Abstract
Background and Purpose: Shockwave lithotripsy (SWL) and ureteroscopy (URS) are minimally invasive treatment alternatives for kidney stones. Although less invasive, SWL subjects the renal parenchyma to a high level of energy and the potential to cause renal injury. The ability to detect renal injury post-SWL in a reliable and noninvasive way would be clinically beneficial. Kidney injury molecule 1 (KIM-1) and N-acetyl-β-D-glucosaminidase (NAG) are two proteins secreted by the kidney into the urine and have been found to be sensitive markers of acute kidney injury in transplant patients. The aim of this work was to measure urinary levels of KIM-1 and NAG in patients with kidney stone who were treated by SWL or URS and in nonstone volunteers.
Patients and Methods: Patients with kidney stones who were treated by SWL (n=50) or URS (n=10) were recruited. Voided urine samples were collected before and 2 to 3 hours after URS and SWL. In addition, further urinary specimens were collected 2 days and 2 weeks post-SWL treatment. Voided urine samples from healthy volunteers were also collected.
Results: Mean KIM-1 values were increased in patients with kidney stones when compared with volunteers. KIM-1 and NAG levels significantly increased post-SWL and returned to baseline within 2 weeks post-SWL. Poor kidney function was significantly associated with increased biomarker activity both in baseline and post-SWL measurements. There was no significant change in urinary KIM-1 and NAG concentrations before and after URS.
Conclusions: Kim-1 and NAG levels significantly increased post-SWL treatment suggesting a potential role for these urinary markers in identifying patients at higher risk of tissue injury.
Introduction
Shockwave lithotripsy (SWL) was introduced in the early 1980s as a minimally invasive alternative to traditional open renal surgery techniques for urinary stones.1 It is currently considered to be an effective first-line modality of therapy for renal and ureteral calculi. SWL is very appealing because of its noninvasive nature, minimal anesthesia requirement, and high level of patient and physician acceptance. Although generally considered less invasive than other stone management options, SWL still subjects the renal parenchyma to high levels of energy, leading to possible renal morphologic and physiologic alterations. There is no existing adequate imaging modality available to assess parenchymal injury, thus creating a need for a potential novel diagnostic test that can reliably detect such injuries.
To date, there is no consistent dependable urinary marker to permit detection of significant renal injury as a result of SWL. The urinary excretion of various constitutive proteins from kidney cells as markers of kidney injury has been explored in the past using multiple enzymes such as N-acetyl-β-D-glucosaminidase (NAG),2 γ glutamyltranspeptidase (GGT)3 and lactate dehydrogenase (LDH).4 Several of these have been shown to be elevated after SWL in animal models, but have been found to be inconsistent and less reliable in human studies.5
Kidney injury molecule 1 (KIM-1) has been found to be a sensitive marker of acute ischemic injury of the proximal tubular cells.6 KIM-1 is excreted in urine and thus has been used as a biomarker for acute kidney injury mostly in the renal transplant population.7 Despite the observation that KIM-1 has outperformed other kidney injury markers of ischemia, it has not yet been evaluated in patients undergoing SWL. The goal of this study was to test urinary KIM-1 and NAG concentration before and after SWL. A cohort of patients undergoing flexible ureteroscopy (fURS) for kidney stones and nonstone volunteer participants served as controls.
Patients and Methods
Participant selection
This study was approved by our Institutional Research Ethics Board. Fifty consecutive patients with renal calculi who were treated in our institution by SWL were included in this study. The Storz Modulith SLX-F2 lithotriptor was used, which features a dual focus and an electromagnetic shockwave generator (Karl Storz Lithotripsy-America, Inc., Kennesaw, GA). Ten additional consecutive patients treated by fURS and 10 volunteers with no history of stone disease were also included in this study. Inclusion and exclusion criteria are detailed in Table 1.
Table 1.
