Abstract
Navigation depends on a network of neural systems that accurately monitor an animal’s directional heading and location in an environment. Within this navigation system are head direction (HD) cells, which discharge as a function of an animal’s directional heading, providing an animal with a neural compass to guide ongoing spatial behavior. Experiments were designed to test this hypothesis by damaging the dorsal tegmental nucleus (DTN), a mid-brain structure that plays a critical role in the generation of the rodent HD cell signal, and evaluating landmark based navigation using variants of the Morris water task. In Experiments 1 and 2, shams and DTN lesioned rats were trained to navigate toward a cued platform in the presence of a constellation of distal landmarks located outside the pool. After reaching a training criteria, rats were tested in three probe trials in which 1) the cued platform was completely removed from the pool, 2) the pool was repositioned and the cued platform remained in the same absolute location with respect to distal landmarks, or 3) the pool was repositioned and the cued platform remained in the same relative location in the pool. In general, DTN-lesioned rats required more training trials to reach performance criterion, were less accurate to navigate to the platform position when it was removed, and navigated directly to the cued platform regardless of its position in the pool, indicating a general absence of control over navigation by distal landmarks. In Experiment 3, DTN and control rats were trained in directional and place navigation variants of the water task where the pool was repositioned for each training trial and a hidden platform was placed either in the same relative location (direction) in the pool or in the same absolute location (place) in the distal room reference frame. DTN-lesioned rats were initially impaired in the direction task, but ultimately performed as well as controls. In the place task, DTN-lesioned rats were severely impaired and displayed little evidence of improvement over the course of training. Together, these results support the conclusion that the DTN is required for accurate landmark navigation.
Introduction
Animals can use a variety of navigational strategies and stimulus types to get from one place to another (Gallistel, 1990; O’Keefe & Nadel, 1978; Sutherland & Hamilton, 2004). For instance, animals can learn to follow cues that form a trail or path to a goal location (Wallace, Hines, & Whishaw, 2002), or simply approach cues located above or besides a goal location (Redhead, Roberts, Good, & Pearce, 1997); a form of navigation often termed cued (or beacon) navigation. Under conditions in which the goal location is not marked by a distinct cue, animals can navigate based on background, or distally located cues (Morris, 1981; Prados & Trobalon, 1998; Roberts & Pearce, 1998; Sutherland & Dyck, 1984). The latter form of navigation, often termed landmark navigation, can be achieved on the basis of the fixed relationship between the goal location and distal visual cues in the environment (i.e., place navigation) (reviewed in Knierim and Hamilton (2011)). In addition, recent work has established that animals can also solve tasks that have traditionally been thought to elicit and require place navigation by navigating in the direction of distal landmarks, often referred to as direction navigation (Blodgett, McCutchan, & Mathews, 1949; Hamilton, Akers, Weisend, & Sutherland, 2007; Skinner et al., 2003; Stringer, Martin, & Skinner, 2005). In these studies, the relative contributions of place and direction to landmark-based navigation have been dissociated by translating the apparatus (e.g., Morris water task pool or T-maze) in relation to the distal visual cues such that the location of a goal is put into conflict with the direction of the goal. Thus, landmark navigation can be distinguished from beacon navigation by the use of two independent strategies – place responses and directional responses. Rodents and humans display clear preferences for directional responses over navigation to precise locations based solely on distal visual cues (Akers, Candelaria-Cook, Rice, Johnson, & Hamilton, 2009; Blodgett et al., 1949; Hamilton et al., 2008; Hamilton et al., 2007; Hamilton, Johnson, Redhead, & Verney, 2009; Skinner et al., 2003; Skinner, Horne, Murphy, & Martin, 2010; Stringer et al., 2005; Weisend et al., 1995; Whyte, Martin, & Skinner, 2009), however, these preferences can be diminished or reversed under some circumstances (Hamilton, Akers, et al., 2009; Hamilton et al., 2008; Skinner et al., 2010). Although cued, place, and directional navigation can be distinguished in a broad range of navigation tasks (Hamilton et al., 2007; Skinner et al., 2003; Valerio et al., 2010), they are not necessarily mutually exclusive (Sutherland & Hamilton, 2004) and have been shown to operate simultaneously, in parallel, or sequentially during trajectories to target locations (Hamilton, Akers, et al., 2009; Hamilton et al., 2007; Hamilton, Rosenfelt, & Whishaw, 2004; Packard & McGaugh, 1996).
The study of the neurobiological basis of navigation has identified a limbic circuit that computes an animal’s spatial location and directional heading in an environment (McNaughton, Battaglia, Jensen, Moser, & Moser, 2006; McNaughton, Chen, & Markus, 1991; Moser, Kropff, & Moser, 2008; Taube, 2007). The hippocampus is strongly linked to spatial processing, particularly place navigation, because neurons in this region display location-specific activity commonly referred to as place cells (O’Keefe & Dostrovsky, 1971). The hippocampal place signal is widely theorized to originate within the primary cortical input to the hippocampus, the entorhinal cortex (Burgess, Barry, & O’Keefe, 2007; McNaughton et al., 2006; Moser et al., 2008). This hypothesis is supported in large part by the finding of grid cells in the dorsal medial region of the entorhinal cortex (Hafting, Fyhn, Molden, Moser, & Moser, 2005; Sargolini et al., 2006), which derive their name from the fact that they discharge at multiple locations in an environment that are organized in a hexagonal grid pattern. A third type of spatial signal, referred to as head direction (HD) cells, fire when an animal passes its head through a particular direction, independent of its location in an environment (Taube, 2007). Although HD cells have been identified throughout the rodent limbic system (reviewed in Taube (2007)), this spatial signal is thought to originate within the reciprocal connectivity of the lateral mammillary and dorsal tegmental nuclei because lesions of either structure completely abolishes HD cell tuning in downstream brain regions (Bassett, Tullman, & Taube, 2007; Blair, Cho, & Sharp, 1998; Sharp & Koester, 2008). A diagram showing the relationships between the HD, grid cell, and place cell circuitry is shown in Figure 1.
Figure 1.
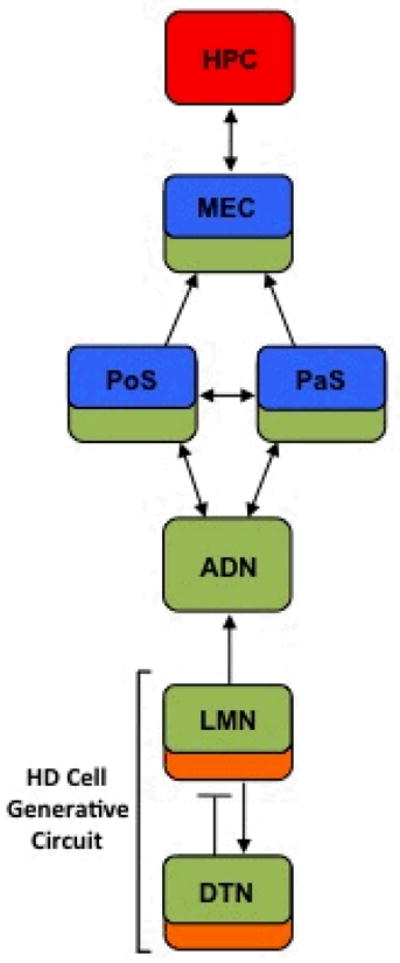
Diagram showing the principal connections between brain regions containing place cells (yellow), grid cells (red), HD cells (green), and angular velocity cells (orange) (see Taube (2007)). Arrows represent the direction of information flow. Solid lines indicate excitatory projections, and lines with bar represent inhibitory projections. ADN, anterodorsal thalamus; DTN, dorsal tegmental nucleus; HPC, hippocampus; LMN, lateral mammillary nuclei; MEC, medial entorhinal cortex; PaS, parasubiculum; PoS, postsubiculum.
The impact of damage to the circuitry involved in computing spatial position has been well documented, with lesions of the hippocampus or entorhinal cortex generally producing large impairments in place navigation (Maaswinkel, Jarrard, & Whishaw, 1999; Morris, Garrud, Rawlins, & O’Keefe, 1982; Parron & Save, 2004; Schenk & Morris, 1985; Steffenach, Witter, Moser, & Moser, 2005; Sutherland, Kolb, & Whishaw, 1982) and directional responding (Stringer et al., 2005) while leaving cued navigation intact. In contrast, however, the role of the HD cell system in navigation has not received similar attention, especially at the level of the lateral mammillary-dorsal tegmental generative circuit. The few lesion studies that have targeted HD cell regions mostly agree that damage to the generative circuitry produce impairments in landmark navigation (Frohardt, Bassett, & Taube, 2006; Vann, 2005, 2011), but have been generally ambiguous as to whether the impairments include directional or place responding. In an effort to address this issue, a recent study by Stackman, Lora, and Williams (2012) examined the role of HD cell circuitry and place cell circuitry on directional and place navigation in the mouse. In the first experiment, these authors replicated previous work in the rat (Hamilton et al., 2007) by demonstrating that mice display a preference for directional responding over place navigation in the Morris water task. In a second experiment, mice were first trained to navigate to a fixed escape platform. Then, a probe trial was conducted with the pool translated in the room after either the HD cell circuitry (i.e., the anterodorsal thalamus; see Figure 1) or the hippocampus was reversibly inactivated via local infusions of muscimol. The probe data revealed a preference for place navigation in the HD circuit-inactivated mice, whereas inactivation of the dorsal hippocampus resulted in no change in preference for directional responding. This study concluded that the recall of place and directional forms of landmark navigation could be distinguished based on selective inactivation of HD and place cell circuitry.
Although the work by Stackman et al (2012) strongly suggests that the HD cell circuit plays a prominent role in the recall of directional landmark information, it is unclear whether this system is necessary for the acquisition of landmark navigation in general (either direction or place). The results of single-unit recording studies supports the possibility that the HD cell system may play a role in acquiring place information as lesions of the HD cell circuit significantly disrupts the specificity of grid cell activity in the entorhinal cortex (Clark, Valerio, & Taube, 2011) and importantly impairs landmark control of place cell activity in the hippocampus (Calton et al., 2003; Clark et al., 2011; reviewed in Yoder, Clark, & Taube, 2011). In the present study, we aimed to address this hypothesis by lesioning the dorsal tegmental nuclei (DTN) and testing animals in variants of the Morris water task that require the use of cued and landmark (directional and place) strategies for accurate navigation (Morris, 1981, 1984). In Experiment 1, we sought to determine whether damage to the DTN disrupts landmark-based navigation as has been reported in previous work using a dry-land maze task (Frohardt et al., 2006). In Experiment 2, we evaluated the relative control of a proximal cue co-localized with the escape platform and directional navigation based on distal landmarks located along the room walls and in the surrounding environment outside of the pool (Figure 2). Experiment 3 evaluated the effects of DTN damage on learning and performance of directional and place responses in a hidden platform variant of the Morris water task (Hamilton et al., 2008).
