Abstract
Background: Improving the carbohydrate quality of the diet by replacing the common cereal staple white rice (WR) with brown rice (BR) could have beneficial effects on reducing the risk for diabetes and related complications. Hence we aimed to compare the effects of BR, WR, and BR with legumes (BRL) diets on 24-h glycemic and insulinemic responses among overweight Asian Indians.
Subjects and Methods: Fifteen overweight (body mass index, ≥23 kg/m2) Asian Indians without diabetes who were 25–45 years old participated in a randomized crossover study. Test meals (nonisocaloric, ad libitum) were identical except for the type of rice and the addition of legumes (50 g/day) and were provided for 5 consecutive days. Glucose profiles were assessed using the Medtronic MiniMed (Northridge, CA) iPro™2 continuous glucose monitoring device. The mean positive change from baseline glucose concentration was calculated as the daily incremental area under the curve (IAUC) on each test day for 5 days and averaged. Fasting serum insulin was measured prior to and at the end of each test diet.
Results: The percentage difference in 5-day average IAUC was 19.8% lower in the BR group than in the WR group (P=0.004). BRL further decreased the glycemic response (22.9% lower compared with WR (P=0.02). The 5-day percentage change in fasting insulin was 57% lower (P=0.0001) for the BR group and 54% lower for the BRL group compared with the 5-day percentage change observed in the WR group. The glycemic and insulinemic responses to the BR and BRL diets were not significantly different.
Conclusions: Consumption of BR in place of WR can help reduce 24-h glucose and fasting insulin responses among overweight Asian Indians.
Introduction
World rice production has grown from 468.5 million tons in the year 2010 to 482.7 metric tons in 2011 (2.6% increase).1 India is the second largest producer of rice in the world, next to China. Cereals such as rice and wheat are staple foods in developing countries, including India.2 Although rice has been a staple food for thousands of years in India,3 advancements in milling technologies to improve yield and shelf life4 have led to highly polished, starchy white rice (WR). Unlike unpolished brown rice (BR), WR lacks phytochemicals such as polyphenols, oryzanol, phytosterols, tocotrienols, tocopherols, and carotenoids, as well as vitamins and minerals that confer protection against heart disease and cancer.5,6
Indian diets are high in carbohydrates, and cereals continue to be the staple food, contributing to two-thirds of the total carbohydrates consumed.7 Traditionally, cereals were processed for consumption by labor-intensive hand-pounding. Today, Asian Indian diets have undergone a rapid nutrition transition, resulting in an increased consumption of refined carbohydrates, unhealthy fats, and foods of animal origin, which are associated with an increased risk of obesity and type 2 diabetes.8 A recent national survey estimated that there were 62.4 and 77.2 million people with diabetes and prediabetes, respectively, in India.9 Obesity (body mass index [BMI], ≥25 kg/m2),10 an independent risk for type 2 diabetes, is also alarmingly high (24.6% of men and 31.2% of women) among the urban Indian adult population.11 Studies on the health impact of refined carbohydrates and of whole cereals are of great importance in the context of the escalating obesity epidemic and related chronic diseases like type 2 diabetes. The glycemic index (GI) values of WR vary and are influenced by several factors, including the degree of processing, amylose content, and cooking time. However, GI values of popular Indian WR varieties were estimated to be higher (in the range of 70–77)12 compared with whole grains such as BR and legumes, which have GI values (according to the 2008 international GI table) ranging from 50 to 87 and 10 to 70, respectively.13
The GI only measures only a 2-h glycemic response. In contrast, measuring a day-long glycemic response may provide additional key information necessary for understanding the role of GI and glycemic load (GL) (the product of both quantity of carbohydrate and GI of the food) in high-carbohydrate diets. Studies have assessed the beneficial effects of low GI diets on 24-h glucose profiles using continuous glucose monitoring (CGM).14–16 However, no CGM study has assessed the effects of replacing cereal staples like rice in general and in particular among Asian Indians. Hence, we aimed to evaluate the effect of replacing WR with BR and BR with legumes (BRL) on the 24-h glycemic response among overweight and obese Asian Indian adults.
Subjects and Methods
Participants
Fifteen volunteers (seven males and eight females) 25–45 years of age with a BMI of ≥23 kg/m2 were recruited from Dr. Mohan's Diabetes Specialties Centre and Madras Diabetes Research Foundation (Gopalapuram, Chennai, India). Volunteers with a BMI of <23 kg/m2,10 known diabetes, or any other medical comorbidity and not on any medication or with fasting blood glucose ≥7 mmol/L (≥126 mg/dL) were excluded from the study.
CGM protocol
The participants were initially screened to ensure that no exclusion criteria applied. Participants were given the full details of the study protocol and viewed a presentation about CGM technology, during which time they were encouraged to ask questions. Informed consent was obtained from all volunteers prior to participation. The study was approved by the institutional ethical committee of the Madras Diabetes Research Foundation and was conducted in accordance with guidelines in the Declaration of Helsinki.
