Abstract
Objective
To determine the prevalence of secondhand smoke (SHS) exposure among infants and young children who received preventive care at pediatric preventative care clinics associated with an urban public hospital. Cotinine, a metabolite of nicotine, has been used to study SHS exposure in population-based studies of children 3 years of age or older.
Design
Retrospective study using a convenience sample.
Setting
Urban county pediatric primary care clinics in San Francisco, California.
Participants
A total of 496 infants and children (mean [SD] age, 2.4 [1.9] years).
Interventions
Discarded plasma samples (which were routinely collected for lead screening) were tested, and medical records were reviewed, for SHS exposure.
Main Outcome Measure
Secondhand smoke exposure based on cotinine plasma level and history of exposure in the medical record.
Results
Thirteen percent of parents reported that their child was exposed to SHS, yet biochemical testing detected cotinine in 55% of samples, at a geometric mean (SD) of 0.23 (3.55) ng/mL. There were no significant sex or age differences. African American children had much higher mean cotinine levels than did Latino children (geometric mean difference, 6.07 ng/mL [95% CI, 4.37 to 8.43 ng/mL]).
Conclusion
In a city with a low smoking rate (12%) and public smoking bans, we documented 55% exposure among infants and young children, using a plasma biomarker, compared with 13% exposure reported by parents. Because SHS is associated with significant respiratory diseases and parents underreport exposure, routine biochemical screening should be considered as a tool to identify and reduce SHS exposure.
Tobacco smoke exposure poses a significant health hazard for children, representing an enormous burden of morbidity and mortality.1,2 Parental reporting of their children’s tobacco smoke exposure may be inaccurate for many reasons (eg, exposures of which they are unaware). Public health measures, such as smoking bans, have reduced secondhand smoke (SHS) exposure among children,3,4 but biochemical testing provides objective documentation of tobacco smoke exposure, which alerts health care providers and parents that exposure has occurred and may help them institute measures to limit or prevent their child’s further exposure, especially among nonsmoking families.4 The most sensitive way to assess exposure is by measurement of biomarkers of tobacco smoke exposure. The most widely used biomarker is cotinine, the major proximate metabolite of nicotine. Cotinine has a relatively long half-life (16 hours) and is a quantitative biomarker of nicotine exposure.5 The National Health and Nutrition Examination Survey (NHANES) has measured plasma cotinine levels in representative samples of the US population over many years. Studies using NHANES data have reported that up to 83% of children and adolescents aged 3 to 18 years had detectable cotinine levels in their plasma.6,7 We are unaware of any systematic studies that included children younger than 3 years of age.
Smoking prevalence remains high in economically disadvantaged populations.8 In the context of a 12% overall smoking prevalence in the city of San Francisco, we recently found that, based on serum cotinine levels, 55% of adults admitted to the San Francisco General Hospital (SFGH) in California were active smokers or heavily exposed to SHS.9 To the best of our knowledge, no such data are available in a general population of children served by urban public pediatric clinics.
Racial differences in the rate of nicotine metabolism are well established in adults.10,11 The hepatic cytochrome P450 enzyme CYP2A6 metabolizes 80% or more of nicotine to cotinine and also metabolizes cotinine to its major metabolite, trans 3′-hydroxycotinine (3HC).12 The ratio of 3HC to cotinine, known as the nicotine metabolite ratio (NMR), is a biomarker for the rate of nicotine clearance.13 A low NMR indicates lower cotinine clearance, which may result in higher cotinine levels due to lower clearance and not just higher SHS exposure. We used the NMR to compare the rates of nicotine metabolism in infants and children of different races, which has not previously been done.
The primary aim of our study was to determine the prevalence of SHS exposure among infants and young children who received primary care at SFGH Medical Center, based on the biomarker of plasma cotinine, using a convenience blood sample. Our other aims were to determine the sensitivity and specificity of parental reporting of SHS history as determined by plasma cotinine level, and to compare the rates of nicotine metabolism estimated using the NMR among children of different race/ethnicity groups.
