Abstract
Trauma to the spinal cord often results not only in sensorimotor but also autonomic impairments. The loss of autonomic control over the cardiovascular system can cause profound blood pressure (BP) derangements in subjects with spinal cord injury (SCI) and may therefore lead to increased cardiovascular disease (CVD) risk in this population. The use of ambulatory blood pressure monitoring (ABPM) allows insights into circadian BP profiles, which have been shown to be of good prognostic value for cardiovascular morbidity and mortality in able-bodied subjects. Past studies in SCI subjects using ABPM have shown that alterations in circadian BP patterns are dependent on the spinal lesion level. Tetraplegic subjects with sensorimotor complete lesions have a decreased daytime arterial BP, loss of the physiological nocturnal BP dip, and higher circadian BP variability, including potentially life-threatening hypertensive episodes known as autonomic dysreflexia (AD), compared with paraplegic and able-bodied subjects. The proposed underlying mechanisms of these adverse BP alterations mainly are attributed to a lost or decreased central drive to sympathetic spinal preganglionic neurons controlling the heart and blood vessels. In addition, several maladaptive anatomical changes within the spinal cord and the periphery, as well as the general decrease of physical daily activity in SCI subjects, account for adverse BP changes.
ABPM enables the identification of adverse BP profiles and the associated increased risk for CVD in SCI subjects. Concurrently, it also might provide a useful clinical tool to monitor improvements of AD and lost nocturnal dip after appropriate treatments in the SCI population.
Key words: : ambulatory blood pressure monitoring, autonomic nervous system, circadian, spinal cord injury, sympathetic
Introduction
More than three decades ago, Millar-Craig and colleagues first described the patterns of circadian variation of arterial blood pressure (BP) using invasive intra-arterial monitoring.1 This technology allowed continuous long-term BP monitoring and provided insights into circadian BP changes. Circadian rhythmicity of BP is characterized by substantial reductions during sleep called a nocturnal dip, a rapid rise upon awakening, and increased variability during daytime in able-bodied, normotensive subjects. The timing and amplitude of these BP fluctuations are influenced by intrinsic factors, such as neurohormonal regulation (autonomic nervous system and renin-angiotensin-aldosterone system), but also extrinsic factors, such as physical activity, emotional state,2 smoking, and/or alcohol consumption.3
With the technological progress to fully automated, non-invasive ambulatory BP monitoring (ABPM), arterial BP nowadays can be non-invasively and continuously assessed at pre-determined intervals over 24 h or longer. Circadian BP changes assessed by ABPM extensively have been studied in many different patient populations, such as in diabetes and chronic kidney disease, and are a valuable tool in the management of hypertensive patients. In addition to the assessment of hypertension, autonomic neuropathy, syncope, hypotension, and the effect of antihypertensive drugs can be evaluated by a circadian BP assessment.4,5 In the field of hypertension, the use of ABPM has demonstrated a number of patterns of BP responses in different time windows, such as the daytime, nocturnal and morning window, that may be relevant for clinical practice.6 Some examples of BP patterns are the following: 1) white-coat hypertension, which is present in most hypertensive patients and can lead to unnecessary prescription of medication5; 2) systo-diastolic hypertension as the most common daytime BP pattern; and 3) nocturnal dipping or non-dipping.
In general, ABPM as an out-of-office non-invasive means of BP monitoring has been shown to be superior to discrete clinic BP monitoring with respect to its prognostic value of cardiovascular morbidity and mortality.7–10 However, the control of BP might be particularly relevant in certain periods of the 24-h profile, such as in the nocturnal and morning windows.5,11 Much of the added benefit of ABPM stems from the ability to measure nocturnal BP and there is compelling evidence suggesting that nocturnal BP is the most sensitive predictor of cardiovascular disease (CVD) outcomes, and thus nocturnal BP measurement is becoming an important part of clinical practice.12–16 The diminution or absence of nocturnal reductions in BP are associated with increased incidence of stroke,17 congestive heart failure,18 and cardiovascular morbidity in able-bodied subjects.19
A plethora of studies related to abnormal fluctuations in circadian BP focus on hypertensive patients with different etiologies. However, since the autonomic nervous system plays a crucial role in BP regulation, subjects with spinal cord injury (SCI) also are prone to develop various adverse pathological BP alterations.20 In addition, CVD has been proposed as the leading cause of morbidity and mortality in the SCI population21–23 and fluctuations in circadian BP might contribute to vascular injury resulting in greater risk for arterial disease in subjects with SCI.24 Accordingly, this review aims firstly to summarize normal BP control and secondly to describe the use of ABPM to assess alterations in BP patterns and the clinical consequences following SCI.
Normal Blood Pressure Regulation
It is relevant to first address the normal physiology of BP control in order to understand alterations occurring after SCI. Mean arterial BP is determined by the product of cardiac output and total peripheral resistance. These two fundamental components are related to cardiac, blood vessel, and blood volume control and influenced by many physiological systems. For example, pressure in the arterial system is regulated on a beat-to-beat basis by the autonomic nervous system, whereas long-term adaptations are controlled by hormones that act on the kidney (renin–angiotensin-aldosterone system.25
Cardiovascular autonomic control
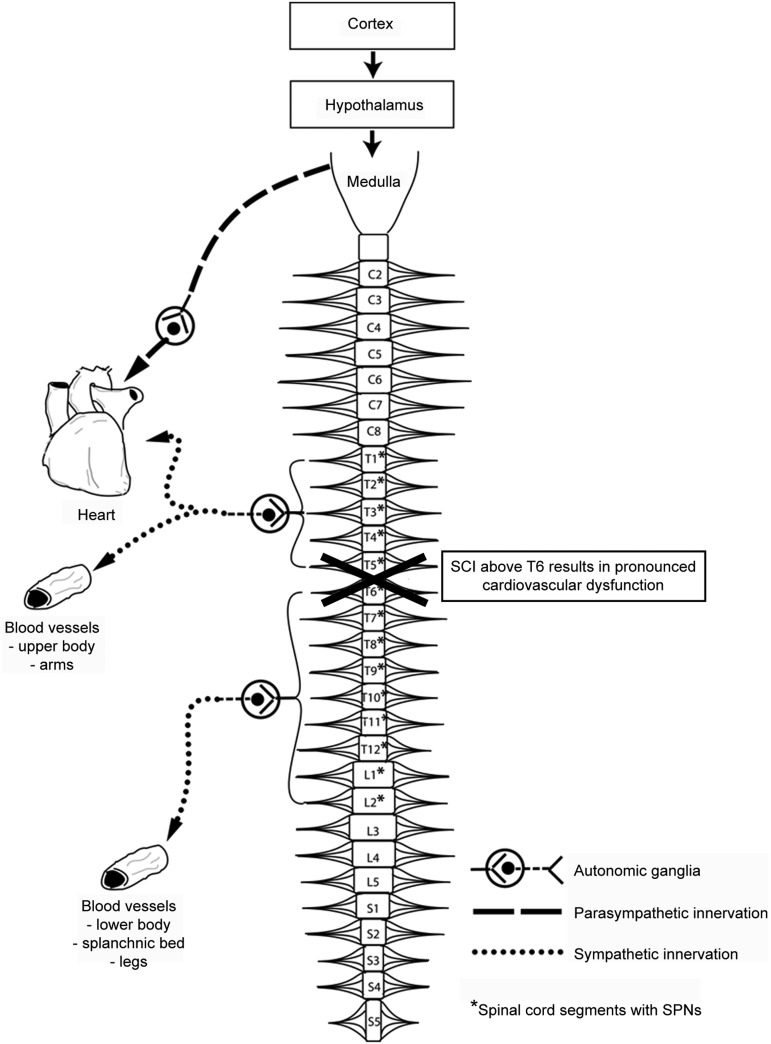
Cardiovascular function is largely controlled by the autonomic nervous system. The sympathetic and the parasympathetic nervous system constitute the autonomic nervous system and they are coordinated by supraspinal centers, such as the cerebral cortex, hypothalamus and brainstem (see Fig. 1). Both, the parasympathetic and the sympathetic systems show similar organization insofar as preganglionic and postganglionic spinal neurons are interposed between the supraspinal centers and the target organs. However, not all target organs are innervated by both systems.
