Some say the world will end in fire, some say in ice. Robert Frost
Most patients with systemic lupus erythematosus (SLE) produce autoantibodies against DNA-protein or RNA-protein autoantigens, such as U1 small nuclear ribonucleoproteins (snRNPs) or cytoplasmic small ribonucleoproteins such as the Ro60/SS-A autoantigen. Accumulating evidence suggests that the nucleic acid components of these antigens are endogenous adjuvants capable of eliciting chronic inflammatory responses via the innate immune receptors toll-like receptor 7 (TLR7) or TLR9 (1, 2). Thus, although strongly associated with autoantibodies, a hallmark of adaptive immunity, SLE increasingly is viewed as a disorder of innate immunity. Along with the TLRs, other cellular sensors, including the NOD-like receptors, detect exogenous pathogen associated molecular signals (PAMPs) or endogenous “danger” associated molecular patterns (DAMPs) generated by cell death or stress (Table 1). These innate immune receptors must be tightly regulated to prevent autoimmune or autoinflammatory disorders.
Selected examples of innate immune receptors implicated in autoimmunity and their endogenous and exogenous ligands
| Receptor | Endogenous ligands | Exogenous ligands |
|---|---|---|
| Toll-like receptors | ||
| TLR2 (PM)* | HMGB1 | Lipoteichoic acid, peptidoglycan (muramyl dipeptide) |
| TLR3 (E) | Cellular dsRNA (e.g. U1 RNA) | Viral dsRNA, poly(I:C) |
| TLR7 (E) | Cellular ssRNA (e.g. U1 RNA) | Bacterial ssRNA, viral ssRNA |
| TLR9 (E) | Cellular dsDNA with unmethylated CpG sequences | Bacterial dsDNA with unmethylated CpG sequences |
| NOD-like receptors | ||
| NLRP3 (C) | Uric acid crystals†, cholesterol† | Muramyl dipeptide§, bacterial RNA§, poly(I:C)§, alum†, silica†, staphylococcal α-hemolysin†, influenza virus† |
| AIM2 (C) | Cellular dsDNA | Bacterial dsDNA (e.g. vaccinia, Francisella) |
Location: PM, plasma membrane; E, endolysosome; C, cytoplasm
Acts via TLR and NFκB, which increases transcription of NLRP3 (“signal 1”)
Acts directly on NLRP3 via ATP and possibly decreased intracellular K+ or pore formation (“signal 2”)
Bold, involved in pristane-induced lupus
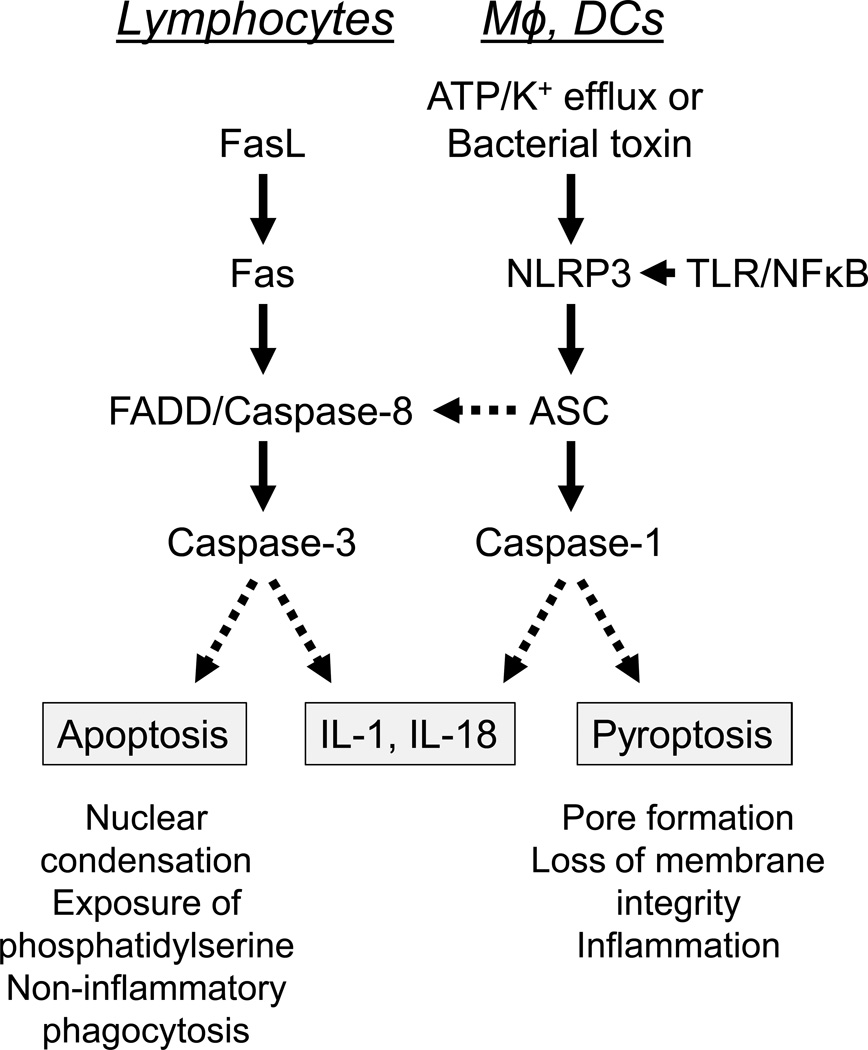
