Abstract
Adenoviruses are efficient gene delivery vectors based on their ability to transduce a wide variety of cell types and drive high-level transient transgene expression. While there have been advances in modifying human adenoviral (HAdV) vectors to increase their safety profile, there are still pitfalls that need to be further addressed. Preexisting humoral and cellular immunity against common HAdV serotypes limits the efficacy of gene transfer and duration of transgene expression. As an alternative, nonhuman AdV (NHAdV) vectors can circumvent neutralizing antibodies against HAdVs in immunized mice and monkeys and in human sera, suggesting that NHAdV vectors could circumvent preexisting humoral immunity against HAdVs in a clinical setting. Consequently, there has been an increased interest in developing NHAdV vectors for gene delivery in humans. In this review, we outline the recent advances and limitations of HAdV vectors for gene therapy and describe examples of NHAdV vectors focusing on their immunogenicity, tropism, and potential as effective gene therapy vehicles.
Introduction
Adenoviruses (AdVs) are nonenveloped viruses with an icosahedral capsid containing a double-stranded linear DNA genome of 26–46 kb (Davison et al., 2003). Since their discovery in 1950s in human cell cultures (Rowe et al., 1953), AdVs have been identified in many species, ranging from fish to humans. Today AdVs form five accepted genera (Mastadenovirus, Aviadenovirus, Siadenovirus, Atadenovirus, and Ichtadenovirus) within the family Adenoviridae. Recently, one more genus was proposed, named Testadenovirus (Doszpoly et al., 2013). So far 57 different AdV serotypes (Lukashev et al., 2008; Walsh et al., 2011) have been isolated from humans (HAdVs) and are classified within the genus Mastadenovirus as 7 species, HAdV A through HAdV G (Jones et al., 2007). While classification was previously based on biological characteristics (erythrocyte agglutination, oncogenicity, and by specific antisera neutralization), phylogenetic relationships have been revised and clarified on the basis of genotype similarity (Bailey and Mautner, 1994; Davison et al., 2003). Accordingly, individual AdV serotypes frequently share characteristics such as tropism and associated host pathologies with other serotypes within their species. The tropism is in large part determined by the capsid proteins that interact with the host cell surface receptors to mediate cell entry. The capsid consists primarily of hexon and penton proteins that form pentameric structures at each of the 12 vertices, securing a trimeric fiber that projects outward. Other viral proteins include the cement proteins (IIIa, VI, VIII, and IX) and the core proteins (TP, V, VII, and mu) that associate with the AdV genome (Vellinga et al., 2005). While the basic structure of these proteins is maintained, variations in the length and composition of the amino acid sequences of these proteins across species and genera cause them to bind to a number of receptors and host factors with variable affinity. Thus, the coxsackie and AdV receptor (CAR), CD46, desmoglein-2 (DSG2), CD80, CD86, vascular cell adhesion molecule-1 (VCAM-1), heparan sulfate proteoglycans (HSPGs), major histocompatibility complex class I-α2 (MHC-I-α2), sialic acid, dipalmitoylphosphatidylcholine, lactoferrin, and others can be utilized by one or more HAdV serotypes to transduce cells (Sharma et al., 2009b; Arnberg, 2012).
Viral vectors remain to be the most promising gene transfer vehicles for gene therapy because of their higher gene-transfer efficiencies when compared with other approaches. Currently, AdV vectors have been used in 23.5% of all clinical trials as they can efficiently transduce a wide variety of dividing and quiescent cells, they very rarely integrate into the host genome minimizing the risk of insertional mutagenesis (Stephen et al., 2010), they have a large packaging capacity up to 36 kb, and they can routinely be produced at high titers. Of the 57 HAdV serotypes, HAdV-5 of species C has been the best characterized and the most widely used in clinical trials so far, mainly in the treatment of cancer but also as a vaccine (Sullivan et al., 2003; Shiver and Emini, 2004).
It has, however, been estimated that over 80% of the adult population have been naturally exposed to the common HAdV serotypes (Garnett et al., 2002). As a result, preexisting humoral and cellular immunity may preclude efficient gene transfer (Kuriyama et al., 1998), and local systemic immune responses against the vector and transgene product may result in a number of clinically undesirable side effects. Severe immune responses against AdV vectors can have serious consequences in humans, which have been exemplified by a fatal case of systemic inflammatory response syndrome that was directly attributed to the systemically administered AdV gene transfer vector (Raper et al., 2003). Moreover, upon systemic administration of recombinant HAdV-5, transduction of immune cells in the liver and spleen leads to immune responses against the AdV vector that can limit its therapeutic efficacy. Eliminating liver tropism and the epitopes involved in viral proteins recognition by neutralizing antibodies (NAbs) has been proposed to reduce these immune responses. Taking advantage of the diversity of AdV capsid protein isoforms and their variety of ligand–receptor interactions, vectors based on low seroprevalent AdVs could potentially enable targeting to different cell types as well as overcoming preexisting immunity. Naturally, nonhuman AdV (NHAdV) species have a low seroprevalence in all human populations and therefore represent a source of potential vectors that could fit this niche. Since the beginning of their development in 1990s (Mittal et al., 1995), recombinant NHAdV vectors have been derived from many different AdV species: canine AdV-2 (CAdV-2), bovine AdV-3 (BAdV-3), porcine AdV-3 (PAdV-3), ovine AdV-7 (OAdV-7), murine AdV-1 (MAdV-1), several simian (SAdVs), and fowl (FAdVs) AdVs. Here we outline the current status of HAdV vectors, summarize the development of NHAdV vectors, and discuss the advantages and shortfalls of each.
Innate and Adaptive Immune Responses Against AdVs
Innate and adaptive immune responses play a critical role in resolving AdV infections. The presence of specific antiviral antibodies significantly increases antibody constant fraction receptor (FcR)-dependent viral internalization in macrophages, triggering an innate immune response to AdVs that leads to type I interferon (IFN) production and caspase-dependent interleukin (IL)-1β maturation (Zaiss et al., 2009). Internalized AdV DNA induces maturation of pro-IL-1β in macrophages dependent on NALP3 and ASC, components of the innate cytosolic molecular complex called the inflammasome, but independent of Toll-like receptors (TLRs) and IFN regulatory factors (Muruve et al., 2008). Later studies reported that this response requires the binding of the arginine-glycine-aspartic acid (RGD) motif in the AdV penton base with macrophage β3 integrins (Di Paolo et al., 2009). Barlan and colleagues also demonstrated that the specific AdV penetration mechanism of the endosomal membranes is required for IL-1β release (Barlan et al., 2011a,b). Macrophages and conventional dendritic cells (cDCs) can detect cytosolic AdV DNA in a TLR9-independent manner (Basner-Tschakarjan et al., 2006; Zhu et al., 2007), while plasmacytoid DCs (pDCs) detect nonmethylated AdV CpG-containing DNA in the endosomes through TLR9. Also, internalized HAdV-5 and HAdV-6 immune complexes have been reported to induce DC maturation via TLR9 agonist motifs present in the AdV genome, whereas HAdV-35, -36, and -26 have a limited potency to induce DC maturation because of having a smaller number of motifs (Perreau et al., 2012). TLR9 signaling pathway is dependent on myeloid differentiation primary response 88 (MyD88) (Yamaguchi et al., 2007; Zhu et al., 2007) and induces the secretion of IL-6 and IL-12 (Yamaguchi et al., 2007). Heparin-sensitive receptors on DCs bind to the AdV shaft fiber region and trigger T cell immune responses (Cheng et al., 2007). HAdV-5-induced natural killer (NK) cell activation relies on T cell contribution in vitro (Pahl et al., 2012), and also appears to be related to the presence of type I IFN in vivo (Zhu et al., 2007, 2008). In contrast to HAdV-5, in response to HAdV-35 pDCs alone are sufficient for NK cell activation in a TLR9-dependent manner, after which NK cells enhance pDC IFN-α secretion (Pahl et al., 2012). This indicates that both pDCs and T cells may be necessary for NK cells' activation. Therefore, it is clear that different AdV serotypes can trigger immune responses in different ways; thus, it is essential to study immune responses in detail to ensure that efficient strategies to circumvent immune responses are developed.
The complement system also plays a critical role in AdV immune responses with effects mediated through the classical and alternative complement pathway. The complement system appears to be related to humoral immune responses to the vector capsid or the encoded transgene (Appledorn et al., 2008). IgM isotype neutralization of virions activates the classical complement pathway hampering receptor–ligand interactions with the target cell (Xu et al., 2013). On the other side, AdV-induced thrombocytopenia has been demonstrated to be dependent on factor B and C3 of the alternative complement pathway, and AdVs mediate induction of the nuclear factor-kappaB (NF-κB) upon binding to C3 (Appledorn et al., 2008). In addition to macrophages, DCs, and NK cells, binding of the CAR protein and αv-integrins on nonimmune cells also activates immune responses (Liu et al., 2005; Schoggins et al., 2005). In particular, CAR-mediated responses have been reported in epithelial cells (Tamanini et al., 2006) and integrin-mediated responses in kidney epithelium-derived (REC) cells, inducing expression of CXCL10 (IP-10) through NF-κB in an RGD motif-dependent manner (Liu et al., 2005).
These anti-AdV innate immune responses are linked to adaptive immune responses through direct interactions and production of cytokines (Ginsberg, 1996; Appledorn et al., 2008). When a cell is infected by AdVs, it releases type I (α and β) IFN. Plasmacytoid DCs, cDCs, and macrophages produce IFN-α upon AdV recognition. The surrounding uninfected cells recognize IFN by IFN receptors and they induce, via the Jak-STAT signaling pathway, the expression of antiviral enzymes such as MxA, 2′,5′-oligoadenylate synthase, and protein kinase R (Katze et al., 2002). Also, type I IFN induces upregulation of costimulatory molecules in DCs such as CD80, CD86, and CD40, leading to DCs' maturation (Vujanovic et al., 2009). Moreover, IFN takes part in B cells' activation for antibody production. Type II IFN (IFN-γ) is an immunoregulatory cytokine secreted by Th1-type T CD4+ cells, T CD8+ cells, and NK cells. IFN-γ induces the expression of major histocompatibility complex class I (MHC-I) on nearly all cells and major histocompatibility complex class II (MHC-II) on professional antigen presenting cells (APCs) promoting antigen presentation to helper T CD4+ cells, and it also activates macrophages (Gattoni et al., 2006).
Dendritic cells have been shown to engulf infected cells and present antigens to naive T CD8+ cells through MHC-I (Albert et al., 1998). At the same time, APCs present antigens to Th1-type T CD4+ cells through MHC-II and upon activation they produce IL-2. If the T CD8+ cell receives both antigen presence and IL-2 signal, it gets activated (Jankovic et al., 2001). Once the T CD8+ cell interacts with an infected cell, mainly recognizing conserved epitopes in hexon protein (Olive et al., 2002; Leen et al., 2004; Tang et al., 2006), it begins a cytotoxic response leading to apoptosis of the infected cell (Schumacher et al., 2004). On the other hand, Th2-type T CD4+ cells induce B cell maturation and isotype switching (Jankovic et al., 2001).