FIG. 1.
Autonomic control of cardiovascular systems. Various nuclei within the medulla oblongata coordinate cardiovascular control and are under control of the cerebral cortex and the hypothalamus. Descending sympathetic projections provide tonic control to spinal sympathetic pre-ganglionic neurons (SPNs) located within the lateral horn of spinal segments T1–L2. Axons of SPNs exit the spinal cord via the ventral root and synapse with post-ganglionic neurons located in the paravertebral ganglia. The post-ganglionic fibers then synapse directly with smooth muscle in the heart and blood vessels. Parasympathetic pre-ganglionic fibers exit the brainstem via the vagus nerve and synapse with post-ganglionic parasympathetic neurons in the cardiac ganglia. Figure adapted with permission from Krassioukov.39
The heart has dual innervation: parasympathetic through cholinergic agonists from the vagal nerve (cranial nerve X) and sympathetic through predominantly adrenergic β-1 receptor activation from the upper thoracic segments of the spinal cord (T1-T5; see Fig. 1). In contrast, most blood vessels have only sympathetic innervation, except the blood vessels of the penile and clitoral cavernous tissue which receive only parasympathetic innervation.26 Blood vessels in the upper portion of the body receive sympathetic innervation from the T1 to T5 spinal sympathetic neurons, while the major vasculature beds in the splanchnic region and lower extremities are under the control of the more caudal T6-L2 sympathetic preganglionic neurons (SPNs; see Fig. 1). The vessels supplying the splanchnic vasculature bed, and particularly the gut, are most important in cardiovascular control. The densely innervated and highly compliant splanchnic vasculature bed is the primary capacitance bed in the human body since it contains approximately one-fourth of the total blood volume at rest.
The dual innervation of the heart and the segmental differences in sympathetic innervation to a variety of vascular beds are particularly important for the understanding of BP and HR control, as well as changes in cardiovascular function following cervical, mid-thoracic, or lower thoracic SCI.27
The mechanisms that constitute the feedback and reflex control of BP are complex and have been extensively studied and characterized. Effective short-term control of BP changes is mediated by baro- and chemoreflexes.28 The cardiovascular centers in the medulla oblongata are not only activated through baro- and chemoreflex loops but also through the limbic system and hypothalamus in response to emotional or psychological stress.29
Other factors influencing BP, which are not further discussed in this review, can either raise BP (e.g., catecholamines, such as epinephrine and norepinephrine, and nicotine) or lower BP (e.g., atrial natriuretic peptide, nitric oxide, and alcohol).
Circadian blood pressure variation
Cardiovascular functions, including BP, show diurnal oscillation that is under control of biological clocks.30 Arterial BP of normotensive able-bodied people shows a peak in the early morning hours and a trough during sleep, which is called a dipper pattern. The sympathetic nervous system is the main contributor to circadian BP oscillations. It remains quiescent during sleep but in the early morning, BP naturally rises sharply in response to activation of the sympathetic nervous system.31 This early morning surge is associated with hemodynamic and neurohormonal changes, including increases in epinephrine levels, renin and aldosterone levels, HR, vascular tone and blood viscosity, and decreases in vagal activity.32–35 The subsequent daily BP rises are rather controlled by direct sympathetic neural input to the heart (from T1-T5 spinal level) and vasculature (T1-T5 for upper body, T6-L2 for lower body) due to increased physical activity and postural changes than by endogenous increase in plasma catecholamines.36 Physical activity has been shown to be the major factor influencing BP during the day.37,38
Since a SCI can lead to profound alterations in autonomic control and might force affected subjects to less physical activity due to paralysis, this devastating condition affects circadian BP control.
Ambulatory Blood Pressure Monitoring following SCI
A SCI diminishes autonomic neural control over cardiovascular function with the most dramatic effects on BP control when the injury occurs above the sixth thoracic segment (T6).39 These high-level injuries eliminate descending autonomic control over the significant portion of the spinal cord that governs the splanchnic vascular bed and lower limb blood vessels that are crucial in regulating BP (see Fig. 1).
The next sections emphasize studies on ABPM as a tool to investigate perturbances of cardiovascular homeostasis after SCI. In general, ABPM following SCI has been used to provide three types of information which are of potential interest in the clinical field: 1) the mean systolic and diastolic arterial BP level; 2) the circadian BP pattern; and 3) BP variability. In the field of SCI research, so far eleven studies have used ABPM to investigate BP behavior in tetraplegic and paraplegic subjects.