Like SLE, systemic autoinflammatory diseases, such as familial Mediterranean fever (FMF) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS), are characterized by recurrent episodes of fever, serositis, arthritis, and skin rashes, but in contrast to SLE autoantibody production is absent. Autoinflammatory diseases are caused by mutations in the components of inflammasomes, multiprotein assemblies that detect DAMPs and PAMPs, serving as a platform for activation of the protease caspase-1 (also known as interleukin-1 converting enzyme, or ICE). This leads to the conversion of pro-interleukin 1 (IL-1) and pro-interleukin 18 (IL-18) to their mature forms (3). Caspase I also can cleave other, less well defined, substrates leading to pyroptosis, a form of programmed cell death that causes the proinflammatory release of cellular constituents (Fig. 1). In contrast, apoptosis resulting from the engagement of Fas by Fas ligand (FasL) is dependent on caspase-3 and usually non-inflammatory in cells of the adaptive immune system (B cells, T cells), though in cells of the innate immune system (macrophages, dendritic cells, neutrophils) Fas signaling can be proinflammatory, stimulating IL-1 and IL-18 production (4, 5) (Fig. 1). In innate immune cells, the Fas-FasL dependent, caspase-1 independent, activation of pro-IL-1 and pro-IL-18 results from TLR-mediated upregulation of Fas expression, leading to FADD-dependent activation of caspase-8 and subsequently caspase-3 (4) (Fig. 1).
Figure.
Caspase activation by the extrinsic (Fas-mediated) apoptosis pathway (left) vs. the NLRP3 inflammasome (right). Left, in lymphocytes, Fas-Fas ligand (FasL) engagement results in the activation of an effector caspase (caspase-3) by an initiator caspase (caspase-8) and the adapter protein FADD. Caspase-3 activation cleaves target molecules that result in apoptosis, a form of programmed cell death without cell lysis. In macrophages (Mφ) and dendritic cells (DCs), TLR signaling upregulates Fas, which then can respond to FasL, leading to caspase-8 activation and caspase-1 independent maturation of pro-IL-1 and pro-IL-18. Right, in macrophages and dendritic cells, the expression of NLRP3 and pro-IL-1 is enhanced by TLR ligands (signal 1) and NLRP3 is activated by ATP and K+ efflux (or by pore formation due to certain bacterial toxins) (signal 2). This leads to the activation of caspase-1 via the adapter protein ASC (apoptosis-associated speck-like protein containing a CARD). Caspase-1 cleaves pro-IL-1 and pro-IL-18 into their mature, biologically active forms. In addition, caspase-1 can cleave other substrates that cause pyroptosis, a form of programmed cell death accompanied by cell lysis. Activation of the NLRP3 inflammasome also can activate apoptosis via ASC and caspase-8.