Inclusion and Exclusion Criteria for Patient Groups
| Inclusion criteria for SWL & fURS patients |
| 1. Patients undergoing SWL or fURS with holmium:yttrium-aluminum-garnet laser treatment for a stone(s) located in the kidney |
| 2. Radiopaque stone |
| 3. Able and willing to give informed consent |
| Inclusion criteria for nonstone volunteers |
| 1. No history of kidney or stone disease |
| 2. Asymptomatic |
| 3. No indwelling ureteral stent |
| 4. Willing to provide medical history information |
| 5. Able and willing to give informed consent |
| Exclusion criteria for SWL & fURS patients |
| 1. Active urinary tract infection |
| 2. Urinary tract obstruction causing hydronephrosis |
| 3. Use of radiologic contrast for treatment guidance |
| 4. Chronic renal failure (eGFR<30, serum creatinine >200 for ≥3 months) |
| 5. Bilateral SWL or URS |
| 6. Ureteral stone |
SWL=shockwave lithotripsy; fURS=flexible ureteroscopy; eGFR=estimated glomerular filtration rate; URS=ureteroscopy.
Data and sample collection
On the day of treatment, patients' medical history, laboratory investigations, vital signs, and SWL or fURS operative data were documented. Estimated glomerular filtration rates (eGFR) were calculated from serum creatinine levels using the Modification of Diet in Renal Disease (MDRD) Study equation,8 and the level of kidney disease was staged according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Classification.9 All included patients provided a spontaneously voided urine sample 1 to 3 hours before and 2 to 3 hours after SWL or fURS treatment. Twenty-three patients provided additional urine samples at 2 days and 2 weeks after SWL. At these follow-up visits, medical history and imaging records were reviewed, and the presence of perinephric/nephric hematoma was documented. Volunteers provided medical history information and one freshly voided urine sample.
Processing of urine samples
All urine samples were checked with a dipstick using Siemens (Bayer) Multistix 8 SG Test Strips and a portable Clinitek Status Urine Chemistry Analyser (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Urine samples were then centrifuged at a relative centrifugal force of 5,000×g (greater than earth gravity), aliquoted, and stored within 2 hours of collection at −80°C. A commercially available colorimetric assay for the determination of NAG in urine (Roche Diagnostics, Basel, Switzerland) was used according to manufacturer references.
Urinary KIM-1 was measured by microbead based sandwich enzyme-linked immunosorbent assay on Luminex® platform (Luminex, Austin, TX) as previously described and validated.10 In summary, anti-human KIM-1 capture antibody (R&D Systems, Minneapolis, MN) was coupled to COOH polystyrene beads (Bio-Rad, Hercules, CA). Coupling occurs by formation of covalent bonds between the COOH group on the beads and the NH2 groups on the antibody proteins. Duplicates of 30 μL of urine along with serially diluted recombinant human KIM-1 standards (13 dilutions) were then incubated with approximately 6000 polystyrene beads for 1 hour. Plates were incubated with biotinylated anti-KIM-1 detection antibody (R&D Systems) for 45 minutes and then incubated for 15 minutes with Streptavidin Phycoerythrin (PE-Streptavidin) (Life Technologies Inc., Grand Island, NY). The amount of KIM-1 in each sample was interpolated by 5 Parameter Logistic nonlinear regression by means of a 13-point standard curve.
Urine creatinine was measured by the Jaffé assay using Randox Daytona Analyzer (Randox Laboratories Ltd, London, United Kingdom). Normalization with urinary creatinine levels was performed to compensate for potential variations in urinary concentrations.
Statistical analyses
An IBM SPSS software ver. 21 (IBM, New York, NY) was used. Normality distribution was tested by the Shapiro-Wilk test. An omnibus test was performed to control for type I error. A chi square, Fisher exact test, or a Student t test was used as appropriate. Given that multiple biomarker measurements were later taken from each patient during follow-up, group comparisons were adjusted using mixed modeling with repeated measures analysis, and the Tukey-Kramer method was used for pairwise comparisons. A univariate linear regression model was used to test relationships with dependent variables. The small number of positive observations among different groups precluded performance of multivariate analyses. Results were considered significant when the P values were <0.05.
Results
Baseline characteristics (Table 2)
Table 2.