The iPro™2 CGM device (iPro2 Professional CGM; Medtronic MiniMed, Northridge, CA) was used to obtain continuous interstitial glucose readings. This evaluation system is based on electrochemical detection of subcutaneous interstitial fluid. The CGM sensor was inserted in the side abdominal area of the body. The CGM system records and stores 288 glucose readings during a 24-h period as the participants perform their routine daily activities. The sensor was calibrated using finger-prick capillary blood glucose measurements taken at fasting and pre- and postmeals (breakfast, lunch, and dinner) during the study period by using a HemoCue® 201+ glucose analyzer (HemoCue Ltd., Ängelholm, Sweden). Interstitial glucose values from CGM have been shown to accurately record the changes and to correlate with plasma glucose values.17 The iPro2 was worn unobtrusively for 5 complete days. The following day participants returned the iPro2 recorder for upload of the data to a Web-based software, which provided a summary of the glucose responses. Participants were encouraged to discuss any concerns they had about any aspect of the dietary intervention or study protocol.
Dietary intervention
Fifteen subjects were randomly allocated to the possible ordering of the BR, WR, and BRL diets in a randomized crossover design. There are six possible orderings of the three diets in this crossover trial. At the time of enrollment, two participants were randomly assigned to BR/WR/BRL, two to BR/BRL/WR, three to WR/BRL/BR, two to WR/BR/BRL, four to BRL/WR/BR, and two to BRL/BR/WR. The test diets aimed to replace rice in two meals (breakfast and lunch) and consisted of typical South Indian breakfast and lunch food choices with suitable accompaniments. Participants were instructed to have their usual dinner (not provided as part of the trial diets). A brief description of these food items is given in Supplementary Tables S1 and S2 (Supplementary Data are available online at www.liebertonline.com/dia). Except for the type of rice and addition of a 50-g portion of legumes in the BRL diet, the menus (breakfast and lunch) were identical for BR, WR, and BRL diets. Participants were instructed to consume the meals ad libitum. Our previous study reported that the mean intake of refined grains in this South Indian population was 333 g/day, of which WR was the major contributor (253 g/day).18 Based on these findings, 200 g of WR replaced with an equal amount of BR was targeted as the minimum quantity to be provided in breakfast and lunch meals together. A washout period of 9 days was given between the diets in order to minimize a potential carryover effect. The test diets were standardized, prepared, and served in the test kitchen at the Madras Diabetes Research Foundation. The nutritional profiles of the test foods included in the meal plan are shown in Supplementary Table S3.
Volunteers were instructed to continue their usual lifestyle and were advised to follow their routine dinner practices during the study and in the washout period. However, they were requested to refrain from smoking and alcohol use as well as strenuous exercise during the study period. Dietary intake during the study period was ascertained with a validated food frequency questionnaire at baseline, and 24-h dietary recalls were collected for 6 consecutive days (1 pretest day and 5 test days) during the intervention period to ensure compliance with the study and to record foods consumed for dinner, which was not part of the test diets. The energy intake and nutrient composition of the diet of the volunteers was determined using EpiNU, an in-house database.
Clinical measurements
Blood pressure was measured using an electronic OMRON machine (Omron Corp., Tokyo, Japan). Anthropometric measurements such as height, weight, and waist circumference were measured, and BMI was assessed for all participants using standardized techniques.19
Blood samples were drawn from an antecubital vein after 10–12 h of fasting to measure serum insulin levels. Insulin levels were assessed at the beginning (Day 1) and at the end (Day 6) of the respective test diet periods using standardized techniques (electrochemiluminescence assay for plasma insulin). The intra- and interassay coefficients of variation for insulin were 1.3% and 1.9%, respectively.
Statistical analysis
Data were analyzed with the Statistical Analysis Systems software package (version 9.2; SAS Institute, Inc., Cary, NC). The effect of BR and BRL diets was considered in relation to the WR diet by evaluating the within-subject differences observed in the positive change from baseline of the incremental area under the curve (IAUC) for interstitial glucose between the BR and BRL diets compared with that of the WR diet. We used mixed linear models to estimate the between-diet differences taking into account repeated measures within participants, with the robust score test to assess the significance of any difference observed.20 We considered the 5-day average IAUC as the primary outcome of this study, based on the CGM measurements. Some 5-min CGM readings were missing during the 5-day study period for some participants, and hence we standardized the summary outcome variables by dividing by the number of CGM measurements available. To determine the daily baseline value for each participant, we used the average of each day's first 2 h of CGM readings under the fasting state. The daily IAUC for each participant was then calculated from the 22 subsequent h of CGM data. The daily positive area under the curve (AUC) was the sum of all observed 5-min CGM measurement standardized by the number of measurements available. Only those measurements greater than the average baseline AUC, standardized to the number of available measurements during this period, were included in the calculation of the daily positive AUC. This daily positive AUC was the dependent variable in the model used to assess the statistical significance of any differences in AUCs observed between the diets and to estimate the percentage difference in daily positive AUCs between diets and their 95% confidence interval (CI). We also assessed the significance of differences in the change of fasting insulin level from baseline of the BR or BRL diet compared with the WR diet using mixed linear models with the robust score test.