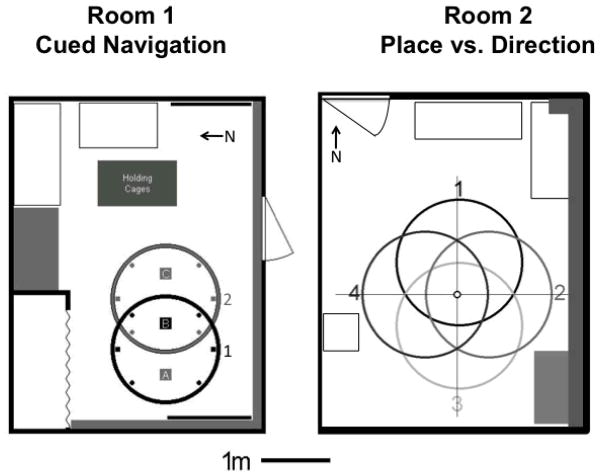
Figure 2.
Layout of the two rooms showing the locations where the pool could be positioned in the room. For room 1, pool positions 1(black) and 2 (gray) represent the same pool positions used by Hamilton et al. (2007)(see their Figure 2). Each pool position was separated by 75 cm (the radius of the pool). The cued platform was located at the black rectangle labeled B; the gray rectangles (locations A and C) mark comparison locations (relative/opposite) used for test trial analyses. The small circles (SW, SE, NW, NE) represent release points used during training trials and the rectangles (north-most and south-most points) represent release points used during test trials; Black indicates release points for pool position 1 and gray indicates release points used for pool position 2. Prominent distal visual cues (e.g., a chalkboard on the west wall) are marked by black or gray rectangles. There were two doors in the room (one of which was covered by a tarp, shown on the east wall) and the other (south wall) was always closed during testing. There were no windows in the room; The curtain shown on the north wall covered a small storage area was always closed. The room was approximately 3.5 m in height and most of the distal stimuli shown here extended at least 1.25 m above the top of the pool. The long gray rectangles mark the location of wooden wiring channels that were located near the top of the room. Room 2, which was used to evaluate the effects of DTN lesions on directional and place navigation to a hidden platform, was comparable to room 1 in terms of layout and content, however, the width of room 2 was larger (~4m) and provided sufficient space for the pool (1.73m diameter) to occupy the four different locations shown here.
Collectively, the findings reported here provide evidence that the DTN and the HD cell circuit play an important role in the acquisition of accurate navigation based on distal landmarks. Importantly, our results indicate that although DTN lesions disrupt the accurate use of both directional and place forms of landmark navigation, impairments in place navigation are more persistent over time compared to directional navigation. Thus, these findings suggest that the DTN is necessary for acquiring and updating place information in relation to distal landmarks, but that other neural systems can acquire directional information in the absence of an intact DTN and HD cell system.
General Methods
Subjects
Subjects were 16 male and 13 female hooded Long-Evans rats (Charles River Laboratories, Wilmington, MA or Harlan, Indianapolis, IN) that were approximately 90 days of age at the beginning of the experiments. All animals were pair-housed in plastic cages on a 12 h light:dark cycle with food and water available ad libitum. Behavioral testing was performed during the light phase. All procedures for the studies reported here were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of New Mexico.
Apparatus
Two separate circular pools and testing rooms were used for evaluation of cued navigation and hidden platform navigation in the Morris water task (see Figure 2). Pool 1 (1.5 m diameter, 48 cm high, white inner wall) was used for cued navigation in room 1. Pool 2 (1.73 m diameter, 61 cm high, grey inner wall) was used for hidden platform training in room 2. Both pools were placed on a wooden frame (48 cm tall) that rested on casters or appliance rollers making it possible to easily reposition the pool when it was filled with water. The escape platform used in both pools was constructed of plastic with a 16 cm × 16 cm top surface and a height of 25 cm. The pools were filled with cool water (22°C) so that the surface of the water was ~2 cm above the top of the platform. The water was made opaque by adding a small amount (~2 oz.) of powdered white tempura paint. The two pools were housed in separate testing rooms. Because the design of the hidden platform task (Experiment 3) required the pool to occupy 8 distinct positions along two axes in the room it was not possible to use room 1 for this aspect of the study. The two testing rooms differed in geometry (see Figure 2) and although the specific cues in each room differed, the rooms were comparable with respect to the quality and number of distal room cues and have previously yielded comparable data regarding the distinction between place and directional navigation (see Hamilton et al. (2008)). In addition to doors, the rooms contained numerous other distal visual cues (e.g., posters, a chalk board, wiring channels, see Figure 2). There were no windows in the testing rooms and the doors were always closed during testing. The top of the pool was approximately 75 cm from the floor and the room walls were approximately 3.5 m tall. Many of the distal visual stimuli were placed high on the walls so that they were not obscured by the pool wall. Behavior was videotaped via an overhead camera and digital camcorder. The digital video was transferred to a Linux workstation for tracking and analysis.
Surgery & Histology
The DTN is divisible into two subnuclei: a ventral region termed the central division, and a dorsal region termed the peri-central division (Paxinos & Watson, 1998). In Nissl-stained coronal sections the central division is expressed as a small, darkly-staining dense grouping of larger neurons and the peri-central division shows up as a larger, more diffuse group of smaller neurons (Figure 3A–B). Previous anatomical work has shown that the central division provides the strongest connectivity with the HD cell circuit via massive projections to the lateral mammillary nuclei (Hayakawa & Zyo, 1984). Thus, although we aimed to produce complete lesions of both DTN subregions, the central division was the primary target in the present study. All rats were initially anaesthetized with a 4% isoflurane/O2 mixture in an induction chamber. Rats were placed in a Kopf stereotaxic instrument (David Kopf Instruments, Tujunga, CA) with 1.5–2% isoflurane delivered via an anesthesia mask to maintain a surgical plane of anesthesia. An incision was made to expose the skull and small holes were then drilled in the skull above the DTN using a dental burr. Electrolytic lesions (n = 16) were produced by first lowering a No. 0 stainless steel insect pin insulated by epoxylite (except for its 1 mm pointed tip) into the DTN. The insect pin was allowed to sit for 1 min before a 0.30 mA current was passed through it for 25 sec (Bassett et al., 2007). The pin was then retracted and lowered into the next lesion site. Lesions were produced at bilateral locations based on coordinates relative to lambda: anterior = +1.0 mm; medial/lateral = +0.5 mm; ventral = −7.0 mm. Sham rats (n = 13) received identical procedures except current was not passed through the electrode.
Figure 3.
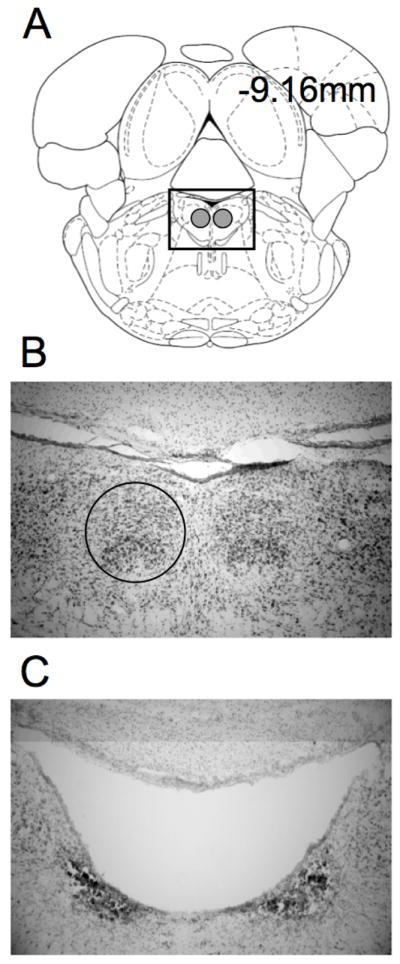
A) Select plate from Paxinos and Watson (1998) showing a section through the DTN at −9.16 mm posterior to bregma. The gray circles show the location of the DTN. B) An enlarged view of the boxed area in A in a representative section from a control rat. The circle outlines the location of the right DTN. C) Representative section from a rat with electrolytic damage of the DTN. Note the complete loss of tissue in the vicinity of the DTN.
At the completion of the experiments, animals were deeply anesthetized with sodium pentobarbital. The rats were then perfused intracardially with saline followed by a 10% formalin solution. Each brain was removed from the skull and was post-fixed in a 10% formalin solution for at least 24 hr. The brains were then cryoprotected in a 20% sucrose solution for 24 hr, and were then frozen and cut coronally at 30 μm sections with a cryostat. Every section was taken through the DTN and mounted on glass microscope slides. Sections were stained with thionin, and examined under light microscopy to evaluate the lesions. To quantify the extent of electrolytic damage to the DTN, digital images were captured at three rostral-caudal levels which included both the central and peri-central subregions of the DTN (−8.8 mm, −9.16 mm, and −9.8 mm posterior to Bregma; Paxinos and Watson (1998)). The area of undamaged tissue in the DTN was calculated at each rostral-caudal level using Image-J software (http://rsbweb.nih.gov/ij/). Tissue was considered undamaged if it contained healthy neurons and few glial cells. Once the area of undamaged tissue was calculated, the area of spared tissue was summed across the three sections and compared with an average area measured in the control rats. The total amount of damage was calculated using the following formula: Tissue damaged = [average area of DTN in control rats (pixels2) - total area of spared DTN tissue in lesioned rats (pixels2) / average area of DTN in control rats (pixels2)] × 100%. Histological analyses were performed blind to the animal’s individual performance in the experiments.