METHODS
STUDY PROCEDURES
This is a study of all infants and children who received pediatric care at SFGH and who had an available leftover blood sample collected for lead screening during a 5-month interval (from November 3, 2009, to March 31, 2010). We retrieved SFGH clinical laboratory samples that originated from the SFGH pediatric clinic. Blood samples for lead screening are obtained as part of standard primary, preventative, and well-child pediatric care. At SFGH, pediatric blood samples to determine lead levels are routinely obtained from children between 9 and 12 months of age and then yearly until 5 years of age. Two children had more than 1 lead sample, and, for this study, we used the first sample to determine their cotinine levels.
San Francisco General Hospital, the county hospital that serves the economically disadvantaged population of San Francisco, has pediatric clinics that serve approximately 10 000 un-duplicated infants and children younger than 18 years of age every year; approximately 77% of pediatric patients attend at least 1 primary care clinic per year. The demographics of the population using the SFGH pediatric clinics are as follows: 59% Latino, 18% African American, 13% Asian, 5% white, and 5% other. The age distribution of this population is as follows: 12% of infants and children from birth to 12 months, 9% from 12 to 23 months, 9% from 24 to 35 months, 19% from 36 to 71 months, and 51% from 6 to 17 years. Virtually all of the children were publicly insured.
Using the name and medical record number on the plasma sample for lead screening, we retrospectively retrieved the subjects’ race/ethnicity, sex, age, insurance status, and lead concentrations from the hospital electronic database. We also retrospectively reviewed all the Child Health and Disabilities Prevention (CHDP) program billing forms stored in the medical records. The CHDP program is the California version of a federal Early Periodic Screening, Diagnosis, and Treatment program that provides funding for primary care services. A CHDP form is filled out at the time of every preventative care visit (usual visit schedule: 1 and 2 weeks; 1, 2, 4, 6, 9, 12, 15, 18, 24, 36, 48, and 60 months; and yearly or biyearly until 18 years). The CHDP billing form includes the question about whether the patient was exposed to passive (secondhand) tobacco smoke and must be answered for reimbursement. The child’s SHS exposure status is obtained from the parent or caregiver. The CHDP forms were analyzed across the patient’s entire history at SFGH. A history of SHS exposure was defined by at least 1 “yes” answer, whereas no history of SHS exposure required all CHDP forms to be marked “no.”
This study did not require informed consent and was approved by the Committee on Human Research at the University of California, San Francisco. Our study used surplus blood samples that were to be discarded; there was no direct patient contact; and after the review of the medical records was completed and data were linked with the sample’s research number, all patient identifiers were deleted from the database and research charts.
SUBSTUDY OF WHEEZING AND ASTHMA
We identified 70 children from our study population with probable wheezing or possible asthma based on electronic prescriptions for asthma-specific medications (eg, β-agonist and/or inhaled steroids). We also identified a control group of 74 children who were matched by date of blood sample collection, race/ethnicity, age, and sex. Individuals were excluded from the control group if they had prescriptions for antibiotics that indicated a respiratory, ear, or sinus infection within 3 months before their blood sample for lead screening was obtained. Group assignment was solely based on electronic prescription data.
ANALYTICAL CHEMISTRY
Samples were analyzed for cotinine and 3HC by liquid chromatography–tandem mass spectrometry.14 The lower limit of quantitation (LOQ) for this assay depends on the volume of plasma available. The volumes available in our study ranged from 0.1 to 1.0 mL, with the LOQ ranging from 0.2 to 0.02 ng/mL, respectively. Overall, for 75% of samples, the LOQ was 0.05 ng/mL or less. The percentage of samples with cotinine levels greater than the LOQ was similar across the volumes of plasma analyzed: LOQ=0.05 ng/mL, with 55% of samples with detectable cotinine; LOQ=0.07 ng/mL, 54%; LOQ=0.1 ng/mL, 55%; LOQ=0.2 ng/mL, 68%.