Results
The experimental protocol was completed by all the study participants, and no one was excluded from the study as long as he or she had at least one reading of CGM data for the BR or BRL diet. All participants had complete data for the WR diet. However, one participant during the BRL diet intervention period had data for 4.5 days, and another six participants had data ranging from 0.5 day to 4.8 days during the BR diet because of technical problems. Baseline BMI and age were similar between those participants missing one or more of the 5-min CGM measurements during the course of this study, compared with those who did not miss any. Missing one or more 5-min CGM measurements was confined to the female participants (P=0.014 by Fisher's exact test). Insulin data were obtained in all 15 volunteers for the three diet intervention periods. The overall baseline mean (SD) for glucose was 47.2 (18.4) mg/dL.
Baseline anthropometric and dietary profiles of study participants are shown in Table 1. Over 50% of the participants were female, and 73% were centrally obese using the Asia Pacific cutoff for excess waist circumference of ≥80 cm for females and ≥90 cm for males.16 Mean BMI and waist circumference of the participants were 27.4±2.3 kg/m2 and 90.4±8.2 cm, respectively. The reported mean energy intake assessed using the food frequency questionnaire was 2,918±842 kcal/day. Carbohydrate was the major contributor of energy, providing 60% of total energy intake, followed by fat (28% of total energy intake) and protein (12% of total energy intake). Refined cereal was the major contributor (39%) of daily energy, followed by fats and oil (13% of total energy intake). In this study group, whole cereals contributed only 2.6% of energy.
Table 1.
Demographic/Anthropometric Characteristics and Dietary Profile of Study Participants (n=15)
| Characteristic | Value |
|---|---|
| Female [% (n)] | 53.3 (8) |
| Age (years) | 33.2±4.6 |
| BMI (kg/m2) | 27.4±2.3 |
| Overweight (BMI <25 kg/m2) [% (n)] | 20.0 (3) |
| Obese (BMI ≥25 kg/m2) [% (n)] | 80.0 (12) |
| Waist circumference (cm) | 90.4±8.2 |
| Central obesity [% (n)]a | 73.3 (11) |
| Food and nutrient output based on FFQ | |
| Energy (kcal/day) | 2,918±842 |
| Carbohydrates (g/day) (%E) | 437.5±123.3 (60.3) |
| Protein (g/day) (%E) | 87.3±27 (11.9) |
| Fat (g/day) (%E) | 90±29.1 (27.7) |
| Dietary fiber (g/day) | 38.6±12.4 |
| Glycemic load | 251.5±76.0 |
| Weighted GI | 63.9±2.5 |
| Refined cereals (g/day) | 326.4±107.1 |
| Cereals whole milled (g/day) | 21±14.8 (2.6%) |
| Millet (g/day) | |
| Milled | 2.6±4.8 |
| Whole | 0.3±0.2 |
| Legumes (g/day) | |
| Split | 64.2±27.3 |
| Whole | 6.4±2.3 |
| Fruits and vegetables (g/day)b | 263.8±94.9 |
| Roots and tubers (g/day) | 156.3±68.1 |
| Milk and its products (g/day)c | 331.9±178.9 |
| Fish and seafoods (g/day) | 31.3±40.5 |
| Meat (g/day) | 16.02±24.6 |
| Egg (g/day) | 17.7±14.9 |
| Poultry (g/day) | 27.1±16.2 |
| Fats and oils (g/day) | 44.1±16.4 |
| Nuts and oilseed (g/day) | 45.8±20.5 |
| Salt (g/day) | 10.8±3.24 |
| Sugar (g/day) | 28.9±18.47 |
Data are mean±SD values except as indicated otherwise.
Central obesity as defined by a waist cirucmference of ≥90 cm in males and ≥80 cm in females.
Fruits and vegetables include fruits, leafy vegetables, and other vegetables.
Milk and its products include milk, cheese, yogurt, and buttermilk.
%E, percentage of total energy; BMI, body mass index; FFQ, food frequency questionnaire; GI, glycemic index.
Dietary intake assessed using multiple 24-h recalls (five recalls per diet period) during the study period is given in Table 2. Although this study was based on an ad libitum dietary regimen, there were no significant differences in the energy, carbohydrate, or protein intake across the three diets during the entire study period. Similarly, no significant differences were observed in intake across the three diets from food groups including fruits and vegetables, roots and tubers, visible fats and oils, dairy, nuts and oilseeds, salt, and sugar. We evaluated the GI of the WR used (BPT raw) in the study and that of BR (BPT parboiled), which were 82.5 and 58.7, respectively. The amounts consumed of dietary fiber, glycemic load, weighted GI, and refined cereals, whole cereal, and legume intake during the BR and BRL diet periods differed significantly from those of the WR diet (P<0.001), partly because of the study design that replaced WR (higher GI and GL and lower fiber) with BR and BRL diets (Table 2). It was also evident that when WR was replaced with BR, the GL of the participants dropped by nearly 20% during both the BR and BRL diets. Whole cereals were the major contributor of the GL in the BR (49.4%) and BRL (48.8%) diets, whereas in the WR diet, refined cereals contributed 72% of GL (data not shown).
Table 2.