Changes in baseline blood pressure
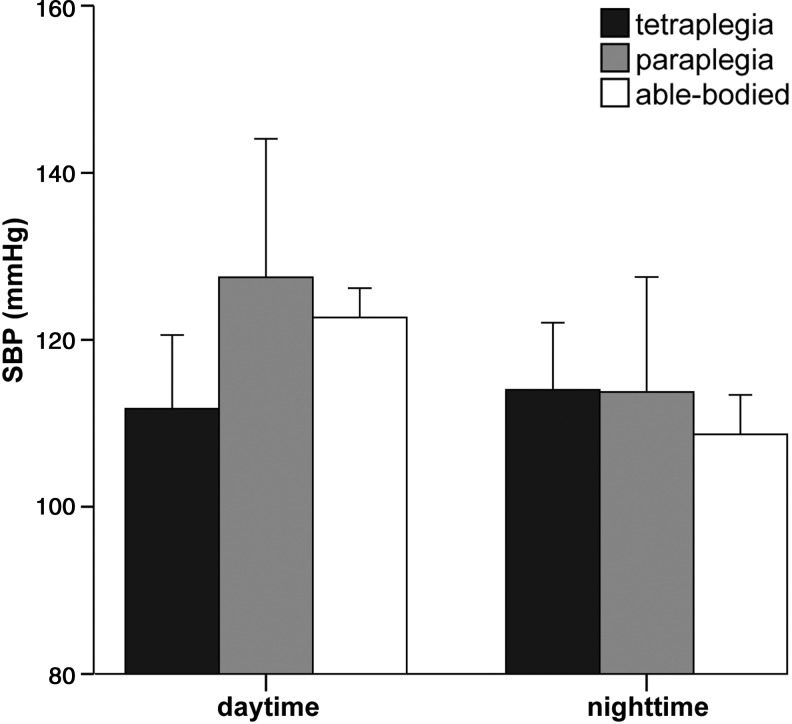
Baseline BP is calculated as either daytime, nighttime, or the whole 24-hour period BP. Daytime mean systolic, diastolic, and arterial pressure are lower in tetraplegic, compared with paraplegic40–42 and able-bodied subjects.42 In contrast, nighttime values of those parameters do not differ in tetraplegic, paraplegic, and able-bodied subjects. The drastic decrease in mean systolic BP (SBP) during daytime in tetraplegic subjects is distinct enough that the mean SBP over the whole 24-hour period is also decreased in tetraplegic versus paraplegic and able-bodied subjects (for values see Table 1).42
Table 1.
Comparison between Daytime SBP, Nighttime SBP and Nocturnal Dip by Lesion Level
| Subjects | Level of lesion | N | AIS | Daytime SBP(mm Hg) | Nighttime SBP (mm Hg) | Nocturnal dip (%) | ABPM device | |
|---|---|---|---|---|---|---|---|---|
| Seabra-Garcez (2012) | tetra/para | C4-T12 | 32 | A,B | 109±1 | 107±1 | 2 | SpaceLabs 90207 |
| Rosado-Rivera (2011) | tetra | C4-C8 | 20 | A,B,C,D | 103±12 | 106±10 | −3 | SpaceLabs 90207 |
| high para | T2-T5 | 10 | A,B | 115±7 | 108±11 | 6 | ||
| low para | T7-T12 | 9 | A | 114±16 | 102±11 | 10 | ||
| able-bodied | 10 | 119±8 | 107±8 | 10 | ||||
| Frisbie (2007) | tetra | C5-C7 | 10 | A | 124±21 | 125±16 | −1 | SpaceLabs 90207 |
| low para | T8-T10 | 5 | A | 151±16 | 133±8 | 12 | ||
| Munakata (1997) | tetra | C4-C7 | 10 | A | 110±3 | 111±6 | −1 | TM 2425 |
| low para | T6-T12 | 9 | A | 127±5 | 114±3 | 10 | ||
| able-bodied | 16 | 123±2 | 105±2 | 15 | ||||
| Nitsche (1996) | tetra | C4-C7 | 11 | A | 110±9 | 114±9 | −4 | SpaceLabs 90207 |
| Curt (1997) | tetra | C2-C8 | 13 | B, C, D | 118±13 | 109±8 | 8 | |
| low para | T6-L4 | 9 | A | 118±12 | 106±13 | 10 | ||
| Krum (1991) | tetra | 10 | 118±7 | 116±6 | 2 | SpaceLabs 90207 | ||
| able-bodied | 10 | 126±3 | 114±3 | 10 |
Data is presented as group mean±standard deviation. Nocturnal dip was calculated as (SBPnighttime – SBPdaytime)/SBPdaytime x 100.42
SBP, systolic blood pressure; AIS, American Spinal Injury Association Impairment Scale; ABPM, ambulatory blood pressure monitoring; tetra, tretraplegic; para, paraplegic; C, cervical; L, lumbar; T, thoracic.
The lower daytime BP levels in tetraplegic subjects (see Fig. 2) are mainly attributed to two factors: 1) loss or reduced central sympathetic drive to the spinal sympathetic circuits resulting in decreased peripheral sympathetic neuronal activity and 2) lower daily physical activity level due to distinct paralysis.41 Additional baroreceptor reflex dysfunction due to interruption of its sympathetic efferent arch has also been suggested to contribute to the generally low baseline BP level in complete tetraplegic subjects.43
FIG. 2.
Differences in daytime and nighttime systolic blood pressure (SBP) between three groups: tetraplegia (C4-C8, American Spinal Injury Association Impairment Scale [AIS] A, n=51), paraplegia (T6-L4, AIS A, n=32), and able-bodied subjects (n=36). Mean±standard deviation of SBP was calculated from values of sensorimotor complete SCI and able-bodied subjects from studies presented in Table 1.
In the able-bodied population, such a pronounced chronic hypotension has been related to higher incidence of depression and fatigue,44 reduced cognitive performance,45,46 and lower self-reported general and social well-being.47,48
Circadian changes in blood pressure after SCI
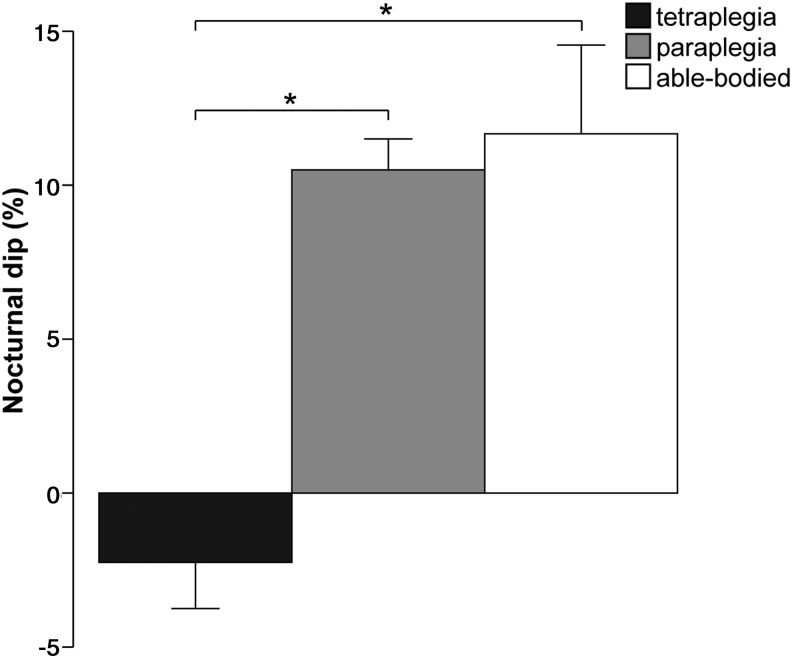
Since daytime resting BP is significantly reduced in tetraplegic subjects but nighttime BP is comparable to paraplegic and able-bodied subjects, the circadian BP rhythm is altered in that tetraplegic subjects show a loss of nocturnal dip (see Table 1 and Fig. 3).41–43,49–54 The level of lesion plays a major role in the preservation of circadian rhythm: SCI subjects with either low thoracic (T6-T12)41,42 or high thoracic lesions (T2-T5)42 show a preserved nocturnal dip, compared with SCI subjects with cervical lesions (C4-C8; see Table 1).