DATA ANALYSIS AND STATISTICS
We used bivariate analyses to test whether cotinine positivity (χ2 test) and absolute measured levels (t test) were different across covariates. We used multivariate analyses to test whether race/ethnicity was associated with cotinine levels (linear regression) and cotinine positivity (logistic regression), controlling for sex and age. We used 2 definitions for cotinine positivity: (1) a cotinine level greater than or equal to the LOQ (detectable cotinine) vs a cotinine level less than the LOQ and (2) a cotinine level greater than or equal to 0.05 ng/mL vs a cotinine level less than 0.05 ng/mL. The value of 0.05 ng/mL was selected for comparability to other studies that have used this as a cutoff for SHS exposure in children.6,15 All tests were 2-tailed and conducted using R software (http://www.r-project.org/).
RESULTS
The demographic characteristics of the 496 infants and children whose data were included in the analysis are shown in Table 1. The subjects’ ages ranged from 8 months to 17 years, with a mean (SD) age of 2.4 (1.9) years. Table 1 includes the numbers and percentages of those with detectable cotinine levels and those with cotinine levels greater than or equal to 0.05 ng/mL, a difference of 22 infants and children between the 2 categorizations. There was no significant difference in the percentage of infants and children who had detectable cotinine levels by age, insurance coverage, or blood lead levels. There was no difference in mean cotinine levels in those with detectable blood lead levels vs those with blood lead levels below the level of detection (geometric mean difference, 1.21 ng/mL [95% CI, 0.76–1.95 ng/mL]), nor was there a significant correlation between lead levels and cotinine levels among those who had both detectable lead and cotinine levels (Pearson r=−0.10 [95% CI, −0.42 to 0.24]). Female children were significantly more likely to have detectable cotinine levels than were male children (Table 1), but sex was not a significant predictor of cotinine level in multivariable linear regression models.
Table 1.
Characteristics of 496 Infants and Children
| Characteristic | No. (%) of Participants | P Valuea | No. (%) of Participants | P Valuea | ||||
|---|---|---|---|---|---|---|---|---|
| Measurable Cotinine in Blood Sample | Cotinine Not Detectable in Blood Sample | Cotinine Level ≥0.05 ng/mL | Cotinine Level <0.05 ng/mL | |||||
| Total | 274 (100) | 222 (100) | 252 (100) | 244 (100) | ||||
| Sex | ||||||||
| Male | 131 (47.8) | 127 (57.2) |
|
.046 | 122 (48.4) | 136 (55.7) |
|
.12 |
| Female | 143 (52.2) | 95 (42.8) | 130 (51.6) | 108 (44.3) | ||||
| Age, y | ||||||||
| <1 | 41 (15.0) | 46 (20.7) |
|
.12 | 38 (15.1) | 49 (20.1) |
|
.42 |
| 1 to <2 | 92 (33.6) | 64 (28.8) | 86 (34.1) | 70 (28.7) | ||||
| 2 to <3 | 59 (21.5) | 47 (21.2) | 53 (21.0) | 53 (21.7) | ||||
| 3 to <4 | 34 (12.4) | 37 (16.7) | 32 (12.7) | 39 (16.0) | ||||
| 4 to <5 | 32 (11.7) | 22 (9.9) | 31 (12.3) | 23 (9.4) | ||||
| 5 to <18 | 16 (5.8) | 6 (2.7) | 12 (4.8) | 10 (4.1) | ||||
| Race/ethnicity | ||||||||
| Latino | 164 (59.9) | 197 (88.7) |
|
<.001 | 148 (58.7) | 213 (87.3) |
|
<.001 |
| African American | 65 (23.7) | 5 (2.3) | 64 (25.4) | 6 (2.5) | ||||
| Asian | 24 (8.8) | 12 (5.4) | 21 (8.3) | 15 (6.1) | ||||
| White, Non-Hispanic | 15 (5.5) | 6 (2.7) | 14 (5.6) | 7 (2.9) | ||||
| Other | 6 (2.2) | 2 (0.9) | 5 (2.0) | 3 (1.2) | ||||
| Insurance coverage | ||||||||
| Medi-Cal managed care | 227 (82.8) | 190 (85.6) |
|
.16 | 208 (82.5) | 209 (85.7) |
|
.18 |
| Publicly insured | 47 (17.2) | 30 (13.5) | 44 (17.5) | 33 (13.5) | ||||
| Uninsured | 0 (0) | 2 (0.9) | 0 (0) | 2 (0.8) | ||||
| History of SHS exposure | ||||||||
| No | 197 (71.9) | 181 (81.5) |
|
.003 | 181 (71.8) | 197 (80.7) |
|
.007 |
| Yes | 48 (17.5) | 18 (8.1) | 44 (17.5) | 22 (9.0) | ||||
| Missing data | 29 (10.6) | 23 (10.4) | 27 (10.7) | 25 (10.2) | ||||
| Lead level | ||||||||
| <2 μg/dL | 239 (87.2) | 187 (84.2) |
|
.41 | 220 (87.3) | 206 (84.4) |
|
.43 |
| ≥2 μg/dL | 35 (12.8) | 35 (15.8) | 32 (12.7) | 38 (15.6) | ||||
Abbreviation: SHS, secondhand smoke.
