. (2005). Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 191, 251–265 [DOI] [PubMed] [Google Scholar]
- 50.Deumens R., Jaken R.J., Marcus M.A., and Joosten E.A. (2007). The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J. Neurosci. Methods 164, 120–130 [DOI] [PubMed] [Google Scholar]
- 51.Vrinten D.H., and Hamers F.F. (2003). 'CatWalk' automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain 102, 203209. [DOI] [PubMed] [Google Scholar]
- 52.Guo Q., Sayeed I., Baronne L.M., Hoffman S.W., Guennoun R., and Stein D.G. (2006). Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp. Neurol. 198, 469–478 [DOI] [PubMed] [Google Scholar]
- 53.Roof R.L., and Hall E.D. (2000). Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388 [DOI] [PubMed] [Google Scholar]
- 54.Garay L.I., Gonzalez Deniselle M.C., Brocca M.E., Lima A., Roig P., and De Nicola A.F. (2012). Progesterone down-regulates spinal cord inflammatory mediators and increases myelination in experimental autoimmune encephalomyelitis. Neuroscience 226, 40–50 [DOI] [PubMed] [Google Scholar]
- 55.Grossman K.J., Goss C.W., and Stein D.G. (2004). Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 1008, 29–39 [DOI] [PubMed] [Google Scholar]
- 56.Lambert J.J., Belelli D., Peden D.R., Vardy A.W., and Peters J.A. (2003). Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 71, 67–80 [DOI] [PubMed] [Google Scholar]
- 57.Labombarda F., Pianos A., Liere P., Eychenne B., Gonzalez S., Cambourg A., De Nicola A.F., Schumacher M., and Guennoun R. (2006). Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology 147, 1847–1859 [DOI] [PubMed] [Google Scholar]
- 58.Ciriza I., Azcoitia I., and Garcia-Segura L.M. (2004). Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J. Neuroendocrinol. 16, 58–63 [DOI] [PubMed] [Google Scholar]
- 59.Bresnahan J.C., Beattie M.S., Todd F.D., 3rd, and Noyes D.H. (1987). A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Exp. Neurol. 95, 548–570 [DOI] [PubMed] [Google Scholar]
- 60.Fehlings M.G., and Tator C.H. (1995). The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 132, 220–228 [DOI] [PubMed] [Google Scholar]
- 61.Schucht P., Raineteau O., Schwab M.E. and Fouad K. (2002). Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 [DOI] [PubMed] [Google Scholar]
- 62.Basso D.M., Beattie M.S., and Bresnahan J.C. (2002). Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: experimental evidence and a review of literature. Restor. Neurol. Neurosci. 20, 189–218 [PubMed] [Google Scholar]
- 63.Azcoitia I., Leonelli E., Magnaghi V., Veiga S., Garcia-Segura L.M., and Melcangi R.C. (2003). Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol. Aging 24, 853–860 [DOI] [PubMed] [Google Scholar]
- 64.Gago N., Akwa Y., Sananes N., Guennoun R., Baulieu E.E., El-Etr M., and Schumacher M. (2001). Progesterone and the oligodendroglial lineage: stage-dependent biosynthesis and metabolism. Glia 36, 295–308 [DOI] [PubMed] [Google Scholar]
- 65.Garay L., Tungler V., Deniselle M.C., Lima A., Roig P., and De Nicola A.F. (2011). Progesterone attenuates demyelination and microglial reaction in the lysolecithin-injured spinal cord. Neuroscience 192, 588–597 [DOI] [PubMed] [Google Scholar]
- 66.Ghoumari A.M., Baulieu E.E., and Schumacher M. (2005). Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience 135, 47–58 [DOI] [PubMed] [Google Scholar]
- 67.Leonelli E., Ballabio M., Consoli A., Roglio I., Magnaghi V., and Melcangi R.C. (2006). Neuroactive steroids: A therapeutic approach to maintain peripheral nerve integrity during neurodegenerative events. J. Mol. Neurosci. 28, 65–76 [DOI] [PubMed] [Google Scholar]
- 68.Huang W.L., King V.R., Curran O.E., Dyall S.C., Ward R.E., Lal N., Priestley J.V., and Michael-Titus A.T. (2007). A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain 130, 3004–3019 [DOI] [PubMed] [Google Scholar]
