Abstract
Fetal growth restriction (FGR) occurs in ∼8% of pregnancies and is a major cause of perinatal mortality and morbidity. There is no effective treatment. FGR is characterized by reduced uterine blood flow (UBF). In normal sheep pregnancies, local uterine artery (UtA) adenovirus (Ad)-mediated overexpression of vascular endothelial growth factor (VEGF) increases UBF. Herein we evaluated Ad.VEGF therapy in the overnourished adolescent ewe, an experimental paradigm in which reduced UBF from midgestation correlates with reduced lamb birthweight near term. Singleton pregnancies were established using embryo transfer in adolescent ewes subsequently offered a high intake (n=45) or control intake (n=12) of a complete diet to generate FGR or normal fetoplacental growth, respectively. High-intake ewes were randomized midgestation to receive bilateral UtA injections of 5×1011 particles Ad.VEGF-A165 (n=18), control vector Ad.LacZ (n=14), or control saline (n=13). Fetal growth/well-being were evaluated using serial ultrasound. UBF was monitored using indwelling flowprobes until necropsy at 0.9 gestation. Vasorelaxation, neovascularization within the perivascular adventitia, and placental mRNA expression of angiogenic factors/receptors were examined using organ bath analysis, anti-vWF immunohistochemistry, and qRT-PCR, respectively. Ad.VEGF significantly increased ultrasonographic fetal growth velocity at 3–4 weeks postinjection (p=0.016–0.047). At 0.9 gestation fewer fetuses were markedly growth-restricted (birthweight >2SD below contemporaneous control-intake mean) after Ad.VEGF therapy. There was also evidence of mitigated fetal brain sparing (lower biparietal diameter-to-abdominal circumference and brain-to-liver weight ratios). No effects were observed on UBF or neovascularization; however, Ad.VEGF-transduced vessels demonstrated strikingly enhanced vasorelaxation. Placental efficiency (fetal-to-placental weight ratio) and FLT1/KDR mRNA expression were increased in the maternal but not fetal placental compartments, suggesting downstream effects on placental function. Ad.VEGF gene therapy improves fetal growth in a sheep model of FGR, although the precise mechanism of action remains unclear.
Introduction
Fetal growth restriction (FGR), which is defined as the failure of a fetus to achieve its genetically predetermined growth potential, affects ∼8% of pregnancies in developed countries (Mandruzzato et al., 2008). It is a leading cause of perinatal mortality (Bernstein et al., 2000) and associated with considerable morbidity in neonatal and later life (Barker, 2006; Halliday, 2009). FGR may complicate genetic syndromes, chromosomal abnormalities, structural anomalies, and congenital infections. In such cases the underlying condition dictates the prognosis. However, >60% of FGR occurs secondary to uteroplacental insufficiency (Ghidini, 1996), which is characterized by reduced uterine blood flow (UBF). Fetal growth in sheep is directly proportional to UBF when experimentally restricted by variable degrees of uterine artery (UtA) embolization (Lang et al., 2003), and data from human FGR pregnancies indicate that UBF is attenuated from midgestation (Konje et al., 2003). There is no effective treatment for uteroplacental FGR. Management constitutes fetal surveillance and deciding when to deliver the baby, balancing the risks of iatrogenic prematurity versus the risks of hypoxic tissue damage. Early delivery does not reduce perinatal mortality as, while it prevents some stillbirths, it results in more neonatal deaths (GRIT Study Group, 2003).
Attenuated UBF directly limits substrate delivery to the developing fetus. Attempts to increase UBF using vasodilators have been unsuccessful (Figueroa and Maulik, 2006), and systemic delivery can even worsen uterine perfusion through widespread maternal vasodilatation (Miller et al., 2009), an effect known as the placental steal phenomenon. To overcome this problem we have developed an approach to locally target the uteroplacental circulation using adenoviruses (Ad) encoding vascular endothelial growth factor (VEGF). VEGF is an important biological agent that induces vasodilatation, angiogenesis, and vascular protection (Holmes and Zachary, 2005). Previous work in normal midgestation sheep pregnancies demonstrated a significant increase in UBF after UtA injection of Ad.VEGF-A165, which was sustained for up to 4 weeks (Mehta et al., 2011). Ad.VEGF-A165 upregulated endothelial nitric oxide synthase (eNOS) expression in the UtA, and Ad.VEGF-A165-treated vessels demonstrated an enhanced vasodilator response to bradykinin and neovascularization within the perivascular adventitia (David et al., 2008).
Herein we tested the hypothesis that an Ad.VEGF-mediated increase in UBF would improve fetal growth in pregnancies complicated by FGR. We used the overnourished adolescent sheep model of FGR because it is characterized by a major reduction in UBF by midgestation and replicates many key features of uteroplacental human FGR without any need for surgical interference (Wallace et al., 2008). Overnourishing pregnant adolescent ewes by offering a high-intake diet throughout gestation paradoxically results in major placental and FGR relative to pregnancies of control-fed adolescent ewes (Wallace et al., 2006a). Overnourishing the young adolescent, who is still growing during pregnancy, results in accelerated maternal tissue deposition with nutrient partitioning away from the gravid uterus at the expense of the fetus. FGR is accompanied by impaired placental vascularization and secretory function and reduced placental expression of VEGF and fms-related tyrosine kinase 1 (FLT1) (Redmer et al., 2005, 2009; Lea et al., 2007). In common with human FGR, asymmetrical growth restriction with preservation of brain growth (brain sparing) and increased umbilical artery (UA) Doppler indices are apparent (Wallace et al., 2000; Carr et al., 2012). A variable response to nutritional manipulation is observed, resulting in a wide range of fetal and placental growth trajectories. Overall ∼52% of pregnancies in overnourished adolescents result in “marked” FGR (with a birthweight more than 2 standard deviations [SDs] below the mean birthweight of normal control fetuses) with an ∼48% reduction in placental and fetal weights (Wallace et al., 2004).
The goal herein was to establish proof of principle for the efficacy and safety of Ad.VEGF on fetal growth in a large animal model of FGR. Thus, our primary aim was to determine the effects of midgestation Ad.VEGF UtA injection on UBF, fetal growth velocity, fetal weight in late gestation, proportions of fetuses demonstrating marked FGR, and fetal body composition, organ weights, and gross morphology. A secondary aim was to investigate potential mechanisms of action by assessing vascular relaxation, neovascularization within the perivascular adventitia, and placental mRNA expression of selected angiogenic factors and receptors.
Materials and Methods
Experimental animals
Animal procedures were approved by the UK Home Office under the Animals (Scientific Procedures) Act 1986 and by local ethics committee review. Ewes were housed in individual pens under natural lighting conditions at the Rowett Institute of Nutrition and Health (57°N, 2°W). Embryos were derived from superovulated donor ewes (Scottish Blackface×Border Leicester) inseminated by a single sire (Dorset Horn) to establish exclusively singleton pregnancies and maximize genetic homogeneity, as described (Wallace et al., 1997). After embryo transfer, the adolescent recipients (Dorset Horn×Mule) were allocated to one of two nutritional treatments: a control intake (C) to promote normal fetoplacental growth (n=12), or a high intake (H) of the same complete diet (∼2×C) to generate FGR (n=45), as described (Wallace et al., 2006b).