Nutrient and Food Intake of Study Participants During Each of the Dietary Intervention Periods (n= 15)
| BR | WR | BRL | |||||
|---|---|---|---|---|---|---|---|
| Nutrient | Median | IQR | Median | IQR | Median | IQR | P valuea |
| Energy (kcal/day) | 2,513 | 634 | 2,607 | 845 | 2,521 | 621 | 0.94 |
| Carbohydrates (g/day) (%E) | 361 (58.2) | 99.9 | 381.2 (60.8) | 144 | 365.5 (58.4) | 110.8 | 0.420 (0.001) |
| Protein (g/day) (%E) | 80.1 (12.5) | 17.2 | 79 (12.0) | 28.6 | 74.8 (12.2) | 18.5 | 0.449 (0.074) |
| Fat (g/day) (%E) | 80.3 (28.1) | 17.7 | 79.1 (25.8) | 24 | 80.2 (28.3) | 18.6 | 0.038 (0.002) |
| Dietary fiber (g/day) | 48.9 | 9.4 | 36.1 | 14.1 | 56 | 13.7 | <0.001 |
| Glycemic load | 183 | 54.7 | 228.2 | 87 | 184.5 | 62.5 | <0.001 |
| Weighted GI | 56.1 | 3.8 | 66.6 | 1.5 | 57.5 | 2.3 | <0.001 |
| Cereals (g/day) (%E) | |||||||
| Refined | 93.7 (13.7) | 93.3 | 298.4 (42.4) | 90.6 | 106.5 (14.8) | 73.6 | <0.001 (<0.001) |
| Whole | 246.2 (34.5) | 58.1 | 0 | 20 | 244.9 (32.0) | 21.5 | <0.001 (<0.001) |
| Legumes (g/day) (%E) | |||||||
| Split | 65.4 (9.6) | 22.5 | 77.6 (10.4) | 29.5 | 24.4 (3.97) | 23.2 | <0.001 (<0.001) |
| Whole | 25.4 (1.3) | 10.1 | 26.2 (0.98) | 16.9 | 59.6 (6.94) | 17.6 | <0.001 (<0.001) |
| Fruits and vegetables (g/day) (%E)b | 256.2 (3.5) | 71.3 | 233 (3.6) | 93.2 | 235.2 (3.07) | 45.6 | 0.282 (0.549) |
| Roots and tubers (g/day) (%E) | 216.5 (5.7) | 51.6 | 191.6 (5.5) | 91.7 | 222.4 (5.56) | 53.0 | 0.936 (0.420) |
| Milk and its products (g/day) (%E)c | 350.9 (9.0) | 149.5 | 350.4 (8.4) | 148.2 | 347.4 (8.86) | 195.1 | 0.819 (0.936) |
| Fish and sea foods (g/day | 0 | 0 | 0 | 15 | 0 | 0 | 0.662 |
| Meat (g/day) | 0 | 0 | — | — | 0 | 0 | 0.368 |
| Eggs (g/day) | 0 | 0 | 0 | 6 | 0 | 9 | 0.852 |
| Poultry (g/day) | 0 | 0 | 0 | 6 | 0 | 0 | 0.779 |
| Fats and oils (g/day) (%E) | 44.2 (16.0) | 21.4 | 44.5 (15.64) | 21.9 | 42.8 (14.9) | 10.5 | 0.55 (0.766) |
| Nuts and oilseeds (g/day) (%E) | 31.3 (5.7) | 13.3 | 27.5 (6.01) | 19.8 | 30.8 (5.9) | 19.1 | 0.189 (0.282) |
| Salt (g/day) | 15.9 | 3.3 | 15 | 4.7 | 15.9 | 2.7 | 0.42 |
| Sugar (g/day) (%E) | 18.2 (3.2) | 9.9 | 22.8 (4.02) | 23.8 | 24.8 (3.4) | 20.7 | 0.819 (0.627) |
Data are average values of five 24-h diet recalls.
P value was tested using Friedman's nonparametric test for significance.
Fruit and vegetables include fruits, leafy vegetables, and other vegetables.
Milk and its product include milk, cheese, yogurt, and buttermilk.
%E, percentage of total energy; BR, brown rice; BRL, brown rice with added legumes; GI, glycemic index; IQR, interquartile range; WR, white rice.