There is substantial evidence that lupus is associated with defects in the regulation of cell death and/or the clearance of dead cells (6). Moreover, inflammation and cell death are linked, for example in the case of the NOD-like receptors (also known as nucleotide-binding domain, leucine-rich repeat containing or NLRs), which mediate inflammasome activation (7). Several types of inflammasomes exist, including NLRP1, NLRP3, IPAF, and AIM2 (8). The best studied is the NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome, which consists of NLRP3, the adapter protein ASC, and procaspase-1. Mutations of NLRP3 inflammasome components are involved in FMF, Muckle-Wells syndrome, and other autoinflammatory diseases (9). Activation of caspase-1 via the NLRP3 inflammasome requires two signals: signal 1 is delivered via TLR or tumor necrosis factor α receptor (TNFR) signaling, which increases the transcription of Pro-IL-1β and NLRP3. Signal 2 directly activates NLRP3 and is provided by ATP or bacterial toxins that form pores in the cell membrane, such as staphylococcal α-hemolysin, altering the intracellular concentration of Na+ and K+ (3). This promotes caspase-1 activation and consequently the cleavage of Pro-IL-1β and Pro-IL-18 to mature IL-1β and IL-18, respectively (Fig. 1). Until recently, IL-1β was thought to be the most important inflammatory mediator and a second closely related cytokine, IL-1α was thought to be released primarily as a consequence of cell injury/death (10). However, there is evidence that IL-1α can be secreted by both caspase-1/inflammasome-dependent (11) and -independent (11, 12) pathways and that it too can promote inflammatory responses (13).
Although SLE is not generally considered an autoinflammatory disorder, recent evidence suggests that NLRP3 inflammasome activation and IL-1 production may be involved in its pathogenesis. IL-1β is expressed at higher levels by macrophages in the renal cortex of MRL/lpr mice with lupus nephritis than in young MRL/lpr mice with normal kidneys (14) and treatment of NZB/W mice with IL-1 accelerates the onset of lupus nephritis (15). Moreover, the NLRP3 inflammasome is activated by immune complexes consisting of U1 snRNPs and anti-Sm/RNP autoantibodies, resulting in K+ efflux and IL-1β production (16). This effect can be reversed by knocking down NLRP3 or inhibiting caspase-1 or TLR7 signaling, suggesting that U1 RNA may provide “signal 1” by interacting with TLR7. The mechanism of K+ efflux (signal 2) remains unclear. DNA-anti-DNA immune complexes activate the NLRP3 inflammasome similarly, but require TLR9 instead of TLR7 (17). The NLRP3 inflammasome also can be activated by DNA in neutrophil extracellular traps (NETs), which is recognized by the DNA-binding antimicrobial peptide LL-37. Interestingly, lupus macrophages are more prone than normal macrophages to NLRP3 activation by NETs/LL-37 (18). Finally, treatment of MRL/lpr mice with Bay11-7082, an NFκB inhibitor with an independent inhibitory effect on the NLRP3 inflammasome, reduces lupus nephritis (19) and anakinra (IL-1 receptor antagonist) may be useful for treating arthritis in SLE patients (20).
In this issue of Arthritis & Rheumatism, Kahlenberg, et al. present compelling new evidence that caspase-1 and inflammasome activation are linked to the pathogenesis of lupus (21). Using an inducible mouse model of SLE, pristane-induced lupus (1), the authors show that autoantibody production, transcription of IFN-I regulated genes (interferon signature), and the development of immune complex-mediated glomerulonephritis all are greatly attenuated in caspase-1 deficient mice. In addition, vascular dysfunction induced by pristane is reduced, consistent with the authors’ previous observations that caspase-1 inhibition improves IL-18-mediated endothelial progenitor cell dysfunction in lupus.
