The concept of cardiac progenitors that persist late into heart development and potentially into postnatal life has ignited the interest of cardiac biologists. Little more than a decade ago, the prevailing notion of heart development centered on a cardiac tube containing immature cardiomyocytes that were committed to form specific segments of the mature heart1. Over the past 15 years, this textbook view was overturned by the modern rediscovery of the pioneering work of Viragh and Chalice and others, which showed that the heart tube grows by addition of new cardiomyocytes to the arterial and venous poles by differentiation of non-cardiomyocyte progenitors2–5. Thus, at the time that the initial heart tube is first visible in the developing embryo through heart looping and the initiation of septation, cardiac progenitors present at both poles of the heart differentiate into cardiomyocytes and thereby significantly contribute to heart growth (Figure panel A).
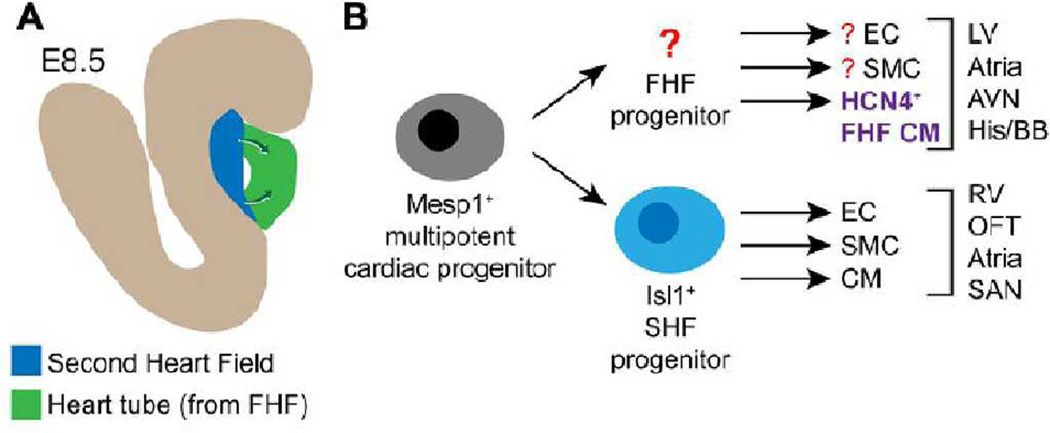
Figure. Cardiac progenitor populations in the developing heart.
A. Illustration of an E8.5 embryo highlighting the heart tube, containing differentiated cardiomyocytes and originating from the first heart field (FHF), and the second heart field (SHF), containing SHF progenitors. SHF progenitors differentiate into cardiac lineages to contribute to the ends of the heart tube (arrows). B. Lineage map of the developing heart. Around the time of gastrulation, a multipotent cardiac progenitor (likely Mesp1+) gives rise to Isl1+ SHF progenitors and possibly a FHF progenitor (yet to be identified). SHF progenitors yield most of the right ventricle (RV), outflow tract (OFT), atria, and sinoatrial node (SAN). FHF cardiomyocyte derivatives, arising from the hypothetical FHF progenitor or directly form Mesp1+ progenitors, express HCN4 at the cardiac crescent stage. These cells ultimately yield most of the left ventricle (LV) and portions of the atria and the cardiac conduction system including the atrioventricular note, bundle of His, and the bundle branches.
These later differentiating progenitors have been referred to as "second heart field progenitors", and reside in the "second heart field" (SHF) located at the arterial and venous pole sof the developing heart. Evans, Cai, and colleagues made the seminal discovery that these SHF progenitors express the transcription factor Islet 1 (ISL1)6. Isl1 is required in the progenitor cells for their normal activity, but is shut off as the progenitors differentiate. ISL1+ SHF progenitors are multipotent and differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells, the major lineages of the heart7. Development of Cre-based reagents allowed investigators to selectively label, isolate, and genetically manipulate these progenitors and their descendants in developing embryos. Isl1-based genetic reagents also proved valuable in isolating these progenitor cells and their descendants from differentiating ES cells, facilitating their in vitro analysis7, 8. Cre-based genetic lineage tracing using most Cre-activated reporters indicated that Isl1Cre-marked SHF progenitors contribute to the right ventricle, the outflow tract, and much of the atria, but little of the left ventricle6. These data suggested that most of these regions of the heart are generated by SHF progenitors following specification of the initial linear heart tube, although an important caveat is that the precise boundaries of the Isl1Cre domain depend on both the specific Isl1Cre allele and the specific Cre-dependent reporter used9, 10.
Relatively less is known about the first differentiating cardiomyocytes of the linear heart tube. By analogy to the terminology used for the SHF, it would be logical to define the "first heart field" as the region of the embryo that houses the "first heart field progenitors", i.e., those cardiac progenitors which first differentiate into the cardiomyocytes that populate the cardiac crescent and linear heart tube (Fig. 1b). By inference based on the Isl1Cre genetic fate map, descendants of the FHF generate most of the left ventricle and a subset of the atria. However, a lack of appropriate markers and genetic reagents has hindered studies of FHF progenitors and their descendants. This gap in knowledge has been particularly acute because these cells most closely match the needs of regenerative applications for acquired heart disease, which primarily affect the left ventricle.
In this issue of Circulation Research, Evans, Liang and colleagues report that the pacemaker channel gene Hcn4 (hyperpolarization activated nucleotide gated cation channel 4) marks the FHF11. Using a suite of Hcn4 knockin alleles (Hcn4LacZ, Hcn4H2BGFP, and Hcn4CreERT2), Liang et al. show that Hcn4 is expressed throughout the cardiac crescent at mouse embryonic day 7.5, in a population distinct from Isl1+ SHF progenitors. Subsequently, Hcn4 is dynamically expressed in portions of the conduction system. Activation of Hcn4CreERT2 with tamoxifen at E7.5 pulse-labeled allowed the fate of their descendants to be traced. As anticipated by inference from Isl1+ SHF progenitor genetic lineage tracing, the Hcn4CreERT2-labeled descendants were found primarily in the left ventricle. Additional descendants were found in the atria, coronary sinus, venous valves, and atrioventricular (AV) and sinoatrial (SA) nodes.
