Abstract
The therapeutic effect of mesenchymal stem cells (MSCs) in tissue repair/regeneration is substantially dampened by the loss of primitive properties and poor engraftment to target organs. In this study, the multipotency and cell sizes of human MSCs, which had been expanded in monolayer culture for several passages, were dramatically restored after an episode of three-dimensional (3D) spheroid culture. Unlike MSCs derived from monolayer, which caused embolism and blindness, MSCs derived from 3D spheroids did not cause vascular obstructions, after intra-carotid artery infusion in rats. Importantly, intra-carotid infusion of 1 million 3D spheroid MSCs in rats 24 h after middle cerebral artery occlusion and reperfusion resulted in engraftment of the cells into the lesion and significant (over 70%) reduction of infarct size along with restoration of neurologic function. Moreover, the enhanced effect of spheroid MSCs was coincided with significantly increased differentiation of the MSCs into neurons and markedly increased number of endogenous glial fibrillary acidic protein–positive neural progenitors in the peri-infarct boundary zone. However, the similarly administered monolayer MSCs resulted in a modest functional improvement. Our results suggest that 3D MSCs, in combination with intra-carotid delivery, may represent a novel therapeutic approach of MSCs for stroke.
Introduction
Stroke is a major cause of morbidity and mortality worldwide. Thrombolytic therapy requires timing administration of alteplase. This makes the majority of patients unable to receive the treatment. Even with the treatment, most patients heal with neurological deficits [1,2]. Therefore, novel therapies to enhance neurogenesis and reduce neurological deficits are required.
Mesenchymal stem cells (MSCs) are self-renewing and expandable [3,4]. They are capable of differentiating into mesoderm- and nonmesoderm-derived tissues [3,5]. Residing in various tissues, MSCs likely participate in the maintenance of stem cell niches and tissue homeostasis [6,7]. Increasing evidence has suggested a profound therapeutic potential of MSCs for a variety of diseases, such as myocardial infarction and strokes [8–10]. Moreover, MSCs show low immunogenicity and transplantation of allogeneic MSCs appear not to cause immune rejections [11]. For these reasons, MSCs are emerging as an extremely promising therapeutic agent and numerous clinical trials for variety of diseases are underway [8].
Recent studies indicate that current expansion methods of MSCs in adherent culture lead to a loss of critical features of the cells [12] and thereby affect their therapeutic effects. MSCs appear as a rare cell population in the bone marrow and other tissues [13]. Therefore, culture expansion of MSCs is an essential procedure to obtain sufficient amounts of cells for clinical therapies and tissue engineering. MSCs are commonly cultured as a two-dimensional monolayer, which facilitates adequate amplification, but is unable to retain the primitive properties of the cells. The cells age quickly, resulting in reduction of valuable abilities such as homing and production of paracrine factors important for tissue repair/regeneration [12]. Increasing evidence suggests that alterations of MSCs in culture are caused by epigenetic changes that are potentially reversible. Recently, MSCs cultured in three-dimensional (3D) spheroids have been found to express much higher levels of several cytokines than monolayer-cultured MSCs, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and angiogenin [14], which are critically involved in tissue repair.
Previous studies indicate that the therapeutic effect of MSCs in tissue repair/regeneration is positively correlated with the number of MSCs engrafted into the injured tissues [10,15]. Extremely low numbers of culture-expanded MSCs could arrive in injured tissues after intravenous infusion due to severe lung vascular entrapment and rapid cell death [16,17]. Though MSCs may release some cytokines to the blood before death, which effectively affect the remote target organ [16], the therapeutic potential of MSCs in tissue regeneration is obviously dampened. Therefore, it is necessary to improve the quality and engraftment of MSCs to achieve maximal therapeutic effect of the cells.
In this study, we found that intra-carotid artery infusion of human MSCs (hMSCs) derived from spheroid culture that significantly reduced the size of hMSCs by 40% resulted in engraftment of hMSCs into the lesion and restored neurologic function after stroke in association with significantly enhanced reduction of infarct volume (by 70%) in rats with middle cerebral artery occlusion (MCAO), compare with hMSCs cultured in monolayer, which caused embolism. In accordance, rats receiving spheroid hMSCs showed profoundly enhanced differentiation of MSCs in the lesion and significantly increased numbers of endogenous glial fibrillary acidic protein (GFAP)–positive neural progenitor cells. Our results indicate a markedly improved therapeutic effect of 3D spheroid hMSCs in neurogenesis and restoration of cerebral function after ischemic stroke.
Materials and Methods
Cell culture
hMSCs were isolated from human placenta as described previously [12] (for details, see Supplementary Materials and Methods; Supplementary Data are available online at www.liebertpub.com/scd). Cells were culture expanded in a growth medium consisting of Dulbecco's modified Eagle's medium (DMEM; Gibco-Invitrogen), 10% fetal bovine serum (FBS; Gibco-Invitrogen), and antibiotics. To form spheroids, passage 5–7 hMSCs were cultured by a hanging-drop method as described previously [18] with modifications. Briefly, 3,000 hMSCs in 35 μL growth medium per drop were plated in hanging drops and incubated for 36 h to form spheroids. Then, the spheroids were transferred to a suspension culture with fresh growth medium and incubated for 24 h. To obtain single cells from spheroids, spheroids were incubated with 0.25% trypsin/ethylenediaminetetraaceticacid for 6–10 min (depending on size of spheroids) with gentle pipetting every 2–3 min.
Measurement of cell size
The size of hMSCs derived from adherent monolayers (mono) or from dissociated spheroids (3D) was determined by microscopy and flow cytometry. For microscopic analysis, single cells from monolayer or 3D spheroids were transferred into chambers of a hemocytometer, respectively, and were observed using ordinary optical microscope. Images were captured using a Ds-Fi1 camera (Nikon). In addition, monolayer hMSCs were labeled with retroviral-enhanced green fluorescent protein, and the cells from 3D culture were labeled with lipophilic fluorochrome 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Sigma-Aldrich). Then, the cells were mixed in equal numbers and the diameter was measured under confocal laser scanning microscope (FV1000; Olympus). For flow cytometric analysis of cell size, 2×105 hMSCs were stained with an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Invitrogen) according to the supplier's instructions. Cell sizes were estimated from the viable population (Annexin V−/PI−) by comparing forward scatter properties of the cells.