Intraperitoneal injection of pristane (2,6,10,14-tetramethylpentadecane), a naturally occurring isoprenoid alkane with adjuvant properties, causes chronic peritoneal inflammation with an influx of neutrophils and inflammatory monocytes followed by the TLR7- and IFN-I dependent production of anti-Sm/RNP (U1 snRNP) and anti-DNA autoantibodies and the onset of immune complex-mediated glomerulonephritis (1). Recent work of Herman, et al. (22) looking at pristane-induced arthritis in rats further supports the conclusion of Kahlenberg, et al. that in addition to its marked enhancement of TLR7 signaling, pristane activates inflammasomes and IL-1/IL-18 production. In the Herman study, pristane stimulated IL-1β and IL-1α when injected into rats and also in cell culture using a human monocyte line (22). IL-1β secretion could be reduced by the sulfonylurea glibenclamide (Glyburide), an ATP-dependent K+ channel blocker. Kahlenberg, et al. now show that in wild-type mice, expression of NLRP3 and caspase-1 are induced by pristane treatment (21). It remains to be determined whether this transcriptional effect is TLR7-mediated, though it has been shown previously that NLRP3 expression is induced via TLR-dependent NFκB signaling (3). Somewhat paradoxically, although the acute (neutrophil) peritoneal inflammatory response to pristane is IL-1α and CXCL5-dependent but NLRP3, caspase-1, and ASC-independent (13), neutrophil influx was enhanced in caspase-1 deficient mice. A likely explanation is provided by two recent reports that IL-1α can be generated by inflammasome-dependent as well as inflammasome-independent pathways (11, 12). IL-1α/CXCL5-dependent neutrophil recruitment to the inflamed peritoneum likely proceeds independently of inflammasomes and caspase-1. Whereas secretion of IL-1α is inflammasome/caspase-1 dependent, its expression on the cell surface requires NFκB but not caspase-1 (12), suggesting that the paradoxically enhanced neutrophil recruitment into the peritoneum of caspase-1 deficient mice may be mediated by cell surface IL-1α.
A key question for future research is how activation of the NALP3 inflammasome is linked to the manifestations of lupus. IFN-I and autoantibody production in lupus have been postulated to be direct effects of interactions of the nucleic acid components of self-antigens, such as U1 snRNPs, with TLR7 (1, 2). TLR7-mediated NFκB generation also can serve as “signal 1” for inflammasome activation. Although it is possible that inflammasome activation enhances the generation of IFN-I in response to TLR7 signaling, that possibility was excluded by Kahlenberg et al. (21). Alternatively, the role of inflammasome activation could be to promote the differentiation of macrophages, dendritic cells, or other antigen-presenting cells, as has been suggested for the AIM2 inflammasome (23). There is considerable evidence that the NLR proteins are a crucial link between innate immunity and cell death signaling (7). Thus, a third and perhaps more likely possibility is that inflammasome function plays a role in generating the TLR7 ligands that stimulate IFN-I production and autoantibody responses. Consistent with that possibility, the number of annexin V+ cells (a marker of either apoptosis or pyroptosis) is decreased in the peritoneum of caspase-1 −/− mice following pristane treatment (21).
Pyroptosis, defined as caspase-1 dependent cell death following inflammasome activation, has so far only been described in macrophages and dendritic cells (24). Cell death occurs via pore formation and loss of membrane integrity, and therefore in contrast to apoptosis, pyroptosis is inflammatory. On the other hand, there is little evidence that the extrinsic (Fas-mediated) apoptosis pathway requires caspase-1, since it is unaffected by the absence of caspase-1 (24, 25). Interestingly, Fas- or Fas ligand- deficient mice are completely resistant to the induction of autoantibodies by pristane (26) in contrast to the substantial, but incomplete, inhibition seen in caspase-1 −/− mice (21). Thus, both caspase-1-dependent (pyroptosis?) (21) and caspase-1-independent/Fas-dependent (apoptosis?) (26) pathways might be involved in disease pathogenesis in this mouse model of lupus. In view of the more profound effect of Fas deficiency, perhaps the extrinsic apoptosis pathway is involved in the initiation of autoantibody production, whereas activation of the inflammasome/pyroptosis pathway maintains autoantibody levels by generating dead cells displaying endogenous TLR7 (or TLR9 or TLR2) ligands. Nucleic acids released from uncleared dead cells may then promote chronic proinflammatory cytokine production and the terminal differentiation of autoantibody-producing B cells. Kallenberg’s novel observations suggest that in some respects, lupus may be viewed as an autoinflammatory disorder caused by abnormal the handling of one’s own dead cells. By promoting cell death, either via apoptosis or pyroptosis, ICE may be fueling the fire of chronic inflammation and autoimmunity.
Acknowledgments
Supported by a research grant from NIH/NIAMS (R01-AR44731)
Footnotes
Conflict of Interest Disclosures: The author declares no competing financial interests.