NAbs, mostly IgG isotype, interact with AdV capsid proteins (Gall et al., 1996; Sumida et al., 2005). It was reported that NAbs recognize mainly the hypervariable regions (HVRs) of the hexon protein (Roberts et al., 2006; Abe et al., 2009; Shiratsuchi et al., 2010; Bradley et al., 2012b) and to a lesser extent the fiber knob domain (Myhre et al., 2007; Bradley et al., 2012a; Yu et al., 2013) and penton base proteins (Yu et al., 2013). AdV neutralization prevents cell attachment and facilitates aggregation and phagocytosis mediated by opsonization (Wohlfart, 1988). FcRs in macrophages and NK cells recognize NAbs bound to AdV antigens, facilitating their phagocytic and cytolytic action, respectively (Gattoni et al., 2006).
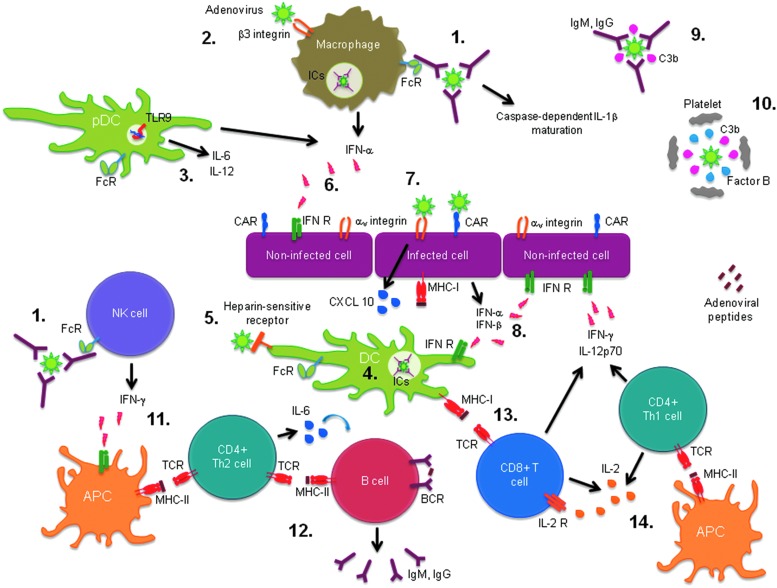
In summary, AdV gene transfer vectors are subjected to antiviral immune responses (Fig. 1) once administered to the patient. This process highly limits their efficiency for gene therapy applications. Therefore, it is critical to understand the immune response to AdVs and have strategies to circumvent antiviral immune responses. These will be discussed in the following sections, focusing on the main limiting factors: NAbs against AdV proteins and CD8+ cytotoxic T lymphocytes (CTLs) against infected cells.
FIG. 1.
Innate and adaptive antiadenoviral immune responses. (1) Activation of macrophages and natural killer (NK) cells by recognition of opsonized adenovirus (AdV) through antibody-constant fraction receptor (FcR). (2) Interleukin (IL)-1β maturation upon binding of arginine-glycine-aspartic acid (RGD) motif in the AdV penton base with macrophage β3 integrins. (3) Induction of IL-6 and IL-12 secretion in plasmacytoid dendritic cell (pDC) upon AdV DNA recognition through Toll-like receptor (TLR)-9. (4) Dendritic cell (DC) maturation through internalized immune complexes (ICs). (5) Triggering of T cell immune responses upon binding of AdV fiber shaft region to heparin-sensitive receptors on DCs. (6) Interferon (IFN)-α production by activated pDCs, conventional DCs (cDCs), and macrophage and IFN-α recognition by uninfected cells leading to a cellular antiviral state. (7) Induction of CXCL10 expression by AdV penton base RGD motif binding to αv integrins. (8) Secretion of IFN-α and β by infected cells. (9) Classical complement pathway activation by virion neutralization with IgM or recognition by IgG. (10) Thrombocytopenia related to alternative complement pathway activation on AdVs. (11) Major histocompatibility complex class II (MHC-II) induction on professional antigen presenting cells (APCs) by IFN-γ signal promoting antigen presentation to helper T CD4+ cells. (12) Induction of B cell maturation and isotype switching through AdV antigen presentation by Th2-type T CD4+ cells. (13) Activation of T CD8+ cells through AdV antigen presentation by DC via MHC-I. (14) IL-2 production by Th1-type T CD4+ cells upon AdV antigen presentation by APCs through MHC-II, which activates T CD8+ cells. BCR, B cell receptor; C3b, complement component 3b; CAR, coxsackie virus and adenovirus receptor; IFN R, interferon receptor; TCR, T cell receptor.
Implications of Antiviral Immune Responses Against HAdV Vectors and Strategies to Circumvent Them
Since the tragic death of a patient participating in a clinical gene therapy trial involving administration of a replication-incompetent HAdV-5 vector (Raper et al., 2003), many efforts have been made to avoid antiviral immune responses against AdV vectors to improve their safety and efficacy. Since the AdV vector recognition by the immune system depends on the existence of antigenic epitopes in the AdV proteins (i.e., NAbs recognize AdV proteins, and CD8+ CTLs recognize infected cells), most of the strategies to circumvent the immunogenicity of HAdV vectors are based on protein modifications.
Functionally significant HAdV-5-specific NAbs are directed primarily against the HAdV-5 hexon protein and to a lesser extent to fiber and penton base proteins (Gall et al., 1996; Sumida et al., 2005; Tian et al., 2011). Despite several groups successfully generated hexon-chimeric HAdV-5 vectors that partially evaded anti-HAdV-5 immune responses (Gall et al., 1998; Roy et al., 1998; Ostapchuk and Hearing, 2001; Wu et al., 2002; Roberts et al., 2006; Bruder et al., 2012), hexon pseudotyping appears to be a limited strategy as it has been reported the formation of many nonviable virions (Youil et al., 2002).
The hexon protein (Rux and Burnett, 2000) contains 7 HVRs (Crawford-Miksza and Schnurr, 1996). Many groups have exchanged HAdV-5 HVRs with those from low-prevalent serotypes and demonstrated that the resultant AdV vector can evade HAdV-5 immunity (Roberts et al., 2006; Abe et al., 2009; Shiratsuchi et al., 2010). Roberts et al. (2006) showed that HAdV-5 with all seven HVRs replaced with the corresponding ones from the rare serotype HAdV-48 could circumvent HAdV-5 NAbs in both mice and rhesus monkeys. Further studies (Bradley et al., 2012b) exchanged only some HVRs and demonstrated that HAdV-5-specific NAbs target multiple HVRs, suggesting that mutation or replacement of all seven HVRs would probably be necessary to evade anti-HAdV-5 immunity. However, in 2012, Coughlan et al. demonstrated that, despite HAdV-5 containing all seven HVRs from HAdV-48, it displayed a decreased hepatocyte transduction and accumulation in Kupffer cells, and it triggered a robust proinflammatory response not present with the wild-type HAdV-48 (Coughlan et al., 2012). In order to generate novel viable HVR-chimeric vectors able to evade immune responses, it would be necessary to use HVRs from other serotypes, and for that it is essential to determine the viability of HVR replacements in AdV vectors based on the structural and biochemical constraints of the different HVRs that limit their manipulation.
Regarding the immunogenicity of the fiber protein, little is known about the epitopes involved in fiber recognition by NAbs, although it is thought that antiknob NAbs represent the major population of antifiber NAbs (Myhre et al., 2007; Bradley et al., 2012a; Yu et al., 2013). It has been reported that fiber pseudotyping of AdV vectors can lead to reduced AdV-associated innate (Schoggins et al., 2005) and adaptive immune responses (Parker et al., 2009; Rogée et al., 2010). Despite the C-terminal and shaft region heterogeneity, the N-terminal region (fiber tail) that is bound to penton base protein presents high similarity among diverse AdV species (Tarassishin et al., 2000). Thus, designing chimeric AdV vectors containing all proteins from HAdV-5 but fibers from other serotypes (mainly species B and D) is a very interesting approach to retarget HAdV-5 to different tissues while circumventing antiviral preexisting immunity. Thus, several chimeric AdV vectors with tropism for different cell types such as HAdV-5/3 (Haviv et al., 2002; Kanerva et al., 2002; Volk et al., 2003; Ulasov et al., 2007), HAdV-5/11 (Havenga et al., 2001; Stone et al., 2005; Wang et al., 2011b), HAdV-5/16 (Havenga et al., 2001), and HAdV-5/35 (Havenga et al., 2001) were generated. Interestingly, HAdV-5/35 and -5/11 were assessed in vivo in murine and nonhuman primate animal models, showing reduced toxicity and limited induction of inflammatory cytokines (Ni et al., 2005). However, other studies showed that HAdV-5/35 presented tropism for CD34+ human hematopoietic stem cells in vitro (Shayakhmetov et al., 2000), monocytes, granulocytes, and blast cells of human bone marrow (Rogozhin et al., 2011), and CD4+ and CD8+ T lymphocytes in vitro (Zhang et al., 2013b). Since transduction of immune cells can lead to immune responses, extensive characterization of chimeric AdV vectors is essential.
Other retargeting strategies such as the truncation or removal of the fiber knob domain and its replacement with foreign trimerization motifs and heterologous peptides fused to the fiber shaft are also recently being assessed (fiber de-knobbing) (Coughlan et al., 2010). Despite that the removal of the fiber knob domain results in a lower number of fiber copies per virion, leading to a less efficient production of the AdV vector, it has been shown that this approach may contribute to evade NAbs (Myhre et al., 2007).
Regarding the adaptive immunity mediated by CD8+ CTLs, the response depends on viral antigenic epitopes loaded in the MHC of the infected cell. High-capacity, helper-dependent (HD) vectors (also called gutless vectors) bypass this problem (Kochanek et al., 1996) as they are devoid of all viral genes, and therefore they are less immunogenic and allow long-term transgene expression (Maione et al., 2001; Ehrhardt and Kay, 2002; Dudley et al., 2004). Furthermore, as in these vectors DNA is constituted of stuffer DNA as part of the vector backbone to maintain optimum vector size, nonmethylated viral CpG-containing DNA (recognized by DCs through TLR9) can be eliminated. However, one study showed that HD AdVs also trigger an innate immune response dependent on TLR9 (Cerullo et al., 2007). Despite the advantages they offer, HD vectors are equally neutralized by preexisting antibodies against capsid proteins and also may present transgene-encoded protein immunogenicity (Tripathy et al., 1996).
On the other hand, the activation of immune responses because of interactions between AdV capsid proteins and receptors in the target cell can limit AdV vector efficacy in gene therapy applications. HAdV-5 binds to the CAR protein on human erythrocytes in the bloodstream, limiting its delivery to the in vivo target tissue and contributing to toxicity (Seiradake et al., 2009). To avoid this interaction, Nicol et al. (2004) described two CAR-binding mutations that abolished the previously described agglutination in human and rat erythrocytes. Moreover, HAdV-5 binding to CAR activates immune responses that can limit its potential as a gene transfer vector (Schoggins et al., 2005; Tamanini et al., 2006). Other interactions such as RGD with macrophage β3 integrins (Cheng et al., 2007; Di Paolo et al., 2009) or the one between the shaft fiber region and the heparin-sensitive receptor from DCs (Cheng et al., 2007) have also been associated with immune responses and, in the case of RGD motif, through IL-1α production (Di Paolo et al., 2009). Interestingly, the mutation of the RGD motif to arginine-glycine-glutamic acid (RGE) resulted in a significant fivefold reduction in spleen uptake, diminishing the antiviral inflammatory response after intravascular administration (Bradshaw et al., 2012). These studies indicate that de-targeting from native receptors could be a useful approach to avoid immune responses against the AdV vector. Nevertheless, further research needs to be done to describe the pathways through which the AdV vectors trigger immune responses. There are more factors involved in cellular recognition that could also be implicated in promotion of immune responses. These include alternative receptors and coreceptors such as FcR (Xu et al., 2008), complement-3 receptor (CR-3) (Xu et al., 2008), HSPGs and low-density lipoprotein receptor-related protein (LRP) (Shayakhmetov et al., 2005), complement receptor-1 (CR1) (Carlisle et al., 2009), scavenging receptor-A (SR-A) (Khare et al., 2012), bridging molecules such as the coagulation factor IX, X, and VII and protein C (Parker et al., 2006), complement component C4-binding protein (C4BP) (Shayakhmetov et al., 2005), and IgM antibodies (Xu et al., 2008). Further studies will have to be done in order to describe their influence on the use of HAdV vectors for gene therapy applications.