Patients Baseline Characteristics
| SWL n=50 (%) | fURS n=10 (%) | Volunteers n=10 (%) | P value | |
|---|---|---|---|---|
| Age | ||||
| Mean | 54 | 51 | 49 | 0.37 |
| Median | 55 | 48 | 48 | |
| Range | 18–79 | 31–72 | 29–58 | |
| Sex | ||||
| Male | 28 (56) | 3 (30) | 5 (50) | 0.31 |
| Female | 22 (44) | 7 (70) | 5 (50) | |
| BMI (kg/m2) category | ||||
| Underweight (<18.5) | 0 | 1 (10) | 0 | 0.15 |
| Normal weight (18.5–24.9) | 12 (24) | 2 (20) | 5 (50) | |
| Overweight (25.0–29.9) | 20 (40) | 2 (20) | 2 (20) | |
| Obese class I (30.0–34.9) | 10 (20) | 1 (10) | 1 (10) | |
| Obese class II (35.0–39.9) | 7 (14) | 3 (30) | 2 (20) | |
| Obese class III (≥40.0) | 1 (2) | 1 (10) | 0 | |
| eGFR (mL/min/1.73m2) before SWL | ||||
| 1 normal (≥90) | 20 (40) | 6 (60) | n/a | 0.43 |
| 2 mild (60–89) | 20 (40) | 3 (30) | ||
| 3 moderate (30–59) | 2 (4) | 1 (10) | ||
| Unknown | 8 (16) | 0 | ||
| Medical history | ||||
| DM | 6 (12) | 3 (30) | 0 | 0.09 |
| HTN | 18 (36) | 4 (40) | 2 (20) | 0.33 |
| CVD | 2 (4) | 1 (10) | 0 | 0.43 |
| Renal influencing medications | 28 (56) | 5 (50) | 5 (50) | 0.52 |
| Previous SWL | 21 (42) | 3 (30) | 0 | 0.72 |
| Time range of previous SWL | 3m–35yrs | 3m–18yrs | n/a | |
| BP on day of SWL | ||||
| Normal (<120/80) | 10 (20) | 5 (50) | n/a | 0.11 |
| Prehypertension (120–39/80–89) | 25 (50) | 5 (50) | ||
| HTN stage 1 (140–159/90–99) | 13 (26) | 0 | ||
| HTN stage 2 (>160/100) | 2 (4) | 0 | ||
| Side | ||||
| Right/Left | 27/23 | 4/6 | n/a | 0.5 |
| Stone location | ||||
| UC | 4 (8) | 1 (10) | n/a | 0.86 |
| MC | 6 (12) | 1 (10) | ||
| LC | 20 (40) | 3 (30) | ||
| RP | 17 (40) | 4 (40) | ||
| UPJ | 3 (10) | 1 (10) | ||
| DJ stent | ||||
| No | 44 (88) | 6 (60) | n/a | 0.47 |
| Presented with in situ stent | 1 (2) | 4 (40) | ||
| Stent inserted day of procedure | 5 (10) | 10 (100) | ||
| Stone largest dimensions | ||||
| Mean | 6 | 8 | n/a | 0.28 |
| Median | 7 | 7 | ||
| Range | 3–17 | 4–16 | ||
| Shockwaves number | ||||
| Mean | 2933 | n/a | n/a | n/a |
| Median | 3000 | |||
| Range | 2000 to 3000 | |||
| Shockwaves energy | ||||
| Mean | 5 | n/a | n/a | n/a |
| Median | 4 | |||
| Range | 3 to 6 | |||
SWL=shockwave lithotripsy; fURS=flexible ureteroscopy; BMI=body mass index; eGFR=estimated glomerular filtration rate; DM=diabetes mellitus; HTN=hypertension; CVD=cardiovascular disease; BP=blood pressure; UC=upper calix; MC=middle calix; LC=lower calix; RP=renal pelvis; UPJ=ureteropelvic junction; DJ=Double-J; n/a=not applicable.