Ultrasound
At 83±0.1 days gestation ewes underwent detailed ultrasound examination including baseline measurements of the fetal abdominal circumference (AC) and biparietal diameter (BPD), and placentome index, as described (Carr et al., 2011). In addition, the deepest vertical pool of amniotic fluid was quantified and a free loop of umbilical cord examined using pulsed wave Doppler ultrasound. Three consecutive UA Doppler waveforms were manually traced, from which the pulsatility index (PI) was determined. UA PI is an indicator of fetoplacental vascular resistance, which relates to fetal well-being, and is the primary surveillance tool in human pregnancies complicated by FGR. Between 98±0.1 and 126±0.3 days of gestation, scans were repeated weekly to assess fetal growth/well-being. All scans were performed by a single operator accredited in advanced obstetric ultrasound (D.J.C.) blind to the treatment allocation using a Logiq 400 CL machine (GE, Milwaukee, WI) with a 5.0 MHz curvilinear probe.
Gene therapy
At 89±0.2 days of gestation ewes underwent a midline laparotomy. Anesthesia was induced with 5 mg/kg intravenous propofol and maintained by inhaled isoflurane. An ultrasonic perivascular flow probe (PSS Series, size 4 or 6 mm; Transonic Systems Inc., Ithaca, NY) was fitted around the main trunk of the UtA supplying the gravid uterine horn. Subsequently, the main trunk of each UtA was occluded and injected with first-generation replication (E1, E3-deleted)-deficient Ads (5×1011 particles in 10 ml normal saline; titer determined at OD260) containing the VEGF-A165 gene (Ad.VEGF) or the β-galactosidase gene (Ad.LacZ), as described (David et al., 2008). High-intake ewes were randomized to receive Ad.VEGF (H+Ad.VEGF, n=18), Ad.LacZ (H+Ad.LacZ, n=14), or saline only (H+Saline, n=13). Control-intake ewes all received saline (C+Saline, n=12). Between 92±0.2 and 130±0.2 days of gestation, UBF was measured continuously for 45 min on alternate days telemetrically, as described (Mehta et al., 2011).
Necropsy
At 131±0.2 days of gestation, ewes and fetuses were euthanized. Necropsy was performed at this stage to strike a balance between allowing sufficient time postinjection to adequately assess the potential impact on fetal growth (a gradual process) while intervening before the onset of labor, which can occur from as early as 135 days gestation in overnourished adolescent dams (Wallace et al., 2006a). Fetuses were dried, weighed, and dissected. Umbilical girth, biparietal head diameter, and lengths of the tibial and femoral bones were measured with callipers. All major internal organs were macroscopically examined and weighed. The uterine arterial tree was carefully dissected and segments were obtained from both gravid and nongravid horns at three levels of branching: main trunk of the UtA (designated UtA1), first branches (UtA2), and second branches (UtA3). Duplicate segments of UtA1–3 were immersion-fixed in neutral buffered formalin saline, processed through an ethanol gradient, and paraffin-embedded for subsequent immunohistochemistry. In a subset of 22 animals (n=4, 7, 5, and 6 from C+Saline, H+Ad.VEGF, H+Saline, and H+Ad.LacZ groups, respectively), additional segments of UtA2–3 were obtained and stored overnight in cold Krebs Ringer buffer pending organ bath analysis. Finally, whole placentomes, comprising both fetal (cotyledonary) and maternal (caruncular) tissues, were counted and weighed. Four representative placentomes were separated into cotyledonary/caruncular components by gentle traction, snap-frozen in isopentane chilled with liquid nitrogen, and stored at −80°C pending RNA extraction. Total placental weight was calculated (total placentome weight+membrane weight).
Laboratory analyses
To assess vascular contraction and relaxation, segments of UtA2/UtA3 from 22 pregnancies were cut into 2–3 mm ring segments and subjected to increasing doses of phenylephrine and bradykinin in an organ bath, as described (David et al., 2008). To assess neovascularization, sections of UtA1–3 from all animals were immunostained with the endothelial cell marker anti–von Willebrand factor (1:200), as described (Mehta et al. 2011). Slides were examined at ×20 magnification to identify the perivascular adventitia. All positively stained structures with a distinct lumen within this layer were counted. The adventitial area was determined by image analysis (Motic Images 2.0; Motic China Group Co. Ltd., Indusxiamen, China). The number of perivascular adventitial blood vessels was examined in absolute terms and per unit adventitial area. Finally, RNA was extracted from separately snap-frozen caruncular/cotyledonary tissues and quantitative real-time reverse transcription polymerase chain reaction used to determine mRNA expression of nine angiogenic factors/receptors using ovine-specific probes/primers, as described (Redmer et al., 2005): VEGF, VEGF receptors 1 (FLT1) and 2 (KDR), nitric oxide synthase (NOS3), fibroblast growth factor 2 (FGF2), angiopoietin 1 (ANG1), angiopoietin 2 (ANG2), endothelial tyrosine kinase (TEK), and nitric oxide receptor soluble guanylate cyclase (SGC). Individual mRNA for each gene of interest was quantified relative to each sample's internal 18S RNA content. For all laboratory analyses the individual investigator was blinded to the treatment group by means of block and sample coding until analysis was complete.
Statistical analyses
During study design a power calculation was performed based on preliminary data showing a 40% increase in UBF after Ad.VEGF delivery in normal sheep pregnancy (David et al., 2008) and prior evaluation of UBF in the overnourished adolescent model (Wallace et al., 2008). Assuming interanimal variability of 143 ml/min, it was estimated that 11 animals per group would be required to detect a 50% increase in UBF (212 ml/min) with 90% power. Study results were analyzed using the Statistical Package for the Social Sciences (SPSS) version 19.0 (SPSS Inc., Chicago, IL). After confirming normality using Q:Q plots, comparisons were made across the four groups using one-way analysis of variance (ANOVA) and post hoc test of least significant difference (LSD). As there were no significant differences between the two different high-intake control groups (H+Ad.LacZ and H+Saline) for any of the parameters studied, these groups were combined as a single group (H+Ad.LacZ/Saline, n=27) for further comparisons, also by ANOVA and post hoc test of LSD. Categorical data were compared using Fisher's exact test. Fetal weight was analyzed in absolute terms (ANOVA) but was additionally analyzed using a categorical approach by determining the numbers of fetuses exhibiting “marked” FGR within each group. An accepted definition of marked FGR is a birthweight that falls >2SD below the genetic potential (Robinson et al., 1979). In this paradigm the latter can be estimated from the contemporaneous control-intake group on a study-by-study basis. The use of embryo transfer and randomization to nutritional treatment on the basis of embryo donor source plus use of a single sire (see above) all helped maximize genetic homogeneity when deriving the singleton pregnancies herein. For the UtA analyses, general linear model was used to assess differences between study groups while taking into account the level of branching (UtA1–3) and uterine horn (gravid/nongravid). All data are presented as mean±standard error of the mean (SEM) unless otherwise stated.