What types of cells in the E7.5 cardiac crescent express Hcn4 and are labeled by Hcn4CreERT2? Are they cardiomyocyte progenitors and if so are they multipotent like Isl1+ progenitors7? Or have they already committed to the cardiomyocyte lineage by the time that they express Hcn4 and are labeled at E7.5? Unfortunately, the study of Liang et al. does not directly address these points. After E9.5 Hcn4H2BGFP was expressed primarily in cardiomyocytes, but whether or not Hcn4 expression is similarly confined to differentiated cardiomyocytes at E7.5 is unclear. Analysis of Hcn4 or Hcn4-driven labels (e.g. H2BGFP or preferably CreERT2) with cardiac progenitor (e.g. Mesp1; Nkx2-5) or cardiomyocyte (e.g. Mlc2a) lineage markers at E7.5 would have been helpful to address this point. Similarly, more detailed analysis of the cell lineages derived from E7.5 Hcn4CreERT2-labeled cells would have provided information about their fate and multipotency. As it is, we can unequivocally say that Hcn4CreERT2 activated at E7.5 marks FHF derivatives (probably cardiomyocytes), but whether or not it marks FHF progenitors is uncertain based on the available data.
Broad expression of Hcn4 in myocardial cells is rapidly extinguished after the linear heart tube stage, so that by E16.5 it is confined to the conduction system, including the SA and AV nodes, the His bundle, the bundle branches, and the Purkinje fibers11. These data, consistent with findings based on a previously reported Hcn4CreERT2 allele12, suggest that Hcn4-driven alleles will be useful tools to label and isolate conduction system cells at later stages of heart development. As pointed out by Liang et al., Tam pulse labeling at these later stages should not be confused with descent from FHF but rather reflects the dynamic nature of Hcn4 expression in different myocardial compartments.
An important use of cardiac lineage markers to be permit isolation of cardiac cell types from pluripotent stem cell differentiation systems, where there is a paucity of anatomical information to facilitate the segregation of populations. It would be desirable, for instance, to differentiate ES cells and isolate FHF progenitors or their derivatives. Whether or not this will be possible with Hcn4 remains to be determined. The dynamic pattern of Hcn4 expression suggests that precisely staged differentiation systems and/or a collection of multiple markers will be needed to permit isolation of the equivalent of the labeled cardiac crescent cells and their derivatives. Interestingly, because Hcn4 is a transmembrane channel, it is possible that its extracellular epitopes could be used as a cell surface marker to permit isolation of Hcn4+ cells without the need for genetic modification.
Over the past decade, considerable advances have been made in elucidating the lineage map of heart development (Figure panel B). Retrospective lineage mapping approaches have defined two distinct cardiac lineages that bifurcate from a common cardiac progenitor probably at or soon after gastrulation13. This common cardiac progenitor, likely marked by Mesp114, 15, differentiates into Isl1+ SHF progenitors, which subsequently differentiate to yield the right ventricle, outflow tract, and portions of the atria and conduction system. Conceptually, the common Mesp1+ progenitor also gives rise to a FHF progenitor, which then differentiates to yield the left ventricle and other portions of the atria and conduction system. Evans, Liang, and colleagues have advanced the field by providing a means to label and isolate these FHF derivatives from embryos. In the future, it will be important to determine if the Hcn4-expressing cells are already committed cardiomyocytes, and if so it will be important to develop means to isolate the hypothetical FHF progenitors. Alternatively, it is possible that Mesp1+ progenitors differentiate directly into committed Hcn4+ cardiomyocytes without a defined progenitor intermediate. It will also be crucial to determine whether and how Hcn4 might be used to isolate bona fide left ventricular cardiomyocytes or their precursors from pluripotent stem cell cultures, and whether the kinetics of human cardiogenesis also permit HCN4 to be used for this purpose in human pluripotent stem cell differentiation.
Acknowledgments
Sources of Funding
WTP was supported by NIH (R01 HL094683 and U01 HL100401), and by charitable support from Gail Federici Smith, Edward Marram, and Karen Carpenter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no competing interests to disclose.
References
- 1.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 2.Viragh S, Challice CE. Origin and differentiation of cardiac muscle cells in the mouse. J Ultrastruct Res. 1973;42:1–24. doi: 10.1016/s0022-5320(73)80002-4. [DOI] [PubMed] [Google Scholar]
- 3.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 4.Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 5.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 6.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang X, Wang G, Lin L, Lowe J, Zhang Q, Bu L, Chen Y-H, Chen J, Sun Y, Evans SM. HCN4 Dynamically Marks the First Heart Field and Conduction System Precursors. Circulation Research. 2013 doi: 10.1161/CIRCRESAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoesl E, Stieber J, Herrmann S, Feil S, Tybl E, Hofmann F, Feil R, Ludwig A. Tamoxifen-inducible gene deletion in the cardiac conduction system. J Mol Cell Cardiol. 2008;45:62–69. doi: 10.1016/j.yjmcc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6:685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- 14.Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3:69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Bondue A, Tannler S, Chiapparo G, Chabab S, Ramialison M, Paulissen C, Beck B, Harvey R, Blanpain C. Defining the earliest step of cardiovascular progenitor specification during embryonic stem cell differentiation. J Cell Biol. 2011;192:751–765. doi: 10.1083/jcb.201007063. [DOI] [PMC free article] [PubMed] [Google Scholar]