Among the SWL group (n=50), four patients had a history of systemic illness including: Williams syndrome (a developmental disorder associated with facial and cardiovascular anomalies), sarcoidosis, Crohn's disease, and ulcerative colitis. All patients were treated with a frequency of 120 shocks per minute using the standard focal zone. Successful stone fragmentation post-SWL treatment was observed in 47 patients. Among the fURS group (n=10), one patient had ulcerative colitis. Renal influencing medications taken by all patient groups are shown in Table 3.
Table 3.
Renal Influencing Medications Taken by the Different Patient Groups
| Patient group | Medication class | Type of medication |
|---|---|---|
| SWL group n=28/50 (56%) |
Antihypertensives | Lisinopril, hydrochlorothiazide, ramilpril, enalapril, irbesartan and/or telmesartan |
| Analgesics | ASA and/or ibuprofen | |
| Oral hypoglycemic | Metformin | |
| Immunosuppressant | Infliximab | |
| fURS group n=5/10 (50%) |
Antihypertensives | Valsartan, ramipril, aliskiren, telmisartan, and/or perindopril |
| Analgesics | Celecoxib and/or ASA | |
| Oral hypoglycemic | Metformin | |
| Volunteer group n=4/10 (40%) |
Antihypertensives | Irbesartan and/or valsartan |
| Analgesics | ASA and/or ibuprofen |
SWL=shockwave lithotripsy; ASA=acetylsalicylic acid; fURS=flexible ureteroscopy.
Urinary biomarker concentrations
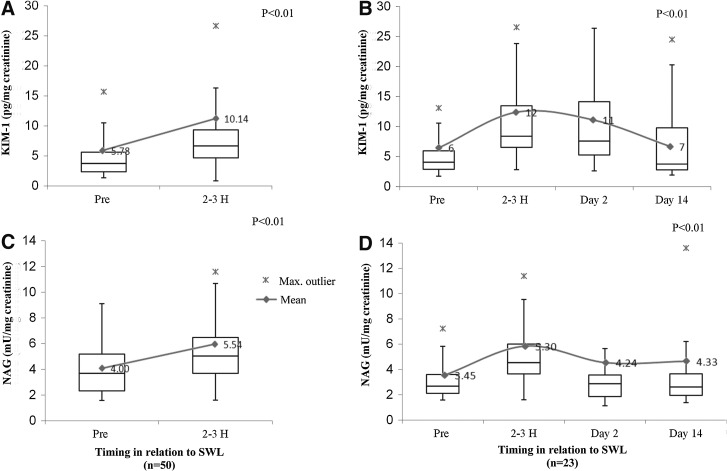
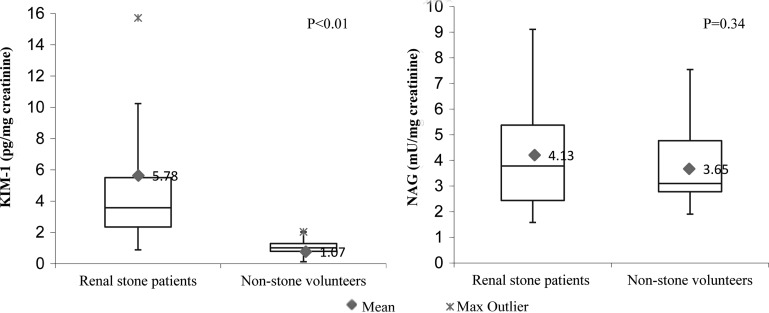
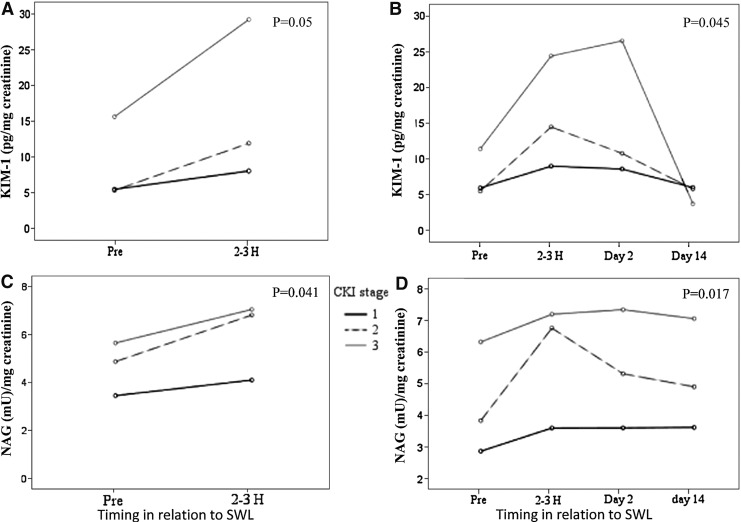
KIM-1 and NAG concentrations of all patients significantly increased in voided urinary specimens collected 2 to 3 hours after SWL when compared with their preprocedure baseline levels (Table 4). Urinary markers returned back to baseline levels within 2 weeks after SWL (P<0.01) (Fig. 1). There were no significant differences detected in urinary KIM-1 and NAG concentrations before and after fURS (Table 4). Baseline preprocedure voided urine samples collected from patients with kidney stones revealed significantly higher KIM-1 concentrations when compared with nonstone volunteers' voided urine (P<0.01). There was no significant change in urinary NAG concentrations documented among the two groups (Table 4, Fig. 