Results
There were no adverse maternal or fetal complications during the surgery or the postoperative recovery period. In particular, there were no adverse fetal responses to the 5 min UtA occlusion. All pregnancies remained viable until the point of necropsy at 131±0.2 days of gestation and no maternal health problems were observed.
Fetoplacental growth and fetal well-being
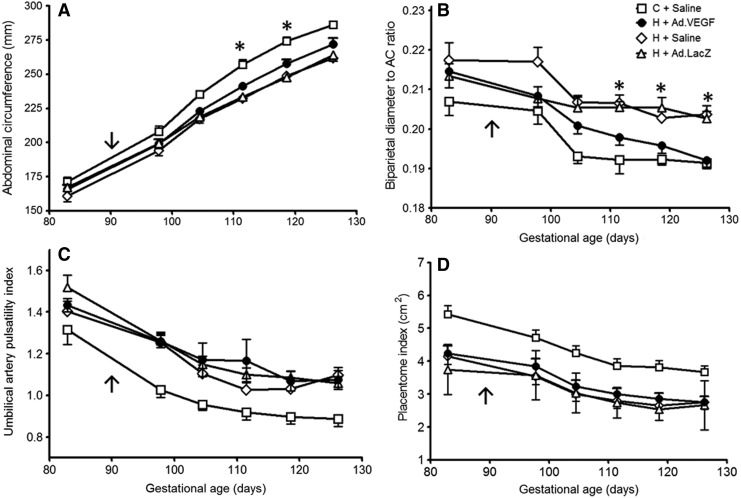
Figure 1A shows serial fetal AC measurements from 98±0.1 until 126±0.3 days of gestation. As expected, fetuses of control-intake ewes grew normally and hence had greater AC measurements compared with the compromised fetuses of high-intake ewes from 105±0.1 days of gestation onward (p<0.001). Compared with equivalent presurgery baseline values, AC measurements increased more in H+Ad.VEGF versus H+Saline/H+Ad.LacZ groups and were greater at 112±0.1 (241±2.8 vs. 233±2.3 and 233±2.5 mm, p=0.031 and p=0.047, respectively) and 119±1 days of gestation (258±3.1 vs. 249±2.3 and 248±2.8 mm, p=0.032 and p=0.016, respectively), corresponding to 22±0.2 and 29±0.2 days postinjection. No treatment differences were evident between high-intake groups at 126±0.3 days of gestation, when AC measurements were more variable. BPD measurements were not different between any groups at any gestational age. Consequently, differences in BPD:AC ratio (an index of fetal brain sparing) were apparent (Fig. 1B). With advancing gestation, BPD:AC ratios diminished progressively in all groups but were lower in C+Saline versus H+Saline and H+Ad.LacZ groups (p<0.001) from 105±0.1 days of gestation onward. BPD:AC ratios within the H+Ad.VEGF group fell faster relative to H+Saline and H+Ad.LacZ and were lower from 112±0.1 days of gestation onward (p<0.001–0.016). By 126±0.1 days of gestation they were similar to the C+Saline group (p=0.807), indicating that fetal brain sparing had been ameliorated by Ad.VEGF treatment.
FIG. 1.
Ultrasound assessment of fetoplacental growth and umbilical blood flow. Serial ultrasound measurements of fetal AC (A), biparietal head diameter:AC ratio (B), umbilical artery pulsatility index (C), and placentome index (D) between 83±0.1 and 126±0.3 days of gestation in singleton-bearing adolescent ewes fed a control (C; open squares, n=12) or a high (H) dietary intake to generate normal and compromised fetoplacental growth, respectively. After baseline measurements at 83±0.1 days of gestation, ewes received bilateral uterine artery injections of Ad.VEGF (closed circles, n=18), Ad.LacZ (open triangles, n=14), or saline (open diamonds for H+Saline, n=13, open squares for C+Saline, n=12) at 89±0.2 days of gestation (indicated by arrow). Asterisks denote gestational time points at which there were significant differences in H+Ad.VEGF relative to H+Saline and H+Ad.LacZ groups (p<0.05 for post hoc comparisons). AC, abdominal circumference; Ad, adenovirus; LacZ, β-galactosidase; VEGF, vascular endothelial growth factor.
Figure 1C shows serial measurements of UA PI throughout the study period. UA PI values fell with advancing gestation and were lower in C+Saline relative to all three high-intake groups from 98±0.1 days of gestation onward (p<0.001–0.038). There were no differences between H+Ad.VEGF and H+Saline or H+Ad.LacZ groups. There were no differences between groups in amniotic fluid measurements (data not shown). Finally, serial measurements of the placentome index are shown in Fig. 1D. The placentome index was greater in C+Saline versus high-intake groups at all time points (p<0.001–0.005) and also decreased with advancing gestation. There were no differences in this two-dimensional ultrasound measure of placental size between the three high-intake groups.
Fetal weight and body composition
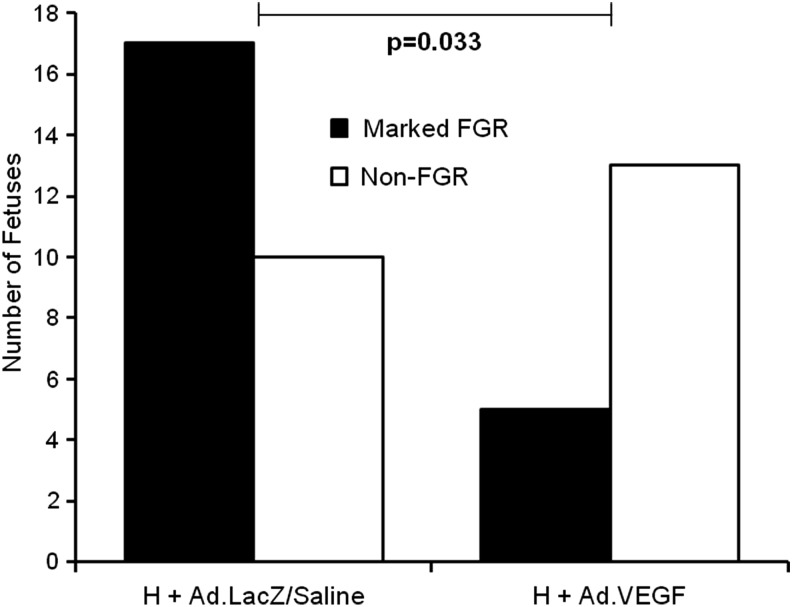
Table 1 shows fetal and placental weights, fetal anthropometric data, and major organ weights at necropsy at 131±0.2 days of gestation. Fetal weight was reduced in all three high-intake groups relative to the normally grown controls (minimum p=0.001 for individual comparisons). When comparing the group means, fetal weight in the H+Ad.VEGF group was not significantly different from that of the H+Saline or H+Ad.LacZ groups in late gestation (p=0.375 and p=0.149, respectively). However, significant differences were observed in the numbers of fetuses exhibiting “marked” FGR (birthweight >2SD below the non-FGR control mean). The mean±SD within the C+Saline group was 5084±431 g, giving a minus 2SD cutoff of 4222 g. Figure 2 illustrates the proportion of fetuses in the H+Ad.VEGF and H+Ad.LacZ/Saline (combined) groups weighing less than or greater than 4222 g (marked FGR and non-FGR, respectively). Significantly fewer fetuses were categorized as markedly FGR in H+Ad.VEGF compared with H+Ad.LacZ/Saline groups.
