Abstract
Sub-retinal implantation of foreign materials is becoming an increasingly common feature of novel therapies for retinal dysfunction. The ultimate compatibility of implants depends not only on their in vitro chemical compatibility, but also on how well the mechanical properties of the material match those of the native tissue. In order to optimize the mechanical properties of retinal implants, the mechanical properties of the mammalian retina itself must be carefully characterized. In this study, the compressive moduli of eye tissues, especially the retina, were probed using a dynamic mechanical analysis instrument in static mode. The retinal compressive modulus was lower than that of the sclera or cornea, but higher than that of the RPE and choroid. Compressive modulus remained relatively stable with age. Conversely, apparent retinal softening occurred at an early age in mice with inherited retinal degeneration. Compressive modulus is an important consideration for the design of retinal implants. Polymer scaffolds with moduli that are substantially different than that of the native tissue in which they will ultimately reside will be less likely to aid in the differentiation and development of the appropriate cell types in vitro and will have reduced biocompatibility in vivo.
Keywords: retinal degeneration, transplantation, implant, retinal modulus
Introduction
Inherited retinal degenerative diseases such as retinitis pigmentosa, Leber congenital amaurosis, and Stargardt disease are characterized by death of the light sensing photoreceptor cells of the outer neural retina and irreversible blindness. For neurodegenerative diseases such as these, drug and/or gene therapy alone may not suffice, especially in patients who have suffered extensive photoreceptor cell loss prior to molecular diagnosis of their gene defect. Under these circumstances, strategies focused on cellular or tissue replacement will be beneficial. Many studies suggest that the use of stem cells to achieve such a goal is now feasible (Barber et al. 2013, Gonzalez-Cordero et al. 2013, Gust and Reh. 2011, Klassen et al. 2004, La Torre et al. 2012, Lakowski et al. 2011, Lamba et al. 2009, Lamba et al. 2010, Ma et al. 2011, MacLaren et al. 2006, Tucker et al. 2010, Tucker et al. 2011, Tucker et al. 2013, West et al. 2012, Yao et al. 2011); however, a major remaining hurdle is the development of an optimal cell transplantation system. Current delivery methods typically result in massive cell loss and limited cellular integration following transplantation. For instance, several studies have shown that following bolus photoreceptor cell injection, less than 0.01% of transplanted cells survive and even fewer actually integrate within the host retina (Klassen et al. 2004, MacLaren et al. 2006). In large part, poor integration can be attributed to the lack of donor cell support following the bolus injection. These results are particularly common when attempting to perform subretinal transplants in late stage retinal degenerative hosts that have lost the majority of their outer retina due to photoreceptor cell death.
In an attempt to increase cellular survival and subsequent integration following retinal progenitor/stem cell (RPC) transplantation, several researchers have designed polymer scaffolds as vehicles for delivery of drugs or cells to the subretinal space (da Silva et al. 2010, Diniz et al. 2013, Hu et al. 2012, Janoria et al. 2007, Pritchard et al. 2010, Tucker et al. 2010, Winter et al. 2008). Most implanted materials are tested for material or chemical biocompatibility (which is clearly an essential property of an implanted polymer) prior to implantation using in vitro culture techniques. However, the mechanical properties of material also play an important role in the ultimate compatibility, efficacy and outcome of the implant in vivo. To overcome potential rejection issues and for stimulation of optimal cellular differentiation and transplant integration, it is generally believed that the mechanical properties of an implanted material should match those of the recipient tissue as closely as possible. Thus, directing stem cell differentiation and cellular proliferation with mechanical cues (Zoldan et al. 2011, Lee et al. 2013) has long been utilized for generating cartilage (Little et al. 2011) and bone (Parekh et al. 2011). More recently this concept has also been applied to materials meant to regenerate softer tissues such as tendons (Kinneberg et al. 2011), cardiac valves (Wang et al. 2013), cardiac muscles (Guillemette et al. 2010, Chen et al. 2008), and neurons (Banerjee et al. 2009, Yao et al. 2013). In order to obtain an optimal match between implant and native tissue, therefore, the mechanical properties of the native tissue need to be thoroughly characterized in normal and diseased states.