Rat MCAO model
Female Sprague–Dawley rats (250–270 g) underwent MCAO. After anesthesia, rats were subjected to transient focal ischemia for 120 min by the intraluminal suture technique as described previously [19], and reperfusion was achieved by withdrawing the suture (for details, see Supplementary Materials and Methods).
Cell transplantation
One day after MCAO, rats were randomly divided into three groups, which received intra-carotid injection of spheroid hMSCs, monolayer hMSCs, or equal volume of phosphate-buffered saline (PBS), respectively. Briefly, anesthesia was reinstituted. A 30G microinjection needle (Hamilton) was inserted through a small puncture in the left common carotid artery into the internal carotid artery after ligation of the pterygopalatine artery with a 6-0 silk suture. hMSCs in monolayer or spheroids were trypsinized and filtered through a cell strainer with 40-μm pores to generate single-cell suspensions immediately before transplantation. Cell sizes were measured by calculating the average diameter of 100 cells in microscopic images and by fluorescence-activated cell sorting analysis. About 1×106 monolayer or 3D spheroid hMSCs in 100 μL PBS (n=20 for each group) or equal volume of PBS (n=15) were injected via the needle in no less than 5 min.
5-bromo-2′-deoxyuridine injection
Rats received intraperitoneal injection of 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) in 0.9% NaCl at 50 mg/kg body weight daily from the day of surgery until the day before sacrifice. For brain tissue collection, rats were deeply anesthetized with 10% chloral hydrate and underwent intracardial perfusion first with PBS, followed by 4% paraformaldehyde (PFA; Sigma-Aldrich) in PBS.
Behavioral tests
Each rat was subjected to a series of behavioral tests [9,20] to evaluate neurologic functions before MCAO and 1, 3, 5, 7, 9, 11, and 14 days after MCAO. Modified neurologic severity score (mNSS) test was performed according to a method reported previously [20] (for details, see Supplementary Materials and Methods).
Adhesive-removal somatosensory test
Somatosensory deficit was conducted both before and after MCAO by adhesive-removal somatosensory test [20]. Two small pieces of adhesive-backed paper dots (113.1 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The rat was then returned to its cage. The time to remove each stimulus from the limb was recorded during five trials per day for every other day. Individual trials were separated by at least 5 min.
2,3,5-triphenyltetrazolium chloride assay
Fourteen days after MCAO, rats were anesthetized and decapitated. Their brains were removed and coronally sectioned into six 2-mm coronal slices, incubated for 30 min in a 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) at 37°C, and fixed with 4% PFA in PBS. Infarct areas were measured by using software Image J and infarct volumes were calculated, which was presented as infarct volume per volume of the entire cerebral hemisphere.
Magnetic resonance imaging
Lesion development was assessed on days 1, 7, and 14 after MCAO using magnetic resonance imaging (MRI, a clinical 3.0 T MR system, Magnetom TIM Trio; Siemens) with a custom rat coil. Rats were anesthetized using the described method in MCAO model. T2-weighted sequences (T2-TSE) were performed at each MRI session consisting of 23 transverse slices (repetition time: 4,000 ms, time to echo: 82 ms, field of view: 42×60 mm2; slice thickness: 1 mm). All sequences were measured with a multicontrast-spin echo (se-mc) sequence. For further processing, MRI sequences were exported as DICOM sequences. Volumes of ischemic lesion as well as ipsilateral and contralateral side ventricles were determined by a blinded investigator using software Image J. Subsequently, each calculated volume was displayed as the percentage of the infarct volume on day 1.
Whole mount of retina
hMSCs in monolayer or spheroids were tryspinized to generate single-cell suspensions and labeled with DiI [5]. Cell viability analysis was performed using Annexin-V-FITC and showed that the DiI staining did not increase cell death. Single-cell suspensions of MSCs were filtered through a cell strainer with 40-μm pores to remove cell aggregates. About 1×106 hMSCs derived from monolayer or spheroids in 100 μL PBS (n=5 for each) were injected into the left internal carotid artery. After 24 h, rats were sacrificed and the left retina was carefully removed, mounted on glass slides, fixed with 4% PFA [21], and visualized under fluorescence microscope (Leica).
Neuronal differentiation
To induce neurogenic differentiation, the cells were grown in DMEM-HG containing 1% FBS, 1% penicillin-streptomycin, and 100 ng/mL bFGF (R&D) for 24 h, followed by supplementing 20 ng/mL epidermal growth factor (Peptech), 20 ng/mL bFGF, 25 ng/mL nerve growth factor (R&D), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), and 1 mM dbcAMP (Sigma-Aldrich) for another 7 days. Total RNA was extracted for real-time polymerase chain reaction (PCR) analysis for the expression of nestin and s100. Immunofluorescence analysis was performed to determine the protein expression of microtubule-associated protein (MAP)-2.
Real-time PCR and immunofluorescence stain
Real-time PCR and immunofluorescence stain were performed with standard protocols (for details, see Supplementary Materials and Methods), and primers are listed in Supplementary Table S1.
Statistical analyses
Values were expressed as mean±standard deviation. Results were analyzed using one-way or repeated-measure analysis of variance with independent variables of treatment groups and days of testing, followed by Bonferroni post-hoc test for multiple comparisons between treatment groups at each time point. A probability (P) value<0.05 was considered significant.