Table 1.
Fetal Anthropometric and Placental Data in Late Gestation
| Necropsy measurement | C+Saline (n=12) | H+Ad.VEGF (n=18) | H+Saline (n=13) | H+Ad.LacZ (n=14) | p |
|---|---|---|---|---|---|
| Fetal weight (g) | 5084±124a | 4395±188b | 4153±202b | 4008±234b | 0.003 |
| Placentome weight (g) | 521±82.5a | 366±96.6b | 372±106.7b | 345±93.6b | <0.001 |
| Placentome number | 71.0±0.78a | 68.6±1.28ab | 66.6±1.12b | 66.8±1.00b | 0.043 |
| Total placental weight (g) | 802±36.1a | 594±33.5b | 595±42.4b | 570±37.2b | <0.001 |
| Fetal to placental weight ratio | 6.45±0.266a | 7.52±0.250b | 7.16±0.254ab | 7.21±0.339ab | 0.018 |
| Biparietal head diameter (mm) | 71.0±0.78a | 68.6±1.28ab | 66.6±1.12b | 66.8±1.00b | 0.043 |
| Umbilical girth (mm) | 363±4.9a | 340±6.2b | 336±7.2b | 328±7.0b | 0.006 |
| Length of femoral bone (mm) | 89.8±1.00a | 85.9±1.42b | 84.4±1.11b | 83.9±1.56b | 0.024 |
| Length of tibial bone (mm) | 107±1.2a | 102±1.9ab | 101±1.4b | 100±1.9b | 0.070 |
| Brain weight (g) | 47.0±0.95 | 44.1±1.58 | 44.8±0.79 | 44.5±1.10 | 0.431 |
| Paired adrenal weight (g) | 0.50±0.023 | 0.46±0.022 | 0.47±0.027 | 0.50±0.032 | 0.666 |
| Pancreas weight (g) | 3.93±0.134 | 3.75±0.197 | 3.63±0.287 | 3.51±0.182 | 0.582 |
| Stomach weight (g) | 32.6±1.46 | 30.2±1.21 | 30.6±0.85 | 28.5±1.26 | 0.171 |
| Small intestine weight (g) | 51.2±2.70 | 44.7±2.33 | 48.1±2.45 | 42.9±2.11 | 0.109 |
| Large intestine weight (g) | 14.5±1.09 | 12.9±0.49 | 13.3±0.59 | 12.4±0.75 | 0.190 |
| Liver weight (g) | 161±7.0a | 134±6.3b | 132±7.8b | 121±9.3b | 0.007 |
| Spleen weight (g) | 9.03±0.415 | 8.10±0.444 | 8.16±0.736 | 7.57±0.598 | 0.374 |
| Paired kidney weight (g) | 27.5±0.77a | 25.1±1.17ab | 23.4±1.14b | 22.0±1.43b | 0.019 |
| Perirenal fat weight (g) | 21.1±0.58a | 18.2±0.93b | 18.9±1.05ab | 17.2±1.09b | 0.055 |
| Heart weight (g) | 36.7±1.20a | 33.6±1.48ab | 30.9±1.29b | 29.6±1.45b | 0.007 |
| Lung weight (g) | 172±10.9a | 158±8.5ab | 142±8.7b | 128±10.8b | 0.021 |
| Thymus weight (g) | 7.15±0.447 | 6.72±0.489 | 5.95±0.581 | 6.10±0.661 | 0.432 |
| Thyroid weight (g) | 0.97±0.021a | 0.77±0.069b | 0.80±0.044b | 0.74±0.048b | 0.025 |
| Carcass weight (g) | 3289±92.2a | 2847±134.7b | 2779±132.6b | 2669±157.7b | 0.021 |
Ad, adenovirus; ANOVA, analysis of variance; LacZ, β-galactosidase; SEM, standard error of the mean; VEGF, vascular endothelial growth factor.
All data are presented as mean±SEM. p-Values reported in table are for overall ANOVA. Mean values within a row with unlike superscripts are significantly different (p≤0.05 for individual comparisons). Individual post hoc comparisons are detailed in the text.
FIG. 2.
Incidence of marked FGR. Number of fetuses delivered by hysterotomy at 131±0.2 days gestation from singleton-bearing adolescent ewes fed a high (H) dietary intake (to compromise fetoplacental growth) with marked FGR (closed bars) or mild FGR (open bars). Marked FGR was defined as a birthweight >2SD below the mean birthweight of the fetuses of 12 contemporaneous control-intake ewes that demonstrating normal fetoplacental growth (<4222 g for the present study). Fetuses with birthweights >4222 g were of similar weight to normally grown controls and therefore termed “non-FGR.” In midgestation H ewes received bilateral uterine artery injections of Ad.VEGF or control treatment (Ad.LacZ/Saline). Proportions were compared using Fisher's exact test. FGR, fetal growth restriction.
Placentome weight and total placental weight were both reduced in control- versus high-intake pregnancies (p<0.001) but were not significantly different between H+Ad.VEGF, H+Saline, and H+Ad.LacZ groups. The fetal-to-placental weight ratio (an index of placental efficiency) was highest in the H+Ad.VEGF group, and greater than normally grown control-intake pregnancies (p=0.009). Physical measurements of the fetus at necropsy, namely, biparietal head diameter, umbilical girth, and long bone lengths, were in agreement with the equivalent ultrasonographic fetal biometry at 126±0.3 days of gestation (r=0.641–0.745, p<0.001, n=57). Postmortem measurements of umbilical girth and femoral length were reduced in all three high-intake groups relative to fetuses of control-intake ewes (p=0.001–0.044), while biparietal head diameters were significantly reduced only in H+Saline and H+Ad.LacZ versus C+Saline groups (p=0.013/p=0.015), H+Ad.VEGF being intermediate. The apparent differences between study groups in absolute weights of liver, kidneys, perirenal fat depots, heart, lungs, and thyroid (Table 1) were no longer significant when expressed per kg fetus (data not shown), indicating a proportionate shift in these indices of fetal size across all high-intake groups. By contrast, despite no differences between groups in absolute brain weight, both relative brain weight and brain:liver weight ratios were increased in H+Ad.LacZ/Saline groups combined compared with the C+Saline group, commensurate with fetal brain sparing (11.2±0.30 vs. 9.3±0.24 g/kg, p<0.001; and 0.37±0.02 vs. 0.30±0.01, p=0.001, respectively). Importantly, both indices of fetal brain sparing were lower after Ad.VEGF treatment (10.1±0.26 g/kg, p=0.009; and 0.33±0.01, p=0.046, respectively). Gross organ morphology was normal in all cases.