Glucose response
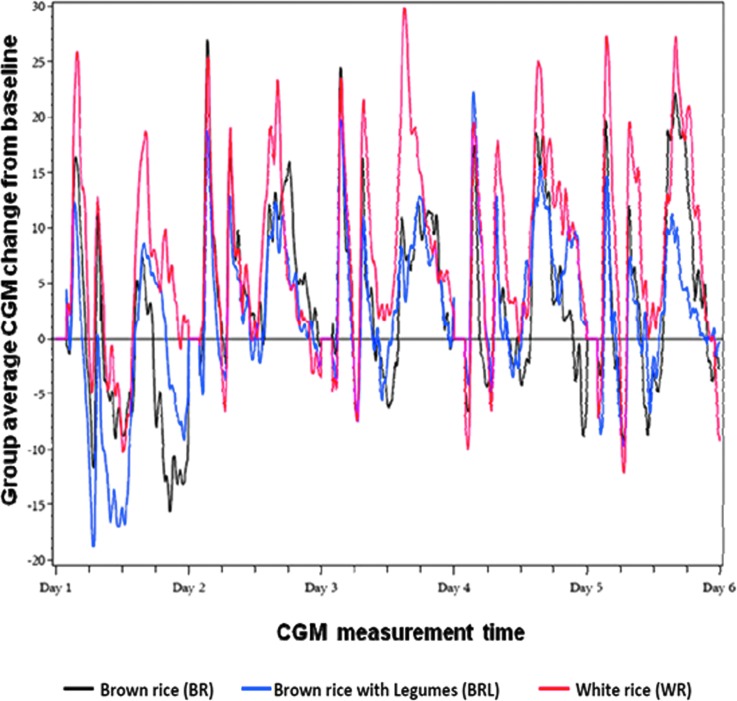
The 5-day average glycemic responses of participants fed with BR, WR, and BRL diet based on the interstitial glucose values are given in Table 3. (Table 3 also gives baseline means for all three diets.) The BR 5-day average glycemic response (IAUC) was significantly lower than that for the WR diet: BR versus WR percentage difference (95% CI), −19.8% (−32.8%, −6.7%) (P=0.004). Adding legumes to the BR diet (the BRL diet) further decreased the glycemic response: BRL versus WR percentage difference (95% CI), −22.9% (−41.8%, −4.0%) (P=0.02). These results are displayed in Figure 1. However, the difference between the BR and BRL diets was not statistically significant.
Table 3.
Metabolic Effects of Brown Rice and Brown Rice with Legumes Diets, Compared with White Rice Diet
| Outcome | Number of observations (number of participants) | WR | BR | BRL | BR vs. WR | BRL vs. WR |
|---|---|---|---|---|---|---|
| Daily IAUC (mg-5 min/dL)a | 44 (15) | |||||
| 5-day mean (95% CI) | 81.8 (67.2, 96.3) | 65.5 (53.8, 77.1) | 63.0 (51.0, 75.1) | |||
| % difference (95% CI) | −19.8% (−32.8%, −6.7%)b | −22.9% (−41.8%, −4.0%)b | ||||
| P value | 0.004 | 0.02 | ||||
| Fasting insulin (μIU/mL) | 45 (15) | |||||
| Baseline mean (95% CI) | 11.9 (9.7, 14.2) | 15.8 (12.4, 19.1) | 16.1 (13.0, 19.2) | |||
| End of respective diet mean (95% CI) | 14.5 (11.7, 17.4) | 11.2 (9.2, 13.1) | 11.2 (9.1, 13.3) | |||
| Change from baseline (95% CI) | +2.6 (−0.32, +5.6) | −4.6 (−7.2, −2.0) | −4.9 (−8.4, −1.4) | |||
| Absolute difference in % change from baseline (95% CI) | −57% (−84%, −30%) | −54% (−88%, −20%) | ||||
| P value | 0.0001 | 0.0009 |
Each daily incremental area under the curve (IAUC) measurement is the positive change from the baseline IAUC between 9:00 a.m. to the next day at 6:55 a.m., where each day's continuous glucose monitoring reading between 7:00 to 8:55 a.m. was used to calculate the baseline value.
Percentage difference in 5-day averages.
BR, brown rice; BRL, brown rice with added legumes; CI, confidence interval; WR, white rice.
FIG. 1.
Average change in interstitial glucose concentrations from baseline of overweight participants fed with brown rice (BR), white rice (WR), and BR with added legumes (BRL) diets (n=15). CGM, continuous glucose monitoring.
Serum insulin response
The within-subject differences of the change in fasting serum insulin levels measured at the beginning (Day 1) and end (Day 5) of the study period for each test diet are shown in Table 3. The percentage change over the 5-day study period in fasting insulin was 57% lower (P=0.0001) for the BR diet and 54% lower for the BRL diet compared with the 5-day percentage change observed in the WR group. The glycemic and insulinemic responses in the BR and BRL diets were not significantly different.
Discussion
The present study was conducted in 15 overweight and/or obese (≥23 kg/m2) Asian Indian adults without diabetes to assess the effects of WR, BR, and BRL on interstitial glucose (using Medtronic iPro2 CGM technology) and insulin responses. The study showed that replacing WR as a staple with a whole grain such as BR or BRL resulted in a significant reduction in the 5-day average IAUC values and fasting serum insulin levels. The BRL diet did not differ statistically in comparison with the BR diet with respect to these parameters. To our knowledge, this is the first study to use CGM technology to evaluate the effect of replacing a cereal staple food such as WR (a refined grain) with either BR (a whole grain) or BRL among obese Asian Indians.