FIG. 3.
Nocturnal dip differences of systolic blood pressure among tetraplegic (C4-C8, American Spinal Injury Association Impairment Scale [AIS] A, n=51), paraplegic (T6-L4, AIS A, n=32), and able-bodied subjects (n=36). Values are shown in mean±standard deviation. Nocturnal dip was calculated as (systolic blood pressure [SBP] nighttime – SBP daytime)/SBP daytime x 10042 from values of sensorimotor complete (AIS A) SCI and able-bodied subjects of studies presented in Table 1. Significant differences in nocturnal SBP dip were found between tetraplegic and paraplegic subjects (p=0.02), and tetraplegic and able-bodied subjects (p=0.03; Mann-Whitney U test).
This indicates that the autonomic supraspinal control over the whole pool of the spinal sympathetic neurons (T1-L2) is required to maintain a circadian BP rhythm in humans. In addition, these findings are in line with the idea that the physiological early morning surge observed in healthy subjects is mediated by the sympathetic nervous system.
Next to the level of lesion, the severity of lesion also affects the circadian BP pattern. Curt and colleagues showed that only subjects with sensorimotor complete (American Spinal Injury Association Impairment Scale-AIS A)55 tetraplegia presented a loss of circadian rhythm, while subjects with sensory incomplete (AIS B, C, D) tetraplegia showed a preserved nocturnal dip.51
The AIS represents sensorimotor impairments after SCI but does not allow any conclusion about the completeness of the autonomic nervous system, which plays a major role in BP control as stated above.56 Therefore, the standard neurological classification of SCI has recently been updated and provided with International Standards to document remaining autonomic function after SCI.56 Only one study so far has focused on autonomic completeness and the influence on circadian BP pattern, which reported that 70% of SCI subjects with autonomically complete lesions (i.e., lack of sympathetic skin responses) have lost circadian BP patterns.51
Not only does ABPM allow monitoring of changes in BP but also in HR. Although the focus of this review is on BP changes after SCI measured by ABPM, the comparison between alterations in HR and BP patterns supports the understanding of involved underlying neuronal mechanisms.
There are inconsistent findings with respect of circadian HR rhythm in the literature. On one side, there are publications presenting preserved circadian HR rhythm,46,48 while other studies report a lost rhythm of circadian HR.45,49
The findings of preserved circadian HR rhythm in tetraplegic subjects, compared with lost circadian BP rhythms in these subjects,50,51 reflects the differences in autonomic control of BP and HR. As the efferent extra-spinal vagal innervation of the heart is still intact in tetraplegic subjects, increased vagal tone appears likely to account for the preserved nocturnal dip in HR in this population.50 The preserved nocturnal HR dip in tetraplegic subjects is interpreted as an adaptive mechanism of the intact parasympathetic cardiac control to a reduced sympathetic outflow after a cervical SCI. HR reductions at night are mediated by increased parasympathetic outflow through the vagal nerve to the heart as well as the withdrawal of sympathetic activity.57 The circadian modulation of vagal activity, with its peak during night, seems to be sufficient to elicit the characteristic nocturnal dip in HR, even after a severe SCI. It is speculated that the physiological nocturnal dip in HR is mainly an effect of increased parasympathetic activity, while the nocturnal dip in BP depends on inhibition of spinal sympathetic outflow by supraspinal centers.43
In contrast, other studies have reported lost circadian HR rhythms after cervical spinal cord lesion.41,49 Their explanation focused on the lost sympathetic drive after a SCI rather than the preserved parasympathetic innervation of the heart; the lack of daytime HR increase in tetraplegic subjects is proposed by a loss of central modulation of cardiac sympathetic nervous activity. The preservation of HR circadian rhythm (i.e., daytime increase) in paraplegic but not in tetraplegic subjects is well explained by the fact that SPNs fibers controlling HR leave the spinal cord at T1-T5 level (see Fig. 1).
Next to alterations of sympatho-excitatory drive after SCI, decreased daily physical activity due to paralysis in affected subjects has been proposed to be another, intuitive, reason for decreased daytime BP (i.e., loss of nocturnal dip).41 However, the observation made by Krum and colleagues that nocturnal dip in BP is markedly diminished in tetraplegic subjects but maintained in neurologically intact orthopedic subjects who were almost totally immobile during the 24-h period suggests that the increased BP during daytime versus nighttime is a consequence of a diurnal variation in sympathetic activity, which is relatively independent of changes in physical activity levels.50
To strengthen the assumption of decreased systemic sympathetic nervous activity after a severe SCI, neurohormal changes have been measured by catecholamine levels in the blood. Tetraplegic subjects with a loss of nocturnal BP dip show simultaneously reduced plasma levels of norepinephrine, compared with paraplegic and able-bodied subjects with preserved nocturnal dip.41,50 In contrast, the plasma level of epinephrine was lower in both, tetraplegic and paraplegic subjects, compared with able-bodied subjects.41 The low plasma level of epinephrine in SCI subjects might be due to a disturbance of sympathoadrenal secretion. However, it seems that central sympathoadrenal drive does not play an essential role in the maintenance of a normal circadian BP rhythm, since paraplegic subjects have a preserved nocturnal dip but low plasma epinephrine levels.41
The loss of circadian BP pattern in tetraplegic subjects was associated with the occurrence of sudden hypertensive events: 91% of SCI tetraplegic subjects with a loss of nocturnal dip in BP showed clinically reported autonomic dysreflexia (AD).51
Blood pressure variability after SCI
A typical daily BP pattern in SCI subjects is characterized by the occurrence of potentially life-threatening hypertensive events due to AD as well as hypotensive events mainly due to changes in position, (i.e., from lying to sitting or standing position).20 These immense BP fluctuations could contribute to increased shear stress of blood vessels and consequently to vascular injury, and predisposing this population to a greater risk for arterial disease.24
ABPM also can be used to assess BP variability within a 24-h period in SCI subjects. It has been reported that tetraplegic subjects who have recently experienced episodes of AD show greater BP and HR variability, assessed as coefficient of variation (CV) within the 24-h period, than able-bodied subjects and SCI subjects who have never reported AD.58