2). An estimated MDRD eGFR<60 mL/min/1.73m2 correlated with a significant increase in baseline urinary KIM-1 and NAG when tested by univariate linear regression (P<0.05) The detected significant increase in urinary KIM-1 and NAG concentrations after SWL significantly correlated with low baseline MDRD eGFR (P<0.05). (Table 5, Fig. 3). No other baseline characteristic variables were found to have a significant association by the univariate linear regression model. All tested predictor variables are shown in Table 6.
Table 4.
Urinary Biomarker Levels Across Study Groups
| KIM-1 | NAG | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean | SE | P value | Mean | SE | P value | |
| Pre-SWL | 50 | 5.78 | 0.8 | <0.001 | 4 | 0.3 | <0.001 |
| 2-3H post-SWL | 50 | 10.14 | 1.4 | 5.53 | 0.4 | ||
| Pre-URS | 10 | 5.78 | 2 | 0.893 | 4.8 | 0.6 | 0.674 |
| 2–3 h post-fURS | 10 | 5.49 | 0.7 | 5.23 | 3.4 | ||
| Pre-SWL or fURS | 60 | 5.78 | 0.7 | <0.01 | 4.13 | 0.3 | 0.344 |
| Volunteers | 10 | 1.07 | 0.1 | 3.65 | 0.4 | ||
KIM-1=kidney injury molecule 1; NAG=N-acetyl-β-D-glucosaminidase; SWL=shockwave lithotripsy; UR=ureteroscopy; fURS=flexible ureteroscopy; SE=standard error.
FIG. 1.
Mean urinary biomarker concentrations in relation to shockwave lithotripsy (SWL) treatment. Treatment arm (A) and (C). Follow-up arm (B) and (D). Pg=pictogram; mU=milliunits; uCr=urinary creatinine; KIM-1=kidney injury molecule 1; NAG=N-acetyl-β-D-glucosaminidase.
FIG. 2.
Mean urinary biomarker concentrations among stone patients and volunteer group. Pg=pictogram; mU=milliunits; uCr=urinary creatinine.
Table 5.
Univariate Linear Regression Testing the Association Between Chronic Kidney Injury Stage and Urinary Biomarker Concentration in the Shockwave Lithotripsy Patient Group
| Dependent variable | Odds ratio | SE | 95% Confidence interval | P value |
|---|---|---|---|---|
| Pre-SWL | ||||
| KIM-1 | 1.961 | 0.616 | 1.035–4.226 | 0.049 |
| NAG | 1.209 | 0.570 | 0.954–2.364 | 0.041 |
| 2–3 h post-SWL | ||||
| KIM-1 | 6.450 | 3.116 | 1.136–12.764 | 0.045 |
| NAG | 1.701 | 0.682 | 1.319–3.084 | 0.017 |
SE=standard error; SWL=shockwave lithotripsy; KIM-1=kidney injury molecule 1; NAG=N-acetyl-β-D-glucosaminidase.
FIG. 3.