Results
3D spheroid culture reduces the size of hMSCs and prevent embolism
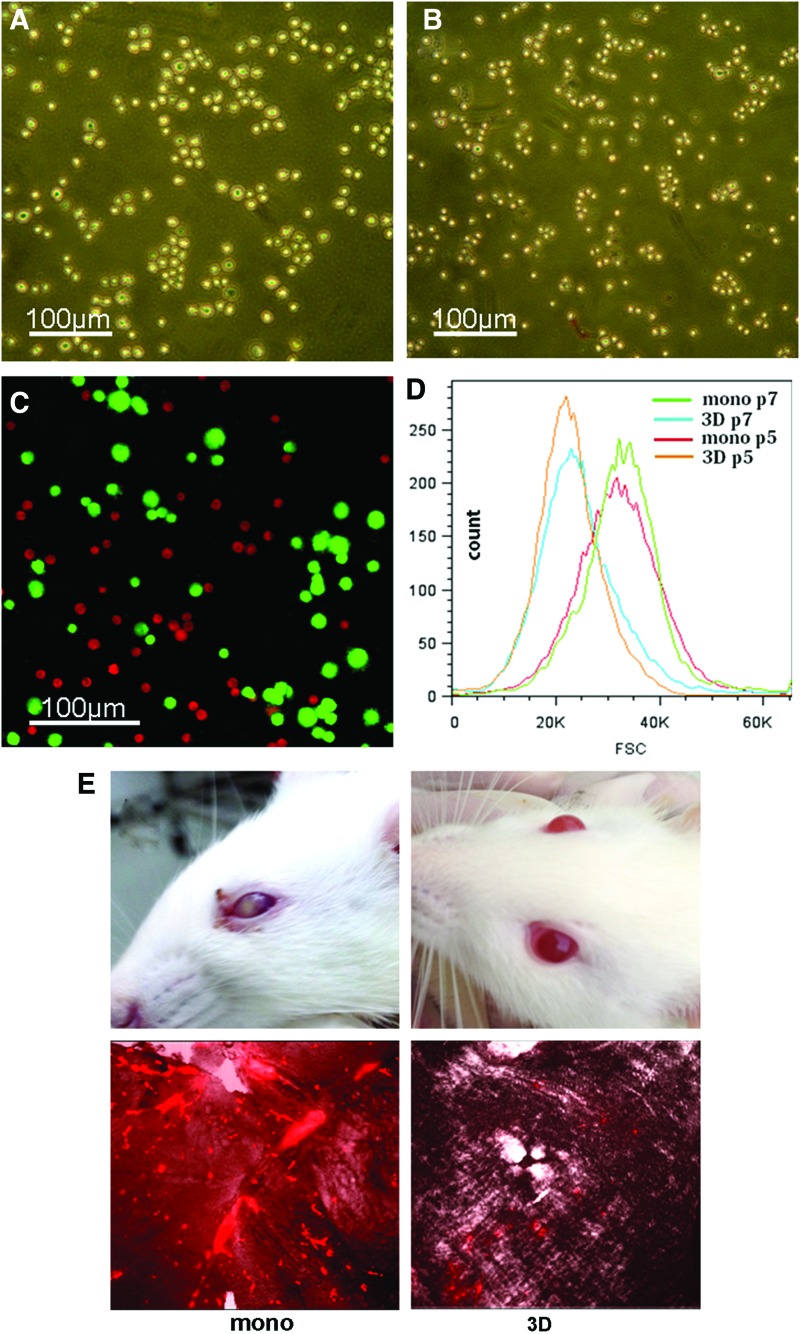
After 60 h of culture in 3D spheroids, the average diameter of hMSCs decreased by 40% from 25.4±5.3 μm to 15.2±3.1 μm (Fig. 1A–D). After being re-seeded in plates, cells became smaller and more 3D. Of 20 rats receiving intra-internal carotid artery injection of monolayer hMSCs, 11 rats developed some degrees of abnormality with their left eyes (the same side with cell injection). Within minutes following the cell injection, the pupil of the eye became dark and unclear. In many of them, the eye quickly became cloudy (Fig. 1E). Meanwhile, the eye lost pupillary reflex, a sign of vision loss. Of 20 rats receiving injection of spheroid hMSCs, none of them showed abnormality with their eyes. Since the eye artery that supplies the retina is a branch of the internal carotid artery in rats [22], we examined whether intra-carotid artery injection of hMSCs led to obstructions of the small arteries in the retina. Twenty-four hours after injection of DiI-labeled single hMSCs prepared from monolayer or spheroids (n=5), whole-mount preparations of the retina were examined under fluorescence microscope for DiI-positive cells in the retina. Surprisingly, we found substantial amounts of DiI-positive hMSCs in the blood vessels of various sizes (probably small to microarteries and -capillaries), whereas only few DiI-positive cells were detected in the retina of rats receiving injection of equal numbers of spheroid hMSCs (Fig. 1E). Meanwhile, we observed a transient worsening of neurologic function in some rats with MCAO shortly following injection of monolayer hMSCs.
FIG. 1.
Mesenchymal stem cell (MSC) size. (A–C) Microscopic images of human MSCs (hMSCs) from monolayer (A) or three-dimensional (3D) spheroids after trypsinization (B). A mixture of single-cell suspensions of monolayer hMSCs expressing enhanced green fluorescent protein (green) and spheroid hMSCs labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; red) (C). (D) Analysis of hMSC size by flow cytometry. The size of hMSCs derived from monolayer (mono) or 3D spheroids (3D) in passage (P) 3 or 7 was estimated by flow cytometry. (E) Embolism following hMSC injection. One million hMSCs derived from monolayer or spheroids were injected into the left carotid artery. Some rats injected with monolayer hMSCs developed blindness with the left eye. The upper left panel showed a blind eye at 3 days post-cell injection. Similarly injected spheroid hMSCs did not cause visible abnormalities in the eyes (upper right panel). Whole-mount preparations of the retina were examined under fluorescence microscope for the presence of DiI-positive cells 24 h after intra-carotid injection of 1 million DiI-labeled hMSCs. In rats injected with monolayer hMSCs, a substantial amount of DiI-positive cells was found in blood vessels of the retina (lower left panel), while in rats injected with spheroid hMSCs, a few DiI-positive cells were detected (lower right panel). Color images available online at www.liebertpub.com/scd
Spheroid hMSCs reduce infarct volume
MRI was applied to analyze the volume of cerebral infarct on days 1 (prior to hMSC transplantation), 7, and 14 post-MCAO. Before MSC transplantation, there were no significant differences in infarct volume among the three groups (Fig. 2A, B; P>0.05, n=6). On day 7, rats treated with 3D spheroid hMSCs showed a 55% decrease in infarct volume relative to the infarct volume at day 1 (considered as 100%), compared with 27.5% reduction in rats treated with monolayer hMSCs and 29.9% reduction in rats treated with PBS (Fig. 2A, B; P<0.01, 3D hMSC group vs. monolayer hMSC group or PBS group; P>0.05, monolayer hMSC group vs. PBS group). On day 14, the remaining infarct volume in 3D hMSC group was reduced to 27.1% (n=6, P<0.01, the infarct volume at day 1 was considered as 100%); however, modest reductions were found in the monolayer hMSC group (remaining infarct volume 57.41%, n=6) and in the PBS group (remaining infarct volume 61.21%, n=5, P>0.05 between monolayer and PBS groups) (Fig. 2A, B). Moreover, we measured infarct areas in coronal slices of the brain with TTC stain 14 days post-MCAO. There was a significant reduction in infarct size in rats treated with 3D spheroid hMSCs, compared with rats treated with monolayer hMSCs (n=6, P<0.01) or PBS (n=5, P<0.01) (Fig. 2C, D).