Although some mechanical properties of the retina were described as early as 1987 (WU et al. 1987), the extremely delicate nature of this neural tissue has limited the extent of its characterization. Attaching retinal samples to displacement probes, for example, is a commonly reported challenge, originally overcome by using synthetic adhesive to bind the retina to the probes. More recent work has addressed this challenge by using mechanical pressure to tightly clamp samples to analysis probes (Chen et al. 2010, Wu et al. 1987), leading to examination of retinal mechanical properties of the retina using uniaxial tension. Some insight into the pathophysiology of retinal tearing was gained following these analyses. For instance, the retina was found to have a relatively short (compared to the choroid) reversible elastic phase followed by a large irreversible plastic deformation (Wollensak and Spoerl. 2004). From these stress and strain studies, the tensile modulus of the retina was estimated to be about 100 kiloPascals (kPa) (Basinger et al. 2009, Chen and Weiland. 2012).
As described, the mechanical properties of the retina have been characterized thus far using tension applied parallel to the isotropic retinal plane. Given the layered nature of the retina, however, mechanical properties are not likely to be the same if characterized using compression perpendicular to the isotropic plane of the retina. For the purpose of developing cell delivery scaffolds, the transverse compressive mechanical properties are a better representation of actual mechanical pressures encountered by cells and tissues during chronic implantation. In this study, the compressive modulus of porcine and murine ocular tissues was examined. The effects of aging and disease on the murine retinal compressive modulus were also characterized. In an effort to begin to identify mechanically appropriate materials for retinal differentiation and transplantation, the transverse compressive modulus values for retinal tissue were compared to relevant synthetic materials.
Materials and Methods
Animals and Dissection
For testing of mouse retinal modulus, C57Bl/6J were used as a wild type control line (stock number 000664; Jackson Laboratories, Bar Harbor, ME). To test the effect of retinal degeneration on mouse retinal modulus we used the mutant line C3H/HeJPde6rd1 (stock number 000659; Jackson Labs), which develops rapid retinal degeneration due to rod photoreceptor cell death. Mice were euthanized using CO2 inhalation, followed by cervical dislocation. Eyes were enucleated and the anterior segment was removed by carefully cutting around the circumference of the eye along the limbus. Once exposed, the lens and vitreous were removed, leaving the posterior cup consisting of the retina, choroid and sclera. Retinas were then carefully separated and maintained in their natural cup-like form with the cupped portion open, facing up in the sample basin. The media was removed to allow surface tension to flatten the tissue and the edges of the retinal cup were gently teased out to their most extreme limit, causing flattening of the retina, without damaging the sample. Each mouse retina measured was treated identically. Pig eyes (Iowa outbred swine, 5 months of age) were obtained from a local slaughterhouse, transported on ice and dissected within two hours of harvest. Whole pig globes were processed in the same manner as mouse eyes above, except the retina was not removed from the choroid. Instead, a 5mm biopsy punch was used to cut out retinal samples for modulus testing, which were then separated from the choroid and other layers. A biopsy punch was also used to isolate specimens of cornea, choroid and sclera for testing. Prior to modulus measurements, all tissues were maintained in 1X Hank’s buffered salt solution (HBSS, 340 mOsm/L, Sigma-Aldrich, St. Louis, MO).
