Abstract
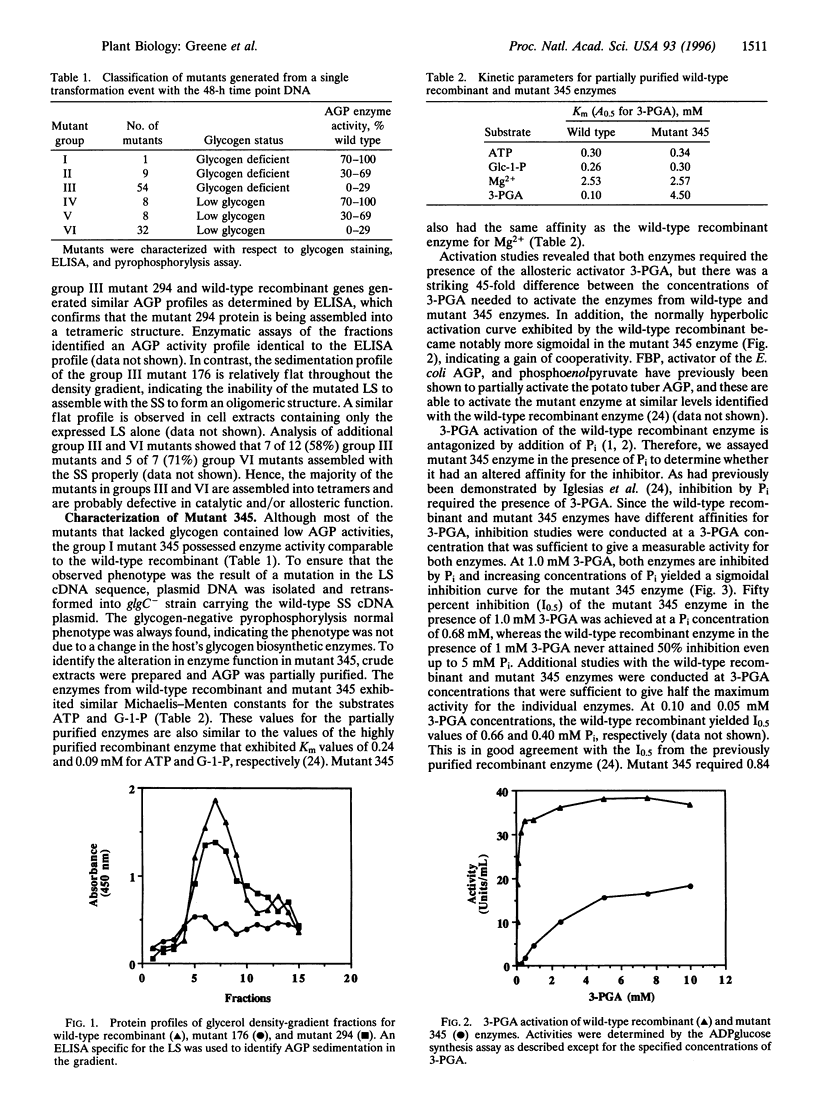
ADPglucose pyrophosphorylase (glucose-1-phosphate adenylyltransferase; ADP:alpha-D-glucose-1-phosphate adenylyltransferase, EC 2.7.7.27) catalyzes a key regulatory step in alpha-glucan synthesis in bacteria and higher plants. We have previously shown that the expression of the cDNA sequences of the potato tuber large (LS) and small (SS) subunits yielded a functional heterotetrameric enzyme capable of complementing a mutation in the single AGP (glgC) structural gene of Escherichia coli. This heterologous complementation provides a powerful genetic approach to obtain biochemical information on the specific roles of LS and SS in enzyme function. By mutagenizing the LS cDNA with hydroxylamine and then coexpressing with wild-type SS in an E. coli glgC- strain, >350 mutant colonies were identified that were impaired in glycogen production. One mutant exhibited enzymatic and antigen levels comparable to the wild-type recombinant enzyme but required 45-fold greater levels of the activator 3-phosphoglycerate for maximum activity. Sequence analysis identified a single nucleotide change that resulted in the change of Pro-52 to Leu. This heterologous genetic system provides an efficient means to identify residues important for catalysis and allosteric functioning and should lead to novel approaches to increase plant productivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball K. L., Preiss J. Evidence for an arginine residue at the allosteric sites of spinach leaf ADPglucose pyrophosphorylase. J Protein Chem. 1992 Jun;11(3):231–238. doi: 10.1007/BF01024861. [DOI] [PubMed] [Google Scholar]
- Ball K., Preiss J. Allosteric sites of the large subunit of the spinach leaf ADPglucose pyrophosphorylase. J Biol Chem. 1994 Oct 7;269(40):24706–24711. [PubMed] [Google Scholar]
- Ballicora M. A., Laughlin M. J., Fu Y., Okita T. W., Barry G. F., Preiss J. Adenosine 5'-diphosphate-glucose pyrophosphorylase from potato tuber. Significance of the N terminus of the small subunit for catalytic properties and heat stability. Plant Physiol. 1995 Sep;109(1):245–251. doi: 10.1104/pp.109.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Meyer C., Remy E., Peterson D., Preiss J. Cloning, expression, and nucleotide sequence of glgC gene from an allosteric mutant of Escherichia coli B. Arch Biochem Biophys. 1992 Jul;296(1):122–128. doi: 10.1016/0003-9861(92)90553-9. [DOI] [PubMed] [Google Scholar]
- Govons S., Vinopal R., Ingraham J., Preiss J. Isolation of mutants of Escherichia coli B altered in their ability to synthesize glycogen. J Bacteriol. 1969 Feb;97(2):970–972. doi: 10.1128/jb.97.2.970-972.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. A., Kaufmann K., Otero J., Preiss J. Biosynthesis of bacterial glycogen. Mutagenesis of a catalytic site residue of ADP-glucose pyrophosphorylase from Escherichia coli. J Biol Chem. 1991 Jul 5;266(19):12455–12460. [PubMed] [Google Scholar]
- Iglesias A. A., Barry G. F., Meyer C., Bloksberg L., Nakata P. A., Greene T., Laughlin M. J., Okita T. W., Kishore G. M., Preiss J. Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem. 1993 Jan 15;268(2):1081–1086. [PubMed] [Google Scholar]
- Isackson P. J., Bertrand K. P. Dominant negative mutations in the Tn10 tet repressor: evidence for use of the conserved helix-turn-helix motif in DNA binding. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6226–6230. doi: 10.1073/pnas.82.18.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Villand P., Lüthi E., Olsen O. A., Preiss J. Insensitivity of barley endosperm ADP-glucose pyrophosphorylase to 3-phosphoglycerate and orthophosphate regulation. Plant Physiol. 1993 Jan;101(1):179–186. doi: 10.1104/pp.101.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Ghosh P., Lee Y. M., Hill M. A., Preiss J. Biosynthesis of bacterial glycogen. Determination of the amino acid changes that alter the regulatory properties of a mutant Escherichia coli ADP-glucose synthetase. J Biol Chem. 1989 Jun 25;264(18):10464–10471. [PubMed] [Google Scholar]