FIG. 2.
Effects of MSCs on infarct volume. (A, B) Infarct volumes were determined by magnetic resonance imaging (MRI) 24 h post-middle cerebral artery occlusion (MCAO) immediately before treatment with hMSCs or phosphate-buffered saline (PBS), and after the treatment on days 7 and 14 post-MCAO (n=6 for monolayer or 3D hMSC group; n=5 for PBS group). Representative images of MRI were shown. White areas on the left side represent the infarcts (A). No statistical differences in infarct volume were found among the three groups 24 h after MCAO before the treatment (B). Significant reductions in infarct volume were found in 3D spheroid hMSC–treated rats 7 and 14 days after MCAO, *P<0.05, **P<0.01. (C, D) 2,3,5-Triphenyltetrazolium chloride stain of coronal sections of the brain for infarct size. Representative images of brain sections at 14 days post-MCAO were shown. Infarcts are shown as pale (unstained) regions that involve the striatum and cortex (C). Infarct area in each section was measured, and infarct volume in each brain was calculated, which was presented as infarct volume per volume of the entire cerebral hemisphere [(D), n=6 in mono and 3D MSC groups, n=5 in PBS group, *P<0.05, **P<0.01]. Color images available online at www.liebertpub.com/scd
Meanwhile, we assessed the number of hMSCs engrafted into the lesion site at 3, 7, and 14 days after cell transplantation. There were significantly more M1281-positive cells in the lesion of rats that received spheroid hMSCs compared with rats that received monolayer hMSCs at 3 days (44.75±4.21 vs. 28.41±0.53, n=5, P<0.05). Although the numbers of hMSCs in the lesion declined in both animal groups, there were significantly more spheroid hMSCs remaining at 7 (37.85±3.73 vs. 23.17±1.17, n=5, P<0.05) and 14 days (25.37±2.31 vs. 17.33±1.82, n=5, P<0.05) than monolayer hMSCs (Fig. 3A, B). Moreover, there were much fewer cells positive for caspase-3 in the infarct border zone in rats treated with spheroid hMSCs, compared with rats treated with PBS or monolayer hMSCs (Fig. 3C).
FIG. 3.
Engraftment of the cells into the ischemic cerebral tissue. Rats with MCAO-received intra-carotid infusion of 1 million monolayer (mono) or 3D spheroid (3D) hMSCs. (A, B) Immunofluorescence stain for human protein showed that there were significantly more M1281-positive cells in the lesion of rats injected with spheroid hMSCs at 3, 7, and 14 days, compared with rats treated with monolayer hMSCs (n=5, *P<0.05). (C) In the infarct border zone, there were more cells stained positive for caspase 3 in rats that received an injection of PBS or monolayer hMSCs at 3 days, compared with rats treated with spheroid hMSCs. Color images available online at www.liebertpub.com/scd
Spheroid hMSCs restore neurologic functions
To examine whether spheroid hMSCs led to enhanced recovery of neurologic functions of the brain after stroke, a battery of functional tests were performed. Functional response was measured for each animal on 1, 3, 5, 7, 9, 11, and 14 days following MCAO. Prior to MCAO, there was no neurological defect in all rats. One day after MCAO and before MSC transplantation, no significant differences were found in neurologic functional defect among three groups. Rats receiving 3D spheroid hMSCs exhibited significantly improved performance in mNSS (Fig. 4A, n=8) and adhesive removal test (Fig. 4B, n=8; Fig. 2C, n=8) from 3 to 14 days after MCAO, compared with monolayer-hMSC-treated rats (P<0.05) and PBS-treated rats (P<0.05) (Fig. 3). There were no significant differences in adhesive-removal test between monolayer-hMSC-treated rats and PBS-treated rats (P>0.05). However, monolayer-hMSC-treated rats (n=8) significantly ameliorated the neurological severity in MCAO rats at 14 days, compared with PBS-treated rats (P<0.05).
FIG. 4.
Effects of MSCs on neurologic outcomes after stroke. Evaluation of neurologic function was performed before and after MCAO at times as indicated in the figure following protocols as described in “Materials and Methods.” (A) Modified neurologic severity score (mNSS). (B) Adhesive-removal test. n=8 for each group; *,#P<0.05: *3D versus PBS; #3D versus mono. Color images available online at www.liebertpub.com/scd
Spheroid hMSCs exhibit enhanced capacity of neuronal differentiation
To examine the capability of neuronal differentiation of hMSCs derived from spheroids or monolayers, we first cultured the cells in neurogenic induction medium. Real-time PCR analysis revealed that spheroid hMSCs expressed higher mRNA levels of neural-specific genes Nestin, MAP-2, and GFAP, upon neurogenic induction for 3 and 7 days (Supplementary Fig. S1B, C), compared with monolayer hMSCs. Immunofluorescence analysis showed that, before the induction, MAP-2-expressing hMSCs were barely detected and nestin expression was found in a small fraction of hMSCs; however, after the induction for 3 days, the number of hMSCs positive for MAP-2 or nestin dramatically increased, and a much higher proportion of nestin- or MAP-2-positive cells were detected in hMSC spheroids than in monolayer cultures (Fig. 5A; Supplementary Fig. S1A).