Polymers
Polydimethylsiloxane (PDMS, SYLGARD® 184 silicone elastomer kit, Dow Corning, Midland, MI) samples were formed using a 10:1 ratio of base to crosslinker. The blend was mixed by vortexing, poured into a petri dish, and cured in an oven for two days at 50 °C. The soft crosslinked poly(ethylene glycol) (PEG) samples were formed using 22.5 weight percent (wt%) PEG dimethacrylate (PEGDMA, MW 875, Sigma-Aldrich, St. Louis, MO), 22.5 wt% PEG methacrylate (PEGMA, MW 500, Sigma-Aldrich) and 0.1 wt% photoinitiator (Irgacure-651,Ciba Specialty Chemicals, Tarrytown, NY), in HBSS. The stiff crosslinked PEG samples were formed using 55 wt% PEGDMA, 22.5 wt% PEGMA, and 0.1 wt% photoinitiator in HBSS. These mixtures were vortexed and then photopolymerized with 365 nm light for 10 minutes in a laminate glass mold. Many other crosslinked PEG formulations with varying composition were prepared in the same manner to further demonstrate the flexibility of using polymeric materials. To prepare the gelatin samples, 4 grams (g) of gelatin crystals (Knox original unflavored gelatin) were mixed with 29 mL of cold HBSS. Boiling HBSS (88 mL) was added to this mixture, and the resulting solution was stirred to homogeneity, poured into a petri dish, and refrigerated for 24 hours. All polymers were stored in HBSS for two days at room temperature, after which disks from each polymer species were punched with a 5 mm biopsy punch.
Stress and Strain Measurement
The mechanical properties of all samples were measured using a dynamic mechanical analysis instrument (DMA Q800 V7.0 Build 113, TA Instruments, New Castle, DE) equipped with a submersion compression clamp in static mode. Prior to each group of measurements, the drive shaft position, clamp mass, clamp offset, and clamp compliance were calibrated according to suggested protocols. Each sample was carefully transferred to the basin of the clamp either in HBSS using a wide-tipped Pasteur pipette (for soft tissue samples, Figures 1A and 1B) or carefully with forceps (for polymer, cornea, and sclera samples). Any excess water or buffer surrounding the sample was removed using a syringe. Once the sample was installed, the top portion of the clamp was gently lowered onto the sample surface, the furnace was closed to maintain constant temperature and prevent disturbances, and a pre-load force of 0.0001 N was applied to the sample. The force was then gradually increased to a final value of 0.2 N at a rate of 0.02 N/min and displacement data were collected every 2 seconds as the sample (which was not confined) was compressed. Crosslinked PEG samples were measured in a fully hydrated state as per the method used for other samples, and also in a semi-hydrated state after initial compression, at which time most liquid had escaped the crosslinked network. All samples were assumed to be cylindrical with a diameter of 5 mm, except the whole mouse retinas, which had a diameter of 3 mm. All retinal thicknesses (mouse and pig) were based on histological measurements of age and strain matched controls (data not shown), while the thicknesses of polymer and more robust tissue samples were measured using the initial displacement of the clamp before testing began. Sample thicknesses are provided in Tables 1-3.
Figure 1. Mechanical analysis of eye tissues in compression.
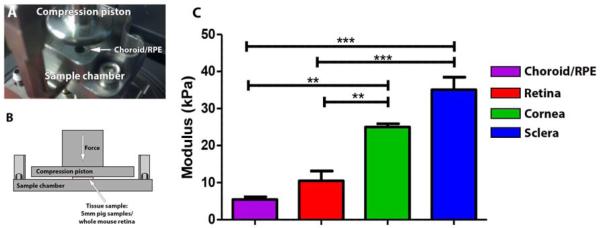
5mm circular punches of the RPE/Choroid (A) are shown in the DMA compression clamp. B: A schematic diagram depicting the compression paradigm/tissue instrument interaction. The mean compressive modulus (C) of porcine corneal and sclera tissue is greater than that of retinal tissue, while the modulus of choroid with retinal pigment epithelium (RPE) is slightly lower. Error bars represent standard error of the mean, **p<0.01, ***p<0.001.
Table 1.