References
- 1.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. SeminImmunol. 2011;23(2):106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nature immunology. 2012;13(4):325–332. doi: 10.1038/ni.2231. Epub 2012/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. Journal of immunology. 2012;189(12):5508–5512. doi: 10.4049/jimmunol.1202121. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchiyama R, Yonehara S, Tsutsui H. Fas-mediated inflammatory response in Listeria monocytogenes infection. Journal of immunology. 2013;190(8):4245–4254. doi: 10.4049/jimmunol.1203059. Epub 2013/03/20. [DOI] [PubMed] [Google Scholar]
- 6.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. Epub 2010/03/10. [DOI] [PubMed] [Google Scholar]
- 7.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. NatRevImmunol. 2008;8(5):372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 8.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. NatClinPractRheumatol. 2008;4(1):34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. Epub 2011/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, et al. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36(3):388–400. doi: 10.1016/j.immuni.2012.01.018. Epub 2012/03/27. [DOI] [PubMed] [Google Scholar]
- 12.Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, et al. Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(44):18055–18060. doi: 10.1073/pnas.1109176108. Epub 2011/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PY, Kumagai Y, Xu Y, Li Y, Barker T, Liu C, et al. IL-1alpha modulates neutrophil recruitment in chronic inflammation induced by hydrocarbon oil. JImmunol. 2011;186(3):1747–1754. doi: 10.4049/jimmunol.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. Journal of immunology. 1988;141(9):3050–3054. Epub 1988/11/01. [PubMed] [Google Scholar]
- 15.Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. Journal of immunology. 1989;143(11):3470–3475. Epub 1989/12/01. [PubMed] [Google Scholar]
- 16.Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, et al. U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. Journal of immunology. 2012;188(10):4769–4775. doi: 10.4049/jimmunol.1103355. Epub 2012/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, et al. Self double-stranded (ds)DNA induces IL-1beta production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies. Journal of immunology. 2013;190(4):1407–1415. doi: 10.4049/jimmunol.1201195. Epub 2013/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. Journal of immunology. 2013;190(3):1217–26. doi: 10.4049/jimmunol.1202388. Epub 2012/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Zhang H, Huang Y, Wang H, Wang S, Zhao C, et al. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-kappaB activation. International immunopharmacology. 2013;17(1):116–122. doi: 10.1016/j.intimp.2013.05.027. Epub 2013/06/19. [DOI] [PubMed] [Google Scholar]
- 20.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Annals of the rheumatic diseases. 2005;64(4):630–633. doi: 10.1136/ard.2004.025858. Epub 2004/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahlenberg JM, Yalavarthi S, Zhao W, Hodgin JB, Reed TJ, Tsuji NM, et al. An essential role for caspase-1 in the induction of murine lupus and its associated vascular damage. Arthritis and rheumatism. 2013 doi: 10.1002/art.38225. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman S, Kny A, Schorn C, Pfatschbacher J, Niederreiter B, Herrmann M, et al. Cell death and cytokine production induced by autoimmunogenic hydrocarbon oils. Autoimmunity. 2012;45(8):602–611. doi: 10.3109/08916934.2012.719948. Epub 2012/08/25. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Cai Y, Xu W, Yin Z, Gao X, Xiong S. AIM2 facilitates the apoptotic DNA-induced systemic lupus erythematosus via arbitrating macrophage functional maturation. Journal of clinical immunology. 2013;33(5):925–937. doi: 10.1007/s10875-013-9881-6. Epub 2013/03/13. [DOI] [PubMed] [Google Scholar]
- 24.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243(1):206–214. doi: 10.1111/j.1600-065X.2011.01044.x. Epub 2011/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng TS, Schlosser SF, Dao T, Hingorani R, Crispe IN, Boyer JL, et al. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13618–13623. doi: 10.1073/pnas.95.23.13618. Epub 1998/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh M, Weintraub JP, Yoshida H, Shaheen VM, Richards HB, Shaw M, et al. Fas and Fas ligand mutations inhibit autoantibody production in pristane-induced lupus. JImmunol. 2000;165:1036–1043. doi: 10.4049/jimmunol.165.2.1036. [DOI] [PubMed] [Google Scholar]