FIG. 5.
Microtubule-associated protein (MAP)-2 expression in hMSCs. (A) Immunofluorescence staining showed that MAP-2 was barely detected in hMSCs before neurogenic induction; after the induction for 3 and 7 days, the proportions of MAP-2-positive cells increased evidently, particularly in spheroid hMSCs. (B, C) In vivo, significantly higher percentages of MAP-2-positive hMSCs in spheroid hMSCs compared with monolayer hMSCs in the ischemic lesion 7 days post-transplantation [(B, C), n=5, **P<0.01]. Color images available online at www.liebertpub.com/scd
To further examine whether spheroid hMSCs exhibited enhanced neuronal differentiation in vivo, tissue sections of the brain were costained with antibodies against neuron-specific protein MAP-2 (or astrocyte-specific protein GFAP) and the human-specific nuclear protein (antibody M1281). We found that a significantly increased proportion of spheroid hMSCs expressed MAP-2, compared with monolayer hMSCs (5.48±8.9% vs. 1.87±0.9%, n=5, P<0.01) (Fig. 5B, C), in the lesion of 7 days. Meanwhile, the proportion of GFAP+/M1281+ cells increased slightly in spheroid hMSCs than in monolayer hMSCs in the tissue (n=5, P<0.05) (Supplementary Fig. S2A, B).
Spheroid hMSCs increase angiogenesis in the infarcted brain
Angiogenesis in the ischemic boundary zone of the brain with MACO was determined on days 3 and 7 post-hMSC transplantation. Immunofluorescence staining with antibodies against Von Willebrand factor (vWF) or α-smooth muscle actin (α-SMA) showed that there was a significant increase in the density of blood vessels in spheroid-hMSC-treated rats at days 3 and 7, compared with monolayer-hMSC-treated animals or PBS-treated animals (Fig. 6A–C, n=5, P<0.01). A modest but significant increase in blood vessel density was also found in monolayer-hMSC-treated animals than in PBS-treated animals at both 3 and 7 (n=5, P<0.05) days following hMSC transplantation. Moreover, double stain for BrdU+/vWF + or BrdU+/α-SMA+ showed that the new blood vessels were largely colocalized in the ischemic boundary zone (Supplementary Fig. S3).
FIG. 6.
Effects of MSCs on angiogenesis. Rats with MCAO received intra-carotid infusion of 1 million monolayer or 3D spheroid hMSCs or equal volume of vehicle PBS 24 h after MCAO. Tissue sections of the brain at 3 days were immunostained for Von Willebrand factor (vWF) [(A), green], and tissue sections at 7 days were immunostained for α-smooth muscle actin (α-SMA) [(B), red], which were subjected to examination under confocal microscope. The total area of vessels per macroscopic field area in the infarct boundary zone was measured [(C), n=5, *P<0.05; **P<0.01]. Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Color images available online at www.liebertpub.com/scd
Since paracrine factors secreted by MSCs may contribute to angiogenesis, we tested the expression levels of anti-inflammatory cytokines [interleukin (IL)-4, IL-10, IL-11, and IL-13] and growth factors [VEGF, bFGF, neurotrophin-3 (NT3), and glial derived neurotrophic factor cell (GDNF)] in monolayer and spheroid hMSCs. Real-time PCR analysis showed that the mRNA levels of IL-10, IL-11, IL-13, VEGF, NT3, and GDNF were significantly higher in spheroid hMSCs than in monolayer hMSCs (Supplementary Fig. S4A, B). Moreover, enzyme-linked immunosorbent assay analysis of the conditioned media indicated that 3D hMSCs secreted greater amounts of VEGF and bFGF (Supplementary Fig. S4C, D).
Spheroid hMSCs enhance proliferation of GFAP-expressing progenitors
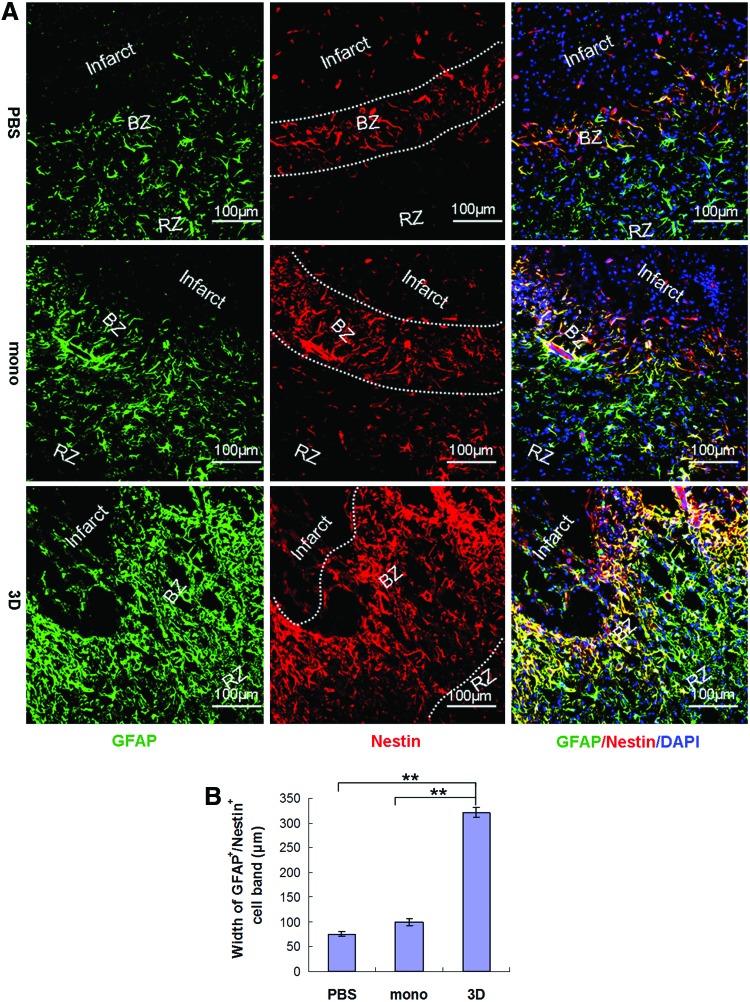