Sample size, mean, standard error of the mean, and average or estimated thickness for each group of porcine tissue samples.
| Sclera | Cornea | Retina | Choroid/RPE | |
|---|---|---|---|---|
| N | 5 | 9† | 17 | 5 |
| Mean | 35.1 | 25.0 | 10.5 | 5.4 |
| S.E.M. | 3.38 | 0.91 | 2.67 | 0.74 |
| z (mm) | 1.78§ | 2.56 | 0.30‡ | - |
One statistical outlier was removed prior to analysis
Based on histological measurements
Sclera, choroid, and RPE were measured together
Table 3.
Sample size, mean, standard error of the mean, and average thickness for each group of polymer samples.
| Soft | Stiff | |||
|---|---|---|---|---|
| Gelatin | PEG | PEG | PDMS | |
| NAnalyzed | 4† | 6 | 6 | 4† |
| Mean | 2.99 | 24.9 | 308.5 | 379.6 |
| S.E.M. | 0.68 | 6.84 | 23.86 | 15.74 |
| z (mm) | 0.39 | 1.18 | 1.22 | 1.61 |
One statistical outlier was removed prior to analysis
Data Analysis
From the recorded displacement (reported in μm) and static force (reported in N) values, the infinitesimal strain (ε) and stress (σ) were calculated as follows:
| (1) |
| (2) |
where D is displacement, D0 is initial displacement (which accounts for any changes brought about by the initial pre-load force), z is the sample thickness, F is static force, A is sample cross-sectional area, and C is a necessary correction factor provided by the instrument manufacturer based on sample geometry.
Modulus is defined as the ratio of stress increase to strain increase in the elastic deformation region. Values of stress at absolute strain values between 0 and 0.1 (0% and 10% deformation) were plotted and the slope estimated using a trend line function (see Supplemental Figure 1). The total number of samples in each group is given in Tables 1-3.
Statistics
Modulus data were analyzed for significance using one-way analysis of variance followed by Tukey’s multiple comparison tests. Differences were considered significant at p-value < 0.05. Statistically significant outliers were rejected if identified using a Q test with 95% confidence limits. Crosslinked PEG moduli were predicted for a range of formulations based on a three-component mixture design (DesignExpert 9.0.0.7, StatEase Inc., Minneapolis, MN) with design points (measured values) at each vertex (n = 3), at each axis third (n = 3), the overall centroid (n = 3), and at three internal check blends (n = 6) for a total of 13 design points and 48 measurements.
Results
Accurately determining the compressive modulus of the retina lays the groundwork for the future design of polymer based cell delivery scaffolds. We began by studying eye tissues isolated from swine, which are structurally similar to analogous human tissues. To test compressive moduli, 5mm biopsy punches of the retina, and (for comparison purposes) the retinal pigment epithelium (RPE)/choroid, sclera, and cornea were placed in a submersion compression clamp connected to a dynamic mechanical analysis instrument (which was employed here in static mode). As shown in Figures 1A and 1B, samples were positioned in the center of the clamp basin and excess fluid was carefully removed. Following proper positioning, an increasing amount of stress was applied and the resulting strain recorded; both of which were used to calculate the modulus. As shown in Figure 1C, of the tissues tested, the highest modulus recorded corresponded to the sclera at 35.1 ± 3.4 kPa. This finding was not surprising considering the fact that this tissue is largely composed of irregularly layered collagen fibrils. The cornea, which is nearly uniform in thickness and largely constructed of perfectly parallel collagen fibrils, exhibited a modulus 25% lower than that of the sclera (25.0 ± 0.9 kPa). The retina, which is primarily composed of neurons and their axons, had a modulus 63% lower than that of the cornea (10.5 ± 2.67 kPa). Lastly, the choroid and RPE combined (a loose vascular connective tissue and neuroepithelial monolayer) had a modulus more than 40% lower than even that of the retina. (5.4 ± 0.74 kPa).
In addition to determining the compressive modulus of the retina of swine, we sought to determine the effects of both aging and retinal degenerative disease on retinal mechanical integrity. For these studies, wild type C57BL/6J and mutant C3H/HeJPde6rd1 mouse retinas were used. As shown in Figure 2 (solid markers), the retinal compressive moduli of wild type C57BL/6J mice does not change significantly from initial values obtained at 5 weeks. This relatively constant modulus is sustained for up to 36 weeks of age. Based on these results, the retinal modulus remains relatively stable under normal aging conditions.