Uterine blood flow
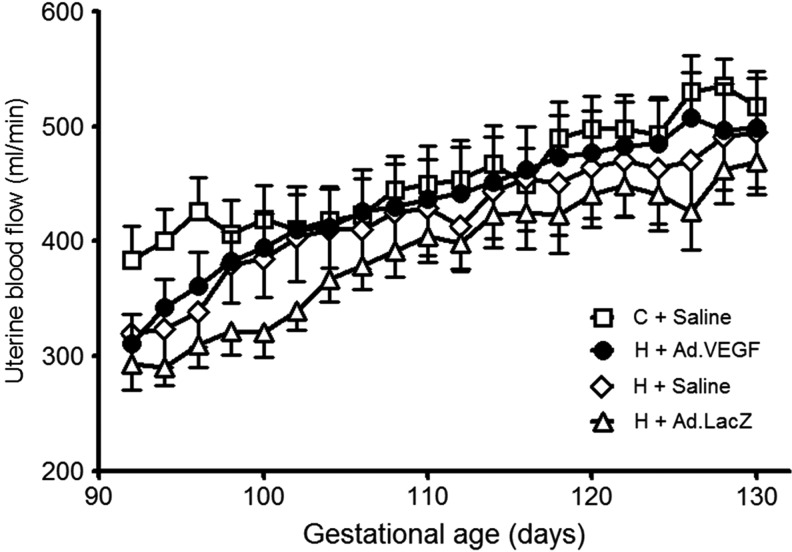
Serial measurements of UBF via the main trunk of the UtA supplying the gravid horn between 92±0.2 and 130±0.2 days of gestation are shown in the Fig. 3. Mean UBF was greater in C+Saline versus H+Saline/H+Ad.LacZ groups at 94±0.1 and 96±0.1 days of gestation (p=0.006–0.042) but not thereafter. UBF measurements in the H+Ad.VEGF group were not significantly different when compared with any other group at any point. The gestational increase in UBF over the study period was not different between C+Saline, H+Ad.VEGF, H+Saline, and H+Ad.LacZ groups in absolute (134±96.3, 188±100.6, 201±115.1, and 176±78.6 ml/min, respectively, p=0.531) or relative terms (41.0±9.72%, 61.1±7.68%, 76.7±19.02%, and 65.4±9.42%, respectively, p=0.248). There were no differences in average UBF between groups (p=0.357 and p=0.565, respectively).
FIG. 3.
In vivo assessment of uterine blood flow. Serial measurements of UBF were recorded between 92±0.2 and 130±0.2 days of gestation using an ultrasonic perivascular flow probe placed around the main trunk of the UtA supplying the gravid horn in singleton-bearing adolescent ewes fed a control (C; open squares, n=12) or a high (H) dietary intake to generate normal and compromised fetal and placental growth trajectories, respectively. UBF determinations were commenced 3 days after bilateral UtA injections of Ad.VEGF (closed circles, n=18), Ad.LacZ (open triangles, n=14), or saline (open diamonds for H+Saline, n=13, and open squares for C+Saline, n=12). Asterisks denote gestational time points at which there were significant differences between the four groups (overall ANOVA p<0.05). UBF, uterine blood flow; UtA, uterine artery.
Vascular reactivity
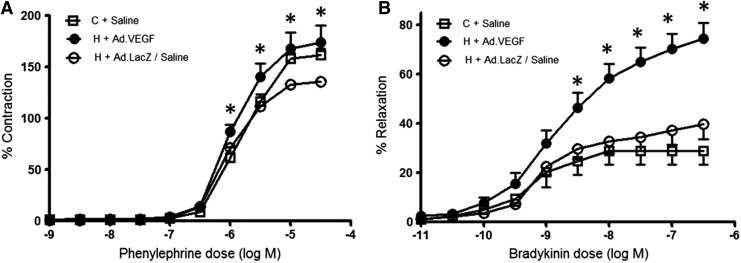
Figure 4A shows the contractile response of UtA2/UtA3 vessel segments to increasing concentrations of phenylephrine in the organ bath when examined on the day after necropsy. Satisfactory dose–response curves were achieved in 76 of 78 vessels examined from a subset of 22 animals. For these vessels the degree of contraction was somewhat greater in H+Ad.VEGF relative to H+Ad.LacZ/Saline groups at phenylephrine concentrations of −6.0 log M upward (p≤0.001–0.04) and was not different to the C+Saline group at any point. The maximum contractile response (EmaxC) was also increased in H+Ad.VEGF versus H+Ad.LacZ/Saline groups (190±18.5 vs. 148±3.2%, p=0.04) and was not significantly different from that of the C+Saline group (p=0.718).
FIG. 4.
Uterine artery vascular reactivity. Dose–response curves to phenylephrine (A) and bradykinin (B), reflecting vascular contraction and relaxation, respectively, for UtA segments (from gravid and nongravid horns at two levels of branching) from 22 singleton-bearing adolescent ewes fed a control (C; open squares) or a high (H) dietary intake to generate normal and compromised fetoplacental growth, respectively, and necropsied at 131±0.2 days of gestation. At 89±0.2 days of gestation, H ewes received bilateral uterine artery injections of Ad.VEGF (closed circles) or Ad.LacZ/Saline (open circles). Asterisks indicate concentrations of bradykinin at which there were significant differences in H+Ad.VEGF versus H+Ad.LacZ/Saline and C+Saline groups (p<0.05 for overall ANOVA and post hoc comparisons).
Figure 4B illustrates the subsequent relaxation response to bradykinin after precontraction with a suboptimal dose of phenylephrine (1 μM) that caused a contraction equivalent to 50–60% of EmaxC. The response to bradykinin was insufficient for analysis in 3 of 14 vessel segments (27.3%) in C+Saline, 7 of 24 (29.2%) in H+Ad.VEGF, 7 of 18 (38.9%) in H+Saline, and 6 of 20 (30.0%) in H+Ad.LacZ groups, which meant that no data from these vessels were available for analysis. For the remaining vessels, vasorelaxation was markedly greater in H+Ad.VEGF compared with all other groups (p≤0.001–0.015) at concentrations of −8.5 log M upward. The maximum relaxation (EmaxR) was higher in H+Ad.VEGF (75±6.2%) relative to both H+Ad.LacZ/Saline (41±6.1%, p≤0.001) and C+Saline (30±6.0%, p≤0.001) groups. There were no significant differences in any indices of vascular relaxation between branches UtA2 and UtA3 (p=0.655) or between those from the gravid versus nongravid horns (p=0.562) and no interactions between these factors and treatment group (p=0.129–0.778).
Neovascularization
Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/hum) shows absolute numbers of blood vessels in the perivascular adventitia, adventitial area, and vessel density (number of vessels per unit adventitial area) stratified by UtA branch. Both vessel number and adventitial area decreased with successive branching (p≤0.001), and consequently there were no differences in vessel density between branches UtA1, 2, and 3 (p=0.113). There were also no differences between vessels harvested from the gravid and nongravid horns. Although there were no differences in absolute vessel number between study groups per individual branch, overall there were more vessels per section in H+Ad.VEGF and H+Ad.LacZ/Saline versus C+Saline groups (142±7.2 and 138±11.0 vs. 105±7.0, p=0.006 and p=0.024, respectively) without any significant differences in adventitial area by group (p=0.846). Consequently, vessel density was significantly increased in high-intake relative to control-intake pregnancies, both across and within different branches (p≤0.001). There was no demonstrable effect of Ad.VEGF.