Morris Water Task
Following a seven-day post-surgical recovery time, all animals completed cued (Experiments 1–2) and hidden (Experiment 3) platform tasks. Each room (1 or 2) was only used for one task and to limit potential order effects the order of the cued navigation and hidden platform experiments was counterbalanced for rats in each lesion group such that approximately half of the animals from each group completed Experiments 3 prior to Experiments 1–2. The remaining animals completed the experiments in order. Preliminary analyses revealed no interactions between task order and lesion type, or other independent variables [all ps > .31], therefore, further analyses or discussion of order effects is not included.
Experiment 1
In Experiment 1, control and DTN lesion rats were trained to swim directly to a platform marked by a distinct cue in the presence of a constellation of distal room cues (i.e., landmarks located outside of the pool). After a pre-determined criterion was reached a probe trial was conducted with the cued platform removed. Prior work from our laboratory (Hamilton et al., 2004) demonstrated that distal cues control the initial selection of the swim trajectory to a conspicuous cue marking the platform location. If such control by distal cues is compromised by DTN damage then intact animals may learn to navigate to a cued platform in the presence of distal cues more rapidly. Further, rats with DTN damage should display greater disruption than controls if the cued platform is removed for a probe trial.
Method
Apparatus
The pool (pool 1) and room (room 1) are described in the General Methods. During cued navigation trials the platform was marked by a conspicuous black sphere (~ 5 in. dia.) positioned approximately 10 in. above the platform.
Cued Platform Morris Water Task
The cued platform protocol included a training phase followed by a no-platform probe trial. During training the pool was in position 1 for half of the rats and in position 2 for the other half (see Figure 2, left panel). The platform was always in position B. For a given sequence of four trials four release points equally spaced around the pool were selected randomly without replacement. Cued platform training continued until the animal took direct trajectories (~4 to 5 sec) to the platform on three consecutive trials, with the constraint that the animals received a minimum of 7 training trials. Thus, the Experiment was conducted in a single test session. Each trial was video-taped and transferred to a Linux workstation for subsequent tracking and analyses. Trials to meet criterion was the primary dependent measure. We also measured average swim speed.
Cued Platform Removal Test
Immediately after meeting criterion, a single probe trial with the platform and cue removed from the pool was conducted. The release point was selected randomly from the two release points furthest from the platform location and was the same as used for the prior training trial. Because we were primarily interested in the initial trajectory, particularly whether the animal swam toward and crossed the platform location, animals were retrieved after they had an opportunity to swim to the platform location. Retrieval of the animal typically occurred within 4–5 seconds after release. The video record of the trial was used to record 1) moment-to-moment position, 2) proximity to the former platform location, 3) heading error (taken when the path length first exceeded 25% of the pool diameter), and 4) the minimum distance from the platform location.
Results
Separate univariate analyses of variance (ANOVAs) are presented for each dependent measure and followed by analyses of simple effects when necessary. Where relevant, analyses of location effects within lesion groups are reported as planned comparisons. All results are significant at p < 0.05 unless otherwise noted. Preliminary analyses revealed that there were no significant main effects for sex or interactions for any dependent measure; so, sex was not included as a factor in the analyses. Estimated effect size () is reported for all significant effects.
Histology
Figure 3 shows a representative section from a DTN and sham lesion animal at a select plate from (Paxinos & Watson, 1998). Based on histological examination, 11 out of 16 lesion animals sustained significant damage to the DTN (>65%), while the remaining 5 rats sustained minimal damage to the DTN. Because the majority of the DTN was spared in the latter 5 animals, they were excluded from further analysis. Overall, 9 of the 11 lesion rats had complete, or near complete, damage of the DTN (range: 92 to 100%), and importantly, had complete damage of the central division (i.e., the subregion producing the primary connectivity with the HD cell circuit). The remaining 2 animals had lesions comprising a smaller portion of the DTN (65 and 75%). In general, the smaller lesion size in the latter two rats was mainly due to sparing found in the peri-central division (i.e, −8.8mm relative to Bregma). Importantly, both animals had animals.
Cued Platform Morris Water Task
Rats with DTN lesions required significantly more training trials to meet criteria than sham controls [F(1, 22) = 4.57, = 0.17; see Figure 4A]. Representative swim paths observed midway through training and during the final training trial are shown in Figures 4B and 4C, respectively. The swim paths taken by DTN-lesioned animals at mid-training were uniformly characterized as having an inaccurate initial trajectory, even though the platform was clearly marked by visible cue. Swim paths by DTN-lesioned animals were relatively direct and comparable to those of control animals by the end of training. Further, average swim speed (cm/sec) was comparable between groups during the final training trial [M(SEM)CONTROL = 30.94(1.47), M(SEM)DTN = 32.73(1.95); p > 0.46].
Figure 4.
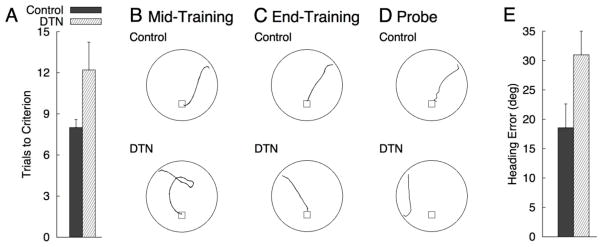
A) Mean (+SEM) trials to reach criterion for Controls and DTN during cued platform training of Experiment 1. B–D) Representative paths from Control and DTN rats at Mid-Training (B), during the final training trial (C), and during the probe trial with the cue and platform removed from the pool (D). Swim paths were taken from animals with median values for path length (panels B–C) and initial heading error during the probe (panel D) within their respective group. E) Mean (+SEM) initial heading error for Controls and DTN during the probe trial of Experiment 1. Heading error (angular deviation from a straight trajectory to the platform) was measured when the animals has traveled path length equal to 25% of the pool diameter.
Cued Platform Removal Test
Representative probe trial swim paths for control and DTN-lesioned rats are shown in Figure 4D. Valid probe trial data were not obtained for one DTN-lesioned rat. Of the 13 control rats, 7 crossed the former cued-platform location and 4 additional controls navigated to within 8 cm of the platform location. Two control rats took initial trajectories toward the platform location but failed to navigate within 25cm of the platform location. Of the 10 DTN-lesioned rats, 4 crossed the former location of the cued-platform and an additional 3 rats swam within 10 cm of the former platform location. Two DTN-lesioned rats never navigated within 25cm of the platform location. The minimum distance from the platform location was comparable for the two groups [M(SEM)CONTROL = 11.39(5.56), M(SEM)DTN = 8.93(4.27); p > 0.70]. There were numerical trends toward superior performance in controls for initial heading error (see Figure 4E), however, this difference did not reach statistical significance [p > 0.09].
Discussion
The results of Experiment 1 with a cued-platform location demonstrate that animals with lesions of the DTN required a significantly greater number of training trials to reach criterion and were less accurate with respect to initial heading during the probe trial. This result is consistent with a previous study showing that electrolytic DTN lesions impaired landmark navigation in a food-carrying task (Frohardt et al., 2006), and is consistent with other studies showing that lesions of the HD cell generative circuit produce appreciable deficits in spatial navigation (e.g., Vann 2005; 2011). Nevertheless, whether the observed landmark impairments observed here involve a disruption in learning the direction vs. place of the cued platform in relation to the distal landmarks is unclear and is the focus of the experiments below.
Experiment 2
Hamilton et al. (2007) trained rats to navigate to a cued platform in the Morris water task and then translated the pool within the distal cue reference frame for a test trial. For this test the cued platform was positioned such that it either remained in the same absolute location in the room or in the same relative location in the pool (i.e., the cued platform was translated with the pool; see Figure 2, left panel). This manipulation revealed that rats navigate quickly and directly to the cued platform in the relative location but take longer, less direct paths to a cued platform in the absolute location, often navigating to the relative location of the platform during training first. These observations suggest that distal cues (i.e., room cues) contribute to the directionality of the swim path even when the platform is marked by a conspicuous proximal cue. In Experiment 2 we used the manipulation of Hamilton et al. (2007) to further evaluate the effects of DTN damage on the control of navigation by distal cues. If control of navigation by distal cues is impaired in DTN-lesioned rats then it is expected sensitivity to the position of the cued platform within the pool and room reference frames during the pool translation test will be reduced. That is, at test DTN rats should navigate directly to the cued platform during the pool translation test regardless of its position in the local apparatus and distal room reference frame.
Method
Apparatus
The pool, cued platform, and distal room environment were the same as used in Experiment 1 (Figure 2, left panel).
Cued-Navigation Relocation Test
Twenty-four hours following the no-platform probe trial of Experiment 1, rats were given 4 additional cued platform training trials in which all four release points were selected randomly and used once without replacement. The only purpose of this training was to counter potential extinction effects associated with the probe trial, and no significant group differences in escape latency were observed for these trials [M(SEM)CONTROL = 3.79(0.257), M(SEM)DTN = 3.83(0.467); p = 0.93]. Each rat completed two test trials in which the pool and/or platform were repositioned. The pool was moved to the position not used for training (see Figure 2, left panel) and the platform either remained in the same relative location in the pool or in the same absolute location in the room. Release points were equidistant between the absolute and relative locations (i.e., N or S, see Figure 2, left panel). Each rat completed both test trials. The order of test trials and the release points were counterbalanced within each lesion group and four additional training trials were provided between the test trials. Latency and path length to navigate to the platform served as dependent measures for the test trials.