Previously it has been shown that the extent of BP variability plus an abnormal circadian BP rhythm (i.e., a loss of nocturnal BP dip) can help differentiate primary hypertension from hypertension secondary to autonomic dysfunction, such as following SCI.53
In addition to a general assessment of BP variability by calculation of CV, ABPM also has been used to describe the occurrence of specific episodes of AD over a 24-h period.51 In this study, authors reported that in 70% of SCI subjects with clinically reported symptoms of AD, such as headache, goose bumps, profuse sweating etc., pathological ABPM recordings, including high peaks of SBP, could be described.51 Severe episodes of AD with SBP up to 224 mm Hg, bradycardia as low as 45 beats per minute, and accompanying symptoms were reported in subjects with sensorimotor complete tetraplegia.51 However, the same group showed that incomplete tetraplegic subjects neither self-reported any AD events nor could ABPM identify such hypertensive events.43
As described above, ABPM has been used to qualitatively describe pathological recordings within circadian BP pattern after SCI.43,51 However, no study has yet objectively quantified the frequency of hypertensive events nor tried to assess hypotensive events, such as orthostatic hypotension (OH), which is a known clinical problem of a severe SCI.59,60
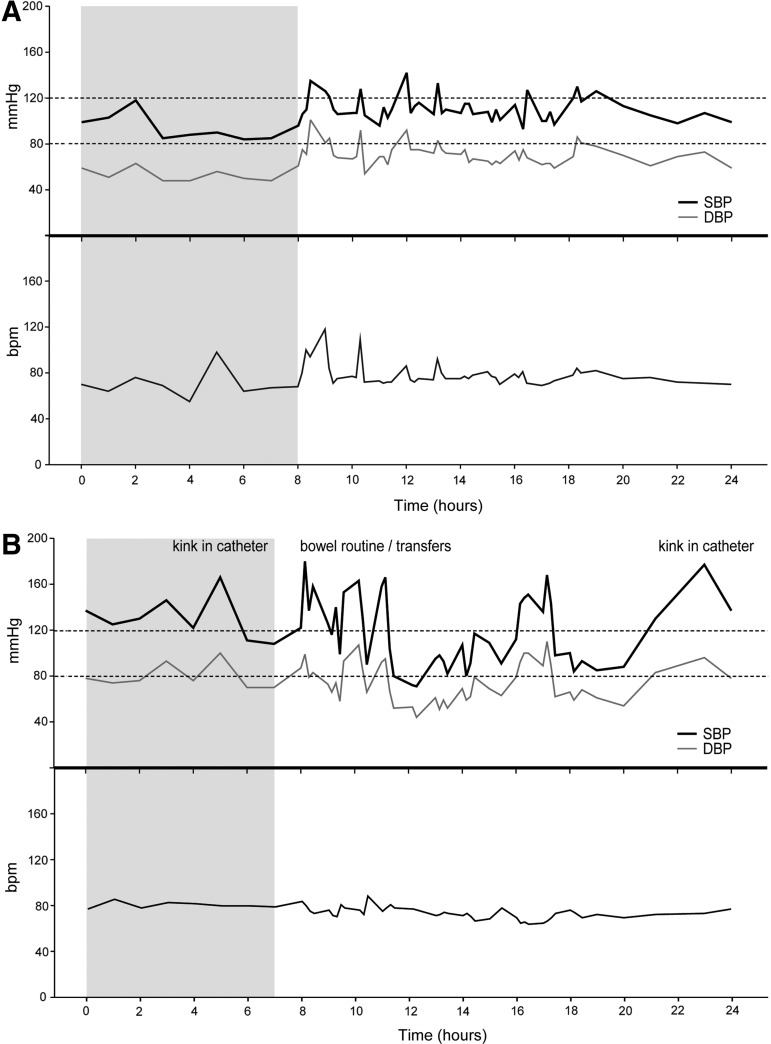
Representative examples of ABPM recordings from our laboratory in intact and subject with SCI are presented in Figure 4.
FIG. 4.
(A) Ambulatory blood pressure monitoring (ABPM) profiles from an able-bodied subject (female, 29 years) with preserved physiological nocturnal dip (>10% drop in systolic blood pressure [SBP] from day- to nighttime) and normal daily blood pressure and heart rate variability due to increased physical bouts, such as walking upstairs. (B) Recordings from a subject with complete tetraplegia (C5, American Spinal Injury Association Impairment Scale B, male, 58 years) showing loss of nocturnal dip, multiple episodes of autonomic dysreflexia and hypotension ranging from 180 to 71 mm Hg SBP. Shaded time section represents nighttime. These ABPM profiles are examples from our laboratory.
The primary mechanisms underlying the increase of BP variability with frequent episodes of AD and OH after SCI include abnormal autonomic control resulting from the loss of supraspinal input to spinal sympathetic circuits.39 Further, numerous subsequent plastic phenomena that occur below the level of SCI possibly contribute to increased BP fluctuations: 1) plastic changes within SPNs61 interneurons,62 2) aberrant dorsal root afferents sprouting,63 3) plastic changes within the peripheral autonomic ganglia,64 and 4) changes in α-adrenoceptors sensitivity.65,66
Clinical Practicability
In the last few years, CVD has emerged as a leading cause of morbidity and mortality in the SCI population, even exceeding pulmonary and renal failures.21–23 ABPM as an out-of-office BP monitoring has been shown in able-bodied subjects to be superior to clinic BP monitoring with respect to its prognostic value of cardiovascular morbidity and mortality.7–10 In particular, the ability to measure nocturnal BP as the most sensitive predictor of CVD outcomes considers ABPM as an important part of clinical practice.12–16
ABPM revealed a persistent hypotension and a lack of an appropriate nocturnal BP dip in tetraplegic subjects, which may predispose them to ischemic brain injury and cognitive deficits as it has been proposed for the able-bodied population.42 In addition, AD, exaggerated BP variability, and disruption of the circadian BP pattern pose potential cardiovascular risk factors that are unique to SCI subjects. ABPM also may improve outpatient management in this regard by further clarifying BP patterns in SCI subjects.53
The use of ABPM enables prediction of the occurrence and severity of autonomic failure (i.e., sympathetic disconnection) in SCI subjects51 and therefore further clarifies underlying neuronal mechanisms of changes in circadian BP patterns. Episodes of daily occurring AD events can hardly be assessed with discrete BP assessments. However, circadian BP patterns give more insight into the occurrence of these potentially life-threatening events and, importantly, can be used to assess the effectiveness of medical and surgical treatments.51
Challenges
Currently available ABPM devices are much smaller, easier to use, and less noisy than previous devices, leading to better acceptability and tolerance in assessed subjects.67 However, compliant use of the devices by subjects sometimes is not possible. Depending on the frequency of measures—generally between 15–30 min while awake—SCI subjects are more or less restricted in their daily activities. Strong movements, for example during wheeling or transfers, can prevent an accurate BP assessment or commonly results in failed readings. Clinical staff should instruct the subjects to ensure they keep the monitored arm steady and at heart level during each reading for the entire 24-hour period.5
ABPM devices produce tactile and sonorous stimuli during BP measurements, which can cause sleep disturbances in some subjects.68,69 The repeated cuff-inflation during overnight BP monitoring (generally every 30–60 min) might cause sleep deprivation resulting in non-physiological BP elevations, and therefore the clinical implications of altered circadian BP patterns in such cases should be interpreted with caution.12
It is encouraged that an activity-diary of the monitoring period is completed by the assessed subject or a caregiver (e.g., in the case of tetraplegia with restricted writing skills) to report sleep and wake times, the timing of BP medication administration and any symptoms potentially related to high or low BP.5 In the SCI population, however, such diaries have seldom been used and if so, only wake and sleep times were noted.42,52 One study used a diary to capture AD symptoms with the goal of identifying silent episodes of AD.53
Since SCI subjects experience significant BP alterations, such as AD and OH, subject diaries should not only capture individual daytime and nighttime BP, and BP medication administration, but also transfers from bed to wheelchair and vice versa, sudden symptoms of OH, such as dizziness, light-headedness, blurred vision and/or nausea, and sudden symptoms of AD, such as headache, heart palpitations, goose bumps and/or profuse sweating. In addition to the report of such events or symptoms in the diary, it might be critical to allow subjects to take additional self-measures in the case of events or symptoms. This may help capture more BP derangements than pre-programmed measures alone.