Mean urinary biomarkers in relation to chronic kidney injury (CKI) stage. Treatment arm (A) and (C). Follow-up arm (B) and (D). Pg=pictogram; mU=milliunits; uCr=urinary creatinine; KIM-1=kidney injury molecule 1; NAG=N-acetyl-β-D-glucosaminidase.
Table 6.
Variables Tested by the Univariate Linear Regression Model (n=50)
| P value | ||
|---|---|---|
| Predictor variables | KIM-1 | NAG |
| Baseline identifiers | ||
| Age | 0.971 | 0.331 |
| Sex | 0.228 | 0.528 |
| BMI category | 0.082 | 0.696 |
| CKI class | 0.049 | 0.041 |
| Past medical history | ||
| HTN | 0.312 | 0.225 |
| DM | 0.474 | 0.559 |
| CVD | 0.870 | 0.614 |
| Renal influencing medications | 0.599 | 0.941 |
| Previous SWL | 0.071 | 0.077 |
| Present stone factors | ||
| Side | 0.225 | 0.725 |
| Location | 0.749 | 0.914 |
| Largest dimension | 0.907 | 0.471 |
| Double-J stent | 0.062 | 0.059 |
| SWL parameters | ||
| BP on day of SWL | 0.911 | 0.201 |
| Number of shocks | 0.061 | 0.066 |
| Frequency | 0.625 | 0.930 |
| Energy | 0.714 | 0.887 |
| Fragmentation | 0.94 | 0.871 |
| Post-SWL | ||
| Proteinuria | 0.172 | 0.298 |
| Microscopic hematuria | 0.795 | 0.875 |
KIM-1=kidney injury molecule 1; NAG=N-acetyl-β-D-glucosaminidase; BMI=body mass index; CKI=chronic kidney injury; HTN=hypertension; DM=diabetes mellitus; CVD=cardiovascular disease; SWL=shockwave lithotripsy; BP=blood pressure.
Discussion
Most of our knowledge about SWL injury to the kidney is based on experimental animal studies where invasive methods were used to assess for tissue damage.11 A broad spectrum of vascular kidney damage has been documented ranging from self-limited hematuria to perinephric/nephric hematomas.11 Although most hematomas resolve, some may cause life-threatening hemodynamic instability and acute renal failure.12 In the long term, animal11 and human13 studies have suggested that these acute hemorrhagic lesions may progress to scar formation and complete atrophy of the renal papillae.
The absence of hematoma detection by conventional imaging techniques does not rule out the occurrence of potentially significant injury to the SWL-treated kidneys. A need exists to find a noninvasive diagnostic test that can reliably identify kidney injuries, especially in certain populations such as children, patients with preexisting renal disease, or those undergoing multiple SWL treatments.
Over the last decade, interest in urinary markers of kidney injury has increased, and transplant scientists predicted a potential role of markers in predicting survival after kidney injury.14 The American Society of Nephrology categorized biomarker development as a top research priority.15 Various urinary proteins like glutathione-S-transferases (GST),16 neutrophil gelatinase-associated lipocalin,17 interleukin (IL)-18,18 GGT,3 LDH,4 and NAG2 have been investigated as potential noninvasive biomarkers for the detection of kidney injury. Many of these markers were found to be unreliable, and the precise indications for their use remain unclear.
In addition, various biologic and metabolic interactions were found to influence their specificity as markers for renal injury. For example, hepatic parenchymal injury increases LDH levels,19 GST needs a specific preservative added to the urine, GGT in urine is unstable after 4 hours and can be altered by the presence of different other urinary components,20 IL-18 is nonspecific and can be elevated in various conditions including sepsis.18 Overall, the investigative role of urinary biomarkers seems to be superior when the etiology and timing of renal injury is known,21 rendering them a potential useful tool in assessing acute kidney injury after SWL.