- Kumar A., Tanaka T., Lee Y. M., Preiss J. Biosynthesis of bacterial glycogen. Use of site-directed mutagenesis to probe the role of tyrosine 114 in the catalytic mechanism of ADP-glucose synthetase from Escherichia coli. J Biol Chem. 1988 Oct 15;263(29):14634–14639. [PubMed] [Google Scholar]
- Larsen C. E., Lee Y. M., Preiss J. Covalent modification of the inhibitor-binding site(s) of Escherichia coli ADP-glucose synthetase. Isolation and structural characterization of 8-azido-AMP-incorporated peptides. J Biol Chem. 1986 Nov 25;261(33):15402–15409. [PubMed] [Google Scholar]
- Leung P., Lee Y. M., Greenberg E., Esch K., Boylan S., Preiss J. Cloning and expression of the Escherichia coli glgC gene from a mutant containing an ADPglucose pyrophosphorylase with altered allosteric properties. J Bacteriol. 1986 Jul;167(1):82–88. doi: 10.1128/jb.167.1.82-88.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C. R., Ghosh P., Nadler S., Preiss J. Cloning, expression, and sequence of an allosteric mutant ADPglucose pyrophosphorylase from Escherichia coli B. Arch Biochem Biophys. 1993 Apr;302(1):64–71. doi: 10.1006/abbi.1993.1181. [DOI] [PubMed] [Google Scholar]
- Morell M., Bloom M., Preiss J. Affinity labeling of the allosteric activator site(s) of spinach leaf ADP-glucose pyrophosphorylase. J Biol Chem. 1988 Jan 15;263(2):633–637. [PubMed] [Google Scholar]
- Nakata P. A., Greene T. W., Anderson J. M., Smith-White B. J., Okita T. W., Preiss J. Comparison of the primary sequences of two potato tuber ADP-glucose pyrophosphorylase subunits. Plant Mol Biol. 1991 Nov;17(5):1089–1093. doi: 10.1007/BF00037149. [DOI] [PubMed] [Google Scholar]
- Okita T. W. Is there an alternative pathway for starch synthesis? Plant Physiol. 1992 Oct;100(2):560–564. doi: 10.1104/pp.100.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Nakata P. A., Anderson J. M., Sowokinos J., Morell M., Preiss J. The Subunit Structure of Potato Tuber ADPglucose Pyrophosphorylase. Plant Physiol. 1990 Jun;93(2):785–790. doi: 10.1104/pp.93.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Volcani B. E. The deoxyribonucleic acid polymerases from the diatom Cylindrotheca fusiformis. Subcellular distribution, exonuclease activity and heterogeneity of the enzymes. Biochem J. 1977 Dec 1;167(3):611–619. doi: 10.1042/bj1670611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. F., Preiss J. Biosynthesis of bacterial glycogen. Incorporation of pyridoxal phosphate into the allosteric activator site and an ADP-glucose-protected pyridoxal phosphate binding site of Escherichia coli B ADP-glucose synthase. J Biol Chem. 1978 Sep 10;253(17):6197–6202. [PubMed] [Google Scholar]
- Parsons T. F., Preiss J. Biosynthesis of bacterial glycogen. Isolation and characterization of the pyridoxal-P allosteric activator site and the ADP-glucose-protected pyridoxal-P binding site of Escherichia coli B ADP-glucose synthase. J Biol Chem. 1978 Nov 10;253(21):7638–7645. [PubMed] [Google Scholar]
- Preiss J., Romeo T. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1994;47:299–329. doi: 10.1016/s0079-6603(08)60255-x. [DOI] [PubMed] [Google Scholar]
- Preiss J., Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–238. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- Smith-White B. J., Preiss J. Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources. J Mol Evol. 1992 May;34(5):449–464. doi: 10.1007/BF00162999. [DOI] [PubMed] [Google Scholar]