In addition to technical challenges, it is important to mention that currently most of the clinical ABPM is conducted by cardiologists, who have great expertise in use and interpretation of 24-hour BP profiles in the general population. However, the rather expensive devices, and data processing software as well as the required know-how about ABPM usage, data analysis and interpretation might challenge a widespread adoption of ABPM in the SCI community. Further, without specialized knowledge on possible events that result in BP alteration among SCI individuals (monitoring BP on bowel day, etc.) and detailed documentation (diary) of symptoms makes this technique more difficult to utilize in monitoring SCI individuals. Finally, without strong documentation of adverse effect of unstable BP on health and quality of life of SCI subjects, insurance might not cover ABPM assessments.
Conclusions
In summary, subjects with SCI exhibit drastic changes in their cardiovascular control due to loss of supraspinal input to spinal sympathetic neurons and due to immobility. Subjects with complete tetraplegia show the most adverse BP changes. Therefore, the outpatient management of BP in this population is becoming increasingly important. In addition, SCI subjects are living longer and independently in outpatient settings, and their risk for CVD is known to be increased. ABPM seems to serve as a helpful, non-invasive tool to investigate altered circadian BP patterns and documentation of events related to episodes of OH and AD. Further, abnormal patterns in ABPM, such as a loss of nocturnal dip, have been shown to be associated with increased risk for CVD in the able-bodied population. Therefore, presence of potentially life-threatening AD events as well as abnormal ABPM patterns, also might pose potential CVD risk factors in the SCI population. This relation is not well documented yet in patients with SCI but will be particularly important for complete tetraplegic subjects who show adverse circadian BP profiles. The use of ABPM in this population might help detect these profiles and monitor the efficacy of various treatment strategies. Although further research is required in this area, we suggest that ABPM should be used as a clinical modality for assessment of BP derangements in subjects with SCI.
Acknowledgments
We would like to thank Cameron Gee for his editorial help. This study was supported by a Swiss National Science Foundation Fellowship for prospective researchers (Dr. M. Hubli, Postdoctoral Fellow, PBEZP3_145704) and a grant from the Canadian Institutes of Health Research (PI. Dr. A. Krassioukov, TCA-118348).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Millar-Craig M.W., Bishop C.N., and Raftery E.B. (1978). Circadian variation of blood-pressure. Lancet 1, 795–797 [DOI] [PubMed] [Google Scholar]
- 2.Cranston W.I. (1964). Diurnal variation in plasma volume in normal and hypertensive subjects. Am. Heart J. 68, 427–428 [DOI] [PubMed] [Google Scholar]
- 3.James G.D. and Pickering T.G. (1993). The influence of behavioral factors on the daily variation of blood pressure. Am. J. Hypertens. 6, 170S–173S [DOI] [PubMed] [Google Scholar]
- 4.Pickering T.G., Hall J.E., Appel L.J., Falkner B.E., Graves J., Hill M.N., Jones D.W., Kurtz T., Sheps S.G., and Roccella E.J. (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Hypertension 45, 142–161 [DOI] [PubMed] [Google Scholar]
- 5.O'Brien E., Asmar R., Beilin L., Imai Y., Mallion J.M., Mancia G., Mengden T., Myers M., Padfield P., Palatini P., Parati G., Pickering T., Redon J., Staessen J., Stergiou G., and Verdecchia P. (2003). European society of hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J. Hypertens. 21, 821–848 [DOI] [PubMed] [Google Scholar]
- 6.O'Brien E. (2011). Twenty-four-hour ambulatory blood pressure measurement in clinical practice and research: a critical review of a technique in need of implementation. J. Intern. Med. 269, 478–495 [DOI] [PubMed] [Google Scholar]
- 7.Dolan E., Stanton A., Thijs L., Hinedi K., Atkins N., McClory S., Den Hond E., McCormack P., Staessen J.A., and O'Brien E. (2005). Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 46, 156–161 [DOI] [PubMed] [Google Scholar]
- 8.Eguchi K., Pickering T.G., Hoshide S., Ishikawa J., Ishikawa S., Schwartz J.E., Shimada K., and Kario K. (2008). Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am. J. Hypertens. 21, 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickering T.G., Shimbo D., and Haas D. (2006). Ambulatory blood-pressure monitoring. N. Engl. J. Med. 354, 2368–2374 [DOI] [PubMed] [Google Scholar]
- 10.Clement D.L., De Buyzere M.L., De Bacquer D.A., de Leeuw P.W., Duprez D.A., Fagard R.H., Gheeraert P.J., Missault L.H., Braun J.J., Six R.O., Van Der Niepen P., and O'Brien E. (2003). Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N. Engl. J. Med. 348, 2407–2415 [DOI] [PubMed] [Google Scholar]
- 11.Eguchi K., Ishikawa J., Hoshide S., Pickering T.G., Schwartz J.E., Shimada K., and Kario K. (2009). Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am. J. Hypertens. 22, 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yano Y. and Kario K. (2012). Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens. Res. 35, 695–701 [DOI] [PubMed] [Google Scholar]
- 13.Fagard R.H., Celis H., Thijs L., Staessen J.A., Clement D.L., De Buyzere M.L., and De Bacquer D.A. (2008). Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 51, 55–61 [DOI] [PubMed] [Google Scholar]
- 14.Boggia J., Li Y., Thijs L., Hansen T.W., Kikuya M., Bjorklund-Bodegard K., Richart T., Ohkubo T., Kuznetsova T., Torp-Pedersen C., Lind L., Ibsen H., Imai Y., Wang J., Sandoya E., O'Brien E., and Staessen J.A. (2007). Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 370, 1219–1229 [DOI] [PubMed] [Google Scholar]