NAG, an enzyme found at the brush border of the proximal tubules, is one of the most commonly studied biomarkers in the context of SWL. Results, however, have been inconsistent, with NAG levels found to be altered by endogenous urea, nephrotoxic agents, impaired glucose tolerance, rheumatoid arthritis, hyperthyroidism, and after exercise, undermining its role as a biomarker.20
KIM-1 is a type I transmembrane protein that appears to play an important role in the clearance of apoptotic debris from the tubular lumen. Despite the fact that KIM-1 outperformed all other tested kidney injury biomarkers,5,10 it had not previously been evaluated in patients undergoing SWL. KIM-1 has been found to be stable in urine for extended periods and not affected by urinary biochemical variations.20 One unit elevation of KIM-1 was associated with 12 times increased odds for ischemic acute tubular necrosis.6
After unifying measurement units, urinary KIM-1 and NAG concentrations measured in the volunteer group of this study were comparable to previously reported levels.10,22 In this work, urinary KIM-1 activity was significantly increased in persons with kidney stones when compared with nonstone volunteers. This can be partially explained by the fact that crystal deposition during stone formation has been shown to be associated with obstruction, extensive cell injury, and production of reactive oxygen species leading to oxidative damage.23,24 In contrast to our study, Nikoobakht and associates25 reported a higher NAG activity among stone patients, and this activity significantly correlated with stone size. In their study, 82% of patients had stones >1 cm, of which 12% were >2 cm. In our study, only 20% of patients had stones >1 cm.
As a regional center for SWL referral, our program serves a large patient population with many patients living a considerable distance from our city. As such, of the 50 SWL patients recruited in this study, it was not possible to obtain 2 day and 14 day urine samples for all subjects.
We have demonstrated a significant increase in urinary KIM-1 and NAG activity in patients with kidney stones undergoing SWL treatment and not fURS. Even though baseline levels were higher in some patients than the post-SWL levels in other patients, it is the increases within patient samples that were consistent and likely valuable to the clinician. While NAG activity findings of this study were comparable with previous reported observations, no previous studies to our knowledge have reported NAG and KIM-1 activity in patients undergoing fURS.
In a trial to identify baseline characteristic variables that may identify patients at risk of significant injury after SWL, we performed a linear regression analysis. The small number of positive observations precluded performance of a multivariate analysis, but on univariate regression, eGFR was the only independent variable that significantly correlated with elevated urinary KIM-1 and NAG activity in baseline specimens and post-SWL treatment. These findings are comparable to those of previous studies indicating increased KIM-1 and NAG activity in association with decreased eGFR.26 Still, this finding should be examined carefully given the limited number of persons with impaired eGFR included in this study.
Tissue damage in SWL was previously found to be dose-dependent,27,28 but because in this study, almost all patients were treated with similar shockwave parameters, there was no significant association with urinary biomarker activity. The presence of a ureteral stent, a history of SWL, and the number of shockwaves used tended to be associated with increased biomarker activity, but this was not statistically significant (P≤0.07).
This study has several limitations that were previously discussed and should be considered as a pilot study. In this work, KIM-1 seemed to outperform NAG and, demonstrating increased activity even in the presence of stones <1 cm, exhibited a greater increase after SWL when compared with NAG. It should also be noted that normalization of urinary biomarkers within 2 weeks after SWL only mirrors the end of cellular injury and enzymatic leak, but does not reflect actual ongoing residual damage. To further identify patients at risk for kidney injury after SWL, larger prospective case controlled studies need to be conducted.
Abbreviations Used
- eGFR
estimated glomerular filtration rate
- fURS
flexible ureteroscopy
- GGT
gamma glutamyltranspeptidase
- GST
glutathione-S-transferases
- IL
interleukin
- KIM-1
kidney injury molecule 1
- LDH
lactate dehydrogenase
- MDRD
Modification of Diet in Renal Disease
- NAG
N-acetyl-β-D-glucosaminidase
- SWL
shockwave lithotripsy
- URS
ureteroscopy
Acknowledgment
Funding provided by Lawson Health Research Institute Internal Research Fund & Ernest B. Fletcher Memorial Fund (St. Joseph's Foundation Endowed Funds).
Disclosure Statement
No competing financial interests exist.