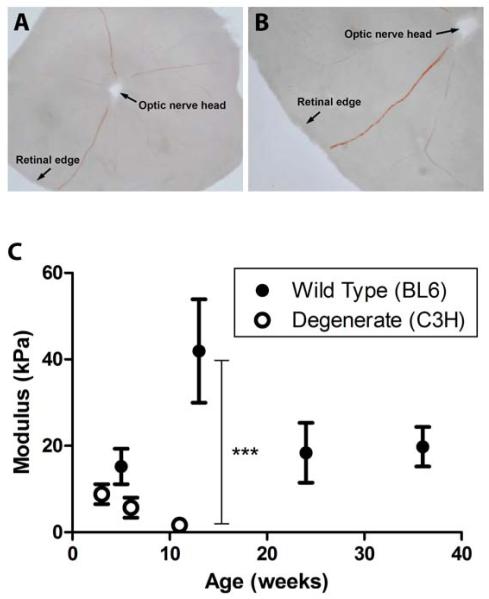
Figure 2. Moduli of whole mouse retinas at different ages and disease states.
A-B: Exemplary whole mouse retina that has been dissected free of the optic cup, placed photoreceptor side down and prepared for compression analysis. As liquid is removed surface tension allows the dissected retina to lay flat (A), edges of the retinal cup are gently teased out to their most extreme limit taking care not to disrupt tissue integrity (B). C: Analysis of the development and degeneration of mouse retina mechanical properties demonstrate that the compressive modulus of mouse retina stays relatively constant in the range of ages examined (wild type C57BL/6J) but rapidly deteriorates with mutation-induced degeneration (C3H/HeJPde6rd1). Error bars represent standard error of the mean,*p<0.05.
Due to the aggressive nature of the retinal degeneration observed in the C3H/HeJPde6rd1 mouse, i.e. significant degeneration observed by 3 weeks of age, these animals were studied immediately post-weaning and at 6 and 12 weeks of age. Unlike wild type mice, the retinal compressive moduli of the C3H/HeJPde6rd1 mouse decreased from initial recordings taken at 3 weeks of age (Figure 2, open markers). By 12 weeks of age, a time in which the C3H/HeJPde6rd1 mice have little to no outer nuclear layer remaining, the retinal modulus was much lower than the previous time point (Figure 2, open markers). Furthermore, the mean modulus of 12-week-old C3H/HeJPde6rd1 retina was reduced by more than 90% compared to the wild type C57BL6 mouse at a similar age (p<0.05). These results demonstrate that retinal degenerative disease significantly affects the mechanical properties of the retina.
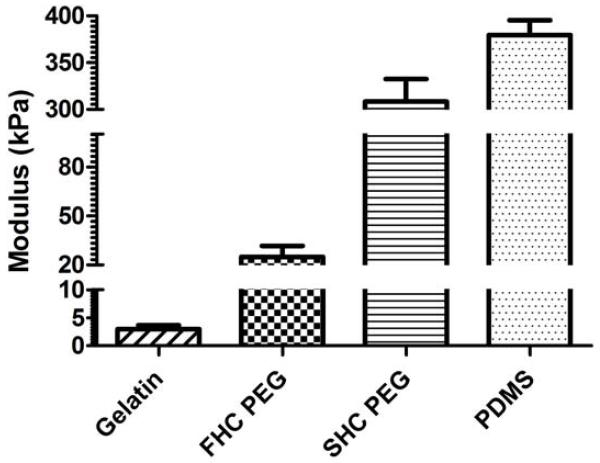
As the ultimate goal of determining retinal modulus is to guide the future design of polymer based cell delivery scaffolds, we next attempted to determine the compressive modulus of a set of polymer materials generated in our lab. The materials chosen (PDMS, crosslinked PEG, and gelatin) were selected based upon their biocompatibility and their previous use in the eye (Del Priore et al. 2004, Khodair et al. 2003, Lim et al. 2004, Lu et al. 2001, Singh et al. 2001). Furthermore, as a group these materials cover a wide spectrum of mechanical properties relevant to biomaterials. The compressive modulus of each polymer is shown in Figure 3. PDMS had the highest modulus (380 ± 16 kPa), followed by semi-hydrated crosslinked PEG (309 ± 24 kPa), fully-hydrated crosslinked PEG (24.9 ± 6.8 kPa) and gelatin (2.99 ± 0.68 kPa). These measured values correspond well with previously reported compressive moduli for the same or similar formulations of each polymer (Armani et al. 1999, Clapper and Guymon. 2007, Wiwatwongwana et al. 2012). Of these polymers, the soft crosslinked PEG and gelatin most closely matched the range of moduli identified for the neural retina. However, it is important to note that the mechanical properties of all three materials can be modified by changing molecular weight and crosslinking density, as demonstrated for crosslinked PEG in Supplemental Figures 2 and 3. Thus, each material has the potential to be used for future cell delivery applications.