To determine whether markedly enhanced reduction of infarct volume and restoration of neurologic functions in spheroid-hMSC-treated rats were associated with increased structural restoration, we examined neurogenesis in the lesion site after MCAO. Immunofluorescence stain of coronal sections of the brain 3 days after cell transplantation showed substantially increased numbers of GFAP-positive cells in the infarct border zone in rats treated with spheroid hMSCs, which were in higher density and formed a broader band around the infarct, compared with rats treated with monolayer hMSCs or PBS (Fig. 7A, B, n=5, P<0.01). Since both mature astrocytes and neural stem cells express GFAP, we examined whether the GFAP+ cells in the boundary zone expressed stem cell markers. Immunofluorescence staining of the tissue sections with a neural stem cell marker nestin and a pluripotent marker Sox2 showed that the highest amount of GFAP+/nestin+ cells were localized to the front of the migrating GFAP+ cells immediately adjacent to the infarct in the boundary zone, whereas in the remote zone, further apart from the infarct area, almost no GFAP+/nestin+ cells were found (Fig. 7A). The GFAP+/nestin+ band around the infarct in rats treated with spheroid hMSCs was much thicker compared with rats treated with PBS (320.59±9.58 μm vs. 76.12±4.67 μm, n=5, P<0.01) or monolayer hMSCs (100.34±7.24 μm, n=5, P<0.01) (Fig. 7A, B). Moreover, we found that in rats treated with spheroid hMSCs, 73.2% of GFAP+ cells expressed Sox2, compared with 54.27±2.81% in rats treated with monolayer hMSCs (n=5, P<0.01) and 56.15±6.69% in rats treated with PBS (n=5, P<0.01) (Supplementary Fig. S5).
FIG. 7.
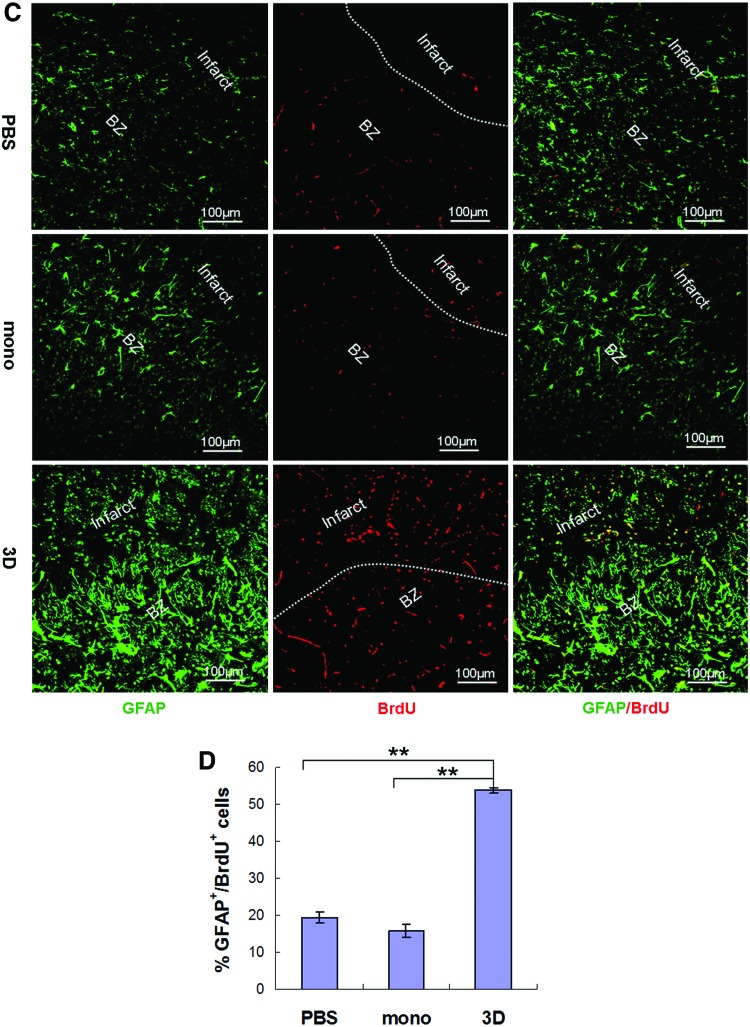
Effects of MSCs on glial fibrillary acidic protein (GFAP)–expressing progenitors. Rats with MCAO received intra-carotid infusion of 1 million monolayer or 3D spheroid hMSCs or equal volume of vehicle PBS 24 h after MCAO. (A, B) Immunofluorescence stain of coronal sections of the brain 3 days after cell transplantation showed a broader boundary zone (BZ) of GFAP-expressing cells around the infarct in rats treated with 3D spheroid hMSCs, compared with rats treated with monolayer (mono) hMSCs, or PBS [(A, B), n=5, **P<0.01]. The GFAP+ cells in the BZ in 3D-hMSC-treated rats were in higher density and formed clusters invading into the infarct (A). Double stain showed that most GFAP-expressing cells were also positive for nestin (A). (C, D) Rats received intraperitoneal injection of BrdU at 50 mg/kg body weight daily from the day of surgery until the day before sacrifice. Immunofluorescence stain of brain tissue sections 3 days post-cell transplantation with antibodies against 5-bromo-2′-deoxyuridine (BrdU) and GFAP showed higher levels of GFAP+/BrdU+ proliferating cells in rats transplanted with spheroid hMSCs, compared with rats treated with PBS (n=5, **P<0.01) or with monolayer hMSCs (n=5, **P<0.01). Color images available online at www.liebertpub.com/scd
To understand whether the GFAP+ cells in the infarct border zone were proliferating, BrdU was injected into rats. Brain tissue sections were immunostained with antibodies against BrdU and GFAP. We found significantly higher levels of GFAP+/BrdU+ proliferating cells in rats that received spheroid hMSCs, compared with rats that received PBS (n=5, P<0.01) or monolayer hMSCs (n=5, P<0.01) at 3 days (Fig. 7C, D). In morphology, the GFAP+/BrdU+ cells in the infarct predominantly had no processes, the cells adjacent to the infarct were largely unipolar with a process directing to the infarct, and the cells further away from the infarct showed more processes.