Apart from genetic engineering of HAdV vectors, other strategies such as chemical modifications by addition of cationic polymers or lipid molecules to shield AdV vectors and prevent them from binding undesired proteins have been widely studied. Several variants of polyethylene glycol (PEG) have been used, but better shielding was found with multivalent copolymers of poly[N-(2-hydroxypropyl)methacrylamide] (pHPMA). Shielding of HAdV vectors with pHPMA showed an increased biological stability of the vector and a longer persistence in blood, decreased liver transduction, and therefore a lower immune response (Green et al., 2004). Furthermore, coated HAdV vectors have been shown to be less vulnerable to recognition by preexisting NAbs in vitro (Romanczuk et al., 1999; Wang et al., 2011a) and by Kupffer cells in the liver (Mok et al., 2005), likely because of the hampered interaction of PEGylated AdVs and Kupffer cell SR-A (Haisma et al., 2009). However, other factors could be affecting liver transduction of the coated HAdV such as the limited size of the sinusoidal endothelial cells' fenestrae (Hofherr et al., 2008). The use of PEGylated HAdV vectors resulted in decreased levels of IL-6 production in vitro (Mok et al., 2005) and of IL-6 and IL-12 in vivo (Croyle et al., 2000). Moreover, coated HAdV vectors induce lower IL-6 levels in serum because of decreased levels of spleen AdV uptake (De Geest et al., 2005). Furthermore, several groups successfully retargeted coated AdV vectors (Parker et al., 2005; Stevenson et al., 2007; Morrison et al., 2008, 2009; Wang et al., 2010). Although in some studies they presented similar levels of antitumoral efficacy (Morrison et al., 2009) or transgene expression (Parker et al., 2005; Stevenson et al., 2007; Morrison et al., 2008; Wang et al., 2010) to unmodified wild-type HAdV-5, others achieved a greater therapeutic effect with negligible side effects (Eto et al., 2010; Yao et al., 2010). Despite the limited retargeting efficiency in comparison with unmodified HAdV-5, the use of PEGylated AdV vectors achieved lower hepatic tropism and reduction in liver and spleen toxicity in vivo. Moreover, evidence highlights the potential of AdV polymer coating strategies to circumvent preexisting immunity to all viral capsid proteins (Romanczuk et al., 1999; Croyle et al., 2000; De Geest et al., 2005; Mok et al., 2005; Haisma et al., 2009; Zeng et al., 2012). Nevertheless, the reported PEG immunogenicity could hamper PEGylated AdVs efficacy (Shimizu et al., 2012).
Despite the advances made in HAdV vectors to evade anti-AdV immune responses, the current strategies are limited by the formation of nonviable virions following molecular engineering and by the limited knowledge and characterization of capsid epitopes motifs responsible for cross-reactivity. Therefore, the use of low-seroprevalent HAdV serotypes has been proposed as an alternative to hexon or fiber-chimeric AdV vectors in order to circumvent preexisting NAbs. More than 15 years ago, several studies showed that the alternate use of AdV vectors from different serotypes, even within the same AdV species, can circumvent anti-AdV humoral immunity (Kass-Eisler et al., 1996; Mastrangeli et al., 1996; Mack et al., 1997; Parks et al., 1999). A study on the presence of NAbs against different HAdV serotypes in the Belgian population reported high seroprevalence toward species A, C, and E, while low seroprevalence toward species B and D, among which, serotypes 11, 34, 35, 50 (species B), 43, and 48 (species D) showed the lowest values (Vogels et al., 2003). This indicates that these serotypes could be potentially used to evade preexisting immunity. Vectors have been developed from multiple HAdV serotypes with lower seroprevalence than HAdV-5: HAdV-11 (Holterman et al., 2004; Stone et al., 2005; Abbink et al., 2007), HAdV-35 (Gao et al., 2003; Sakurai et al., 2003, 2009; Seshidhar Reddy et al., 2003; Vogels et al., 2003; Barouch et al., 2004; Wu and Tikoo, 2004; Abbink et al., 2007; Brouwer et al., 2007; Sakurai, 2008; McVey et al., 2010; Geisbert et al., 2011), HAdV-49 (Lemckert et al., 2006; Abbink et al., 2007), HAdV-26, -48, and -50 (Abbink et al., 2007; Geisbert et al., 2011), HAdV-7 (Nan et al., 2003), and HAdV-6 (Capone et al., 2006). However, since the presence of NAbs depends on AdV exposure, which is closely related to geographical location, seroprevalence studies in different specific populations are very important. Moreover, better characterization of such serotypes in terms of tropism and specificity, biodistribution, and toxicity is yet required to design safe HAdV vectors.
Use of Nonhuman Adenoviral Vectors
The idea about NHAdVs as vectors appeared in 1990s, with the assumption that they would be more useful for vaccines and gene therapeutic approaches in human medicine than HAdV-based vectors. Design and characterization of NHAdV vectors was reviewed earlier (Bangari and Mittal, 2006), with a focus on vaccine vectors. In this review we focus on the progress in the development of NHAdV vectors (Table 1) for gene therapy.
Table 1.
Most Popular Nonhuman Adenovirus Used as a Vector
| Species | Genus | Serotype |
|---|---|---|
| Canine adenovirus A | Mastadenovirus | CAdV-2 |
| Bovine adenovirus B | Mastadenovirus | BAdV-3 |
| Porcine adenovirus A | Mastadenovirus | PAdV-3 |
| Human adenovirus E | Mastadenovirus | ChAdV-68/Pan 9/SAdV-25 |
| ChAdV-5/Pan 5/SAdV-22 | ||
| ChAdV-6/Pan 6/SAdV-23 | ||
| ChAdV-7/Pan 7/SAdV-24 | ||
| Human adenovirus G | Mastadenovirus | SAdV-7 |
| Murine adenovirus A | Mastadenovirus | MAdV-1 |
| Fowl adenovirus A | Aviadenovirus | FAdV-1 |
| Fowl adenovirus C | Aviadenovirus | FAdV-10 |
| Fowl adenovirus D | Aviadenovirus | FAdV-9 |
| Fowl adenovirus E | Aviadenovirus | FAdV-8 |
| Ovine adenovirus D | Atadenovirus | OAdV-7 |
Different species of nonhuman adenovirus are listed, specifying the genus to which they belong and the serotypes from which the vectors have been constructed.
Canine AdV vectors
Canine AdV (genus Mastadenovirus, species CAdV A)-derived vectors are currently the best described NHAdV vectors. Up to 90% of the residues involved in CAdV-2 fiber tail-penton base interactions are conserved compared with HAdV-5 (Schoehn et al., 2008). CAdV-2 binds to CAR (Tomko et al., 1997; Soudais et al., 2000), but it does not contain an integrin-interacting RGD motif in the penton base (Chillon and Kremer, 2001) in contrast with HAdV-5 (Greber, 2002). CAdV-2 penton base appears to be ∼20% shorter than that of HAdV-5 (Schoehn et al., 2008). In addition, CAdV-2 has shorter protuberances on the top of the penton base, mainly where RGD motif is contained in HAdVs, and a shorter N-terminal region. The hypervariable loops in the hexon protein are shorter in CAdV-2 and the protruding structures containing the epitopes recognized by antihexon HAdV-5 antibodies are absent, while the fiber is more complex since the shaft region contains two bends (Schoehn et al., 2008). Also, protein IX and IIIa are shorter in CAdV-2 (Schoehn et al., 2008). Production of CAdV-2 vectors started more than 15 years ago (Klonjkowski et al., 1997) in an attempt to avoid the inhibitory effect of the preexisting humoral and cellular immunity against HAdVs when used in the clinics. Later, it was shown that CAdV-2 vectors rarely cause neither the activation and proliferation of T cells nor the maturation of DCs (Perreau and Kremer, 2006). The advantage of these vectors over HAdV vectors is that CAdV-2 mainly interacts with CAR (Soudais et al., 2000) and they present specific tropism for neurons in the central nervous system (Soudais et al., 2001) with great capacity for axonal transport in vivo. Taking into account that CAdV-2 vector development was recently reviewed (Bru et al., 2010), the following data will focus on the more recent trials performed with the mentioned vector. Until very recently, the production of CAdV-2 vectors was based on dog kidney (DK) cell lines (Kremer et al., 2000), which is not accepted by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA). To circumvent this problem, CAdV-2 E1-transcomplementing cell lines, based on Madin-Darby canine kidney (MDCK) cells accepted by the FDA and EMA for the production of vaccines, have been developed (Fernandes et al., 2013a). CAdV-2 vector DNA replication in the MDCK-E1 cell line was shown to correlate with the expression levels of the transcomplementing E1A and E1B. While Cre recombinase expression in MDCK-E1-Cre cell lines (for HD CAdV-2 vectors production) can impair cell growth, controlled expression of Cre was determined not to result in negative effects on viral production (Fernandes et al., 2013b). CAdV-2-based vectors are ideally suited for treating neurological disorders, but because of vector titer limitations, large volumes are necessary for applicability in the clinics. Therefore, better purification and scalable production according to good manufacturing practice (GMP) are being currently developed (Segura et al., 2012; Fernandes et al., 2013a), and the improvement of producer cell lines has increased vector titers (Ibanes and Kremer, 2013). In a comparative study on HD HAdV, HD CAdV-2, and a VSV-G lentiviral vector, the transduction efficiency and transgene expression in human midbrain neuroprogenitor cells was assessed. HD CAdV-2 was determined to have the most promising vector profile: equal transduction efficacy, no negative effect to neuronal development, and milder induced immune response (Piersanti et al., 2013). HD CAdV-2 was also used as a vector in order to evaluate a gene therapy approach to correct the neurodegenerative lysosomal storage disorder mucopolysaccharidosis type IIIa (Lau et al., 2012). It was shown to effectively transduce neurons and provide long-term transgene expression; however, its use in vivo was limited to discrete areas of the brain, indicating a need to find a way to increase transgene expression throughout the whole brain. Furthermore, in a clinically relevant stroke model in rats, cortical CAdV-2 vector injection transduced neurons at a greater level and covered a larger region than lentiviral vectors (Ord et al., 2013), reducing infarct volume and improving neurologic recovery. For all the potential of CAdV-2 vectors, there ultimately remains the need for further understanding of the effects of CAdV-2 vectors on human cells before they are able to enter human clinical trials.