Results
Cued-Navigation Relocation Test
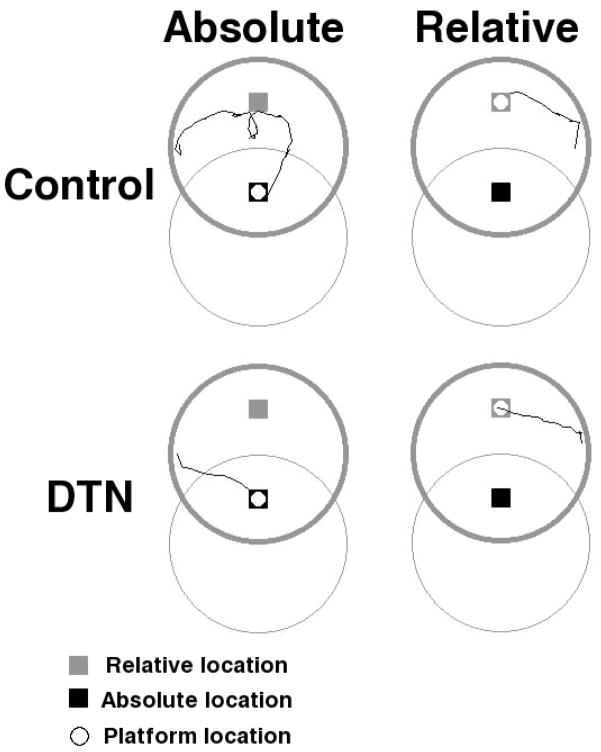
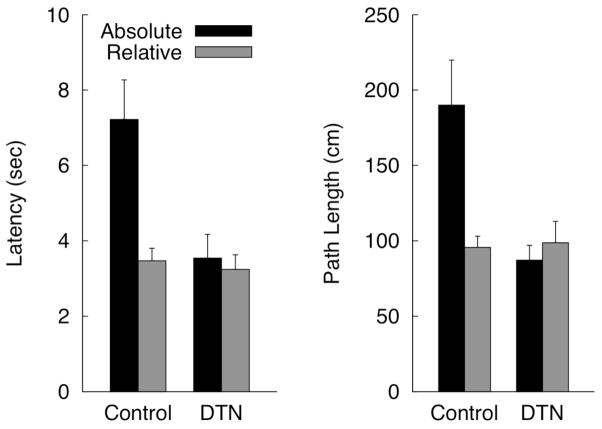
Representative swim paths from each combination of group and relocation test type (absolute and relative location) are shown in Figure 5. Mean latency and path length for each group to navigate to the cued platform in the absolute or relative location during the pool relocation test trial are shown in Figure 6. Control rats navigated quickly and directly to the cued platform in the relative location, but took longer, generally indirect paths to the cued platform when it was in the absolute location. In contrast, DTN-lesioned rats navigated quickly and directly to the cued platform regardless of whether it was in the relative or absolute location. ANOVAs with lesion group as a between-subjects factor and location (relative v. absolute) as a within-subjects factor revealed significant Group X Location interactions for latency [F(1, 22) = 10.73, = 0.33] and path length [F(1, 22) = 12.48, = 0.36]. Analyses of simple effects confirmed that control rats navigated to the relative location faster than the absolute location [F(1, 12) = 21.12, = 0.64] and took shorter path lengths to arrive at the relative location compared to the absolute location [F(1, 12) = 15.29, = 0.56]. In contrast, no significant location effects were observed for DTN-lesioned rats [both ps > 0.47].
Figure 5.
Representative swim paths from the pool and cued platform relocation tests in Experiment 2. Paths were selected from animals with median values for latency to navigate to the platform within their respective condition. The thin circular lines indicate the pool location during training and the thick circular lines indicate the pool location for the test.
Figure 6.
Mean (+SEM) latency (A) and path length (B) for Controls and DTN rats to navigate to the cued platform in the Relative and Absolute location during the two pool relocation tests of Experiment 2.
Discussion
The results of Experiment 2 replicated previous work (Akers, Candelaria-Cook, Rice, Johnson, & Hamilton, 2011; Hamilton et al., 2007) demonstrating that intact rats swim longer paths and take a greater amount of time to reach a cued platform when it is in the absolute position compared to the relative position. This result suggests that the directionality of the swim trajectories of intact rats is strongly controlled by distal environmental cues. Rats with DTN lesions on the other hand swam directly toward the cued platform regardless of its relative or absolute position. As a result, there was little difference between the relative and absolute probe conditions with respect to swim latency or path length, indicating a failure of DTN-lesioned rats to adopt a directional navigation strategy in the presence of a proximal cue marking the goal. Together, these findings support the conclusions of Stackman et al (2012) suggesting that the HD cell circuit is necessary for the expression of direction-based landmark navigation. It is important to consider, however, that lesion animals may have acquired directional landmark information, but a simpler and preferred source of information (i.e., a cue directly marking the platform) may have overshadowed directional information. In Experiment 3 we aimed to address this possibility by testing DTN-lesioned rats in a hidden platform variant of the Morris water task, and sought to determine the relative impact of DTN lesions on the acquisition of directional and place navigation strategies.
Experiment 3
In Experiment 3 we tested the impact of DTN lesions on the acquisition of directional and place navigation by repositioning the pool on each trial and training rats to swim to a hidden platform that was either maintained in the same relative location within the repositioned pool, or always remained in the absolute location relative to the distal cues (Figure 7). Using this procedure on intact animals, Hamilton et al. (2008) found a strong preference for directional responding when the platform was maintained in the relative position in the pool. When the platform was maintained in the same absolute location a preference for place navigation was observed, however, this response was learned more slowly than directional responding. In Experiment 3 we used this method to evaluate the effects of DTN damage on place and directional responding in the absence of a cued platform. If DTN lesions and damage to the HD cell circuit selectively disrupts processing of directional landmark information, then learning in the directional responding (Relative) condition should be impaired and acquisition in the place responding (Absolute) condition should be more rapid. If, however, DTN damage causes general disruptions in the control of navigation by distal visual cues, then we would expect impairments in both directional and place responding.
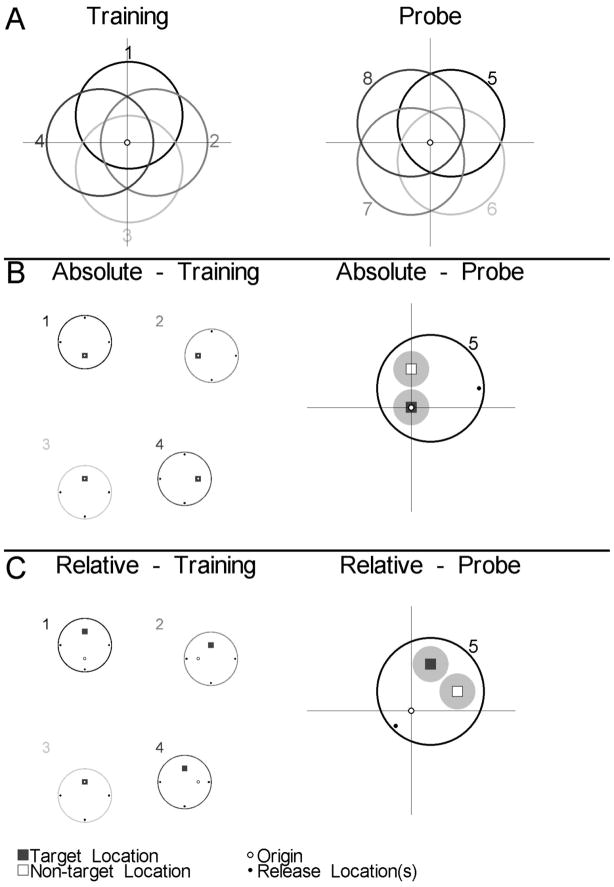
Figure 7.
A) Pool positions used during hidden platform training (positions 1–4) and the no-platform probe trial (positions 5–8) to compare place and directional navigation in Experiment 3. For all panels of this figure, the point marked by the open circle where the two lines cross was designated as the room origin. B) LEFT: The four pool positions and platform locations used during training for all rats in the Absolute condition. RIGHT: Pool position, release point, and analysis regions used during the probe trial for one rat from the Absolute condition. The black square and surrounding circle represent the target region and the open rectangle and surrounding circle represent the non-target region. Small black circles mark the training and probe release points for panels B and C. C) LEFT: The four pool positions used during training for all rats in the Relative condition. The platform locations shown here represent the locations used for the 2 animals trained with the platform in the north region of the pool. RIGHT: Pool position, release point, and analysis regions used during the probe trial for one rat from the Relative condition (north location).
Method
Apparatus
Room 2 and pool 2 were used for Experiment 3 (Figure 2, right panel).
Place and Direction Hidden Platform Morris Water Tasks
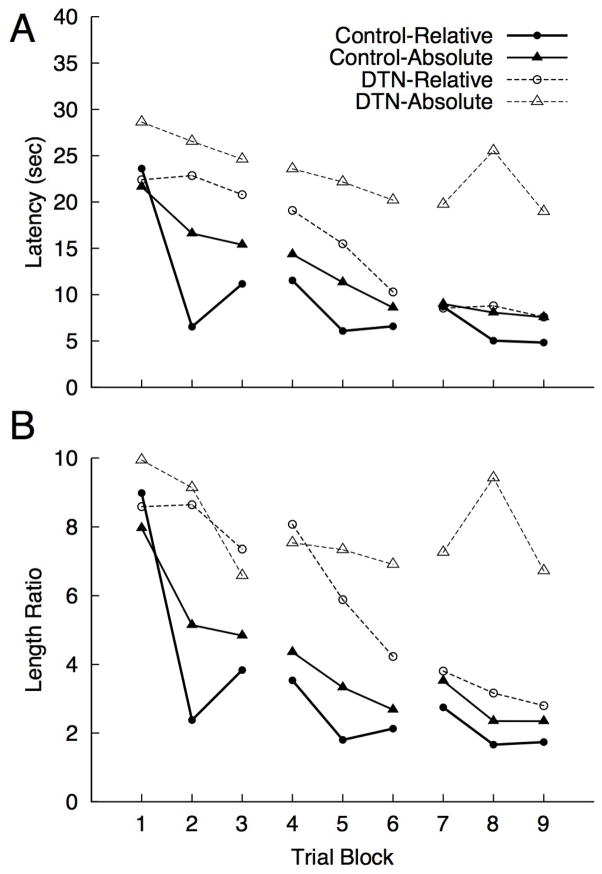
The place versus direction protocol included a hidden platform training phase followed by a single no-platform probe trial with the pool repositioned in the environment. All rats received 12 hidden-platform training trials on each of three days (3 blocks of 4 trials per day). For each trial the pool was repositioned to one of four locations (see Figure 7A, left). The pool positions and platform locations for each group during training and testing are described using x, y coordinates (cm units). Using (0,0) as an arbitrarily selected central point around which the pool was repositioned, the four locations at which the pool was centered during training were: location 1 (0,43.25), location 2 (43.25,0), location 3 (0,−43.25), and location 4 (−43.25,0). Rats were assigned to either the Absolute group or Relative group. For the Absolute group the platform was always in the same absolute location in the room as shown in Figure 7B; note that the platform occupied four separate relative locations in the pool (one for each pool position) during training. For the Relative group the platform was always in the same relative location within the pool; note that for each rat in the Relative group the platform was located at four separate places in the room (one per pool position) during training. A roughly equal number of rats from each lesion condition were trained with the platform in the north, south, east and west regions of the pool (see Figure 7C). During each training block of four trials each pool location was used once and the order of pool positions followed a pseudorandom sequence. Rats were released from one of three release points (directly opposite, to the left, or to the right of the platform) with each release point being used once for each pool position during a given daily session of 12 trials (3 blocks of 4 trials). For each trial, we measured the latency to navigate to the platform and length ratio (path length: minimum possible path length to reach the platform). The results were analyzed using a multivariate approach to repeated measures with Trial Block (4 trials), Lesion and Task as factors.