SI conversion factor: To convert lead to micromoles per liter, multiply by 0.0483.
Calculated from χ2 tests comparing subject who tested positive for cotinine with those who tested negative, using the described cutoff measures.
HISTORY OF SHS EXPOSURE
There was a significant difference in the number of subjects with detectable cotinine levels by history of SHS exposure (Table 1). Cotinine levels were significantly higher among those with a history of SHS exposure compared with those without (geometric mean difference, 2.68 ng/mL [95% CI, 1.68–4.28 ng/mL]; data not shown). The sensitivity of parental reporting of SHS exposure for identifying subjects with detectable plasma cotinine levels was 19.6%, whereas the specificity was 91%. Among participants with detectable cotinine levels and no history of SHS exposure, the mean number of CHDP forms per child was 7 (range, 1–16).
RACE/ETHNICITY
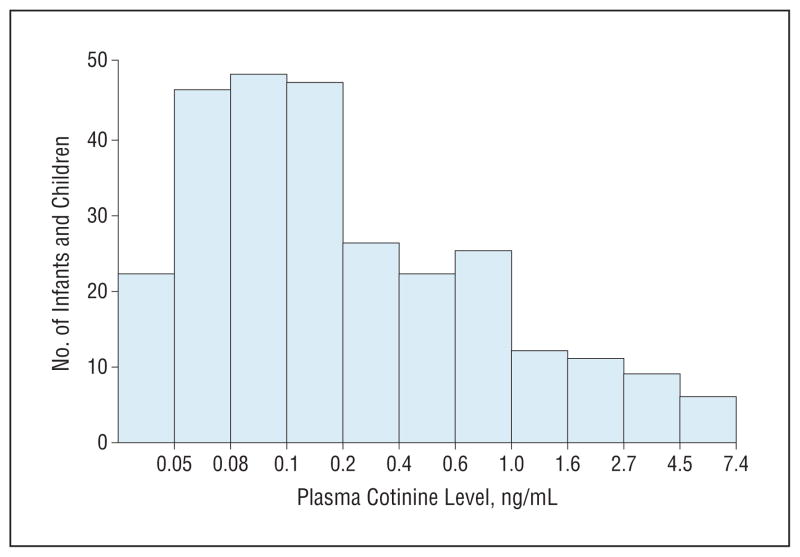
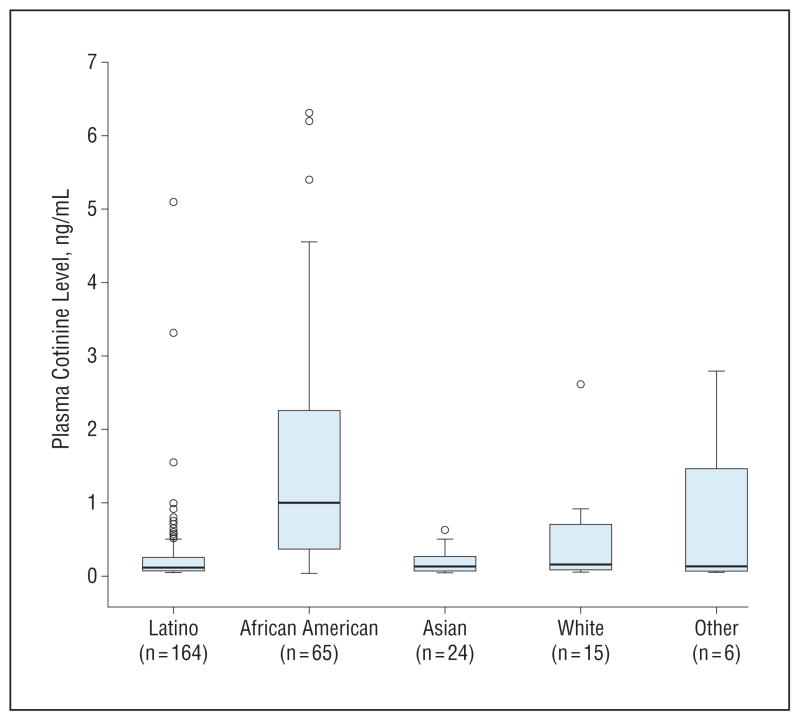
Figures 1 and 2 display data for those children with detectable cotinine levels. Table 2 gives the plasma cotinine levels by race/ethnicity. As seen in Figure 1, 61% had cotinine levels of less than 0.25 ng/mL, whereas 14% had cotinine levels of 1 ng/mL or greater. Figure 2 displays box plots of cotinine levels by race/ethnicity, showing that African Americans had much higher levels compared with Latinos, Asians, whites, and other races. Nearly 93% of African Americans had detectable cotinine levels compared with 45% of Latinos (Table 1), and the mean cotinine level among African American children was also significantly higher than those of other races/ethnicities (Table 2). Latinos had the lowest mean cotinine level compared with other races/ethnicities, but the difference was only significant in comparison with African Americans, whose mean level was 6 times that of Latinos after controlling for age and sex (Table 2). Relative to Latinos, the adjusted odds ratio for detectable cotinine levels in African Americans was 16.0 (95% CI, 6.3–40.7), whereas it was 2.4 (95% CI, 1.16–4.98) for Asians and 2.9 (95% CI, 1.08–7.61) for whites.