Bovine AdV vectors
Bovine AdVs (genus Mastadenovirus, species BAdV A, B, C; genus Atadenovirus, species BAdV D) were the first NHAdVs used for vectoring purposes. Mittal and colleagues substituted the nonessential E3 region of BAdV-3 (BAdV B species) with the firefly luciferase reporter gene, and luciferase expression lasted at least 6 days postinfection in human embryonic kidney (HEK)-293 cells (Mittal et al., 1995). BAdV-3 might be a better option to target a specific tissue or organ, since its cell entry is independent of CAR (Bangari et al., 2005b) and other primary receptors of HAdV-5 (Bangari et al., 2005a). BAdV-3 uses sialic acid molecules as a primary receptor to enter into host cells (Li et al., 2009) and it can efficiently transduce different cell types of various species. However, it shows limitations in transducing human cell lines (Rasmussen et al., 1999; Wu and Tikoo, 2004; Bangari et al., 2005b). To circumvent this problem, capsid proteins (fiber and pIX) were genetically modified to increase the ability of BAdV-3 to transduce nonbovine cells (Zakhartchouk et al., 2007). Studies on BAdV-3 vectors in vivo biodistribution in a mouse model showed efficient transduction of the heart, kidney, and lung in addition to liver and spleen with a longer duration and higher levels of transgene expression than HAdV-5 vectors (Sharma et al., 2009a). In a mouse model of breast cancer, the biodistribution of BAdV-3 vector was comparable to that of HAdV-5 vectors, the vector had the ability to efficiently transduce tumor and systemic tissues in the presence of HAdV-5 immunity, and it evaded sequestration by Kupffer cells (Tandon et al., 2012). Also, it has been shown that there is minimal cross-neutralization by preexisting anti-HAdV immunity in humans (Bangari et al., 2005b). These results show the suitability of BAdV-3 vectors as a delivery system for cancer gene therapy while circumventing preexisting antivector immunity. Moreover, BAdV-3 and HAdV-5 vectors have a comparable biological behavior in human, bovine, porcine, and mouse cell lines and both genomes persist at high levels in vector-permissive cell lines in the absence of immune pressure, proving BAdV-3 vectors' safety for their use as gene delivery vehicles (Sharma et al., 2011).
Porcine AdV vectors
The idea of using PAdV (genus Mastadenovirus, species Porcine A, B, C) vectors was published over 20 years ago (Tuboly et al., 1993). A few years later it was discovered that the virus can enter but is unable to replicate in dog, sheep, bovine, and human cells (Reddy et al., 1999a). Soon after, PAdV-3 (PAdV A species) vectors were constructed with the long-term objective to develop replication-competent PAdV-3 as a live gene transfer vector to induce a mucosal immune response in pigs (Reddy et al., 1999b). Recombinant PAdV-3 is an effective delivery system in pigs (Hammond and Johnson, 2005), and it is often studied together with BAdV-3 vectors, showing similar properties: lack of cross-neutralization with preexisting anti-HAdV immunity in humans (Bangari and Mittal, 2004; Bangari et al., 2005b), CAR- and integrin-independent cell entry (Bangari and Mittal, 2005; Bangari et al., 2005b), similar safety profile and biological characteristics (Sharma et al., 2011), efficient transduction of several cell types of various species with PAdV-3 having an advantage in specific targeting of the breast tissue (Bangari et al., 2005b), and the vector genomes remain as linear episomes (Sharma et al., 2009a). In contrast with BAdV-3 vectors, the genome levels of PAdV-3 vector were shown to be comparable or lower than those of HAdV-5 vectors in vivo in a mouse model, except in the case of the heart, where the levels were comparable or higher (Sharma et al., 2009a). The specificity of PAdV vectors for distinct tissues is very interesting for the design of vectors for targeted gene delivery.
Simian AdV vectors
Simian AdVs are grouped into several human (certain HAdV species contain simian or ape AdVs, based on species-demarcation criteria such as phylogenetic distance and nucleotide composition) and simian AdV species within the genus Mastadenovirus. The first gene transfer vector derived from chimpanzee AdV-68 (ChAdV-68/Pan 9/SAdV-25; species HAdV E) was able to grow in HEK293-complementing cells without being neutralized by antibodies against HAdV serotypes (Farina et al., 2001). Since neutralizing seroprevalence to ChAdV-68 in different regions of the world is significantly lower than that to HAdV-5 (Xiang et al., 2006; Ersching et al., 2010; Zhang et al., 2013a), that makes it a good candidate for application in humans. Interestingly, a single surface loop defines a major neutralization site for the ChAdV-68 hexon (Pichla-Gollon et al., 2007), which when mutated, permitted the virus to escape from neutralization by polyclonal antisera obtained from animals in vitro. This was actually the first neutralizing site identified for any AdV, which was later very important for the successful application of the vectors in the clinics. Gene delivery by ChAdV-68 is CAR dependent (Cohen et al., 2002), and unlike the E1 of HAdV-5, the flanking sequences of ChAdV-68 are nonhomologous with cell-derived E1, preventing the formation of replication-competent viruses (Xiang et al., 2002). Development of vectors derived from chimpanzee AdVs Pan 5 (ChAdV-5/SAdV-22), Pan 6 (ChAdV-6/SAdV-23), and Pan 7 (ChAdV-7/SAdV-24), members of HAdV E species, started in 2004 (Roy et al., 2004). The vectors transduced skeletal muscle with the same efficiency as HAdV-5 vectors, but without being neutralized by human sera, and the neutralization domains showed to be different in Pan 6 and Pan 7 both in vitro and in vivo.
One study investigated the importance of neutralizing determinants on capsid proteins by using chimeric vectors with different combinations of Pan 7 and Pan 6 penton base, and hexon and fiber proteins (Roy et al., 2005), reporting that both hexon and fiber have neutralization epitopes and that in vivo transduction was more affected by antihexon antibodies, with no effect of antipenton base NAbs. Other studies on seroprevalence rates of various ChAdVs were carried out, with Pan 6 and Pan 7 seeming promising as gene therapy vectors although depending on the region where the potential patients live (Jian et al., 2013). A tropism-modified derivative of Pan 7 showed several advantages over HAdV-5 for the use in gene therapy: lower degree of inactivation, diminished liver transduction caused by poor stability of FX-Pan 7 complexes, greater gene delivery efficiency than the wild-type control Pan 7 vector in vitro, and higher transduction in vivo of the Her2 (human epidermal growth factor receptor type 2)-expressing human tumor cells injected intravenously in mice but not statistically significant compared with the control (Belousova et al., 2010). The last finding indicates that there is a need to improve the vector to achieve the desired levels of target-specific transduction. Pan 6 vector demonstrated a better capacity than HAdV-5 vector in transduction of brain tumor cells and glioma cells, indicating that it is a promising vector candidate for the brain tumor therapy (Skog et al., 2007). Twenty E1-deleted vectors were generated from different AdVs isolated from chimpanzees, bonobos, and gorillas, of which 12 could be propagated, rescued, and expanded in HEK293 cells (Roy et al., 2011b). SAdV-7 (HAdV G species) was proposed for vectoring a few years earlier (Purkayastha et al., 2005b), and was subsequently constructed as an E1-deleted vector that could be complemented by HAdV-5 E1 genes in HEK293 cells (Roy et al., 2011a). The latter observation was surprising since SAdVs from HAdV G species are phylogenetically quite distant from HAdV C members.
Recently, it was shown that humans have low seroreactivity against simian-derived (SAdV-11, -16) or chimpanzee-derived (ChAdV-3, -63) AdV vectors compared with HAdV vectors (rHAdV-5, -28, -35) across multiple geographic regions (Quinn et al., 2013). Chimpanzees and macaques elicited a systemic humoral but not systemic cellular immune response to endogenous AdVs. The structure of the E3 locus, involved in modulating the host's response to infection, is smaller in HAdVs than in ape AdVs, which may impact the ability of the ape AdVs to evade host immune detection and elimination (Calcedo et al., 2009). ChAdV-68 showed the ability to transduce immature and mature human DCs, followed by secretion of IFN-α and IL-6 but not IL-12 or tumor necrosis factor (TNF)-α, and transduced immature DCs could stimulate proliferation of autologous T lymphocytes (Varnavski et al., 2003). Therefore, ChAdV-68 could be used as a vector for transduction of human DCs.
Fowl AdV vectors
Construction of FAdV (genus Aviadenovirus, species FAdV A to FAdV E) vectors started with the demonstration that recombinant FAdV-10 (FAdV C species) could be used to express an antigen to induce a protective immune response in pathogen-free chickens (Sheppard et al., 1998). After the identification of FAdV-9 (species FAdV D) regions that are nonessential for the virus (Ojkic and Nagy, 2001, 2003; Corredor and Nagy, 2010a), FAdV-9 recombinants demonstrated wild-type growth kinetics, virus titers, cytopathic effect and plaque morphology (Corredor and Nagy, 2010a), expression in avian and mammalian cells, and lack of replication in mammalian cells, indicating their applicability as gene delivery vehicles for mammalian systems (Corredor and Nagy, 2010b). FAdV-8 (species FAdV E) vectors were also constructed and showed efficacious in vivo delivery of antibody fragments against pathogenic infectious bursal disease virus (Greenall et al., 2010). FAdV-1 (species FAdV A) or CELO (Chicken Embryo Lethal Orphan) vectors have been widely used. The genomic regions that could be deleted were evaluated more than 10 years ago and a cosmid-based strategy was developed for generating CELO vectors (Michou et al., 1999; François et al., 2001). FAdV-1 has several interesting properties: it interacts with CAR and its capsid architecture allows changes in its tropism (Tan et al., 2001), it transduces mammalian cells as efficiently as HAdV-5 vector, and it has a potential for mammalian gene transfer applications, especially because it has larger DNA packaging capacity and greater physical stability (Michou et al., 1999). It was shown that FAdV-1 is able to deliver p53 transgene into various tissues in vivo restoring p53 function in human tumor cell xenografts in mice and leading therefore to the inhibition of tumor growth (Logunov et al., 2004). Moreover, FAdV-1 encoding the human IL-2 gene can successfully produce biologically active recombinant IL-2 in vitro, in ovo, and in vivo, and it is capable of increasing the median survival time of mice carrying melanoma tumors (Shmarov et al., 2002; Cherenova et al., 2004). Also, recombinant FAdV-1 encoding the HSV-1 thymidine kinase showed cytotoxicity in carcinoma cell lines and suppressed tumor growth and increase median of survival of mice with melanoma tumors (Shashkova et al., 2005), demonstrating its efficacy for the gene delivery to tumor cells in vitro and in vivo. Furthermore, one study showed that FAdV-1 successfully enhanced expression levels of secreted alkaline phosphatase reporter gene in transduced mammalian cells in vitro and in vivo (Tutykhina et al., 2008). Finally, PEGylated and retargeted with fibroblast growth factor, FAdV-1 showed increased levels of binding and internalization in a variety of human cell lines (Stevenson et al., 2006), with transgene expression being greater than the unmodified version in PC-3 human prostate cells, and being fully resistant to inhibition by human serum in vitro (Stevenson et al., 2006). All together indicates that retargeting of CELO virus to human cells via disease-specific receptors is possible, while avoiding preexisting humoral immunity.