- 15.Hansen T.W., Li Y., Boggia J., Thijs L., Richart T., and Staessen J.A. (2011). Predictive role of the nighttime blood pressure. Hypertension 57, 3–10 [DOI] [PubMed] [Google Scholar]
- 16.Ben-Dov I.Z., Kark J.D., Ben-Ishay D., Mekler J., Ben-Arie L., and Bursztyn M. (2007). Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 49, 1235–1241 [DOI] [PubMed] [Google Scholar]
- 17.O'Brien E., Sheridan J., and O'Malley K. (1988). Dippers and non-dippers. Lancet 2, 397. [DOI] [PubMed] [Google Scholar]
- 18.Ingelsson E., Bjorklund-Bodegard K., Lind L., Arnlov J., and Sundstrom J. (2006). Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 295, 2859–2866 [DOI] [PubMed] [Google Scholar]
- 19.Verdecchia P., Schillaci G., Gatteschi C., Zampi I., Battistelli M., Bartoccini C., and Porcellati C. (1993). Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation 88, 986–992 [DOI] [PubMed] [Google Scholar]
- 20.Krassioukov A. and Claydon V.E. (2006). The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog. Brain Res. 152, 223–229 [DOI] [PubMed] [Google Scholar]
- 21.Garshick E., Kelley A., Cohen S.A., Garrison A., Tun C.G., Gagnon D., and Brown R. (2005). A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 43, 408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers J., Lee M., and Kiratli J. (2007). Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am. J. Phys. Med. Rehabil. 86, 142–152 [DOI] [PubMed] [Google Scholar]
- 23.Groah S.L., Nash M.S., Ward E.A., Libin A., Mendez A.J., Burns P., Elrod M., and Hamm L.F. (2011). Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J. Cardiopulm. Rehabil. Prev. 31, 73–80 [DOI] [PubMed] [Google Scholar]
- 24.Steins S.A., Johnson M.C., and Lyman P.J. (1995). Cardiac rehabilitation in patients with spinal cord injuries. Phys. Med. Rehabil. Clin. 6, 263–296 [Google Scholar]
- 25.Singh M., Mensah G.A., and Bakris G. (2010). Pathogenesis and clinical physiology of hypertension. Cardiol. Clin. 28, 545–559 [DOI] [PubMed] [Google Scholar]
- 26.Krassioukov A. and Weaver L.C. (1996). Anatomy of the autonomic nervous system. In: Physical Medicine and Rehabilitation: State of the Art Reviews. Teasell R.W. and Baskerville V.B. (eds). Hanley & Belfus, Inc.: Philadelphia, pps. 1–14 [Google Scholar]
- 27.West C.R., Mills P., and Krassioukov A.V. (2012). Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord 50, 484–492 [DOI] [PubMed] [Google Scholar]
- 28.Prasad K. (2000). Blood pressure and its control mechanism. In: Textbook of Angiology. Chang J. (ed). Springer: New York, pps. 46–54 [Google Scholar]
- 29.Gasperin D., Netuveli G., Dias-da-Costa J.S., and Pattussi M.P. (2009). Effect of psychological stress on blood pressure increase: a meta-analysis of cohort studies. Cad. Saude Publica. 25, 715–726 [DOI] [PubMed] [Google Scholar]
- 30.Maemura K., Takeda N., and Nagai R. (2007). Circadian rhythms in the CNS and peripheral clock disorders: role of the biological clock in cardiovascular diseases. J. Pharmacol. Sci. 103, 134–138 [DOI] [PubMed] [Google Scholar]
- 31.Pickering T.G. and James G.D. (1993). Determinants and consequences of the diurnal rhythm of blood pressure. Am. J. Hypertens. 6, 166S–169S [DOI] [PubMed] [Google Scholar]
- 32.Furlan R., Guzzetti S., Crivellaro W., Dassi S., Tinelli M., Baselli G., Cerutti S., Lombardi F., Pagani M., and Malliani A. (1990). Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and rr variabilities in ambulant subjects. Circulation 81, 537–547 [DOI] [PubMed] [Google Scholar]
- 33.Dodt C., Breckling U., Derad I., Fehm H.L., and Born J. (1997). Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension 30, 71–76 [DOI] [PubMed] [Google Scholar]
- 34.Linsell C.R., Lightman S.L., Mullen P.E., Brown M.J., and Causon R.C. (1985). Circadian rhythms of epinephrine and norepinephrine in man. J. Clin. Endocrinol. Metab. 60, 1210–1215 [DOI] [PubMed] [Google Scholar]
- 35.Panza J.A., Epstein S.E., and Quyyumi A.A. (1991). Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 325, 986–990 [DOI] [PubMed] [Google Scholar]
- 36.Schofl C., Becker C., Prank K., von zur Muhlen A., and Brabant G. (1997). Twenty-four-hour rhythms of plasma catecholamines and their relation to cardiovascular parameters in healthy young men. Eur. J. Endocrinol. 137, 675–683 [DOI] [PubMed] [Google Scholar]
- 37.Mansoor G.A., White W.B., McCabe E.J., and Giacco S. (2000). The relationship of electronically monitored physical activity to blood pressure, heart rate, and the circadian blood pressure profile. Am. J. Hypertens. 13, 262–267 [DOI] [PubMed] [Google Scholar]
- 38.Clark L.A., Denby L., Pregibon D., Harshfield G.A., Pickering T.G., Blank S., and Laragh J.H. (1987). A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J. Chronic Dis. 40, 671–681 [DOI] [PubMed] [Google Scholar]
- 39.Krassioukov A. (2009). Autonomic function following cervical spinal cord injury. Respir. Physiol. Neurobiol. 169, 157–164 [DOI] [PubMed] [Google Scholar]
- 40.Frisbie J.H. (2007). Unstable baseline blood pressure in chronic tetraplegia. Spinal Cord 45, 92–95 [DOI] [PubMed] [Google Scholar]
- 41.Munakata M., Kameyama J., Kanazawa M., Nunokawa T., Moriai N., and Yoshinaga K. (1997). Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: Simultaneous evaluation of autonomic nervous activity and physical activity. J. Hypertens. 15, 1745–1749 [DOI] [PubMed] [Google Scholar]
- 42.Rosado-Rivera D., Radulovic M., Handrakis J.P., Cirnigliaro C.M., Jensen A.M., Kirshblum S., Bauman W.A., and Wecht J.M. (2011). Comparison of 24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J. Spinal Cord Med. 34, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nitsche B., Perschak H., Curt A., and Dietz V. (1996). Loss of circadian blood pressure variability in complete tetraplegia. J. Hum. Hypertens. 10, 311–317 [PubMed] [Google Scholar]