Figure 1.
Histogram of plasma cotinine levels for subjects with detectable cotinine levels. Data on the x-axis are plotted on a logarithmic scale.
Figure 2.
Box plots of plasma cotinine levels in subjects with cotinine levels greater than or equal to the limit of quantitation, by race/ethnicity.
Table 2.
Plasma Cotinine Levels by Race/Ethnicity
| Race/Ethnicty | No. of Subjects | Geometric Mean (SD) Cotinine Level, ng/mL | Multiplicative Factora Change in Cotinine (95% CI) |
|---|---|---|---|
| All | 274 | 0.23 (3.55) | |
| Latino | 164 | 0.14 (2.48) | 1 [Reference] |
| African American | 65 | 0.87 (3.33) | 6.01 (4.49–8.05) |
| Asian | 24 | 0.13 (2.32) | 0.93 (0.60–1.44) |
| White | 15 | 0.22 (3.42) | 1.52 (0.89–2.61) |
| Other | 6 | 0.23 (5.70) | 1.58 (0.69–3.62) |
Derived from linear regression and representing the multiplicative change in cotinine concentration relative to Latinos after controlling for age and sex.
SUBSTUDY OF WHEEZING AND ASTHMA
Among the 70 individuals on medications for wheezing/asthma and the 74 matched controls, there were no significant differences in the number of subjects who tested positive for cotinine, in cotinine levels between the 2 groups, or in self-reported SHS exposure (data not shown).
NICOTINE METABOLITE RATIO
Table 3 displays the mean differences for the NMR by race/ethnicity. Because blood levels of 3HC are typically much lower than those of cotinine, as cotinine levels approach their LOQ, 3HC levels will more likely be below their LOQ. Only 32% of participants with a detectable cotinine level had a detectable 3HC level in their plasma. African Americans had the highest mean cotinine level (Table 2) and the largest proportion of subjects with detectable 3HC levels (72%; Table 3), whereas Latinos had the lowest mean cotinine level and the lowest proportion of subjects with detectable 3HC levels (15%). Compared with Latinos, children from all other racial/ethnic groups had a lower mean NMR, but only African Americans had a significantly lower mean NMR after adjusting for age and sex (mean difference, −0.39 [95% CI, −0.05 to −0.73]).