Ovine AdV vectors
The production of OAdV (genus Mastadenovirus, species OAdV A, B; genus Atadenovirus, species OAdV D) vectors started more than 15 years ago (Xu et al., 1997) after determining the nonessential regions within the genome (Vrati et al., 1996). Surprisingly, much larger insertions than expected are tolerated, and portions of sequences can be deleted without affecting virus viability (Xu et al., 1997). The primary receptor for OAdV-7 (OAdV D species) is not CAR, as it was shown that OAdV-7 does not compete with HAdV-5 for the entry into cells (Xu and Both, 1998; Voeks et al., 2002). Recombinant OAdVs can infect a variety of nonovine cells such as rabbit, human, and murine cells but cannot replicate within them (Khatri et al., 1997; Hofmann et al., 1999; Löser et al., 2000; Xu et al., 2000; Kümin et al., 2002; Lockett and Both, 2002). They are not neutralized by polyclonal serum against HAdV-5 (Xu and Both, 1998) and they can overcome preexisting humoral immunity against HAdVs in vivo (Hofmann et al., 1999). A cosmid-based system proved useful for efficient generation of recombinant OAdVs and vectors could be rescued in an ovine fetal skin fibroblastic producer cell line (Löser et al., 2003). OAdV-7 carrying purine nucleoside phosphorylase (PNP), which converts the prodrug fludarabine into its activated form 2-fluoroadenine, was effective in vivo against prostate cancer progression upon prodrug addition (Voeks et al., 2002; Martiniello-Wilks et al., 2004). Moreover, recombinant OAdV-7 expressing ovalbumin showed efficacy in inducing antitumor response in a mouse model (Tang et al., 2012). OAdVs' biosafety profile and their application as gene delivery vectors were reviewed in the last decade (Both, 2004).
Murine AdV vectors
The production of MAdV (genus Mastadenovirus, species Murine A, B, C) mutants started with the MAdV-1 (MAdV A species) E3-deleted recombinant (Beard and Spindler, 1996; Cauthen et al., 1999) and E1-deleted recombinant (Ying et al., 1998). MAdV-1 presents mouse endothelial cell tropism (Charles et al., 1998; Kajon et al., 1998; Lenaerts et al., 2005), but it can also infect human endothelial cells (Nguyen et al., 1999) and it displays higher affinity for primary human smooth muscle cells than recombinant HAdV-5 (Lenaerts et al., 2009). MAdV-1 lacks the RGD motif in the penton base, but it contains it in the fiber knob domain (Raman et al., 2009). The RGD motif was reported to play a role in MAdV-1 infection through αv integrins, which act as a receptor for the virus, and it was shown that cell surface heparan sulfate glycosaminoglycans are involved in MAdV-1 infection (Raman et al., 2009). Conversely, the primary attachment of MAdV-1 is independent of CAR (Lenaerts et al., 2006). Distribution of MAdV-1 to the liver is markedly lower in intravenously injected immunodeficient mice than that observed with recombinant HAdV-5 (Lenaerts et al., 2009). Moreover, MAdV-1 has been used as an oncolytic vector, proving that it is a suitable murine homolog model to test the mechanism of action of murine oncolytic AdV vectors in an immunocompetent, tumor-bearing host (Robinson et al., 2009). Furthermore, MAdV-1 has preference for ovarian carcinoma cell lines, probably because of their increased expression of the enzyme involved in HSPG biosynthesis, therefore having the potential to be used in the oncolytic treatment of ovarian cancer (Lenaerts et al., 2012).
Concluding Remarks and Future Perspectives
The main limitation of clinically applied HAdV-based vectors today is their failure in achieving high levels of efficient transduction in their target tissues. This is primarily because of the preexisting NAbs leading to the rapid clearance of circulating vector and cellular immune responses resulting in the elimination of transduced cells, greatly limiting the expression of the transgene. These immunological responses most likely stem from the high frequency of HAdV exposure in multiple human populations across the globe, which results in immunological memory against HAdV serotypes that most of today's vectors are based on. In order to circumvent this, several strategies have been developed for HAdVs, including molecular engineering of chimeric vectors or the use of low-seroprevalent HAdV vectors.
As an alternative, NHAdV vectors have been developed and are being tested in various nonhuman and human cell lines. Most of the NHAdV vectors have two important features that make them more appropriate than HAdV vectors: there is no or very low pathogenicity for their natural host, and there is no presence of preexisting NAbs, CD4+ T cells, and CD8+ CTLs against them. However, it should be noted that caution must be applied when using nonhuman primate AdVs, since their ability to infect human cells and their structural similarity could potentially result in anti-HAdV T-cell and/or NAb cross-reactivity with nonhuman primate AdV antigens. Different NHAdVs have specific tropism for different cell types, which makes them applicable in treating different diseases especially when the targeting of specific cells is required, and therefore they could extend the range of potential target tissues. Furthermore, the inability of NHAdVs to co-replicate with wt HAdVs, as well as the fact that they are replication defective in human cells, makes their vectors much safer than HAdV vectors. Many of the NHAdVs are able to tolerate longer sequences of exogenous DNA than HAdVs. For some of the NHAdVs, there are low-cost propagation systems available, for example, chicken embryos for CELO virus (Laver et al., 1971; Michou et al., 1999). Despite these advantages, there are still many steps necessary before using the NHAdV vectors in the clinics. The first challenge is to find the appropriate cell lines for efficient production. There are also safety issues compared with HAdVs such as the possible occurrance of interspecies adaptation. For example, HAdV-4 is the only HAdV member of HAdV E species that is very similar to SAdVs from that group, indicating its zoonotic origin and molecular adaptation to its new host (Purkayastha et al., 2005a; Dehghan et al., 2013). As AdVs have coevolved with their hosts (Benkö and Harrach, 2003; Davison et al., 2003), there is a possibility that humans and monkeys could be infected with the same AdVs, a concern when considering chimpanzee or simian AdV vectors for use in humans. Nevertheless, NHAdV vectors present great potential as effective gene therapy vehicles and will hopefully be part of the successful AdV vectors used in the clinics in the coming years.
Acknowledgments
We would like to thank all members of the “ADVance” international training network (Adenoviruses as Clinical Treatments; FP7 ITN, EU grant agreement ref. 290002) and in particular Andrew Baker and Eric J. Kremer for their advice and encouragement to write this review.
Author Disclosure Statement
No competing financial interests exist.
References
- Abbink P., Lemckert A.A., Ewald B.A., et al. (2007). Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81, 4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe S., Okuda K., Ura T., et al. (2009). Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5. J. Gene Med. 11, 570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., and Bhardwaj N. (1998). Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 [DOI] [PubMed] [Google Scholar]
- Appledorn D.M., McBride A., Seregin S., et al. (2008). Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 15, 1606–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N. (2012). Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol. Sci. 33, 442–448 [DOI] [PubMed] [Google Scholar]
- Bailey A., and Mautner V. (1994). Phylogenetic relationships among adenovirus serotypes. Virology 205, 438–452 [DOI] [PubMed] [Google Scholar]
- Bangari D.S., and Mittal S.K. (2004). Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 105, 127–136 [DOI] [PubMed] [Google Scholar]
- Bangari D.S., and Mittal S.K. (2005). Porcine adenovirus serotype 3 internalization is independent of CAR and alphavbeta3 or alphavbeta5 integrin. Virology 332, 157–166 [DOI] [PubMed] [Google Scholar]
- Bangari D.S., and Mittal S.K. (2006). Development of nonhuman adenoviruses as vaccine vectors. Vaccine 24, 849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari D.S., Sharma A., and Mittal S.K. (2005a). Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem. Biophys. Res. Commun. 331, 1478–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari D.S., Shukla S., and Mittal S.K. (2005b). Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem. Biophys. Res. Commun. 327, 960–966 [DOI] [PubMed] [Google Scholar]
- Barlan A.U., Danthi P., and Wiethoff C.M. (2011a). Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology 412, 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlan A.U., Griffin T.M., McGuire K.A., and Wiethoff C.M. (2011b). Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D.H., Pau M.G., Custers J.H., et al. (2004). Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172, 6290–6297 [DOI] [PubMed] [Google Scholar]
- Basner-Tschakarjan E., Gaffal E., O'Keeffe M., et al. (2006). Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 8, 1300–1306 [DOI] [PubMed] [Google Scholar]
- Beard C.W., and Spindler K.R. (1996). Analysis of early region 3 mutants of mouse adenovirus type 1. J. Virol. 70, 5867–5874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousova N., Mikheeva G., Xiong C., et al. (2010). Development of a targeted gene vector platform based on simian adenovirus serotype 24. J. Virol. 84, 10087–10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkö M., and Harrach B. (2003). Molecular evolution of adenoviruses. Curr. Top. Microbiol. Immunol. 272, 3–35 [DOI] [PubMed] [Google Scholar]
- Both G.W. (2004). Ovine atadenovirus: a review of its biology, biosafety profile and application as a gene delivery vector. Immunol. Cell Biol. 82, 189–195 [DOI] [PubMed] [Google Scholar]
- Bradley R.R., Lynch D.M., Iampietro M.J., et al. (2012a). Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J. Virol. 86, 625–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.R., Maxfield L.F., Lynch D.M., et al. (2012b). Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions. J. Virol. 86, 1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw A.C., Coughlan L., Miller A.M., et al. (2012). Biodistribution and inflammatory profiles of novel penton and hexon double-mutant serotype 5 adenoviruses. J. Control. Release 164, 394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer E., Havenga M.J., Ophorst O., et al. (2007). Human adenovirus type 35 vector for gene therapy of brain cancer: improved transduction and bypass of pre-existing anti-vector immunity in cancer patients. Cancer Gene Ther. 14, 211–219 [DOI] [PubMed] [Google Scholar]
- Bru T., Salinas S., and Kremer E.J. (2010). An update on canine adenovirus type 2 and its vectors. Viruses 2, 2134–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder J.T., Semenova E., Chen P., et al. (2012). Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses. PLoS One 7, e33920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R., Vandenberghe L.H., Roy S., et al. (2009). Host immune responses to chronic adenovirus infections in human and nonhuman primates. J. Virol. 83, 2623–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone S., Meola A., Ercole B.B., et al. (2006). A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J. Virol. 80, 1688–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle R.C., Di Y., Cerny A.M., et al. (2009). Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 113, 1909–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauthen A.N., Brown C.C., and Spindler K.R. (1999). In vitro and in vivo characterization of a mouse adenovirus type 1 early region 3 null mutant. J. Virol. 73, 8640–8646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo V., Seiler M.P., Mane V., et al. (2007). Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15, 378–385 [DOI] [PubMed] [Google Scholar]
- Charles P.C., Guida J.D., Brosnan C.F., and Horwitz M.S. (1998). Mouse adenovirus type-1 replication is restricted to vascular endothelium in the CNS of susceptible strains of mice. Virology 245, 216–228 [DOI] [PubMed] [Google Scholar]