- 44.Barrett-Connor E. and Palinkas L.A. (1994). Low blood pressure and depression in older men: a population based study. BMJ 308, 446–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duschek S., Matthias E., and Schandry R. (2005). Essential hypotension is accompanied by deficits in attention and working memory. Behav. Med. 30, 149–158 [DOI] [PubMed] [Google Scholar]
- 46.Duschek S. and Schandry R. (2007). Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin. Auton. Res. 17, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilgrim J.A., Stansfeld S., and Marmot M. (1992). Low blood pressure, low mood? BMJ 304, 75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosengren A., Tibblin G., and Wilhelmsen L. (1993). Low systolic blood pressure and self perceived wellbeing in middle aged men. BMJ 306, 243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goswami R., Krishan K., Suryaprakash B., Vaidyanathan S., Rao K., Rao M.S., Goswami A.K., Goel A.K., and Sharma P.L. (1985). Circadian desynchronization in pulse rate, systolic and diastolic blood pressure, rectal temperature and urine output in traumatic tetraplegics. Indian J. Physiol. Pharmacol. 29, 199–206 [PubMed] [Google Scholar]
- 50.Krum H., Louis W.J., Brown D.J., Jackman G.P., and Howes L.G. (1991). Diurnal blood pressure variation in quadriplegic chronic spinal cord injury patients. Clin. Sci. 80, 271–276 [DOI] [PubMed] [Google Scholar]
- 51.Curt A., Nitsche B., Rodic B., Schurch B., and Dietz V. (1997). Assessment of autonomic dysreflexia in patients with spinal cord injury. J. Neurol. Neurosurg. Psychiatry 62, 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casiglia E., Pizziol A., Piacentini F., Biasin R., Onesto C., Tikhonoff V., Prati R., Palatini P., and Pessina A.C. (1999). 24-hour leg and forearm haemodynamics in transected spinal cord subjects. Cardiovasc. Res. 41, 312–316 [DOI] [PubMed] [Google Scholar]
- 53.Tolbert G. and Tuck M.L. (2004). Ambulatory blood pressure monitoring in persons with chronic spinal cord injury. J. Spinal Cord Med. 27, 476–480 [DOI] [PubMed] [Google Scholar]
- 54.Seabra-Garcez J.D., Matos-Souza J.R., Goulart D., Pithon K.R., Abib E., Etchebehere M., Cliquet A., Jr., and Nadruz W., Jr., (2012). Ambulatory blood pressure is associated with subclinical atherosclerosis in spinal cord injury subjects. Int. J. Cardiol. 154, 89–90 [DOI] [PubMed] [Google Scholar]
- 55.Kirshblum S.C., Burns S.P., Biering-Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt-Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krassioukov A., Biering-Sorensen F., Donovan W., Kennelly M., Kirshblum S., Krogh K., Alexander M.S., Vogel L., and Wecht J. (2012). International standards to document remaining autonomic function after spinal cord injury. J. Spinal Cord Med. 35, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claydon V.E. and Krassioukov A.V. (2008). Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am. J. Physiol. Heart Circ. Physiol. 294, 668–678 [DOI] [PubMed] [Google Scholar]
- 58.Krum H., Howes L.G., Brown D.J., and Louis W.J. (1989). Blood pressure variability in tetraplegic patients with autonomic hyperreflexia. Paraplegia 27, 284–288 [DOI] [PubMed] [Google Scholar]
- 59.Sidorov E.V., Townson A.F., Dvorak M.F., Kwon B.K., Steeves J., and Krassioukov A. (2008). Orthostatic hypotension in the first month following acute spinal cord injury. Spinal Cord 46, 65–69 [DOI] [PubMed] [Google Scholar]
- 60.Claydon V.E. and Krassioukov A.V. (2006). Orthostatic hypotension and autonomic pathways after spinal cord injury. J. Neurotrauma 23, 1713–1725 [DOI] [PubMed] [Google Scholar]
- 61.Krassioukov A.V. and Weaver L.C. (1995). Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin. Exp. Hypertens. 17, 361–373 [DOI] [PubMed] [Google Scholar]
- 62.Krassioukov A.V., Johns D.G., and Schramm L.P. (2002). Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J. Neurotrauma 19, 1521–1529 [DOI] [PubMed] [Google Scholar]
- 63.Krenz N.R., Meakin S.O., Krassioukov A.V., and Weaver L.C. (1999). Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J. Neurosci. 19, 7405–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramer L.M., van Stolk A.P., Inskip J.A., Ramer M.S., and Krassioukov A.V. (2012). Plasticity of trpv1-expressing sensory neurons mediating autonomic dysreflexia following spinal cord injury. Front Physiol. 3, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alan N., Ramer L.M., Inskip J.A., Golbidi S., Ramer M.S., Laher I., and Krassioukov A.V. (2010). Recurrent autonomic dysreflexia exacerbates vascular dysfunction after spinal cord injury. Spine J. 10, 1108–1117 [DOI] [PubMed] [Google Scholar]
- 66.Arnold J.M., Feng Q.P., Delaney G.A., and Teasell R.W. (1995). Autonomic dysreflexia in tetraplegic patients: evidence for alpha-adrenoceptor hyper-responsiveness. Clin. Auton. Res. 5, 267–270 [DOI] [PubMed] [Google Scholar]
- 67.Mallion J.M., Baguet J.P., and Mancia G. (2006). European society of hypertension scientific newsletter: clinical value of ambulatory blood pressure monitoring. J. Hypertens. 24, 2327–2330 [DOI] [PubMed] [Google Scholar]
- 68.Mallion J.M., de Gaudemaris R., Baguet J.P., Azzouzi L., Quesada J.L., Sauzeau C., Siche J.P., Tremel F., and Boutelant S. (1996). Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press. Monit. 1, 197–203 [PubMed] [Google Scholar]
- 69.Dimsdale J.E., Coy T.V., Ancoli-Israel S., Clausen J., and Berry C.C. (1993). The effect of blood pressure cuff inflation on sleep. A polysomnographic examination. Am. J. Hypertens. 6, 888–891 [DOI] [PubMed] [Google Scholar]