Placental analyses
Table 2 shows the mRNA expression of nine angiogenic factors/receptors evaluated separately in the maternal and fetal placental compartments. In caruncular tissues, mRNA expression of endogenous VEGF and its two main receptors (FLT1 and KDR) was reduced in H+Ad.LacZ/Saline relative to C+Saline groups (p=0.012, p=0.031, and p=0.039, respectively). Although endogenous VEGF expression was not significantly influenced by Ad.VEGF treatment, expression of both FLT1 and KDR was increased in H+Ad.VEGF versus H+Ad.LacZ/Saline groups (p=0.028 and p=0.034, respectively) and was similar in magnitude to control-intake pregnancies. In cotyledonary tissues, mRNA expression of ANG2 and TEK was significantly increased in both H+Ad.VEGF and H+Ad.LacZ/Saline compared with C+Saline groups (ANG2: p=0.032 and p=0.003; TEK: p=0.018 and p=0.045, respectively). There was no significant effect of Ad.VEGF on expression of any genes in the fetal placental compartment.
Table 2.
Placental Expression of Angiogenic Factors/Receptors
| Placental compartment and gene of interest | C+Saline (n=12) | H+Ad.VEGF (n=18) | H+Saline/Ad.LacZ (n=27) | p |
|---|---|---|---|---|
| Maternal caruncle | ||||
| VEGF | 27.3±3.04a | 23.7±1.75ab | 20.3±1.26b | 0.036 |
| FLT1 | 18.8±2.26a | 18.1±2.36a | 12.5±1.36b | 0.031 |
| KDR | 23.8±2.40a | 23.2±2.52a | 17.4±1.43b | 0.040 |
| NOS3 | 28.6±2.82 | 37.6±3.25 | 33.5±2.65 | 0.185 |
| FGF2 | 15.7±1.54 | 13.8±1.28 | 12.5±1.15 | 0.280 |
| ANG1 | 24.0±1.622 | 21.1±1.65 | 20.1±1.48 | 0.291 |
| ANG2 | 14.0±1.17 | 14.7±0.85 | 12.4±0.69 | 0.110 |
| TEK | 21.6±2.63 | 22.9±1.51 | 19.8±1.33 | 0.370 |
| SGC | 16.5±1.52 | 16.2±1.15 | 13.6±0.96 | 0.138 |
| Fetal cotyledon | ||||
| VEGF | 26.5±3.53 | 30.6±2.67 | 30.9±2.59 | 0.579 |
| FLT1 | 15.1±2.42 | 13.0±1.50 | 14.6±1.44 | 0.699 |
| KDR | 17.0±2.44 | 17.6±1.59 | 18.4±1.80 | 0.877 |
| NOS3 | 16.1±2.17 | 21.5±3.01 | 19.6±1.87 | 0.383 |
| FGF2 | 9.9±1.07 | 10.6±0.58 | 11.3±0.69 | 0.430 |
| ANG1 | 21.9±1.64 | 20.9±0.94 | 23.1±0.90 | 0.312 |
| ANG2 | 13.0±0.79a | 16.0±0.73b | 16.9±0.80b | 0.010 |
| TEK | 14.0±0.79a | 16.8±0.71b | 16.2±0.61b | 0.049 |
| SGC | 8.9±0.90 | 9.3±0.81 | 8.3±0.40 | 0.517 |
ANG1, angiopoietin 1; ANG2, angiopoietin 2; FGF2, fibroblast growth factor 2; FLT1, fms-related tyrosine kinase 1 (VEGF receptor 1); KDR, kinase insert domain receptor (VEGF receptor 2); NOS3, nitric oxide synthase; SGC, soluble guanylate cyclase (nitric oxide receptor); TEK, endothelial tyrosine kinase; VEGF, vascular endothelial growth factor.
p-Values shown are for overall ANOVA. Mean values within a row with unlike superscripts are significantly different (p<0.05 for individual post hoc comparisons). All data are presented as mean±SEM.
Discussion
The most important finding of this study is that Ad.VEGF treatment midpregnancy improves prenatal growth velocity in a well-characterized ovine paradigm of uteroplacental FGR. This is apparent from the greater AC measurements 3 and 4 weeks after Ad.VEGF treatment compared with inactive treatments controlling for surgical intervention±adenoviral infection in the putatively FGR pregnancies. The AC is the most sensitive ultrasound marker of longitudinal fetal growth in human and sheep pregnancy and correlates strongly with birthweight (Degani, 2001; Nahum and Stanislaw, 2003; Carr et al., 2011). Fetal growth was by no means completely normalized, given that the growth trajectories of Ad.VEGF-treated growth-restricted fetuses remained significantly below those of the normally growing fetuses of the contemporaneous control-fed ewes. However, from a clinical perspective, it is not necessary to normalize fetal growth in order to produce benefit. Very modest increases in fetal growth, enough to delay delivery by a few days or weeks, can greatly improve survival and morbidity at the threshold of viability (Costeloe et al., 2000). In infants born at 26 weeks of gestation, 1-year survival is 78% at birthweights between 601 and 700 g compared with 51% between 501 and 600 g (Morse et al., 2006), and survival at 6 years with only mild disability is 2.7 times higher (24% vs. 9%) at 25 weeks relative to 24 weeks of gestation (Marlow et al., 2005).
Despite the improvement in fetal growth velocity observed herein, fetal weight per se was not increased in Ad.VEGF-treated versus Ad.Saline/LacZ-treated high-intake pregnancies. This likely reflects the heterogeneous response to nutritional manipulation, such that only half of overnourished adolescent pregnancies result in marked FGR. It is revealing that significantly fewer fetuses were categorized as markedly growth-restricted at necropsy after Ad.VEGF treatment and the incidence of marked FGR was notably less than expected from historical data for overnourished adolescent dams delivering spontaneously close to term (27.8% vs. 52%) (Wallace et al., 2004).
Uteroplacental FGR is usually asymmetrical (Cox and Marton, 2009). Faced with a reduced substrate supply, the fetus prioritizes growth of important organs such as the brain (Peebles, 2004). This “brain sparing” effect results in higher ultrasound BPD:AC ratios and brain:liver weight ratios. While this is generally regarded as an adaptive fetal response to maintain cerebral perfusion, there is increasing evidence that this phenomenon may confer an increased risk of brain injury, as children born after FGR with antenatal evidence of brain sparing have more cognitive and behavioral problems (Scherjon et al., 2000; Roza et al., 2008; Figueras et al., 2011). In this study there was significantly less brain sparing in H+Ad.VEGF versus H+Ad.LacZ/Saline groups, suggesting that Ad.VEGF treatment reduced the need for such fetal adaptation.