Table 3.
Mean Difference in 3HC, Cotinine, and NMR for Subjects With Detectable 3HC and Cotininea
| Race/Ethnicity | No. of Subjects | 3HC Level (95% CI), ng/mL | Cotinine Level (95% CI), ng/mL | 3HC/Cotinine NMR (95% CI) |
|---|---|---|---|---|
| Latino | 25 | 0.13 | 0.28 | 0.69 |
| African American | 47 | 2.71 (1.17–6.27) | 4.94 (2.30–10.61) | −0.39 (−0.05 to −0.73) |
| Asian | 7 | 0.53 (0.12–2.25) | 0.64 (0.17–2.40) | −0.20 (−0.78 to 0.38) |
| White | 7 | 1.38 (0.32–5.89) | 1.79 (0.48–6.68) | −0.29 (−0.87 to 0.29) |
| Other | 2 | 1.64 (0.14–19.78) | 1.69 (0.17–16.32) | −0.22 (−1.22 to 0.78) |
Abbreviations: NMR, nicotine metabolite ratio; 3HC, 3′-hydroxycotinine.
Mean difference calculated using Latinos as the reference group, adjusted for age and sex.
COMMENT
We present novel data on the prevalence of tobacco smoke exposure among infants and children receiving preventative care at pediatric clinics within a major urban public hospital. Approximately 70% of study participants were younger than 3 years, an age group that, to our knowledge, has not previously been studied with cotinine-validated SHS exposure. More than 55% of children were exposed to tobacco smoke, even though smoking rates are low in San Francisco (<12%) (http://www.chis.ucla.edu/main/DQ3/output.asp?_rn=0.1304285), and public smoking bans include parks, playgrounds, and the SFGH campus. The prevalence and magnitude of exposure were higher among African American children than infants and children of every other racial/ethnic group. Parental reporting of SHS exposure predicts a higher level of exposure compared with no history of SHS exposure, but the parents of most children who had cotinine-validated SHS exposure repeatedly denied exposure. Finally, we found that the rate of nicotine metabolism in children, estimated using the NMR, is slower, on average, among African Americans than among Latinos or whites, which is consistent with previous observations in adults.10,12
Much progress has been achieved in tobacco control in the United States in the past 30 years, and the prevalence of active and passive smoking has decreased substantially.2,8 Although the prevalence of active smoking has declined to an average of 21% in the United States8 and less than 12% in San Francisco, cotinine-validated SHS exposure in children remains high, as indicated in our study and by NHANES studies, which have found that, between 1999–2000 and 2007–2008, the percentage of children aged 3 to 11 years with cotinine levels of 0.05 ng/mL or higher decreased from 64.9% to 53.6%.16 Likewise, absolute cotinine levels have also decreased.6,7,15,16 In the 1988–1991 survey, the geometric mean for those 4 to 11 years of age was approximately 0.3 ng/mL and had decreased to 0.12 ng/mL for those 3 to 5 years of age by the 2003–2006 survey.2,6 In comparison, our geometric mean was 0.23 ng/mL, which falls between the levels reported in the 1991–1994 and 1999–2000 NHANES reports. To put the cotinine levels into perspective, cotinine plasma cut points that optimally separate nonsmokers from smokers range from a recent estimate17 of 3 ng/mL to a previous estimate18 of 14 ng/mL. Twelve of our subjects (median age, 2.1 years; range, 1.0–5.2 years) had levels above 3 ng/mL (maximum level, 6.3 ng/mL), consistent with light active smoking.
Our study found striking differences in cotinine levels by race/ethnicity. The low cotinine levels among Latinos and the high levels among African Americans are consistent with what is reported in the pediatric and adult literature.2,10 A 2003–2006 NHANES study of 3- to 19-year-olds found that African Americans had a mean cotinine level that was nearly 5 times higher than that of Mexican Americans,6 whereas, in our study, the disparity approached a factor of 6.