Discussion
The therapeutic effect of MSCs in tissue repair/regeneration is significantly affected by their repair capacity and the number of cells engrafted into the target tissue [10,15]. Inappropriate culture expansion impairs the properties of MSCs critical for tissue repair and regeneration. Previous studies indicate that the homing efficiency of culture-expanded MSCs is poor. Upon intravenous infusion, over 80% of MSCs are entrapped in lungs, where most of them die in 24 h, and only <1% of MSCs are detected in the ischemic heart or brain [16,17]. Though a beneficial effect of intravenously administered MSCs in improving functional recovery of stroke has been observed [9,23], controversial results have been reported with the reduction of infarct size [24]. Low levels of engraftment of MSCs in the injured cerebral tissue particularly limit their regenerative potential through differentiation into neural cells. Therefore, to achieve maximal effect of MSCs in tissue regeneration, both the culture condition and the administration approach have to be improved. Previous studies have shown that MSCs cultured in 3D spheroids exhibit upregulated expression of paracrine factors important for tissue regeneration, but unaffected engraftment into the infarcted myocardium after intravenous infusion [14,16,18]. To increase engraftment, we injected the cells through the internal carotid artery. This led to a substantial engraftment of the cells into the injured cerebral tissue. We found more hMSCs derived from spheroids in the lesion than hMSCs derived from monolayer culture. This is probably due to improved response of spheroid hMSCs to chemokines in the ischemic cerebral tissue due to increased expression of chemokine receptors on the surface of spheroid MSCs such as CXCR4 [18,25].
Neurogenesis is an ideal therapeutic strategy to restore the lost structure and function of the cerebrum after stroke. MSCs have been shown to acquire phenotypes of neural lineage cells in vitro upon chemical inductions in numerous studies [26]; however, low frequency of neuronal differentiation of MSCs has been found in vivo [27]. We proposed that this might be largely due to reduced neurogenic differentiation ability of MSCs after culture expansion. MSCs expanded in monolayer culture undergo spontaneous differentiation toward osteoblasts [12]. Recent studies suggest that 3D spheroid culture increases the multipotency of MSCs [18,28]. In this study, we found that spheroid hMSCs had increased capacity of neurogenic differentiation in vitro and in vivo. Notably, over 5% of spheroid hMSCs engrafted into the injured cerebrum expressed MAP-2, a neuron-specific gene. In consideration of significantly increased abundance of spheroid MSCs in the lesion, neurons derived from MSCs may account for a part in neurogenesis.
We found that spheroid hMSCs significantly increased the number of GFAP-expressing neural progenitors in the ischemic lesion. They were predominantly localized to the ischemic boundary zone after MCAO, expressed nestin and Sox2, features of neural stem cells [29–31], and were actively proliferating. Several lines of evidence indicate that GFAP-expressing astrocyte-like cells in the brain are the principal source of constitutive neurogenesis in rodents and humans [29,32], which are capable of differentiating into neurons, oligodendrocytes, and astrocytes [31,32]. Neurogenesis following stroke has been observed in many studies [33]. However, this appears not to change the outcome of stroke. Increasing evidence indicates that while stroke injury is necessary to activate quiescent endogenous neural stem cells, local non-permissive cues, the inflammatory response and poor blood supply affect the survival and maturation of the newly formed neurons [31,33]. In this study, we observed a substantial enhancement in GFAP-positive progenitor cells 3 and 14 days following transplantation of spheroid MSCs, along with a remarkable reduction in infarct size and functional restoration. This may largely attribute to the enhancement of anti-inflammatory effect of spheroid MSCs. Previous studies indicate that 3D spheroid culture profoundly upregulates the expression of anti-inflammatory cytokines such as tumor necrosis factor-α stimulated gene/protein-6 (TSG-6) but not influence the expression of proinflammatory cytokines such as IL-6 [14,16,18]. These results suggest that besides generating new neurons, MSCs in the lesion release paracrine factors that provide a favorable niche for neurogenesis.
We observed a modest effect of monolayer MSCs in improving functional recovery of stroke, which were substantially less effective than spheroid MSCs. Monolayer MSCs significantly improved angiogenesis at 3 and 7 days and mNSS after the first week, but failed to reduce the infarct size. This is in agreement with several previous studies, where modest benefit of monolayer-cultured MSCs in improving functional outcomes but not the infarct size of stroke has been observed [20,24]. On the other hand, it has been suggested that repeated intravenous injections of monolayer MSCs or injection of non-culture-passaged primary MSCs may reduce the infarct size [34,35]. In our study, the benefit of monolayer MSCs was likely dampened by their side effect in vascular obstruction. Intra-arterial injection of monolayer MSCs likely caused embolism and microinfarction. We noticed a transient worsening of neurologic function and severe obstruction of retinal arteries and blindness in some animals following intra-carotid injection of monolayer MSCs (average diameter 25 μm). This is in agreement with previous studies where intracoronary delivery of MSCs (average diameter 20 μm) expanded in monolayer culture caused acute myocardial infarction in dogs and reduction of coronary blood flow in pigs [36,37]. It has been recognized that MSCs become substantially enlarged upon monolayer culture expansion [18,36]. In consistence with previous findings [18], we found that 3D spheroid culture significantly reduced the size of MSCs ∼40% (from average diameter 25 μm to average dimmer 15 μm). Cell size has been suggested as a critical parameter of vascular obstructions in a recent study [38]. Our results indicate that 3D-spheroid-cultured MSCs are safer and more effective than conventionally monolayer-cultured MSCs for intra-artery administration.