Figure 3. Compressive modulus of various polymers.
The compressive modulus of gelatin and fully-hydrated crosslinked PEG (FHC PEG) most closely approximate the transverse compressive modulus observed for healthy retinas, where semi-hydrated crosslinked PEG (SHC PEG) and fully hydrated PDMS moduli are much higher. Error bars represent standard error of the mean.
Discussion
Since the development of the first intraocular lens (See Moore et al. 2011 for a discussion of the first poly(methyl methacrylate)-based Ridley lens), the use of polymer based materials for the treatment of blinding eye diseases has been widely studied. To date a variety of different polymer materials, both biodegradable and nonbiodegradable, have been used in the eye for applications ranging from retinal stimulation to drug/cell delivery. For example, biodegradable poly(lactic-co-glycolic acid) (PLGA) microspheres have been shown to be well tolerated and to maintain their ability to provide sustained release of incorporated drug following intravitreal injection (Checa-Casalengua et al. 2011, Jiang et al. 2007, Yao et al. 2011). When used for cell delivery, PLGA based scaffolds have been shown to increase the survival and integrative capacity of retinal stem cells following transplantation (Tomita et al. 2005, Tucker et al. 2010). As a strategy to treat age related macular degeneration, nondegradable parylene-C based films have been used as a vehicle for the delivery of stem cell derived RPE cells (Diniz et al. 2013, Lu et al. 2012, Ribeiro et al. 2013). In comparison to bolus RPE cell injections, delivery of RPE cells on parylene-C scaffolds drastically increased donor cell survival and viability following transplantation (Diniz et al. 2013).
Despite these encouraging results, none of these polymers are well tolerated by the host retina (Diniz et al. 2013, Tucker et al. 2010). In the case of the electrospun PLGA systems, the negative effects observed could in large part be explained by the inherent rigidity of the scaffold and its mechanical incongruity with the remaining host retinal tissue (Tomita et al. 2005, Tucker et al. 2010), resulting in injury to the delicate retinal neurons. These findings point to the need for newly developed polymer systems to more closely mimic the physical properties of the native tissue in which they will eventually be placed. Achieving material biomimicry is not only important for permanent implantation, but also in situations where the role of the polymer is to enhance cellular differentiation and development in vitro.