Our original hypothesis was that increased fetal growth would occur secondary to a therapeutic increase in UBF; however, we demonstrated no effect of Ad.VEGF on UBF in the growth-restricted pregnancies, despite obvious effects on fetal growth and the incidence of marked FGR. However, since we also failed to detect significant differences in UBF between control- and high-intake pregnancies (other than for a small difference in UBF at 94–96 days gestation) the lack of a measurable effect of Ad.VEGF on UBF is perhaps unsurprising. The failure to detect significant differences between nutritional groups is in contrast with previous studies in this paradigm in which UBF has been quantified using two different methods. UBF measured using indwelling flowprobes directly linked to a flowmeter (nontelemetrically) was reduced by 42% in high-intake pregnancies at 88 days gestation and by 30% (on average) over a subsequent 50-day period (Wallace et al., 2008), while UBF measured by the Fick principle in chronically instrumented sheep was reduced by 36% at 130 days gestation in high-intake versus control-intake pregnancies (Wallace et al., 2002). The reason for this discrepancy in a key feature of our experimental paradigm is unclear. Variability in initial UBF among the high-intake ewes in the present study was ∼157 ml/min, well below the 212 ml/min derived from our original study using nontelemetric equipment and used for the power calculation. It is also noteworthy that the increase in flow from midgestation to late gestation in normal pregnancies in the present study (41%) was markedly lower than the 2-fold increase reported previously over the same period in this model (Wallace et al., 2008), as well as the 2.5–3.5-fold increase demonstrated by earlier cross-sectional studies in sheep (Meschia, 1983) and other mammals (Reynolds and Redmer, 1995; Reynolds et al., 1986), and serially in humans (Konje et al., 2003). Both aspects indicate with hindsight that the telemetric Physiogear system was not functioning optimally and probably lacked the required sensitivity and precision to detect both the expected differences between nutritional groups and the impact of Ad.VEGF treatment. In addition, the assumption of a 50% increase in UBF after Ad.VEGF treatment was based on a previous study in normally developing pregnancies with a very different experimental design, having compared effects of Ad.VEGF injection into one UtA with Ad.LacZ injection into the opposite UtA of the same animal (Mehta et al., 2011). The increase in UBF was expressed as a percentage change from baseline values within the same animal using flow probes, which has been determined over 4–7 days before gene therapy administration. By contrast, the present study compared UBF between animals and in absolute terms.
In support of the hypothesis that VEGF treatment improves fetal growth velocity in FGR by causing vascular changes that increase blood flow, Ad.VEGF-transduced vessels demonstrated significant differences in vascular reactivity when examined in the organ bath, including an enhanced contractile response to phenylephrine relative to Ad.LacZ/Saline-treated vessels, which was similar in magnitude to vessels harvested from control-intake pregnancies. These results contrasted with our previous organ bath findings in UtA branches from normal sheep pregnancies, which demonstrated a reduced contractile response in Ad.VEGF-transduced vessels at both short-term and long-term time points: 4–7 days and 30–45 days posttreatment, respectively (David et al., 2008; Mehta et al., 2011). Moreover, there was a strikingly enhanced relaxation response to bradykinin relative to all other groups. We previously demonstrated increased vasodilatation in the short-term only (David et al., 2008) and hypothesized that the longer-term effects might be mediated in part by adventitial neovascularization (Mehta et al., 2011). In the present study we demonstrated no significant effect of Ad.VEGF on angiogenesis. Notably, however, there was increased neovascularization in all high-intake groups relative to the control-intake group. This may reflect an adaptive response aimed at optimizing UBF in compromised pregnancies. Irrespective, on the background of enhanced neovascularization secondary to high nutritional intakes, any further effect of Ad.VEGF delivery would be more difficult to detect. The observation herein that Ad.VEGF enhanced both vasoconstriction and vasorelaxation may be explained by the fact that VEGF has vasoprotective properties and can induce prolonged endothelial cell survival (Zachary et al., 2000). The observation that Ad.VEGF-transduced vessels were significantly more contractile than Saline/Ad.LacZ-treated vessels from both control- and high-intake animals may reflect improved overall vascular function secondary to VEGF overexpression, with relative degradation of the vessels (particularly the endothelium) in the absence of Ad.VEGF transduction.
Alternatively, downstream placental effects may have been responsible for mediating the beneficial effects of Ad.VEGF on fetal growth. In this study we showed an upregulation of both VEGF receptors on the maternal side of the placenta. The lack of significant changes on the fetal side is in keeping with the absence of any effect on UA Doppler indices herein, reflecting umbilical blood flow, and previous observations that Ads do not cross the ovine placenta (Mehta et al., 2011). Moreover, placental efficiency was also increased after Ad.VEGF treatment.
This study provides the first proof of principle of Ad.VEGF therapy to treat FGR, although the exact mechanism remains far from clear. The main aim of this work was to evaluate efficacy, and the long-term endpoint required (42 days after vector injection) unfortunately limited the extent of any thorough mechanistic assessment, as it is known that adenoviral expression is no longer detectable by 30 days postinjection. Nevertheless, the relative strengths of our study lie in the use of a well-characterized large-animal FGR model, which replicates the key features of human FGR secondary to uteroplacental insufficiency. However, no animal model can completely represent the human condition. The main limitation of this work lies in the differences between human (hemochorial) and ovine (syndesmochorial) placentation, which lacks the trophoblastic invasion of the spiral arteries that is considered essential for the maternal adaptation to human pregnancy and underlies FGR and pre-eclampsia. Furthermore, while this model features an early insult on uteroplacental development, the resultant FGR is relatively late-onset, equivalent to the third trimester of human pregnancy. Evaluation of Ad.VEGF therapy in early-onset FGR is needed. While there is clearly a long way to go with respect to potential clinical translation, we have already secured funding via the EU Framework Programme 7 to conduct full reproductive toxicology studies, a bioethical study and a phase I/IIa study in women with severe early-onset FGR, who will be recruited around 22–26 weeks of gestation. The UtA in such cases will be accessed via transfemoral catheterization under X-ray guidance (an established interventional radiological procedure) rather than via a laparotomy.
In summary, midgestation delivery of Ad.VEGF gene therapy in FGR pregnancy induced by overnourishment of adolescent sheep dams resulted in a significant increase in fetal growth velocity at 3 and 4 weeks posttreatment. Despite no significant differences in overall fetal weight, fewer fetuses exhibited marked FGR in late gestation after Ad.VEGF treatment, and there appeared to be an attenuated fetal brain sparing effect. Ad.VEGF-transduced UtA demonstrated enhanced vascular reactivity and there was also upregulation of VEGF receptors FLT1 and KDR in the maternal placental compartment, suggesting downstream effects on placental function. Our results support the further experimental evaluation of Ad.VEGF as a potential therapy for FGR.
Supplementary Material
Acknowledgments
We would like to thank Dr. Graham Horgan (Biomathematics and Statistics Scotland, Rowett Institute of Nutrition and Health) for statistical support. Funding for the study was received from the following sources:
• The Wellcome Trust (D.J.C.)
• Wellbeing of Women (D.J.C.)
• The Scottish Government (J.M.W., R.P.A., J.S.M.)
• UCL/UCLH NIHR Comprehensive Biomedical Research Centre (A.L.D., D.M.P.)
• The British Heart Foundation (I.C.Z.)
• Ark Therapeutics Oy, Kuopio, Finland (supplied Ad vectors free of charge)
Author Disclosure Statement
No competing financial interests exist.