Hidden Platform Removal Test
After the final training trial on day 3, a single 30 s probe trial without the platform was conducted with the pool in one of four novel locations (pool locations 5–8, see Figure 7A, right) that were not used during training: location 5 (30.5,30.5), location 6 (30.5,−30.5), location 7 (−30.5,−30.5), and location 8 (−30.5,30.5). The goal of the probe trial was to evaluate how well each group learned to navigate to a particular target location in the pool. Therefore, the probe trial dependent measures were taken for the target location (place or direction within the pool) and a single non-target comparison location rotated 90 deg (around the center of the pool) to the left or right of the target location (see Figure 7B and 7C). For the Absolute group the target location was always at (0,0). For the Relative group the target location was always the same relative location in the pool used during training, which was 43.25 cm to the north, south, east, or west of the pool coordinates given above. The non-target location was 43.25 cm to the east or west of the pool center for rats trained with the platform in the north or south, and 43.25 cm to the north or south of the pool center for rats trained with the platform in the east or west. The release point for the probe trial was always on the opposite side of the pool and equidistant from the two critical locations. For analysis purposes four dependent measures were evaluated for the target and non-target locations. We measured the number of times each critical location was crossed and the average distance from each location during the probe trial were measured. The latter measure was adapted from the goal proximity measure of Gallagher, Burwell, and Burchinal (1993). We also measured the latency to enter and the amount of time spent in a circular region (66 cm in diameter) centered on each of the critical locations were also measured.
As a training measure, the latency data was analyzed with Lesion, Task, and trial Block as factors using the multivariate approach to repeated measures for effects that included the Block factor. A multivariate ANOVA (MANOVA) was performed for the four probe trial dependent measures with Lesion, Task, and Location (target vs. non-target) as factors.
Results
Place and Direction Hidden Platform Morris Water Task
Mean escape latencies and length ratio per trial block for each combination of group and task type (Relative and Absolute) are shown in Figures 8A and 8B, respectively. The overall pattern of latency and length ratio means suggest the control group learned both tasks, with faster learning in the Relative condition. DTN-lesioned rats appeared to learn in the Relative condition to comparable levels as controls, albeit at a slightly slower rate, while failing to learn in the Absolute condition to the same level or at the same rate as controls. A significant multivariate three-way interaction was observed [Wilks’ λ = 0.05, F(16, 5)] = 5.98, = 0.95]. Follow-up analyses revealed a significant Block X Lesion effect in the Absolute condition for latency [Wilks’ λ = 0.039, F(8, 4) = 12.17, = 0.96] and length ratio [Wilks’ λ = 0.072, F(8, 4) = 6.48, = 0.92], whereas no significant Block X Lesion interactions were observed for the Relative condition [ps > 0.33]. Thus, the three-way interaction can be understood as a significant difference in the pattern of Lesion effects across trial Blocks in the Absolute condition that was not observed in the Relative condition. The significant Lesion X Block interaction for the Absolute condition was followed with separate comparisons of Lesion effects for each level of the trial Block factor. These analyses revealed a significant Lesion effect [DTN > control] for Block 8 latency [F(1, 11)] = 5.95, = 0.35] and length ratio [F(1, 11)] = 7.34, = 0.40]. The Lesion effect for length ratio during Block 2 approached significance [F(1, 11)] = 4.69, p = .053 = 0.30]. None of the other Block effects were significant [all ps > 0.063]. We also note that there were significant reductions in latency and length ratio (Block effects) observed for control rats in the Absolute condition [ps < 0.004], whereas Block effects were not observed for DTN rats in this condition [ps > 0.47].
Figure 8.
Mean latency (A), path and length ratio (B; path length: minimum possible path length) per trial block for Controls and DTN-lesioned rats to navigate the hidden platform in the Relative and Absolute conditions in Experiment 3. Trial blocks 1–3 were conducted on day 1, blocks 4–6 on day 2, and blocks 7–9 on day 3.
Hidden Platform Removal Test
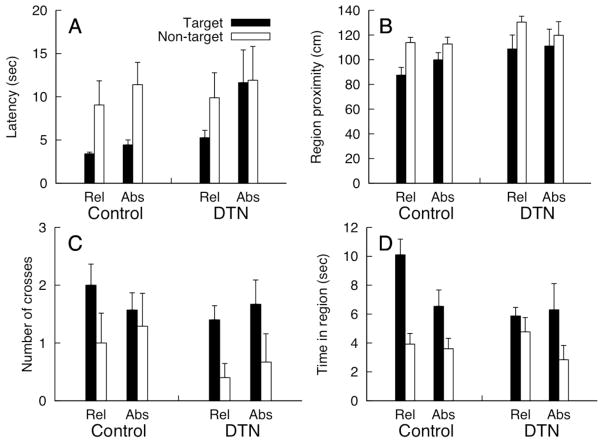
Representative probe trial swim paths for control and DTN-lesioned animals from Relative and Absolute conditions are shown in Figure 9, and the mean (+SEM) probe trial measures are shown in Figure 10. Learning to navigate to either a relative location in the pool or the absolute location in the room should be accompanied by successful discrimination between the target and non-target locations when the pool is placed in a novel location in the room. A MANOVA including the four probe trial dependent measures with Lesion, Task (Relative vs. Absolute), and Location (Target vs. Non-Target) as factors revealed a significant three-way interaction [Wilks’ λ = 0.526, F(4, 17)] = 3.83, = 0.47]. To simplify presentation of the results we present the results of a series of ANOVAs that evaluate discrimination between target and non-target locations within each combination of lesion and task for each dependent measure.
Figure 9.
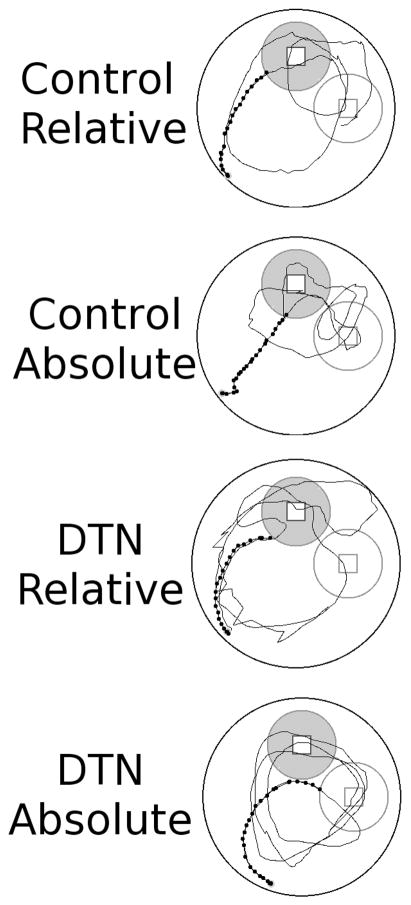
Representative swim paths from the no-platform probe trial of Experiment 3. Paths were selected from animals with median values for latency to navigate to the target region during the probe trial within their respective condition. The black circles mark the path from the release point until either the target (grey circle) or non-target (white circle) region was first entered. For purposes of comparison the paths have been rotated and/or flipped so that the target and non-target regions are represented in the same spatial locations across all conditions.
Figure 10.
Probe trial dependent measures (Mean + SEM) for Controls and DTN-lesioned rats in the Relative and Absolute conditions of Experiment 3. Black bars represent mean values for the target location and white bars represent values for a single, non-target comparison region located 90 deg from the target location (see n. 4). A) Latency to enter the 66 cm diameter circular region around the two locations of interest. B) Average distance from the two critical locations. C) Number of times each critical location was crossed. D) Time spent in each of the two critical circular regions.
Control rats in the Relative condition spent significantly more time in the target region than the non-target region [F(1, 5) = 17.81, = 0.78] and navigated significantly closer to the target location [F(1, 5) = 20.92, = 0.81]. Control rats in the Relative condition also navigated to the target region faster than the non-target region and crossed the target location more frequently, however, both effects failed to reach significance [both ps > .09]. DTN-lesioned rats in the Relative condition navigated to the target region faster than the non-target region, spent more time in the target region, navigated closer to the target region, and crossed the target region more frequently, however, only the latter effect was significant [F(1, 4) = 10.00, = 0.71; all other ps > 0.10]. These outcomes indicate both groups discriminated the target and non-target regions; comparisons of dependent measures for the target region also revealed that the Control group outperformed the DTN-lesioned group, spending more time in the target region than DTN rats [F(1, 9) = 5.08, = 0.36] and navigating closer to the target location [F(1, 9) = 5.26, = 0.37].
Control rats in the Absolute condition navigated to the target region faster than the non-target region [F(1, 6) = 8.14, = 0.58], and navigated significantly closer to the target location [F(1, 6) = 26.14, = 0.81]. Although controls in the Absolute condition spent more time in the target region and crossed the target region more frequently than the non-target region, neither of these effects reached significance [both ps > 0.29]. DTN-lesioned rats in the Absolute location had comparable means for latency to the regions of interest and region proximity [both ps > 0.21]. Although there were numerical trends toward faster latency to the target region and a greater number of target location crosses, neither of these effects were significant [both ps > 0.11]. Further, there were no differences between lesion groups for any dependent measures [all ps > .06].
Discussion
The results of Experiment 3 evaluated the impact of DTN lesions in two variants of the Morris water task. In one variant, the hidden platform remained in a fixed position in relation to the distal cues, but in the other group the platform occupied the same position relative to the pool coordinates. Regardless of the training conditions, however, rats with DTN lesions were generally slower to learn the location of the platform. Interestingly, DTN-lesioned rats had greater difficulty learning to use distal landmarks for place navigation (Absolute condition) compared to directional navigation (Relative condition). Indeed, by the end of the 9-block training period, DTN rats trained in the relative platform condition reached comparable swim latencies and length ratios to control animals. During the no-platform probe trial control and DTN-lesioned rats navigated more directly and preferentially navigated in the target region, however, controls displayed better performance. In contrast, escape latencies of DTN-lesioned rats trained in the absolute platform condition failed to reach control levels and did not discriminate the target and non-target regions. Together, these results suggest that the DTN, and thereby the HD cell system, is necessary for the acquisition of accurate place responding, but has a more transient role in the acquisition of directional navigation strategies. Importantly, these results suggest that other neural systems can acquire directional landmark information in the absence of an intact DTN and HD cell system.