- Cheng C., Gall J.G., Kong W.P., et al. (2007). Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 3, e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherenova L.V., Logunov D.Y., Shashkova E.V., et al. (2004). Recombinant avian adenovirus CELO expressing the human interleukin-2: characterization in vitro, in ovo and in vivo. Virus Res. 100, 257–261 [DOI] [PubMed] [Google Scholar]
- Chillon M., and Kremer E.J. (2001). Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum. Gene Ther. 12, 1815–1823 [DOI] [PubMed] [Google Scholar]
- Cohen C.J., Xiang Z.Q., Gao G.P., et al. (2002). Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 83, 151–155 [DOI] [PubMed] [Google Scholar]
- Corredor J.C., and Nagy E. (2010a). A region at the left end of the fowl adenovirus 9 genome that is non-essential in vitro has consequences in vivo. J. Gen. Virol. 91, 51–58 [DOI] [PubMed] [Google Scholar]
- Corredor J.C., and Nagy E. (2010b). The non-essential left end region of the fowl adenovirus 9 genome is suitable for foreign gene insertion/replacement. Virus Res. 149, 167–174 [DOI] [PubMed] [Google Scholar]
- Coughlan L., Alba R., Parker A.L., et al. (2010). Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses 2, 2290–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan L., Bradshaw A.C., Parker A.L., et al. (2012). Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol. Ther. 20, 2268–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford-Miksza L., and Schnurr D.P. (1996). Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 70, 1836–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A., Yu Q.C., and Wilson J.M. (2000). Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene Ther. 11, 1713–1722 [DOI] [PubMed] [Google Scholar]
- Davison A.J., Benko M., and Harrach B. (2003). Genetic content and evolution of adenoviruses. J. Gen. Virol. 84, 2895–2908 [DOI] [PubMed] [Google Scholar]
- De Geest B., Snoeys J., Van Linthout S., et al. (2005). Elimination of innate immune responses and liver inflammation by PEGylation of adenoviral vectors and methylprednisolone. Hum. Gene Ther. 16, 1439–1451 [DOI] [PubMed] [Google Scholar]
- Dehghan S., Seto J., Liu E.B., et al. (2013). Computational analysis of four human adenovirus type 4 genomes reveals molecular evolution through two interspecies recombination events. Virology 443, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo N.C., Miao E.A., Iwakura Y., et al. (2009). Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31, 110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doszpoly A., Wellehan J.F., Childress A.L., et al. (2013). Partial characterization of a new adenovirus lineage discovered in testudinoid turtles. Infect. Genet. Evol. 17, 106–112 [DOI] [PubMed] [Google Scholar]
- Dudley R.W., Lu Y., Gilbert R., et al. (2004). Sustained improvement of muscle function one year after full-length dystrophin gene transfer into mdx mice by a gutted helper-dependent adenoviral vector. Hum. Gene Ther. 15, 145–156 [DOI] [PubMed] [Google Scholar]
- Ehrhardt A., and Kay M.A. (2002). A new adenoviral helper-dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo. Blood 99, 3923–3930 [DOI] [PubMed] [Google Scholar]
- Ersching J., Hernandez M.I., Cezarotto F.S., et al. (2010). Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys. Virology 407, 1–6 [DOI] [PubMed] [Google Scholar]
- Eto Y., Yoshioka Y., Ishida T., et al. (2010). Optimized PEGylated adenovirus vector reduces the anti-vector humoral immune response against adenovirus and induces a therapeutic effect against metastatic lung cancer. Biol. Pharm. Bull. 33, 1540–1544 [DOI] [PubMed] [Google Scholar]
- Farina S.F., Gao G.P., Xiang Z.Q., et al. (2001). Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75, 11603–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P., Peixoto C., Santiago V.M., et al. (2013a). Bioprocess development for canine adenovirus type 2 vectors. Gene Ther. 20, 353–360 [DOI] [PubMed] [Google Scholar]
- Fernandes P., Santiago V.M., Rodrigues A.F., et al. (2013b). Impact of E1 and Cre on adenovirus vector amplification: developing MDCK CAV-2-E1 and E1-Cre transcomplementing cell lines. PLoS One 8, e60342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François A., Eterradossi N., Delmas B., et al. (2001). Construction of avian adenovirus CELO recombinants in cosmids. J. Virol. 75, 5288–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J., Kass-Eisler A., Leinwand L., and Falck-Pedersen E. (1996). Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70, 2116–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J.G., Crystal R.G., and Falck-Pedersen E. (1998). Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 72, 10260–10264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Robbins P.D., and Gambotto A. (2003). Human adenovirus type 35: nucleotide sequence and vector development. Gene Ther. 10, 1941–1949 [DOI] [PubMed] [Google Scholar]
- Garnett C.T., Erdman D., Xu W., and Gooding L.R. (2002). Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 76, 10608–10616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni A., Parlato A., Vangieri B., et al. (2006). Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts). Clin. Ther. 157, 377–386 [PubMed] [Google Scholar]
- Geisbert T.W., Bailey M., Hensley L., et al. (2011). Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J. Virol. 85, 4222–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H.S. (1996). The ups and downs of adenovirus vectors. Bull. NY Acad. Med. 73, 53–58 [PMC free article] [PubMed] [Google Scholar]
- Greber U.F. (2002). Signalling in viral entry. Cell. Mol. Life Sci. 59, 608–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N.K., Herbert C.W., Hale S.J., et al. (2004). Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 11, 1256–1263 [DOI] [PubMed] [Google Scholar]
- Greenall S.A., Tyack S.G., Johnson M.A., and Sapats S.I. (2010). Antibody fragments, expressed by a fowl adenovirus vector, are able to neutralize infectious bursal disease virus. Avian Pathol. 39, 339–348 [DOI] [PubMed] [Google Scholar]
- Haisma H.J., Boesjes M., Beerens A.M., et al. (2009). Scavenger receptor A: a new route for adenovirus 5. Mol. Pharm. 6, 366–374 [DOI] [PubMed] [Google Scholar]
- Hammond J.M., and Johnson M.A. (2005). Porcine adenovirus as a delivery system for swine vaccines and immunotherapeutics. Vet. J. 169, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenga M.J., Lemckert A.A., Grimbergen J.M., et al. (2001). Improved adenovirus vectors for infection of cardiovascular tissues. J. Virol. 75, 3335–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviv Y.S., Blackwell J.L., Kanerva A., et al. (2002). Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 62, 4273–4281 [PubMed] [Google Scholar]
- Hofherr S.E., Shashkova E.V., Weaver E.A., et al. (2008). Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 16, 1276–1282 [DOI] [PubMed] [Google Scholar]
- Hofmann C., Löser P., Cichon G., et al. (1999). Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73, 6930–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterman L., Vogels R., van der Vlugt R., et al. (2004). Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 78, 13207–13215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanes S., and Kremer E.J. (2013). Canine adenovirus type 2 vector generation via I-Sce1-mediated intracellular genome release. PLoS One 8, e71032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Liu Z., and Gause W.C. (2001). Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22, 450–457 [DOI] [PubMed] [Google Scholar]
- Jian L., Zhao Q., Zhang S., et al. (2013). The prevalence of neutralising antibodies to chimpanzee adenovirus type 6 and type 7 in healthy adult volunteers, patients with chronic hepatitis B and patients with primary hepatocellular carcinoma in China. Arch. Virol. DOI 10.1007/s00705-013-1828-y [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jones M.S., Harrach B., Ganac R.D., et al. (2007). New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 81, 5978–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajon A.E., Brown C.C., and Spindler K.R. (1998). Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J. Virol. 72, 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A., Mikheeva G.V., Krasnykh V., et al. (2002). Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 8, 275–280 [PubMed] [Google Scholar]
- Kass-Eisler A., Leinwand L., Gall J., et al. (1996). Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 3, 154–162 [PubMed] [Google Scholar]
- Katze M.G., He Y., and Gale M. (2002). Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675–687 [DOI] [PubMed] [Google Scholar]
- Khare R., Reddy V.S., Nemerow G.R., and Barry M.A. (2012). Identification of adenovirus serotype 5 hexon regions that interact with scavenger receptors. J. Virol. 86, 2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri A., Xu Z.Z., and Both G.W. (1997). Gene expression by atypical recombinant ovine adenovirus vectors during abortive infection of human and animal cells in vitro. Virology 239, 226–237 [DOI] [PubMed] [Google Scholar]
- Klonjkowski B., Gilardi-Hebenstreit P., Hadchouel J., et al. (1997). A recombinant E1-deleted canine adenoviral vector capable of transduction and expression of a transgene in human-derived cells and in vivo. Hum. Gene Ther. 8, 2103–2115 [DOI] [PubMed] [Google Scholar]
- Kochanek S., Clemens P.R., Mitani K., et al. (1996). A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc. Natl. Acad. Sci. USA 93, 5731–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E.J., Boutin S., Chillon M., and Danos O. (2000). Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J. Virol. 74, 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümin D., Hofmann C., Rudolph M., et al. (2002). Biology of ovine adenovirus infection of nonpermissive cells. J. Virol. 76, 10882–10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S., Tominaga K., Kikukawa M., et al. (1998). Inhibitory effects of human sera on adenovirus-mediated gene transfer into rat liver. Anticancer Res. 18, 2345–2351 [PubMed] [Google Scholar]
- Lau A.A., Rozaklis T., Ibanes S., et al. (2012). Helper-dependent canine adenovirus vector-mediated transgene expression in a neurodegenerative lysosomal storage disorder. Gene 491, 53–57 [DOI] [PubMed] [Google Scholar]
- Laver W.G., Younghusband H.B., and Wrigley N.G. (1971). Purification and properties of chick embryo lethal orphan virus (an avian adenovirus). Virology 45, 598–614 [DOI] [PubMed] [Google Scholar]
- Leen A.M., Sili U., Vanin E.F., et al. (2004). Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood 104, 2432–2440 [DOI] [PubMed] [Google Scholar]
- Lemckert A.A., Grimbergen J., Smits S., et al. (2006). Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J. Gen. Virol. 87, 2891–2899 [DOI] [PubMed] [Google Scholar]
- Lenaerts L., Verbeken E., De Clercq E., and Naesens L. (2005). Mouse adenovirus type 1 infection in SCID mice: an experimental model for antiviral therapy of systemic adenovirus infections. Antimicrob. Agents Chemother. 49, 4689–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts L., Daelemans D., Geukens N., et al. (2006). Mouse adenovirus type 1 attachment is not mediated by the coxsackie-adenovirus receptor. FEBS Lett. 580, 3937–3942 [DOI] [PubMed] [Google Scholar]
- Lenaerts L., McVey J.H., Baker A.H., et al. (2009). Mouse adenovirus type 1 and human adenovirus type 5 differ in endothelial cell tropism and liver targeting. J. Gene. Med. 11, 119–127 [DOI] [PubMed] [Google Scholar]
- Lenaerts L., van Dam W., Persoons L., and Naesens L. (2012). Interaction between mouse adenovirus type 1 and cell surface heparan sulfate proteoglycans. PLoS One 7, e31454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Bangari D.S., Sharma A., and Mittal S.K. (2009). Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology 392, 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., White L.R., Clark S.A., et al. (2005). Akt/protein kinase B activation by adenovirus vectors contributes to NFkappaB-dependent CXCL10 expression. J. Virol. 79, 14507–14515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockett L.J., and Both G.W. (2002). Complementation of a defective human adenovirus by an otherwise incompatible ovine adenovirus recombinant carrying a functional E1A gene. Virology 294, 333–341 [DOI] [PubMed] [Google Scholar]