Cereal grains, including WR, are staple foods in India and contribute to nearly half of the daily caloric intake.7 Because of polishing, the outer bran and germ layers of WR have been removed, resulting in the loss of many vital nutrients and phytonutrients, including fiber, which is found in the bran. Thus, polished WR is left with the starchy endosperm composed of mostly easily digestible carbohydrates. Today both raw and parboiled rices are highly polished compared with the traditional parboiling of undermilled rice to retain some micronutrients. In the state of Tamilnadu both raw and parboiled rices are consumed, as evident from our previous study.7 A recent study from our group indicated that the polishing of BR proportionately decreased its protein, fat, dietary fiber, vitamin, γ-oryzanol, polyphenols, and total antioxidant activity and increased the available carbohydrate content.5
Several prospective cohort studies conducted in Western populations have shown an inverse association between consumption of whole grains and risk of type 2 diabetes,21–23 and a recent meta-analysis has shown beneficial effects of whole grain consumption on the risk of diabetes, cardiovascular disease, and weight gain.24 Substitution of BR for WR significantly lowered the risk of developing diabetes in U.S. men and women.25 A study by Jang et al.26 has shown that replacing WR with whole grain and legume powder significantly reduced fasting glucose and insulin levels. Incorporation of legumes as part of a low GI diet improved glycemic control in participants with type 2 diabetes.27 The present study also found a similar result, with a significant reduction in fasting insulin levels when WR was replaced with either BR alone or BRL.
Several studies have shown that habitual diets with a high GL may lead to an increased glycemic response and insulin resistance.28,29 Rice, mostly in the form of a refined grain, may contribute to a high GL among Asian Indians. A recent meta-analysis of cohort studies by Hu et al.30 found a significant relationship between WR consumption and the risk of type 2 diabetes, especially among Asian populations consuming greater quantities of rice compared with Western populations. An earlier study from India found that high dietary GL and GI mainly derived from refined grains were associated with an increased risk for type 2 diabetes and low high-density lipoprotein cholesterol levels in Asian Indians.17,31 Low GI diets, on the other hand, have consistently been found to favorably influence the glucose profile.15,32 BR was shown to lower glycemic response and to have a lower GI compared with WR.6 In the present study, the GL and weighted GI significantly decreased when BR or BRL was substituted for WR. The GL in these diets was mainly derived from whole cereals, whereas refined cereals in the WR diet contributed the bulk of the GL. The lower glycemic and insulinemic response elicited by BR and BRL diets may be attributed to the lower GI and GL provided by BR and legumes.
The particle size of foods has been shown to influence the glycemic response. For example, cooking ground rice resulted in a significantly higher glycemic response compared with whole rice.33 Broken rice has a high GI (86±8) mainly because of an increase in the amount of cracking within the grain resulting in increased gelatinization and digestibility.34 In the present study, the glycemic response was found to be higher after breakfast meals compared with that after lunch. This may be attributed to the particle size of rice as most of the breakfast food choices (idly, upma, oothappam, and kitchidi) irrespective of whether BR or WR was made out of rice grits, whereas whole rice was served in lunch meals.
The studies by Henry et al.14,15 have shown significant reductions in 1-day interstitial glucose IAUC with low GI bread and beverages using CGM, whereas Powers et al.35 have shown differences in the CGM glucose response between moderate versus high carbohydrate lunch meals. Brynes et al.36 showed that initiating a low GI diet had significantly reduced the glycemic profile of both healthy subjects and participants at risk for heart disease.15 However, the present study's results are unique and to our knowledge the first of its kind to evaluate the effect of replacing WR, a refined grain, with BR, a whole grain, and added legumes for breakfast and lunch over longer periods than the studies mentioned above. A meal-based crossover intervention study by Powers et al.35 to determine the glycemic response with varying carbohydrate (48 vs. 92.5 g/day) content using CGM showed that there were significant differences in the glycemic response to the two meals. The high carbohydrate meal resulted in a significantly higher 4-h AUC (787.1±172.6 vs. 718.2±156.1 mg×4 h/dL [43.7±9.6 vs. 39.9±8.7 mmol×4 h/L]; P=0.036). However, this study did not mention the GI and GL of the moderate and high carbohydrate meals. It is interesting to note in our present study despite similar total carbohydrate intake across the three diets, the significant reduction in IAUC could be due to the GI and GL of the test diets, which vary significantly. Although the meals in our study by design were not isocaloric, participants were advised to eat their usual quantity in both test meals and dinner meals at home. However, the average of five 24-h diet recalls (Table 2) indicated that the total calorie, carbohydrate (g/day), and protein (g/day) intakes across the three diets were not different, despite significantly higher fat intake (g/day) during the BR and BRL diet periods. Hence reduction in IAUC of BR and BRL diets compared with the WR diet could also be attributed to the source of carbohydrate (WR vs. BR).
The study had some limitations, including a small sample size. Because the baseline fasting insulin values were quite different between treatments (WR, 11.9 μIU/mL; BR, 15.8 μIU/mL; and BRL, 16.1 μIU/mL), the levels during treatment (WR, 14.5 μIU/mL; BR, 11.2 μIU/mL; and BRL, 11.2 μIU/mL) might, at least partly, reflect regression to the mean; imbalances such as these and more are not uncommon in small trials. Studies with larger sample sizes and conducted for longer durations are needed to assess the long-term health effects of replacing cereal staples such as WR with whole grains such as BR. Women were most likely to be missing one or more 5-min CGM measurement. Gender-related barriers to CGM will be explored in future research. A longer-term feeding trial is our most compelling and conclusive recommendation to further prove this concept in this cultural context. It should be noted that although the subjects were somewhat young, they were obese or overweight, putting them at high risk for type 2 diabetes and other chronic diseases. In addition, diabetes risk increases in Asian Indians earlier compared with whites.37 Hence, we expect that the findings of this study will be generalizable to the populations such as Asian Indians who are at risk for type 2 diabetes at younger ages than occurs in European and North American populations.