We used MSCs derived from human placenta. Placenta-derived MSCs exhibit typical features of MSCs, such as cell morphology, the expression of surface antigens, and differentiation capacities into adipocytes, chondrocytes, and osteoblasts [12]. In addition, we found that placenta MSCs demonstrated similar alterations in 3D spheroids in cell size and in the expression of cytokines to bone-marrow-derived MSCs [14,18]. Therefore, the benefit of spheroid culture found in placenta MSCs is likely to apply to MSCs derived from other tissue sources.
In conclusion, unlike MSCs grown in monolayer culture, MSCs cultured in 3D spheroids did not cause vascular obstructions after intra-carotid infusion, and resulted in substantial engraftment into the ischemic cerebral lesion in rats. Spheroid MSCs, but not monolayer MSCs, profoundly reduced infarct size and restored neurologic function after stroke. In addition, a significantly increased number of spheroid MSCs in the lesion differentiated into MAP-2-expressing neuron-like cells. Moreover, transplantation of spheroid MSCs markedly increased number of GFAP-positive progenitor cells in the infarct boundary zone. Our results indicate that spheroid MSCs are superior to monolayer MSCs in safety and therapeutic efficacy, and suggest that spheroid MSCs, in combination with intra-carotid administration, may represent a novel therapeutic method of MSCs for stroke.
Supplementary Material
Acknowledgments
This work was supported by grants from Natural Science Foundation of China (no. 30971496, U1032003, and 31371404), the “863 Projects” of Ministry of Science and Technology of People's Republic of China (no. 2011AA020118), and Shenzhen Science and Technology Innovation Committee (JC201005280597A, GJHZ20120614194251967, and JCYJ20130402145002397) to Y.W.
Author Disclosure Statement
The authors of this article have no conflicts of interest to disclose.
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, et al. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZG. and Chopp M. (2009). Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 8:491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prockop DJ. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74 [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM. (2006). MSC: a coming of age in regenerative medicine. Cytotherapy 8:194–195 [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Chen L, Scott PG. and Tredget EE. (2007). Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain G, Fox J, Ashton B. and Middleton J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749 [DOI] [PubMed] [Google Scholar]
- 7.Keating A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709–716 [DOI] [PubMed] [Google Scholar]
- 8.Salem HK. and Thiemermann C. (2010). Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M. and Chopp M. (2001). Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005–1011 [DOI] [PubMed] [Google Scholar]
- 10.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS. and Dzau VJ. (2003). Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195–1201 [DOI] [PubMed] [Google Scholar]
- 11.Uccelli A, Moretta L. and Pistoia V. (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736 [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Liu C, Xie Z, Song P, Zhao RC, Guo L, Liu Z. and Wu Y. (2011). Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One 6:e20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Zhao RC. and Tredget EE. (2010). Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 28:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS. and Doronin SV. (2007). Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 25:1761–1768 [DOI] [PubMed] [Google Scholar]
- 15.Wu Y. and Zhao RC. (2012). The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev 8:243–250 [DOI] [PubMed] [Google Scholar]
- 16.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P. and Prockop DJ. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toma C, Wagner WR, Bowry S, Schwartz A. and Villanueva F. (2009). Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res 104:398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H. and Prockop DJ. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A 107:13724–13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longa EZ, Weinstein PR, Carlson S. and Cummins R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Chen J, Wang L, Lu M. and Chopp M. (2001). Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56:1666–1672 [DOI] [PubMed] [Google Scholar]
- 21.Powner MB, Vevis K, McKenzie JA, Gandhi P, Jadeja S. and Fruttiger M. (2012). Visualization of gene expression in whole mouse retina by in situ hybridization. Nat Protoc 7:1086–1096 [DOI] [PubMed] [Google Scholar]
- 22.Stevens WD, Fortin T. and Pappas BA. (2002). Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke 33:1107–1112 [DOI] [PubMed] [Google Scholar]
- 23.Honmou O, Onodera R, Sasaki M, Waxman SG. and Kocsis JD. (2012). Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med 18:292–297 [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdan S. and Diez-Tejedor E. (2013). Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potapova IA, Brink PR, Cohen IS. and Doronin SV. (2008). Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem 283:13100–13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tropel P, Platet N, Platel JC, Noel D, Albrieux M, Benabid AL. and Berger F. (2006). Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24:2868–2876 [DOI] [PubMed] [Google Scholar]
- 27.Quinn C. and Flake AW. (2008). In vivo differentiation potential of mesenchymal stem cells: prenatal and postnatal model systems. Transfus Med Hemother 35:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng NC, Wang S. and Young TH. (2012). The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 33:1748–1758 [DOI] [PubMed] [Google Scholar]
- 29.Garcia AD, Doan NB, Imura T, Bush TG. and Sofroniew MV. (2004). GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 7:1233–1241 [DOI] [PubMed] [Google Scholar]
- 30.Miller FD. and Gauthier-Fisher A. (2009). Home at last: neural stem cell niches defined. Cell Stem Cell 4:507–510 [DOI] [PubMed] [Google Scholar]
- 31.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T. and Gotz M. (2008). Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A 105:3581–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, et al. (2004). Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740–744 [DOI] [PubMed] [Google Scholar]
- 33.Arvidsson A, Collin T, Kirik D, Kokaia Z. and Lindvall O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970 [DOI] [PubMed] [Google Scholar]
- 34.Leu S, Lin YC, Yuen CM, Yen CH, Kao YH, Sun CK. and Yip HK. (2010). Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med 8:63–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H. and Kocsis JD. (2006). Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 199:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vulliet PR, Greeley M, Halloran SM, MacDonald KA. and Kittleson MD. (2004). Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 363:783–784 [DOI] [PubMed] [Google Scholar]
- 37.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M. and Wilensky RL. (2006). A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27:1114–1122 [DOI] [PubMed] [Google Scholar]
- 38.Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW. and Walczak P. (2013). Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab 33:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.