General Discussion
Our findings provide consistent evidence that the DTN and the HD cell circuit play an important role in the acquisition of navigational strategies based on distal landmarks. In Experiment 1, animals were trained to swim directly to a platform marked by a salient landmark, but with distal landmarks available in the surrounding environment outside of the pool. Previous work using this task has shown that intact animals utilize distal cues for their initial trajectory (Akers et al., 2009; Hamilton et al., 2007; Hamilton et al., 2004), even though a cue directly marks the platform location. Here, we showed that DTN lesion rats required a significantly greater number of training trials to reach criterion (Figure 4A), and displayed impaired initial heading (see e.g., Figure 4B). Furthermore, during the probe trial in which the cued platform was completely removed from the pool, DTN-lesioned rats displayed less accurate swim trajectories to the platform location (Figure 4D and 4E), suggesting that DTN-lesioned rats failed to acquire landmark information. This conclusion is supported further by the results of Experiment 2 in which the pool was repositioned and the cued platform remained in the absolute position relative to the distal room cues (Figure 2, left panel). Whereas control animals swam longer paths to the absolute position compared to the relative position (Figures 5 and 6), rats with DTN lesions swam directly toward the cued platform in the absolute location. Thus, these finding suggest that for control animals the directionality of swim trajectories was strongly influenced by distal landmarks, but in lesion rats the distal landmarks exerted little control over navigation. In Experiment 3, deficits in navigation based on distal landmarks was observed in a hidden platform variant of the Morris water task in which rats were trained to swim to a submerged platform in a pool that was moved to one of four positions within the testing room. For one group of animals, the platform remained in a fixed position in relation to the distal cues, thereby requiring rats to acquire a place navigation strategy, but in the other group the platform occupied the same position relative to the pool coordinates, thereby requiring rats to acquire a directional strategy. In both conditions, DTN lesion rats were slow to learn to navigate to the platform on the basis of the distal cues (Figure 8), suggesting again that the DTN and HD cell system is generally important for acquiring landmark navigation strategies.
A second important conclusion of the present work is that the DTN is only transiently involved in acquiring directional landmark information. This conclusion is largely supported by the results of Experiment 3 in which lesion rats showed a persistent impairment in place navigation, but could eventually accomplish directional navigation comparable to control animals (see Figures 8, 9, 10). One possible explanation for this finding is that directional navigation involves a simpler strategy of approaching or avoiding a small set of distal landmarks, or possibly even a single cue (Hamilton et al., 2007), and is therefore less sensitive to DTN damage. On the other hand, place navigation may require a more complex set of associations involving the formation of several directional trajectories based on distal cues, or it may involve the association of a broad configuration of distal landmarks that enable localization on a “cognitive map” (O’Keefe & Nadel, 1978), and is therefore more sensitive to lesions of the DTN. Alternatively, place navigation may require the acquisition of multiple conditional discriminations (Skinner et al., 2003). For instance, in the absolute location task of Experiment 3, rats may learn to make differential directional responses based on their starting position. That is, if the pool is moved to position 1, then rats may learn to swim towards the distal cues marking the south quadrant, or if the pool is at position 2, then rats learn to swim towards the distal cues marking the west quadrant. Using view information from distinct start points as conditional cues was recently demonstrated by Skinner et al. (2003) who showed that performance on a plus maze variant of the place task was drastically improved by increasing the spatial distance between start points, which likely reduced the number of overlapping features and enhanced discriminability between the starting positions. Horne, Martin, Harley, and Skinner (2007) demonstrated similar improvements by placing unique texture cues at each start point. Nonetheless, regardless of how these forms of landmark navigation might be acquired, the conclusion that place responses may require a more challenging landmark solution is consistent with behavioral work using Morris water task variants (Hamilton et al., 2008) and dry land maze tasks (Blodgett et al., 1949; Skinner et al., 2003) showing directional navigation is learned more readily than place dispositions.
In light of the considerations above, it is possible that animals in Experiment 1 and 2 also acquired directional information from distal landmarks, but that this information was overshadowed by a simpler strategy of approaching a cue directly associated with the platform. This notion is consistent with a previous lesion study by Whishaw, Mittelman, Bunch, and Dunnett (1987) demonstrating that rats with medial striatum lesions learn to use distal cues for navigation. If an alternative strategy, however, is available, such as navigating toward a cue co-localized with the goal, distal cues then failed to capture strong control over behavior.
DTN, HD Cell Circuitry, and Navigation
The role of the DTN in spatial navigation has received little attention, however, several studies using single-unit physiology in behaving animals have demonstrated that the DTN play a prominent role in determining an animal’s sense of spatial orientation (Sharp, Blair, & Cho, 2001; Taube, 2007). In particular, work by Sharp, Tinkelman, and Cho (2001) and Bassett and Taube (2001) have shown that the DTN contains a large population of neurons sensitive to angular head velocity, and a smaller population of neurons sensitive to HD. Angular head velocity cells reportedly account for ~75% of the total cell population, and importantly, is a source of self-movement information that can be integrated over time to determine an animal’s current HD (Clark & Taube, 2012). Moreover, lesion work has demonstrated that damage to the DTN completely abolishes the HD signal in the anterodorsal thalamus (Bassett et al., 2007), a brain structure thought to be “downstream” in the HD signal-processing pathway (see Figure 1; Clark and Taube (2012)).
Frohardt et al. (2006) addressed the hypothesis that the DTN contributes to spatial orientation mechanisms and navigation by testing animals with DTN damage in a food-carrying paradigm, which required rats to search for large food pellets in an open-field, and then directly return to a home location (a secure box) to consume the food (Maaswinkel et al., 1999; Maaswinkel & Whishaw, 1999; Parron & Save, 2004; Whishaw & Tomie, 1997). In one variant of the food-carrying task, Frohardt and colleagues evaluated navigation to the home location while animals were blindfolded, thereby removing visual landmark information. Because external cues were not useful in this version of the task, rats had to rely on their ability to keep track of their directional heading – a process often referred to as path integration or dead reckoning (Etienne & Jeffery, 2004). In a second variant of the task, the blindfolds were removed and the rats were allowed to use landmarks to navigate to the home refuge. The authors reported that lesioned animals showed marked impairments in navigation when the animals were blindfolded, indicating that this region is required for accurate path integration. Importantly, and consistent with the results of the present study, DTN lesions also impaired the use of landmarks to localize the refuge location. However, because landmark-based testing was carried out across only three days, and because there were no probe tests to determine the strategy used by the rats, it was unclear whether landmark impairments stemmed from the inability to form a directional vs. place response.
The DTN sends a strong projection to the lateral mammillary nuclei, which reciprocates this input by sending large projections back to the DTN (Gonzalo-Ruiz, Sanz-Anquela, & Spencer, 1993; Hayakawa & Zyo, 1990; Wirtshafter & Stratford, 1993). The DTN receives additional input from the habenula, interpeduncular nuclei, and nucleus prepositus hypoglossi (reviewed in Taube (2007)), and lesion studies including the habenula (Lecourtier, Neijt, & Kelly, 2004) or interpeduncular nuclei (Clark & Taube, 2009) have reported impairments in hidden platform variants of the Morris water task, suggesting that damage to these areas also impairs landmark navigation. Most notable are studies demonstrating that lesions of the lateral mammillary nuclei impair landmark-based navigation (Vann, 2005, 2011). Interestingly, lesions of the lateral mammillary nuclei also completely abolish HD cell activity in afferent brain regions (Bassett et al., 2007; Blair, Cho, & Sharp, 1999), suggesting that the structure, along with the DTN, has an important role in generating the HD cell signal. Vann (2005, 2011) reported that damage to the lateral mammillary nuclei produced impairments in hidden platform variants of the Morris water task; however, the impairments did not persist across training. Indeed, by the end of training, rats had similar swim latencies and path lengths to reach the hidden platform. Furthermore, rats with lateral mammillary lesions were found to be unimpaired in a spatial alternation T-maze task, which is typically sensitive to disruption of the hippocampus and associated limbic regions (Aggleton, Neave, Nagle, & Hunt, 1995). Although these findings suggest that the HD cell system is not necessary for navigation in these spatial tasks, the transience of impairments in these studies point to the possibility that animals adopted a directional strategy by the end of training. This hypothesis is supported by the results of the present study demonstrating that directional navigation can be accomplished after extended training in animals with large lesions of the DTN. The results of the present study could also account for some of the mild impairments after permanent lesions to other regions of the HD cell circuit (Taube, Kesslak, & Cotman, 1992; Vann, Aggleton, & Maguire, 2009). Finally, although DTN lesions abolish the HD signal in the anterior thalamus (Bassett et al., 2007), it is unclear whether other areas of the HD cell circuits are similarly affected. Furthermore, it is unclear whether the results of the present study reflect some lesion-induced compensatory or covert functional changes (e.g., altered immediate early gene expression) in other areas limbic regions (Amin et al., 2010; Jenkins, Vann, Amin, & Aggleton, 2004). These questions should be addressed in future studies.
Complementary to the lesion work discussed above, Valerio et al. (2010) investigated the role of the HD cell system in landmark navigation by taking advantage of the observation that when rats are positioned in an upside-down orientation, HD cells display a dramatic change in their activity, including a complete loss or marked reduction of directional-specific firing (Calton & Taube, 2005). Following this observation, Valerio et al. (2010) tested animals in a task modeled after the Morris water task and the Barnes hole board task (Barnes, 1979), but designed the experiment so that it could be accomplished in an inverted orientation. The apparatus was a circular (1.2 m diameter) wire mesh suspended from the ceiling with four holes cut uniformly in the mesh, with one of the holes allowing access to the top surface of the platform which the rats could go through to ‘right’ themselves. In one experiment, Valerio and colleagues trained the animals to navigate from one- or two-start points and after the rats reached a set criterion, probe tests were conducted in which possible olfactory/tactile or visual cues were manipulated. Although these probe tests determined that distal visual cues gained the strongest control over navigation, it was revealed that their performance was markedly attenuated when navigating to the escape hole from a novel start point at the periphery or even from the maze center which was also located ~45cm from the escape hole. This absence of flexibility while navigating upside-down was confirmed in a third experiment where their view of the distal room cues was eliminated by surrounding the apparatus with floor-to-ceiling curtains. These findings suggested that the animals did not learn to place navigate, but instead learned two separate trajectories to the target hole, likely defined by the direction of the distal landmarks from their start location they started from. Further, these results suggested that inverted navigation primarily involved a simple directional strategy based on visual landmarks, rather than a strategy defined by the absolute location of the escape hole. In another study, Gibson, Butler, and Taube (2013) showed that HD cells continued to display a general loss of direction-specific activity when the rats were inverted despite extensive training in this task. Together, these findings support the hypothesis that the HD cell system is not required for navigation when a relatively simple directional strategy will suffice, but is required when a place strategy based on multiple distinct trajectories is needed.