The strengths of our study include the randomized crossover study design in which the three diets were given to all participants in a random order, allowing us to fully control for within-subject differences in responses. The BR was milled under a food technologist's supervision specifically for this study, as authentic BR is not presently available in the Indian market. The test kitchen at the center where the study was conducted had excellent facilities for standardizing the test diet meals and was conveniently located for study participants. By using the iPro2 model CGM system, a noninvasive glucose monitoring tool, participants were able to continue their routine activities while wearing the CGM device; hence the results are likely to be more representative of real-life situations.
This is a unique study and to our knowledge is the first of its kind to demonstrate the importance of how a simple dietary change—replacing WR with BR or BRL—in overweight adults who are at risk for obesity-related metabolic disorders such as diabetes in India can have a significant effect on glucose and insulin responses. The study also highlights CGM as a potential tool to provide valuable information on glycemic excursions throughout the day that may be otherwise missed by more conventional monitoring. As rice continues to be the staple food of Asian Indians, lowering the GL of their diet by encouraging them to eat less may not be feasible. Furthermore, at least in the Asian Indian and South Asian context, which represents over one-sixth of the world's population, the present study suggests that improving the quality of the cereal staple in the diet by substituting BR for WR and reducing the overall dietary GL may offer substantial health benefits in this population who are at a greater risk for type 2 diabetes and heart disease at an early age.
Supplementary Material
Acknowledgments
We thank the Heinz Nutrition Foundation of India and the Fogarty International Center at National Institutes of Health (grant 1R03TW008726-01) for funding this study.
Author Disclosure Statement
No competing financial interests exist.
V. Mohan is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. V. Mohan conceived the concept of the study, initiated the first draft, and helped to write the results and discussion of the manuscript. K.K. and J.H. conceived the concept of the study, contributed to the interpretation of data, and reviewed the manuscript. S.V. conducted the methods of the study and helped to write the results and discussion. K.A., S.R., and M.R.R. helped to conduct the methods of the study. R.G. helped in writing the manuscript. B.H. helped in data analysis and wrote the statistical analysis. D.S. helped in data analysis, interpretation of data, and revision of manuscript. K.P. helped in data analysis. N.M.W. contributed to the interpretation of data and manuscript writing. V. Malik contributed to the interpretation of data and manuscript writing. W.W., F.B.H., and R.M.A. contributed to the interpretation of data and reviewed the manuscript.
References
- 1.Trade and Market Division, Food and Agriculture Organization: Rice market summary. In: Food Outlook: Global Market Analysis. Rome: Food and Agriculture Organization, 2012:4 [Google Scholar]
- 2.Global Perspective Studies Unit, Food and Agriculture Organization: Structural Changes in the Commodity Composition of Food Consumption. World Agriculture: Towards 2030/2050. Interim Report: Prospects for Food, Nutrition, Agriculture and Major Commodity Groups. Rome: Food and Agriculture Organization, 2006:21–22 [Google Scholar]
- 3.Achaya KT. The Illustrated Food of India A–Z. New Delhi: Oxford University Press, 2009 [Google Scholar]
- 4.Chattopadhyay PK: Postharvest technology for rice in India: a changing scenario. In: Toriyama K, Heong KL, Hardy B, eds. Rice Is Life: Scientific Perspectives for the 21st Century. Proceedings of the World Rice Research Conference held in Tokyo and Tsukuba, Japan, 4–7 November 2004 Los Baños, the Philippines: International Rice Research Institute/Tsukuba, Japan: Japan International Research Center for Agricultural Sciences, 2005:294–296 [Google Scholar]
- 5.Shobana S, Malleshi NG, Sudha V, Spiegelman D, Hong B, Hu FB, Willett WC, Krishnaswamy K, Mohan V: Nutritional and sensory profile of two Indian rice varieties with different degrees of polishing. Int J Food Sci Nutr 2011;62:800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinesh Babu P, Subhasree RS, Bhakyaraj R, Vidhyalakshmi R: Brown rice—beyond the color reviving a lost health food—a review. Am Eur J Agron 2009;2:67–72 [Google Scholar]
- 7.Radhika G, Sathya RM, Ganesan A, Saroja R, Vijayalakshmi P, Sudha V, Mohan V: Dietary profile of urban adult population in South India in the context of chronic disease epidemiology (CURES-68). Public Health Nutr 2010;12:1–8 [DOI] [PubMed] [Google Scholar]
- 8.Popkin BM: Global nutrition dynamics: the world is shifting rapidly toward a diet linked with non-communicable diseases. Am J Clin Nutr 2006;84:289–298 [DOI] [PubMed] [Google Scholar]