There are 2 likely reasons for the higher cotinine levels in African Americans. The first is that they have greater SHS exposure.19 The second is that their cotinine clearance rates are slower compared with Latinos and whites, which results in a greater accumulation of cotinine in African Americans.10 It is well documented that, compared with other racial/ethnic groups, African Americans adults metabolize cotinine more slowly and have higher cotinine levels for any given level of exposure to nicotine.10,20 In our study, Latinos had a significantly higher mean NMR compared with African Americans (mean difference, 0.39 [95% CI, 0.05–0.73]), indicating a more rapid metabolism of nicotine and cotinine compared with African Americans. The low NMR that we observed among African Americans supports the idea that a slower cotinine metabolism may contribute to the higher cotinine levels in African American children.
The lack of concordance between parental reporting of SHS exposure and cotinine levels may be partially due to parental misreporting combined with a limited history of possible SHS exposure obtained by the medical provider. It is also likely that both parents and providers have a limited understanding of the various sources of SHS exposure. Some parents may consider that SHS exposure only occurs if the child is present when there is active smoking, or they discount or are unaware of SHS exposure that occurs outside the home. For example, elevated air nicotine levels have been documented in nonsmoking residencies in multiunit apartment buildings where tobacco smoke odor is present,21 and higher cotinine levels have been found among children living in multiunit housing compared with children living detached dwellings.7
The 2007 US Surgeon General’s report, Children and Secondhand Smoke Exposure, found that SHS exposure from maternal and/or parental smoking is causally related to sudden infant death syndrome, impaired lung function, respiratory illnesses, middle ear disease, and asthma in their infants and/or children, and that no level of SHS exposure is safe.2 Recent studies indicate a link between pediatric mental disorders and SHS exposure.22,23 Although cotinine is a biomarker for tobacco smoke, the toxicity of SHS is mainly due to toxicants other than nicotine, including oxidant chemicals, nitrosamines, volatile gases, aromatic and heterocyclic amines, and other chemicals.24
The goal of pediatric SHS control is to prevent the morbidity and mortality due to SHS exposure. Besides public health measures, such as public smoking bans,3,4 prevention of exposure requires educating all parents about the hazards of SHS exposure so that they can identify the sources of SHS exposure, with the goal of eliminating these sources, before morbidity develops in their children. The most sensitive way to assess exposure is by measurement of biomarkers of tobacco smoke exposure. Parents who do not smoke cannot prevent exposure if they are unaware of it. Families with an active smoker can still mitigate exposure by never allowing smoking in the home or automobile.4,25 In our study, 55% of children had detectable levels of cotinine in their plasma. If universal childhood cotinine testing were mandated, it would provide an unequivocal signal to providers that the child is exposed to a hazardous environment and that intervention is necessary. Data indicate that parents, both nonsmokers and smokers, support testing of their children for SHS exposure.26
In conclusion, we present novel data on biochemically measured SHS exposure in children from infancy to 18 years old in an urban public hospital pediatric clinic. We found particularly high levels of exposure among African American children. Parental reports of SHS exposure substantially underestimate the biochemically verified smoke exposure. Underreporting might be intentional, parents might not recognize sources of SHS exposure, or medical providers do not properly assess SHS exposure. Although the discrepancy between parental reporting and biochemical assessment highlights the need for better screening questions in the primary care setting, we believe that a biochemical assessment is necessary to fully assess exposure. Our data indicate that biochemical assessment of SHS would result in considerably enhanced detection of exposure and ultimately could result in more effective interventions to prevent SHS-related diseases in children. Research on the use of routine cotinine screening in primary care to reduce childhood exposure is warranted.
Acknowledgments
Funding/Support: This work was supported by the University of California, San Francisco, Bland Lane Center of Excellence on Secondhand Smoke funded by the Flight Attendants Medical Research Institute, National Cancer Institute Training grant CA-113710, and National Institutes of Drug Abuse grant DA012353.
Footnotes
Financial Disclosure: Dr Benowitz has been a paid consultant for several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies.
Additional Contributions: We thank Faith Allen, MD, for data management; Minjiang Duan, MD, and Lisa Yu, BS, for analytical chemistry; and Marc Olmsted, MA, for editorial assistance.