References
- 1.Chaussy C, Eisenberger F, Forssmann B. Extracorporeal shockwave lithotripsy (ESWL): A chronology. J Endourol 2007;21:1249–1253 [DOI] [PubMed] [Google Scholar]
- 2.Wellwood JM, Ellis BG, Price RG, et al. Urinary N-acetyl- beta-D-glucosaminidase activities in patients with renal disease. Br Med J 1975;3:408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endre ZH, Pickering JW. New markers of acute kidney injury: Giant leaps and baby steps. Clin Biochem Rev 2011;32:121–124 [PMC free article] [PubMed] [Google Scholar]
- 4.Plummer DT, Ngaha EO, Wright PJ, et al. The sensitivity of urinary enzyme measurements for detecting renal injury. Curr Probl Clin Biochem 1979;(9):71–87 [PubMed] [Google Scholar]
- 5.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 2010;28:478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002;62:237–244 [DOI] [PubMed] [Google Scholar]
- 7.van Timmeren MM, Vaidya VS, van Ree RM, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 2007;84:1625–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Greene T, Sarnak MJ, et al. Effect of dietary protein restriction on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 2006;48:879–888 [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002;39(suppl 1):S1–S266 [PubMed] [Google Scholar]
- 10.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 2008;1:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman R, Hackett R, Senior D, et al. Pathologic effects of ESWL on canine renal tissue. Urology 1987;29:194–200 [DOI] [PubMed] [Google Scholar]
- 12.Tuteja AK, Pulliam JP, Lehman TH, Elzinga LW. Anuric renal failure from massive bilateral renal hematoma following extracorporeal shock wave lithotripsy. Urology 1997;50:606–608 [DOI] [PubMed] [Google Scholar]
- 13.Skolarikos A, Alivizatos G, de la Rosette J. Extracorporeal shock wave lithotripsy 25 years later: Complications and their prevention. Eur Urol 2006;50:981–990 [DOI] [PubMed] [Google Scholar]
- 14.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 2008;73:1008–1016 [DOI] [PubMed] [Google Scholar]
- 15.American Society of Nephrology. American Society of Nephrology Renal Research Report. J Am Soc Nephrol 2005;16:1886–1903 [DOI] [PubMed] [Google Scholar]
- 16.Branten AJ, Mulder TP, Peters WH, et al. Urinary excretion of glutathione S transferases alpha and pi in patients with proteinuria: Reflection of the site of tubular injury. Nephron 2000;85:120–126 [DOI] [PubMed] [Google Scholar]
- 17.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14:2534–2543 [DOI] [PubMed] [Google Scholar]
- 18.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 2004;43:405–414 [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Schöb O, Pugmire JE, et al. Glycohydrolases as markers of hepatic ischemia-reperfusion injury and recovery. Hepatology 1996;24:157–162 [DOI] [PubMed] [Google Scholar]
- 20.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 2006;290:F517–F529 [DOI] [PubMed] [Google Scholar]
- 21.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol Dial Transplant 2013;28:254–273 [DOI] [PubMed] [Google Scholar]
- 22.Makise J, Saito E, Obuchi M, et al. Improved kinetic rate assay of urinary N-acetyl-beta-D-glucosaminidase with 2-chloro-4-nitrophenyl-N-acetyl-beta-D-glucosaminide as substrate. Clin Chem 1990;36:319–322 [PubMed] [Google Scholar]
- 23.Evan AP, Lingeman JE, Coe FL, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003;111:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: Effect of antioxidants. Urol Res 2003;31:3–9 [DOI] [PubMed] [Google Scholar]
- 25.Nikoobakht MR, Pourmand G, Amidi A, et al. Selective evaluation of two urinary enzymes (NAG and AAP) before and after unnilateral shockwave lithotripsy. Acta Medica Iranica 2006;44:299–304 [Google Scholar]
- 26.Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connors BA, Evan AP, Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourol 2006;20:607–611 [DOI] [PubMed] [Google Scholar]
- 28.Willis LR, Evan AP, Connors BA, et al. Shockwave lithotripsy: Dose-related effects on renal structure, hemodynamics, and tubular function. J Endourol 2005;19:90–101 [DOI] [PubMed] [Google Scholar]