Stackman et al. (2012) reported a study in which they trained mice to navigate to a fixed, hidden platform in the Morris water task and subsequently inactivated the anterodorsal thalamus (i.e., a component of HD cell circuitry; see Figure 1) via local infusions of muscimol for a probe trial. This manipulation resulted in a preference for place navigation over directional responding. The results of the present study are largely consistent with these findings in suggesting that the HD cell circuit is involved in landmark navigation. However, the results of the present study suggests that the DTN is involved in the acquisition of place information, and that other neural systems can obtain directional information in the absence of an intact HD cell system. It is likely that the different findings in the present work and that of Stackman et al. (2012) are primarily due to differences in the acute vs. chronic nature of the effects, and the examination of effects on training vs. test data. In the Stackman et al. study, the results of acute anterodorsal thalamic or hippocampal inactivation clearly demonstrate that both responses are acquired after training. Under normal circumstances when the pool is not translated both forms of responding may contribute to behavior, possibly competing, cooperating, or operating in parallel. During the test with pool translation the directional response preferentially controls behavior, however, with the directional system compromised via anterodorsal thalamus inactivation place responses that are controlled by unaffected circuitry preferentially control behavior. An additional point of contrast with the present lesion effects is that the acute effects observed in the Stackman et al. study might exclusively reflect a weakening of competition between systems, while the long-term consequences of damage to the HD generative circuit likely reflecting competition between systems and the direct effects of damage to the circuits of interest. Further, given the patterns of data observed with lesions, other circuits that are unaffected by widespread HD cell disruption could contribute to the sparing and compensation of directional responding controlled by distal landmarks.
An additional consideration is that the generative HD signals affected by DTN damage may have consequences for both directional and place navigation, as experimental and theoretical work suggests that place cells may derive their directionality and specificity, in part, from HD signals (Calton et al., 2003; Knierim & Hamilton, 2011). Indeed, it has been argued elsewhere that a normal flow of self-movement information to downstream thalamic and cortical regions is required to generate a stable spatial framework for landmark learning (Alyan & Jander, 1994; Dudchenko, Goodridge, Seiterle, & Taube, 1997; Gibson, Shettleworth, & McDonald, 2001; Knierim, Kudrimoti, & McNaughton, 1995; Martin, Harley, Smith, Hoyles, & Hynes, 1997; McNaughton et al., 1996). This possibility would also explain why landmark navigation impairments are observed after DTN lesions despite the fact that the DTN is several synapses removed from the cortical and limbic circuitry frequently linked to landmark processing.
In conclusion, the findings of the present study demonstrate that DTN lesions produce strong impairments in navigation based on distal landmarks in three experiments using cued and hidden platform variants of the Morris water task. In general, DTN lesioned animals were capable of acquiring a directional response after extensive training, but showed persistent impairments in place navigation, which is consistent with recent evidence suggesting that the HD cell system is critical for navigating to specific places in an environment (Gibson et al., 2013; Valerio et al., 2010), and with the results of single-unit physiology experiments demonstrating that an intact HD cell system is necessary for the firing specificity of place and grid cells and the accurate orientation of place cells in relation to landmarks (Calton et al., 2003; Clark et al., 2011). Nevertheless, that widespread alterations of HD cell activity, as would be expected with DTN damage, can impair both forms of landmark navigation, suggests that directional and place navigation share some dependence on the distributed HD cell network. Future work should be directed at exploring the generality of the present findings to other regions of the HD cell, place cell, and grid cells systems, as well as understanding the behavioral and neural mechanisms that underlie and distinguish directional and place forms of landmark navigation.
Acknowledgments
The authors thank Travis Johnson for assistance with data collection. This research was supported in part by funding from the Quad-L Foundation (DAH), NIH grants AA015356 (DAH), NS053907 (JST), DC009318 (JST), and an NSERC postgraduate fellowship (BJC).
References
- Aggleton JP, Neave N, Nagle S, Hunt PR. A Comparison of the Effects of Anterior Thalamic, Mamillary Body and Fornix Lesions on Reinforced Spatial Alternation. Behavioural Brain Research. 1995;68:91–101. doi: 10.1016/0166-4328(94)00163-a. [DOI] [PubMed] [Google Scholar]
- Akers KG, Candelaria-Cook FT, Rice JP, Johnson TE, Hamilton DA. Delayed Development of Place Navigation Compared to Directional Responding in Young Rats. Behavioral Neuroscience. 2009;123:267–275. doi: 10.1037/a0014594. [DOI] [PubMed] [Google Scholar]
- Akers KG, Candelaria-Cook FT, Rice JP, Johnson TE, Hamilton DA. Cued Platform Training Reveals Early Development of Directional Responding among Preweanling Rats in the Morris Water Task. Developmental Psychobiology. 2011;53:1–12. doi: 10.1002/dev.20480. [DOI] [PubMed] [Google Scholar]
- Alyan S, Jander R. Short-Range Homing in the House Mouse, Mus Musculus: Stages in the Learning of Directions. Animal Behaviour. 1994;48:285–298. [Google Scholar]
- Amin E, Wright N, Poirier GL, Thomas KL, Erichsen JT, Aggleton JP. Selective Lamina Dysregulation in Granular Retrosplenial Cortex (Area 29) after Anterior Thalamic Lesions: An in Situ Hybridization and Trans-Neuronal Tracing Study in Rats. Neuroscience. 2010;169:1255–1267. doi: 10.1016/j.neuroscience.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory Deficits Associated with Senescence - Neurophysiological and Behavioral-Study in the Rat. Journal of Comparative and Physiological Psychology. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Taube JS. Neural Correlates for Angular Head Velocity in the Rat Dorsal Tegmental Nucleus. Journal of Neuroscience. 2001;21:5740–5751. doi: 10.1523/JNEUROSCI.21-15-05740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the Tegmentomammillary Circuit in the Head Direction System Disrupt the Head Direction Signal in the Anterior Thalamus. Journal of Neuroscience. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Cho JW, Sharp PE. Role of the Lateral Mammillary Nucleus in the Rat Head Direction Circuit: A Combined Single Unit Recording and Lesion Study. Neuron. 1998;21:1387–1397. doi: 10.1016/s0896-6273(00)80657-1. [DOI] [PubMed] [Google Scholar]
- Blair HT, Cho JW, Sharp PE. The Anterior Thalamic Head-Direction Signal Is Abolished by Bilateral but Not Unilateral Lesions of the Lateral Mammillary Nucleus. Journal of Neuroscience. 1999;19:6673–6683. doi: 10.1523/JNEUROSCI.19-15-06673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett HC, McCutchan K, Mathews R. Spatial Learning in the T-Maze: The Influence of Direction, Turn, and Food Location. Journal of Experimental Psychology. 1949;39:800–809. doi: 10.1037/h0058978. [DOI] [PubMed] [Google Scholar]
- Burgess N, Barry C, O’Keefe J. An Oscillatory Interference Model of Grid Cell Firing. Hippocampus. 2007;17:801–812. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal Place Cell Instability after Lesions of the Head Direction Cell Network. Journal of Neuroscience. 2003;23:9719–9731. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Taube JS. Degradation of Head Direction Cell Activity During Inverted Locomotion. Journal of Neuroscience. 2005;25:2420–2428. doi: 10.1523/JNEUROSCI.3511-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Deficits in Landmark Navigation and Path Integration after Lesions of the Interpeduncular Nucleus. Behavioral Neuroscience. 2009;123:490–503. doi: 10.1037/a0015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Vestibular and Attractor Network Basis of the Head Direction Cell Signal in Subcortical Circuits. Frontiers in Neural Circuits. 2012;6 doi: 10.3389/fncir.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Valerio S, Taube JS. Disrupted Grid and Head Direction Cell Signal in the Entorhinal Cortex and Parasubiculum after Lesions of the Head Direction System. from Society for Neuroscience 2011 [Google Scholar]
- Dudchenko PA, Goodridge JP, Seiterle DA, Taube JS. Effects of Repeated Disorientation on the Acquisition of Spatial Tasks in Rats: Dissociation between the Appetitive Radial Arm Maze and Aversive Water Maze. Journal of Experimental Psychology-Animal Behavior Processes. 1997;23:194–210. doi: 10.1037//0097-7403.23.2.194. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Jeffery KJ. Path Integration in Mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Bassett JP, Taube JS. Path Integration and Lesions within the Head Direction Cell Circuit: Comparison between the Roles of the Anterodorsal Thalamus and Dorsal Tegmental Nucleus. Behavioral Neuroscience. 2006;120:135–149. doi: 10.1037/0735-7044.120.1.135. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of Spatial-Learning Impairment in Aging - Development of a Learning Index for Performance in the Morris Water Maze. Behavioral Neuroscience. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Gibson BM, Butler WN, Taube JS. The Head-Direction Signal Is Necessary for Flexible, Cognitive Mapping, but Not for Learning a Spatial Habit. Current Biology. 2013 doi: 10.1016/j.cub.2013.06.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BM, Shettleworth SJ, McDonald RJ. Finding a Goal on Dry Land and in the Water: Differential Effects of Disorientation on Spatial Learning. Behavioural Brain Research. 2001;123:103–111. doi: 10.1016/s0166-4328(01)00196-6. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Sanz-Anquela JM, Spencer RF. Immunohistochemical Localization of Gaba in the Mammillary Complex of the Rat. Neuroscience. 1993;54:143–156. doi: 10.1016/0306-4522(93)90390-2. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a Spatial Map in the Entorhinal Cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Redhead ES. Evidence for a Shift from Place Navigation to Directional Responding in One Variant of the Morris Water Task. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:271–278. doi: 10.1037/a0013260. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Sutherland RJ, Redhead ES. The Relative Influence of Place and Direction in the Morris Water Task. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:31–53. doi: 10.1037/0097-7403.34.1.31. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, Sutherland RJ. How Do Room and Apparatus Cues Control Navigation in the Morris Water Task? Evidence for Distinct Contributions to a Movement Vector. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:100–114. doi: 10.1037/0097-7403.33.2.100. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Johnson TE, Redhead ES, Verney SR. Control of Rodent and Human Spatial Navigation by Room and Apparatus Cues. Behavioural Processes. 2009;81:154–169. doi: 10.1016/j.beproc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Rosenfelt CS, Whishaw IQ. Sequential Control of Navigation by Locale and Taxon Cues in the Morris Water Task. Behavioural Brain Research. 2004;154:385–397. doi: 10.1016/j.bbr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Comparative Anatomical Study of the Tegmentomammillary Projections in Some Mammals - a Horseradish-Peroxidase Study. Brain Research. 1984;300:335–349. doi: 10.1016/0006-8993(84)90844-8. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Fine-Structure of the Lateral Mammillary Projection to the Dorsal Tegmental Nucleus of Gudden in the Rat. Journal of Comparative Neurology. 1990;298:224–236. doi: 10.1002/cne.902980207. [DOI] [PubMed] [Google Scholar]