- Logunov D.Y., Ilyinskaya G.V., Cherenova L.V., et al. (2004). Restoration of p53 tumor-suppressor activity in human tumor cells in vitro and in their xenografts in vivo by recombinant avian adenovirus CELO-p53. Gene Ther. 11, 79–84 [DOI] [PubMed] [Google Scholar]
- Löser P., Hillgenberg M., Arnold W., et al. (2000). Ovine adenovirus vectors mediate efficient gene transfer to skeletal muscle. Gene Ther. 7, 1491–1498 [DOI] [PubMed] [Google Scholar]
- Löser P., Hofmann C., Both G.W., et al. (2003). Construction, rescue, and characterization of vectors derived from ovine atadenovirus. J. Virol. 77, 11941–11951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev A.N., Ivanova O.E., Eremeeva T.P., and Iggo R.D. (2008). Evidence of frequent recombination among human adenoviruses. J. Gen. Virol. 89, 380–388 [DOI] [PubMed] [Google Scholar]
- Mack C.A., Song W.R., Carpenter H., et al. (1997). Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8, 99–109 [DOI] [PubMed] [Google Scholar]
- Maione D., Della Rocca C., Giannetti P., et al. (2001). An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. USA 98, 5986–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniello-Wilks R., Dane A., Voeks D.J., et al. (2004). Gene-directed enzyme prodrug therapy for prostate cancer in a mouse model that imitates the development of human disease. J. Gene Med. 6, 43–54 [DOI] [PubMed] [Google Scholar]
- Mastrangeli A., Harvey B.G., Yao J., et al. (1996). “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 7, 79–87 [DOI] [PubMed] [Google Scholar]
- McVey D., Zuber M., Ettyreddy D., et al. (2010). Characterization of human adenovirus 35 and derivation of complex vectors. Virol. J. 7, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou A.I., Lehrmann H., Saltik M., and Cotten M. (1999). Mutational analysis of the avian adenovirus CELO, which provides a basis for gene delivery vectors. J. Virol. 73, 1399–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S.K., Prevec L., Graham F.L., and Babiuk L.A. (1995). Development of a bovine adenovirus type 3-based expression vector. J. Gen. Virol. 76 (Pt 1), 93–102 [DOI] [PubMed] [Google Scholar]
- Mok H., Palmer D.J., Ng P., and Barry M.A. (2005). Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 11, 66–79 [DOI] [PubMed] [Google Scholar]
- Morrison J., Briggs S.S., Green N., et al. (2008). Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol. Ther. 16, 244–251 [DOI] [PubMed] [Google Scholar]
- Morrison J., Briggs S.S., Green N.K., et al. (2009). Cetuximab retargeting of adenovirus via the epidermal growth factor receptor for treatment of intraperitoneal ovarian cancer. Hum. Gene Ther. 20, 239–251 [DOI] [PubMed] [Google Scholar]
- Muruve D.A., Pétrilli V., Zaiss A.K., et al. (2008). The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 [DOI] [PubMed] [Google Scholar]
- Myhre S., Henning P., Granio O., et al. (2007). Decreased immune reactivity towards a knobless, affibody-targeted adenovirus type 5 vector. Gene Ther. 14, 376–381 [DOI] [PubMed] [Google Scholar]
- Nan X., Peng B., Hahn T.W., et al. (2003). Development of an Ad7 cosmid system and generation of an Ad7deltaE1deltaE3HIV(MN) env/rev recombinant virus. Gene Ther. 10, 326–336 [DOI] [PubMed] [Google Scholar]
- Nguyen T., Nery J., Joseph S., et al. (1999). Mouse adenovirus (MAV-1) expression in primary human endothelial cells and generation of a full-length infectious plasmid. Gene Ther. 6, 1291–1297 [DOI] [PubMed] [Google Scholar]
- Ni S., Bernt K., Gaggar A., et al. (2005). Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 16, 664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol C.G., Graham D., Miller W.H., et al. (2004). Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 10, 344–354 [DOI] [PubMed] [Google Scholar]
- Ojkic D., and Nagy E. (2001). The long repeat region is dispensable for fowl adenovirus replication in vitro. Virology 283, 197–206 [DOI] [PubMed] [Google Scholar]
- Ojkic D., and Nagy E. (2003). Antibody response and virus tissue distribution in chickens inoculated with wild-type and recombinant fowl adenoviruses. Vaccine 22, 42–48 [DOI] [PubMed] [Google Scholar]
- Olive M., Eisenlohr L., Flomenberg N., et al. (2002). The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 13, 1167–1178 [DOI] [PubMed] [Google Scholar]
- Ord E., Shirley R., McClure J., et al. (2013). Combined antiapoptotic and antioxidant approach to acute neuroprotection for stroke in hypertensive rats. J. Cereb. Blood Flow Metab. 33, 1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., and Hearing P. (2001). Pseudopackaging of adenovirus type 5 genomes into capsids containing the hexon proteins of adenovirus serotypes B, D, or E. J. Virol. 75, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl J.H., Verhoeven D.H., Kwappenberg K.M., et al. (2012). Adenovirus type 35, but not type 5, stimulates NK cell activation via plasmacytoid dendritic cells and TLR9 signaling. Mol. Immunol. 51, 91–100 [DOI] [PubMed] [Google Scholar]
- Parker A.L., Fisher K.D., Oupicky D., et al. (2005). Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J. Drug Target. 13, 39–51 [DOI] [PubMed] [Google Scholar]
- Parker A.L., Waddington S.N., Nicol C.G., et al. (2006). Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108, 2554–2561 [DOI] [PubMed] [Google Scholar]
- Parker A.L., Waddington S.N., Buckley S.M., et al. (2009). Effect of neutralizing sera on factor x-mediated adenovirus serotype 5 gene transfer. J. Virol. 83, 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R., Evelegh C., and Graham F. (1999). Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 6, 1565–1573 [DOI] [PubMed] [Google Scholar]
- Perreau M., and Kremer E.J. (2006). The conundrum between immunological memory to adenovirus and their use as vectors in clinical gene therapy. Mol. Biotechnol. 34, 247–256 [DOI] [PubMed] [Google Scholar]
- Perreau M., Welles H.C., Pellaton C., et al. (2012). The number of Toll-like receptor 9-agonist motifs in the adenovirus genome correlates with induction of dendritic cell maturation by adenovirus immune complexes. J. Virol. 86, 6279–6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichla-Gollon S.L., Drinker M., Zhou X., et al. (2007). Structure-based identification of a major neutralizing site in an adenovirus hexon. J. Virol. 81, 1680–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersanti S., Astrologo L., Licursi V., et al. (2013). Differentiated neuroprogenitor cells incubated with human or canine adenovirus, or lentiviral vectors have distinct transcriptome profiles. Plos One 8, e69808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha A., Ditty S.E., Su J., et al. (2005a). Genomic and bioinformatics analysis of HAdV-4, a human adenovirus causing acute respiratory disease: implications for gene therapy and vaccine vector development. J. Virol. 79, 2559.–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha A., Su J., Carlisle S., et al. (2005b). Genomic and bioinformatics analysis of HAdV-7, a human adenovirus of species B1 that causes acute respiratory disease: implications for vector development in human gene therapy. Virology 332, 114.–129 [DOI] [PubMed] [Google Scholar]
- Quinn K.M., Da Costa A., Yamamoto A., et al. (2013). Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 190, 2720–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S., Hsu T.H., Ashley S.L., and Spindler K.R. (2009). Usage of integrin and heparan sulfate as receptors for mouse adenovirus type 1. J. Virol. 83, 2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper S.E., Chirmule N., Lee F.S., et al. (2003). Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158 [DOI] [PubMed] [Google Scholar]
- Rasmussen U.B., Benchaibi M., Meyer V., et al. (1999). Novel human gene transfer vectors: evaluation of wild-type and recombinant animal adenoviruses in human-derived cells. Hum. Gene Ther. 10, 2587–2599 [DOI] [PubMed] [Google Scholar]
- Reddy P.S., Idamakanti N., Babiuk L.A., et al. (1999a). Porcine adenovirus-3 as a helper-dependent expression vector. J. Gen. Virol. 80 (Pt 11), 2909.–2916 [DOI] [PubMed] [Google Scholar]
- Reddy P.S., Idamakanti N., Hyun B.H., et al. (1999b). Development of porcine adenovirus-3 as an expression vector. J. Gen. Virol. 80 (Pt 3), 563.–570 [DOI] [PubMed] [Google Scholar]
- Roberts D.M., Nanda A., Havenga M.J., et al. (2006). Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441, 239–243 [DOI] [PubMed] [Google Scholar]
- Robinson M., Li B., Ge Y., et al. (2009). Novel immunocompetent murine tumor model for evaluation of conditionally replication-competent (oncolytic) murine adenoviral vectors. J. Virol. 83, 3450–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogée S., Grellier E., Bernard C., et al. (2010). Influence of chimeric human-bovine fibers on adenoviral uptake by liver cells and the antiviral immune response. Gene Ther. 17, 880–891 [DOI] [PubMed] [Google Scholar]
- Rogozhin V.N., Logunov D.Y., Shchebliakov D.V., et al. (2011). An efficient method for the delivery of the interleukin-2 gene to human hematopoietic cells using the fiber-modified recombinant adenovirus. Acta Nat. 3, 100–106 [PMC free article] [PubMed] [Google Scholar]
- Romanczuk H., Galer C.E., Zabner J., et al. (1999). Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice. Hum. Gene Ther. 10, 2615–2626 [DOI] [PubMed] [Google Scholar]
- Rowe W.P., Huebner R.J., Gilmore L.K., et al. (1953). Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 84, 570–573 [DOI] [PubMed] [Google Scholar]
- Roy S., Shirley P.S., McClelland A., and Kaleko M. (1998). Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 72, 6875–6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Gao G.P., Lu Y., et al. (2004). Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum. Gene Ther. 15, 519–530 [DOI] [PubMed] [Google Scholar]
- Roy S., Clawson D.S., Calcedo R., et al. (2005). Use of chimeric adenoviral vectors to assess capsid neutralization determinants. Virology 333, 207–214 [DOI] [PubMed] [Google Scholar]
- Roy S., Clawson D.S., Adam V.S., et al. (2011a). Construction of gene transfer vectors based on simian adenovirus 7. J. Gen. Virol. 92, 1749.–1753 [DOI] [PubMed] [Google Scholar]
- Roy S., Medina-Jaszek A., Wilson M.J., et al. (2011b). Creation of a panel of vectors based on ape adenovirus isolates. J. Gene Med. 13, 17.–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux J.J., and Burnett R.M. (2000). Type-specific epitope locations revealed by X-ray crystallographic study of adenovirus type 5 hexon. Mol. Ther. 1, 18–30 [DOI] [PubMed] [Google Scholar]
- Sakurai F. (2008). Development of a replication-incompetent adenovirus vector derived from subgroup B adenovirus serotype 35. Yakuga. Zasshi 128, 1751–1761 [DOI] [PubMed] [Google Scholar]
- Sakurai F., Mizuguchi H., and Hayakawa T. (2003). Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Ther. 10, 1041–1048 [DOI] [PubMed] [Google Scholar]
- Sakurai F., Nakamura S.I., Akitomo K., et al. (2009). Adenovirus serotype 35 vector-mediated transduction following direct administration into organs of nonhuman primates. Gene Ther. 16, 297–302 [DOI] [PubMed] [Google Scholar]
- Schoehn G., El Bakkouri M., Fabry C.M., et al. (2008). Three-dimensional structure of canine adenovirus serotype 2 capsid. J. Virol. 82, 3192–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins J.W., Nociari M., Philpott N., and Falck-Pedersen E. (2005). Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J. Virol. 79, 11627–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher L., Ribas A., Dissette V.B., et al. (2004). Human dendritic cell maturation by adenovirus transduction enhances tumor antigen-specific T-cell responses. J. Immunother. 27, 191–200 [DOI] [PubMed] [Google Scholar]
- Segura M.M., Puig M., Monfar M., and Chillón M. (2012). Chromatography purification of canine adenoviral vectors. Hum. Gene Ther. Methods 23, 182–197 [DOI] [PubMed] [Google Scholar]
- Seiradake E., Henaff D., Wodrich H., et al. (2009). The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 5, e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshidhar Reddy P., Ganesh S., Limbach M.P., et al. (2003). Development of adenovirus serotype 35 as a gene transfer vector. Virology 311, 384–393 [DOI] [PubMed] [Google Scholar]