- 69.Stirling D.P., Khodarahmi K., Liu J., McPhail L.T., McBride C.B., Steeves J.D., Ramer M.S., and Tetzlaff W. (2004). Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 24, 2182–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rabchevsky A.G., Sullivan P.G. and Scheff S.W. (2007). Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia 55, 831–843 [DOI] [PubMed] [Google Scholar]
- 71.Coleman M.P., and Perry V.H. (2002). Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 25, 532–537 [DOI] [PubMed] [Google Scholar]
- 72.Flygt J., Djupsjo A., Lenne F., and Marklund N. (2013). Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. Eur. J. Neurosci. 38, 2153–2165 [DOI] [PubMed] [Google Scholar]
- 73.Nguyen T., Mehta N.R., Conant K., Kim K.J., Jones M., Calabresi P.A., Melli G., Hoke A., Schnaar R.L., Ming G.L., Song H., Keswani S.C., and Griffin J.W. (2009). Axonal protective effects of the myelin-associated glycoprotein. J. Neurosci. 29, 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magnuson D.S., Trinder T.C., Zhang Y.P., Burke D., Morassutti D.J., and Shields C.B. (1999). Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 156, 191–204 [DOI] [PubMed] [Google Scholar]
- 75.Basso D.M. (2000). Neuroanatomical substrates of functional recovery after experimental spinal cord injury: implications of basic science research for human spinal cord injury. Phys. Ther. 80, 808–817 [PubMed] [Google Scholar]
- 76.Reier P.J., Golder F.J., Bolser D.C., Hubscher C., Johnson R., Schrimsher G.W., and Velardo M.J. (2002). Gray matter repair in the cervical spinal cord. Prog. Brain Res. 137, 49–70 [DOI] [PubMed] [Google Scholar]
- 77.Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., and Joosten E.A. (2005). The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma 22, 214–225 [DOI] [PubMed] [Google Scholar]
- 78.Barriere G., Leblond H., Provencher J., and Rossignol S. (2008). Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J. Neurosci. 28, 3976–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarac F. (2008). Some historical reflections on the neural control of locomotion. Brain Res. Rev. 57, 13–21 [DOI] [PubMed] [Google Scholar]
- 80.Guertin P.A. (2009). The mammalian central pattern generator for locomotion. Brain Res. Rev. 62, 45–56 [DOI] [PubMed] [Google Scholar]
- 81.Pearson K.G., and Rossignol S. (1991). Fictive motor patterns in chronic spinal cats. J. Neurophysiol. 66, 1874–1887 [DOI] [PubMed] [Google Scholar]
- 82.Bareyre F.M., Kerschensteiner M., Raineteau O., Mettenleiter T.C., Weinmann O., and Schwab M.E. (2004). The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 [DOI] [PubMed] [Google Scholar]
- 83.Alsina B., Vu T., and Cohen-Cory S. (2001). Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat. Neurosci. 4, 1093–1101 [DOI] [PubMed] [Google Scholar]
- 84.Hendriks W.T., Eggers R., Ruitenberg M.J., Blits B., Hamers F.P., Verhaagen J., and Boer G.J. (2006). Profound differences in spontaneous long-term functional recovery after defined spinal tract lesions in the rat. J. Neurotrauma 23, 18–35 [DOI] [PubMed] [Google Scholar]
- 85.Joosten E.A., Veldhuis W.B., and Hamers F.P. (2004). Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J. Neurosci. Res. 77, 127–142 [DOI] [PubMed] [Google Scholar]
- 86.Deumens R., Koopmans G.C., Honig W.M., Hamers F.P., Maquet V., Jerome R., Steinbusch H.W., and Joosten E.A. (2006). Olfactory ensheathing cells, olfactory nerve fibroblasts and biomatrices to promote long-distance axon regrowth and functional recovery in the dorsally hemisected adult rat spinal cord. Exp. Neurol. 200, 89–103 [DOI] [PubMed] [Google Scholar]
- 87.Thomas A.J., Nockels R.P., Pan H.Q., Shaffrey C.I., and Chopp M. (1999). Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine 24, 2134–2138 [DOI] [PubMed] [Google Scholar]
- 88.Fee D.B., Swartz K.R., Joy K.M., Roberts K.N., Scheff N.N., and Scheff S.W. (2007). Effects of progesterone on experimental spinal cord injury. Brain Res. 1137, 146–152 [DOI] [PubMed] [Google Scholar]
- 89.Cadotte D.W., and Fehlings M.G. (2011). Spinal cord injury: a systematic review of current treatment options. Clin. Orthop. Relat. Res. 469, 732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]