References
- Barker D.J. (2006). Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 49, 270–283 [DOI] [PubMed] [Google Scholar]
- Bernstein I.M., et al. (2000). Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am. J. Obstet. Gynecol. 182, 198–206 [DOI] [PubMed] [Google Scholar]
- Carr D.J., et al. (2011). Ultrasonographic assessment of growth and estimation of birthweight in late gestation fetal sheep. Ultrasound Med. Biol. 37, 1588–1595 [DOI] [PubMed] [Google Scholar]
- Carr D.J., et al. (2012). Fetoplacental biometry and umbilical artery Doppler velocimetry in the overnourished adolescent model of fetal growth restriction. Am. J. Obstet. Gynecol. 207, 141.e6–141.e15 [DOI] [PubMed] [Google Scholar]
- Costeloe K., et al. (2000). The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 106, 659–671 [DOI] [PubMed] [Google Scholar]
- Cox P., and Marton T. (2009). Pathological assessment of intrauterine growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 23, 751–764 [DOI] [PubMed] [Google Scholar]
- David A.L., et al. (2008). Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene. Ther. 15, 1344–1350 [DOI] [PubMed] [Google Scholar]
- Degani S. (2001). Fetal biometry: clinical, pathological, and technical considerations. Obstet. Gynecol. Surv. 56, 159–167 [DOI] [PubMed] [Google Scholar]
- Figueras F., et al. (2011). Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet. Gynecol. 38, 288–294 [DOI] [PubMed] [Google Scholar]
- Figueroa R., and Maulik D. (2006). Prenatal therapy for fetal growth restriction. Clin. Obstet. Gynecol. 49, 308–319 [DOI] [PubMed] [Google Scholar]
- Ghidini A. (1996). Idiopathic fetal growth restriction: a pathophysiologic approach. Obstet. Gynecol. Surv. 51, 376–382 [DOI] [PubMed] [Google Scholar]
- GRIT Study Group. (2003). A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG 110, 27–32 [DOI] [PubMed] [Google Scholar]
- Halliday H.L. (2009). Neonatal management and long-term sequelae. Best Pract. Res. Clin. Obstet. Gynaecol. 23, 871–880 [DOI] [PubMed] [Google Scholar]
- Holmes D.I., and Zachary I. (2005). The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 6, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konje J.C., Howarth E.S., Kaufmann P., and Taylor D.J. (2003). Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG 110, 301–305 [PubMed] [Google Scholar]
- Lang U., et al. (2003). Uterine blood flow—a determinant of fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 110Suppl 1, S55–S61 [DOI] [PubMed] [Google Scholar]
- Lea R.G., et al. (2007). The expression of ovine placental lactogen, StAR and progesterone-associated steroidogenic enzymes in placentae of overnourished growing adolescent ewes. Reproduction 133, 785–796 [DOI] [PubMed] [Google Scholar]
- Mandruzzato G., et al. (2008). Intrauterine restriction (IUGR). J. Perinat. Med. 36, 277–281 [DOI] [PubMed] [Google Scholar]
- Marlow N., et al. (2005). Neurologic and developmental disability at six years of age after extremely preterm birth. N. Engl. J. Med. 352, 9–19 [DOI] [PubMed] [Google Scholar]
- Mehta V., et al. (2011). Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A(165) in the uterine arteries of pregnant sheep. Gene Ther. 19, 925–935 [DOI] [PubMed] [Google Scholar]
- Meschia G. (1983). Circulation to female reproductive organs. In Handbook of Physiology, Sect. 2, Vol. III, Part 1. Shepherd J.T. and Abboud F.M., eds. (American Physiological Society, Bethesda, MD: ) pp241–269 [Google Scholar]
- Miller S.L., Loose J.M., Jenkin G., and Wallace E.M. (2009). The effects of sildenafil citrate (Viagra) on uterine blood flow and well being in the intrauterine growth-restricted fetus. Am. J. Obstet. Gynecol. 200, 102.e1–102.e7 [DOI] [PubMed] [Google Scholar]
- Morse S.B., et al. (2006). Racial and gender differences in the viability of extremely low birth weight infants: a population-based study. Pediatrics 117, e106–e112 [DOI] [PubMed] [Google Scholar]
- Nahum G.G., and Stanislaw H. (2003). Ultrasonographic prediction of term birth weight: how accurate is it? Am. J. Obstet. Gynecol. 188, 566–574 [DOI] [PubMed] [Google Scholar]
- Peebles D.M. (2004). Fetal consequences of chronic substrate deprivation. Semin. Fetal. Neonatal. Med. 9, 379–386 [DOI] [PubMed] [Google Scholar]
- Redmer D.A., et al. (2005). Influence of maternal nutrition on messenger RNA expression of placental angiogenic factors and their receptors at midgestation in adolescent sheep. Biol. Reprod. 72, 1004–1009 [DOI] [PubMed] [Google Scholar]
- Redmer D.A., et al. (2009). Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction 137, 749–757 [DOI] [PubMed] [Google Scholar]
- Reynolds L.P., and Redmer D.A. (1995). Uteroplacental vascular development and placental function. J. Anim. Sci. 73, 1839–1851 [DOI] [PubMed] [Google Scholar]
- Reynolds L.P., Ferrell C.L., Robertson D.A., and Ford S.P. (1986). Metabolism of the gravid uterus, fetus and utero-placenta at several stages of gestation in cows. J. Agric. Sci. 106, 437–444 [Google Scholar]
- Robinson J.S., Kingston E.J., Jones C.T., and Thorburn G.D. (1979). Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J. Dev. Physiol. 1, 379–398 [PubMed] [Google Scholar]
- Roza S.J., et al. (2008). What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am. J. Epidemiol. 168, 1145–1152 [DOI] [PubMed] [Google Scholar]
- Scherjon S., Briet J., Oosting H., and Kok J. (2000). The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics 105, 385–391 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Da Silva P., Aitken R.P., and Cruickshank M.A. (1997). Maternal endocrine status in relation to pregnancy outcome in rapidly growing adolescent sheep. J. Endocrinol. 155, 359–368 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., et al. (2000). Relationship between nutritionally-mediated placental growth restriction and fetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta 21, 100–108 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., et al. (2002). Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1027–R1036 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Aitken R.P., Milne J.S., Hay W.W., Jr. (2004). Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus. Biol. Reprod. 71, 1055–1062 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., et al. (2006a). Nutritional modulation of adolescent pregnancy outcome—a review. Placenta 27Suppl A, S61–S68 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Milne J.S., Redmer D.A., and Aitken R.P. (2006b). Effect of diet composition on pregnancy outcome in overnourished rapidly growing adolescent sheep. Br. J. Nutr. 96, 1060–1068 [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Milne J.S., Matsuzaki M., and Aitken R.P. (2008). Serial measurement of uterine blood flow from mid to late gestation in growth restricted pregnancies induced by overnourishing adolescent sheep dams. Placenta 29, 718–724 [DOI] [PubMed] [Google Scholar]
- Zachary I., Mathur A., Yla-Herttuala S., and Martin J. (2000). Vascular protection: a novel nonangiogenic cardiovascular role for vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 20, 1512–1520 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.