Author Contributions: Study concept and design: Dempsey, Meyers, Fuentes-Afflick, and Benowitz. Acquisition of data: Dempsey, Meyers, Wu, and Jacob. Analysis and interpretation of data: Dempsey, Oh, Nguyen, Fuentes-Afflick, Wu, and Benowitz. Drafting of the manuscript: Dempsey, Meyers, Oh, Fuentes-Afflick, and Benowitz. Critical revision of the manuscript for important intellectual content: Dempsey, Oh, Nguyen, Fuentes-Afflick, Wu, Jacob, and Benowitz. Statistical analysis: Dempsey, Meyers, Oh, Nguyen, and Fuentes-Afflick. Obtained funding: Fuentes-Afflick, Jacob, and Benowitz. Administrative, technical, and material support: Fuentes-Afflick, Wu, Jacob, and Benowitz. Study supervision: Dempsey, Jacob, and Benowitz.
References
- 1.US Dept of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2006. Respiratory effects in children from exposure to secondhand smoke; pp. 257–420. [Google Scholar]
- 2.US Dept of Health and Human Services. Children and Secondhand Smoke Exposure: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2007. [Google Scholar]
- 3.Dove MS, Dockery DW, Connolly GN. Smoke-free air laws and secondhand smoke exposure among nonsmoking youth. Pediatrics. 2010;126(1):80–87. doi: 10.1542/peds.2009-3462. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar PC, Haw SJ, Currie DB, Zachary R, Currie CE. Smoking restrictions in the home and secondhand smoke exposure among primary schoolchildren before and after introduction of the Scottish smoke-free legislation. Tob Control. 2009;18 (5):409–415. doi: 10.1136/tc.2009.030627. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 6.Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003–2006. Pediatrics. 2009;124(5):1299–1305. doi: 10.1542/peds.2009-0880. [DOI] [PubMed] [Google Scholar]
- 7.Wilson KM, Klein JD, Blumkin AK, Gottlieb M, Winickoff JP. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics. 2011;127(1):85–92. doi: 10.1542/peds.2010-2046. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged >or=18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135–1140. [PubMed] [Google Scholar]
- 9.Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P., III Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol. 2009;170(7):885–891. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL, Pérez-Stable EJ, Herrera B, Jacob P., III Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94(2):108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 12.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey D, Tutka P, Jacob P, III, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879 (3–4):267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Vital signs: nonsmokers’ exposure to secondhand smoke—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59(35):1141–1146. [PubMed] [Google Scholar]
- 16.US Dept of Health and Human Services. Prevalence of exposure to secondhand smoke. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2006. pp. 127–164. [Google Scholar]
- 17.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77(11):1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson SE, Kahn RS, Khoury J, Lanphear BP. The role of air nicotine in explaining racial differences in cotinine among tobacco-exposed children. Chest. 2007;131 (3):856–862. doi: 10.1378/chest.06-2123. [DOI] [PubMed] [Google Scholar]
- 20.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13(9):772–783. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraev TA, Adamkiewicz G, Hammond SK, Spengler JD. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control. 2009;18(6):438–444. doi: 10.1136/tc.2009.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamer M, Ford T, Stamatakis E, Dockray S, Batty GD. Objectively measured secondhand smoke exposure and mental health in children: evidence from the Scottish Health Survey. Arch Pediatr Adolesc Med. 2011;165(4):326–331. doi: 10.1001/archpediatrics.2010.243. [DOI] [PubMed] [Google Scholar]
- 23.Bandiera FC, Richardson AK, Lee DJ, He JP, Merikangas KR. Secondhand smoke exposure and mental health among children and adolescents. Arch Pediatr Adolesc Med. 2011;165(4):332–338. doi: 10.1001/archpediatrics.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Dept of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2006. Assessment of exposure to secondhand smoke; pp. 83–126. [Google Scholar]
- 25.US Dept of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2006. Control of secondhand smoke exposure; pp. 569–666. [Google Scholar]
- 26.Winickoff JP, Tanski SE, McMillen RC, et al. Acceptability of testing children for tobacco-smoke exposure: a national parent survey. Pediatrics. 2011;127(4):628–634. doi: 10.1542/peds.2010-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]