- Sharma A., Bangari D.S., Tandon M., et al. (2009a). Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology 386, 44.–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Li X., Bangari D.S., and Mittal S.K. (2009b). Adenovirus receptors and their implications in gene delivery. Virus Res. 143, 184.–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Bangari D.S., Vemula S.V., and Mittal S.K. (2011). Persistence and the state of bovine and porcine adenoviral vector genomes in human and nonhuman cell lines. Virus Res. 161, 181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova E.V., Cherenova L.V., Kazansky D.B., and Doronin K. (2005). Avian adenovirus vector CELO-TK displays anticancer activity in human cancer cells and suppresses established murine melanoma tumors. Cancer Gene Ther. 12, 617–626 [DOI] [PubMed] [Google Scholar]
- Shayakhmetov D.M., Papayannopoulou T., Stamatoyannopoulos G., and Lieber A. (2000). Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J. Virol. 74, 2567–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov D.M., Gaggar A., Ni S., et al. (2005). Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79, 7478–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard M., Werner W., Tsatas E., et al. (1998). Fowl adenovirus recombinant expressing VP2 of infectious bursal disease virus induces protective immunity against bursal disease. Arch. Virol. 143, 915–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Ichihara M., Yoshioka Y., et al. (2012). Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol. Pharm. Bull. 35, 1336–1342 [DOI] [PubMed] [Google Scholar]
- Shiratsuchi T., Rai U., Krause A., et al. (2010). Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J. Clin. Invest. 120, 3688–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver J.W., and Emini E.A. (2004). Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55, 355–372 [DOI] [PubMed] [Google Scholar]
- Shmarov M.M., Cherenova L.V., Shashkova E.V., et al. (2002). Eukaryotic vectors of Celo avian adenovirus genome, carrying GFP and human IL-2 genes. Mol. Gen. Mikrobiol. Virusol. 2, 30–35 [PubMed] [Google Scholar]
- Skog J., Edlund K., Bergenheim A.T., and Wadell G. (2007). Adenoviruses 16 and CV23 efficiently transduce human low-passage brain tumor and cancer stem cells. Mol. Ther. 15, 2140–2145 [DOI] [PubMed] [Google Scholar]
- Soudais C., Boutin S., Hong S.S., et al. (2000). Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 74, 10639–10649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais C., Laplace-Builhe C., Kissa K., and Kremer E.J. (2001). Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15, 2283–2285 [DOI] [PubMed] [Google Scholar]
- Stephen S.L., Montini E., Sivanandam V.G., et al. (2010). Chromosomal integration of adenoviral vector DNA in vivo. J. Virol. 84, 9987–9994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Boos E., Herbert C., et al. (2006). Chick embryo lethal orphan virus can be polymer-coated and retargeted to infect mammalian cells. Gene Ther. 13, 356–368 [DOI] [PubMed] [Google Scholar]
- Stevenson M., Hale A.B., Hale S.J., et al. (2007). Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 14, 335–345 [DOI] [PubMed] [Google Scholar]
- Stone D., Ni S., Li Z.Y., et al. (2005). Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 79, 5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Geisbert T.W., Geisbert J.B., et al. (2003). Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424, 681–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida S.M., Truitt D.M., Lemckert A.A., et al. (2005). Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174, 7179–7185 [DOI] [PubMed] [Google Scholar]
- Tamanini A., Nicolis E., Bonizzato A., et al. (2006). Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J. Virol. 80, 11241–11254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P.K., Michou A.I., Bergelson J.M., and Cotten M. (2001). Defining CAR as a cellular receptor for the avian adenovirus CELO using a genetic analysis of the two viral fibre proteins. J. Gen. Virol. 82, 1465–1472 [DOI] [PubMed] [Google Scholar]
- Tandon M., Sharma A., Vemula S.V., et al. (2012). Sequential administration of bovine and human adenovirus vectors to overcome vector immunity in an immunocompetent mouse model of breast cancer. Virus Res. 163, 202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Olive M., Pulmanausahakul R., et al. (2006). Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology 350, 312–322 [DOI] [PubMed] [Google Scholar]
- Tang R., Li K., Wilson M., et al. (2012). Potent antietumor immunity in mice induced by vaccination with an ovine atadenovirus vector. J. Immunother. 35, 32–41 [DOI] [PubMed] [Google Scholar]
- Tarassishin L., Szawlowski P., Kidd A.H., and Russell W.C. (2000). An epitope on the adenovirus fibre tail is common to all human subgroups. Arch. Virol. 145, 805–811 [DOI] [PubMed] [Google Scholar]
- Tian X., Su X., Li H., et al. (2011). Construction and characterization of human adenovirus serotype 3 packaged by serotype 7 hexon. Virus Res. 160, 214–220 [DOI] [PubMed] [Google Scholar]
- Tomko R.P., Xu R., and Philipson L. (1997). HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94, 3352–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S.K., Black H.B., Goldwasser E., and Leiden J.M. (1996). Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 2, 545–550 [DOI] [PubMed] [Google Scholar]
- Tuboly T., Nagy E., and Derbyshire J.B. (1993). Potential viral vectors for the stimulation of mucosal antibody responses against enteric viral antigens in pigs. Res. Vet. Sci. 54, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutykhina I.L., Shmarov M.M., Logunov D.I., et al. (2008). Construction of the vector based on the CELO avian adenovirus genome providing enhanced expression of secreted alkaline phosphatase gene in a non-permissive system in vitro and in vivo. Mol. Gen. Mikrobiol. Virusol. 4, 26–30 [PubMed] [Google Scholar]
- Ulasov I.V., Rivera A.A., Han Y., et al. (2007). Targeting adenovirus to CD80 and CD86 receptors increases gene transfer efficiency to malignant glioma cells. J. Neurosurg. 107, 617–627 [DOI] [PubMed] [Google Scholar]
- Varnavski A.N., Schlienger K., Bergelson J.M., et al. (2003). Efficient transduction of human monocyte-derived dendritic cells by chimpanzee-derived adenoviral vector. Hum. Gene Ther. 14, 533–544 [DOI] [PubMed] [Google Scholar]
- Vellinga J., Van der Heijdt S., and Hoeben R.C. (2005). The adenovirus capsid: major progress in minor proteins. J. Gen. Virol. 86, 1581–1588 [DOI] [PubMed] [Google Scholar]
- Voeks D., Martiniello-Wilks R., Madden V., et al. (2002). Gene therapy for prostate cancer delivered by ovine adenovirus and mediated by purine nucleoside phosphorylase and fludarabine in mouse models. Gene Ther. 9, 759–768 [DOI] [PubMed] [Google Scholar]
- Vogels R., Zuijdgeest D., van Rijnsoever R., et al. (2003). Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77, 8263–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk A.L., Rivera A.A., Kanerva A., et al. (2003). Enhanced adenovirus infection of melanoma cells by fiber-modification: incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biol. Ther. 2, 511–515 [DOI] [PubMed] [Google Scholar]
- Vrati S., Macavoy E.S., Xu Z.Z., et al. (1996). Construction and transfection of ovine adenovirus genomic clones to rescue modified viruses. Virology 220, 200–203 [DOI] [PubMed] [Google Scholar]
- Vujanovic L., Whiteside T.L., Potter D.M., et al. (2009). Regulation of antigen presentation machinery in human dendritic cells by recombinant adenovirus. Cancer Immunol. Immunother. 58, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.P., Seto J., Liu E.B., et al. (2011). Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J. Clin. Microbiol. 49, 3482–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I.J., Jhuang M.C., Chen Y.H., et al. (2010). Chitosan modification of adenovirus to modify transfection efficiency in bovine corneal epithelial cells. PLoS One 5, e12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Chan L.W., Johnson R.N., et al. (2011a). The transduction of Coxsackie and Adenovirus Receptor-negative cells and protection against neutralizing antibodies by HPMA-co-oligolysine copolymer-coated adenovirus. Biomaterials 32, 9536.–9545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.Y., Liu S.H., Li X., et al. (2011b). Study on construction of chimeric adenovirus vector Ad5/11 carrying human eGFP and endostatin-K5 and its experimental investigation in vitro. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 27, 143.–145 [PubMed] [Google Scholar]
- Wohlfart C. (1988). Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62, 2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., and Tikoo S.K. (2004). Altered tropism of recombinant bovine adenovirus type-3 expressing chimeric fiber. Virus Res. 99, 9–15 [DOI] [PubMed] [Google Scholar]
- Wu H., Dmitriev I., Kashentseva E., et al. (2002). Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J. Virol. 76, 12775–12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z., Gao G., Reyes-Sandoval A., et al. (2002). Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76, 2667–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z., Li Y., Cun A., et al. (2006). Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 12, 1596–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.Z., and Both G.W. (1998). Altered tropism of an ovine adenovirus carrying the fiber protein cell binding domain of human adenovirus type 5. Virology 248, 156–163 [DOI] [PubMed] [Google Scholar]
- Xu Z.Z., Hyatt A., Boyle D.B., and Both G.W. (1997). Construction of ovine adenovirus recombinants by gene insertion or deletion of related terminal region sequences. Virology 230, 62–71 [DOI] [PubMed] [Google Scholar]
- Xu Z.Z., Nevels M., MacAvoy E.S., et al. (2000). An ovine adenovirus vector lacks transforming ability in cells that are transformed by AD5 E1A/B sequences. Virology 270, 162–172 [DOI] [PubMed] [Google Scholar]
- Xu Z., Tian J., Smith J.S., and Byrnes A.P. (2008). Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J. Virol. 82, 11705–11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Qiu Q., Tian J., et al. (2013). Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat. Med. 19, 452–457 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Kawabata K., Koizumi N., et al. (2007). Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 18, 753–762 [DOI] [PubMed] [Google Scholar]
- Yao X., Yoshioka Y., Morishige T., et al. (2010). Adenovirus vector covalently conjugated to polyethylene glycol with a cancer-specific promoter suppresses the tumor growth through systemic administration. Biol. Pharm. Bull. 33, 1073–1076 [DOI] [PubMed] [Google Scholar]
- Ying B., Smith K., and Spindler K.R. (1998). Mouse adenovirus type 1 early region 1A is dispensable for growth in cultured fibroblasts. J. Virol. 72, 6325–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youil R., Toner T.J., Su Q., et al. (2002). Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 13, 311–320 [DOI] [PubMed] [Google Scholar]
- Yu B., Dong J., Wang C., et al. (2013). Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology 437, 118–123 [DOI] [PubMed] [Google Scholar]
- Zaiss A.K., Vilaysane A., Cotter M.J., et al. (2009). Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J. Immunol. 182, 7058–7068 [DOI] [PubMed] [Google Scholar]
- Zakhartchouk A.N., Wu Q., and Tikoo S.K. (2007). Construction of capsid-modified recombinant bovine adenovirus type 3. Methods Mol. Med. 130, 91–106 [DOI] [PubMed] [Google Scholar]
- Zeng Q., Han J., Zhao D., et al. (2012). Protection of adenovirus from neutralizing antibody by cationic PEG derivative ionically linked to adenovirus. Int. J. Nanomed. 7, 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Huang W., Zhou X., et al. (2013a). Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J. Med. Virol. 85, 1077.–1084 [DOI] [PubMed] [Google Scholar]
- Zhang W.F., Wu F.L., Shao H.W., et al. (2013b). Chimeric adenoviral vector Ad5F35L containing the Ad5 natural long-shaft exhibits efficient gene transfer into human T lymphocytes. J. Virol. Methods 194, 52.–59 [DOI] [PubMed] [Google Scholar]
- Zhu J., Huang X., and Yang Y. (2007). Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81, 3170–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Huang X., and Yang Y. (2008). A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther. 16, 1300–1307 [DOI] [PubMed] [Google Scholar]