- 9.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Bhansali A, Joshi SR, Joshi PP, Yajnik CS, Dhandhania VK, Nath LM, Das AK, Rao PV, Madhu SV, Shukla DK, Kaur T, Priya M, Nirmal E, Parvathi SJ, Subhashini S, Subashini R, Ali MK, Mohan V: Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia 2011;54:3022–3027 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization: The Asia Pacific Perspective. Redefining Obesity and Its Treatment. Melbourne: International Diabetes Institute for the International Association for the Study of Obesity and International Obesity Task Force, 2000 [Google Scholar]
- 11.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V: Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47). Eur J Clin Nutr 2009; 63:259–267 [DOI] [PubMed] [Google Scholar]
- 12.Shobana S, Kokila A, Lakshmipriya N, Subhashini S, Ramya Bai M, Mohan V, Malleshi NG, Anjana RM, Henry CJK, Sudha V: Glycemic index of three Indian rice varieties. Int J Food Sci Nutr 2011;63:178–183 [DOI] [PubMed] [Google Scholar]
- 13.Atkinson FS, Foster-Powell K, Brand-Miller JC: International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry CJK, Lightowler HJ, Tydeman EA, Skeath R: Use of low-glycaemic index bread to reduce 24-h blood glucose: implications for dietary advice to non-diabetic and diabetic participants. Int J Food Sci Nutr 2006;57:273–278 [DOI] [PubMed] [Google Scholar]
- 15.Henry CJK, Newens KJ, Lightowler HJ: Low-glycaemic index sweetener-based beverages reduce 24-h glucose profiles in healthy adults. J Hum Nutr Diet 2009;22:77–80 [DOI] [PubMed] [Google Scholar]
- 16.Brynes AE, Adamson J, Dornhorst A, Frost GS: The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br J Nutr 2005;93:179–182 [DOI] [PubMed] [Google Scholar]
- 17.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV: Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycaemia and hyperinsulinemia? Diabetes Care 2002;25:889–893 [DOI] [PubMed] [Google Scholar]
- 18.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V: Refined grain consumption and the metabolic syndrome in urban Asian Indians (CURES57). Metabolism 2009;58:675–681 [DOI] [PubMed] [Google Scholar]
- 19.Deepa M, Pradeepa R, Rema M, Anjana M, Deepa R, Shanthirani S: The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I). J Assoc Phys India 2003;51:863–870 [PubMed] [Google Scholar]
- 20.Fitzmaurice GM, Laird NM, Ware JH: Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, 2004 [Google Scholar]
- 21.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF: Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr 2002;76:390–398 [DOI] [PubMed] [Google Scholar]
- 22.Newby PK, Maras J, Bakun P, Muller D, Ferrucci L, Tucker KL: Intake of wholegrains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr 2007;86:1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munter de JS, Hu FB, Spiegelman D, Franz M, van Dam RM: Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S: Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012;142:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q, Spiegelman D, van Dam R, Holmes M, Malik V, Willett W, Hu F: White rice, brown rice and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang Y, Lee JH, Kim OY, Park HY, Lee SY: Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler Thromb Vasc Biol 2001;21:2065–2071 [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DJ, Kendall CW, Augustin LS, Mitchell S, Sahye-Pudaruth S, Blanco Mejia S, Chiavaroli L, Mirrahimi A, Ireland C, Bashyam B, Vidgen E, de Souza RJ, Sievenpiper JL, Coveney J, Leiter LA, Josse RG: Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2012;172:1653–1660 [DOI] [PubMed] [Google Scholar]
- 28.Ludwig DS: The glycemic index. JAMA 2002;287:2414–2423 [DOI] [PubMed] [Google Scholar]
- 29.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF: Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004;27:538–546 [DOI] [PubMed] [Google Scholar]
- 30.Hu EA, Pan A, Malik V, Sun Q: White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 2012;344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V: Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br J Nutr 2009;102:1498–1506. Erratum in: Br J Nutr 2010;103:1851–1852. [DOI] [PubMed] [Google Scholar]
- 32.Philippou E, McGowan BM, Brynes AE, Dornhorst A, Leeds AR, Frost GS: The effect of a 12-week low glycaemic index diet on heart disease risk factors and 24 h glycaemic response in healthy middle-aged volunteers at risk of heart disease: a pilot study. Eur J Clin Nutr 2008;62:145–149 [DOI] [PubMed] [Google Scholar]
- 33.O'Dea K, Snow P, Nestel P: Rate of starch hydrolysis in vitro as a predictor of metabolic responses to complex carbohydrate in vivo. Am J Clin Nutr 1981;34:1991–1993 [DOI] [PubMed] [Google Scholar]
- 34.Chan HMS, Brand-Miller JC, Holt SHA, Wilson D, Rozman M, Petocz P: The glycaemic index values of Vietnamese foods. Eur J Clin Nutr 2001;55:1076–1083 [DOI] [PubMed] [Google Scholar]
- 35.Powers MA, Cuddihy RM, Wesley D, Morgan B: Continuous glucose monitoring reveals different glycemic responses of moderate vs high-carbohydrate lunch meals in people with type 2 diabetes. J Am Diet Assoc 2010;110:1912–1915 [DOI] [PubMed] [Google Scholar]
- 36.Brynes AE, Mark Edwards C, Ghatei MA, Dornhorst A, Morgan LM, Bloom SR, Frost GS: A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Br J Nutr 2003;89:207–218 [DOI] [PubMed] [Google Scholar]
- 37.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C: Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res 2007;125:217–230 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.