- Horne MR, Martin GM, Harley CW, Skinner DM. Where Am I? Distal Cue Use Requires Sensitivity to Start Location Change in the Rat. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:92–99. doi: 10.1037/0097-7403.33.2.92. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Vann SD, Amin E, Aggleton JP. Anterior Thalamic Lesions Stop Immediate Early Gene Activation in Selective Laminae of the Retrosplenial Cortex: Evidence of Covert Pathology in Rats? European Journal of Neuroscience. 2004;19:3291–3304. doi: 10.1111/j.0953-816X.2004.03421.x. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Hamilton DA. Framing Spatial Cognition: Neural Representations of Proximal and Distal Frames of Reference and Their Roles in Navigation. Physiological Reviews. 2011;91:1245–1279. doi: 10.1152/physrev.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place Cells, Head Direction Cells, and the Learning of Landmark Stability. Journal of Neuroscience. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH. Habenula Lesions Cause Impaired Cognitive Performance in Rats: Implications for Schizophrenia. European Journal of Neuroscience. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Jarrard LE, Whishaw IQ. Hippocampectomized Rats Are Impaired in Homing by Path Integration. Hippocampus. 1999;9:553–561. doi: 10.1002/(SICI)1098-1063(1999)9:5<553::AID-HIPO9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Whishaw IQ. Homing with Locale, Taxon, and Dead Reckoning Strategies by Foraging Rats: Sensory Hierarchy in Spatial Navigation. Behavioural Brain Research. 1999;99:143–152. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- Martin GM, Harley CW, Smith AR, Hoyles ES, Hynes CA. Spatial Disorientation Blocks Reliable Goal Location on a Plus-Maze but Does Not Prevent Goal Location in the Morris Maze. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:183–193. doi: 10.1037//0097-7403.23.2.183. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Weaver KL. Deciphering the Hippocampal Polyglot: The Hippocampus as a Path Integration System. Journal of Experimental Biology. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path Integration and the Neural Basis of the ‘Cognitive Map’. Nature Reviews Neuroscience. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Chen LL, Markus EJ. Dead Reckoning, Landmark Learning, and the Sense of Direction: A Neurophysiological and Computational Hypothesis. Journal of Cognitive Neuroscience. 1991;3:192–202. doi: 10.1162/jocn.1991.3.2.190. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial Localisation Does Not Require the Presence of Local Cues. Learning and Motivation. 1981;12:239–260. [Google Scholar]
- Morris RGM. Developments of a Water-Maze Procedure for Studying Spatial-Learning in the Rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place Navigation Impaired in Rats with Hippocampal Damage. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Annual Review of Neuroscience. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The Hippocampus as a Spatial Map: Preliminary Evidence from Unit Activity in the Freely-Moving Rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford, England: Oxford University Press; 1978. [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of Hippocampus or Caudate Nucleus with Lidocaine Differentially Affects Expression of Place and Response Learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Parron C, Save E. Evidence for Entorhinal and Parietal Cortices Involvement in Path Integration in the Rat. Experimental Brain Research. 2004;159:349–359. doi: 10.1007/s00221-004-1960-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Prados J, Trobalon JB. Locating an Invisible Goal in a Water Maze Requires at Least Two Landmarks. Psychobiology. 1998;26:42–48. [Google Scholar]
- Redhead ES, Roberts A, Good M, Pearce JM. Interaction between Piloting and Beacon Homing by Rats in a Swimming Pool. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:340–350. doi: 10.1037//0097-7403.23.3.340. [DOI] [PubMed] [Google Scholar]
- Roberts ADL, Pearce JM. Control of Spatial Behavior by an Unstable Landmark. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:172–184. [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive Representation of Position, Direction, and Velocity in Entorhinal Cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Schenk F, Morris RGM. Dissociation between Components of Spatial Memory in Rats after Recovery from the Effects of Retrohippocampal Lesions. Experimental Brain Research. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Cho JW. The Anatomical and Computational Basis of the Rat Head-Direction Cell Signal. Trends in Neurosciences. 2001;24:289–294. doi: 10.1016/s0166-2236(00)01797-5. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Koester K. Lesions of the Mammillary Body Region Severely Disrupt the Cortical Head Direction, but Not Place Cell Signal. Hippocampus. 2008;18:766–784. doi: 10.1002/hipo.20436. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Tinkelman A, Cho JW. Angular Velocity and Head Direction Signals Recorded from the Dorsal Tegmental Nucleus of Gudden in the Rat: Implications for Path Integration in the Head Direction Cell Circuit. Behavioral Neuroscience. 2001;115:571–588. [PubMed] [Google Scholar]
- Skinner DM, Etchegary CM, Ekert-Maret EC, Baker CJ, Harley CW, Evans JH, Martin GM. An Analysis of Response, Direction, and Place Learning in an Open Field and T Maze. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:3–13. [PubMed] [Google Scholar]
- Skinner DM, Horne MR, Murphy KEA, Martin GM. Rats’ Orientation Is More Important Than Start Point Location for Successful Place Learning. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:110–116. doi: 10.1037/a0015773. [DOI] [PubMed] [Google Scholar]
- Stackman R, Lora J, Williams S. Directional Responding of C57bl/6j Mice in the Morris Water Maze Is Influenced by Visual and Vestibular Cues and Is Dependent Upon the Anterior Thalamic Nuclei. Journal of Neuroscience. 2012;32:10211–10225. doi: 10.1523/JNEUROSCI.4868-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial Memory in the Rat Requires the Dorsolateral Band of the Entorhinal Cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Stringer KG, Martin GM, Skinner DA. The Effects of Hippocampal Lesions on Response, Direction, and Place Learning in Rats. Behavioral Neuroscience. 2005;119:946–952. doi: 10.1037/0735-7044.119.4.946. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Dyck RH. Place Navigation by Rats in a Swimming Pool. Canadian Journal of Psychology. 1984;38:322–347. [Google Scholar]
- Sutherland RJ, Hamilton DA. Rodent Spatial Navigation: At the Crossroads of Cognition and Movement. Neuroscience and Biobehavioral Reviews. 2004;28:687–697. doi: 10.1016/j.neubiorev.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial Mapping: Definitive Disruption by Hippocampal or Frontal Cortical Damage in the Rat. Neuroscience Letters. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Taube JS. The Head Direction Signal: Origins and Sensory-Motor Integration. Annual Review of Neuroscience. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Kesslak JP, Cotman CW. Lesions of the Rat Postsubiculum Impair Performance on Spatial Tasks. Behavioral and Neural Biology. 1992;57:131–143. doi: 10.1016/0163-1047(92)90629-i. [DOI] [PubMed] [Google Scholar]
- Valerio S, Clark BJ, Chan JH, Frost CP, Harris MJ, Taube JS. Directional Learning, but No Spatial Mapping by Rats Performing a Navigational Task in an Inverted Orientation. Neurobiology of Learning and Memory. 2010;93:494–505. doi: 10.1016/j.nlm.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD. Transient Spatial Deficit Associated with Bilateral Lesions of the Lateral Mammillary Nuclei. European Journal of Neuroscience. 2005;21:820–824. doi: 10.1111/j.1460-9568.2005.03896.x. [DOI] [PubMed] [Google Scholar]
- Vann SD. A Role for the Head-Direction System in Geometric Learning. Behavioural Brain Research. 2011;224:201–206. doi: 10.1016/j.bbr.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What Does the Retrosplenial Cortex Do? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Whishaw IQ. Quantification of a Single Exploratory Trip Reveals Hippocampal Formation Mediated Dead Reckoning. Journal of Neuroscience Methods. 2002;113:131–145. doi: 10.1016/s0165-0270(01)00489-7. [DOI] [PubMed] [Google Scholar]
- Weisend MP, Klein RL, Hoesing JM, Astur RS, Koerner A, McDonald RJ, Sutherland RJ. Morris Water Task: Which Cues Define Locations? Society for Neuroscience Abstracts. 1995;21:1939–1939. [Google Scholar]
- Whishaw IQ, Mittelman G, Bunch ST, Dunnett SB. Impairments in the Acquisition, Retention and Selection of Spatial Navigation Strategies after Medial Caudate-Putamen Lesions in Rats. Behavioural Brain Research. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Perseveration on Place Reversals in Spatial Swimming Pool Tasks: Further Evidence for Place Learning in Hippocampal Rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Whyte JT, Martin GT, Skinner DM. An Assessment of Response, Direction and Place Learning by Rats in a Water T-Maze. Learning and Motivation. 2009;40:376–385. [Google Scholar]
- Wirtshafter D, Stratford TR. Evidence for Gabaergic Projections from the Tegmental Nuclei of Gudden to the Mammillary Body in the Rat. Brain Research. 1993;630:188–194. doi: 10.1016/0006-8993(93)90656-8. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Taube JS. Origins of Landmark Encoding in the Brain. Trends in Neurosciences. 2011;34:561–571. doi: 10.1016/j.tins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]