To date, the majority of published studies have focused on determining the structural properties of the retina in the horizontal dimension, i.e., perpendicular to the path of light in the living tissue. In one such uniaxial tension study, Chen and coworkers found the modulus of the retina to be approximately 100 kPa, while that of the choroid and sclera were about 2.4 megaPascals (MPa) and 16.6 MPa, respectively (Chen et al. 2010). They also found that the direction in which the sample was cut significantly affected the tensile modulus (Chen et al. 2010). In a later study, these and similar differences were shown to be attributed to the presence and size of blood vessels in the tissue (Chen and Weiland. 2010). Although mechanical properties can be uniform with respect to direction for most synthetic materials, this isotropy does not translate to complex anisotropic biological tissues, especially those that are layered, such as the retina. Rather, a compressive force applied to the surface of the retina will deform the structures within and between the synaptic and nuclear layers in a much different way than a force applied along the length of the layers. For the purpose of developing cell delivery scaffolds, this transverse compressive stress and strain behavior would appear to be a better representation of the actual mechanical pressures encountered during chronic implantation, i.e. the pressure generated by placing a foreign material into the subretinal space is of a compressive nature rather than a tensile or shear force. Likewise, the modulus of the polymer or tissue that a developing or transplanted cell will perceive is more similar to that obtained under a transverse compressive rather than a uniaxial tensile force. As suspected, in the current study, the modulus of the retina was found to be much lower than those obtained in a lateral direction. As indicated above, this is likely due to interconnectivity of the plexiform layers, the nerve fiber layer, the vascular system and the inner and outer limiting membranes.
In summary, this study helps lay the foundation for appropriate material selection and modulation for applications involving retinal cell differentiation and retinal transplantation.
Supplementary Material
Supplemental Figure 1. Representative compressive stress/strain profile of the retina including a schematic of modulus calculation. As modulus is defined as the ratio of stress increase to strain increase in the elastic deformation region, values of stress at strain values between 0% and 10% deformation were plotted and the slope estimated using a trend line function.
Supplemental Figure 2. Measured and predicted moduli (in kPa) of fully hydrated crosslinked PEG as a function of sample composition. The effect of varying saline (5 - 80 wt%), PEGMA (10 - 85 wt%), and PEGDMA (10 – 85 wt%) content on the compressive modulus of the resulting polymer was predicted based on a mixture design model created using the square root of measured design points shown with either 3 or 6 replicates at each point, as indicated in the image.
Supplemental Figure 3. Measured and predicted moduli (in kPa) of semi-hydrated crosslinked PEG as a function of sample composition. The effect of varying saline (5 - 80 wt%), PEGMA (10 - 85 wt%), and PEGDMA (10 – 85 wt%) content on the compressive modulus of the resulting polymer was predicted based on a mixture design model created using the measured design points shown with either 3 or 6 replicates at each point, as indicated in the image.
Highlights.
Estimate of modulus is critical for biomaterial selection and transplantation.
Compressive moduli of sclera and cornea were higher than that of the retina.
Compressive modulus of the RPE and choroid was lower than that of the retina.
Tensile retinal modulus is 10 fold higher than compressive retinal modulus.
Retinal degeneration affects retinal compressive modulus.
Table 2.
Sample size before and after outlier and aberrant data rejection, mean, standard error of the mean, and estimated thickness for each group of murine retina samples
| Wild Type (BL6) |
Degenerate (C3H) |
||||||
|---|---|---|---|---|---|---|---|
| 5 wks | 13 wks | 24 wks | 36 wks | 3 wks | 6 wks | 11 wks | |
| NInitial | 6 | 5 | 5 | 6 | 9† | 6† | 6† |
| Mean | 15.2 | 41.9 | 18.4 | 19.8 | 8.8 | 8.4 | 2.5 |
| S.E.M. | 4.08 | 11.99 | 6.91 | 4.58 | 2.29 | 2.79 | 0.82 |
| z (μm) | 225‡ | 225‡ | 225‡ | 225‡ | 180‡ | 150‡ | 100‡ |
One statistical outlier was removed prior to analysis
Based on histological measurements
Acknowledgments
The authors would like to gratefully acknowledge sources of financial support, including the National Science Foundation, the National Institute of Health New Innovators Award Program, and the Stephen A. Wynn Institute for Vision Research. The authors would also like to thank Emily Kaalberg for her assistance with live animal handling.
Grant Support: NIH Directors New Innovator Award 1-DP2-OD007483-01; NEI EY017451; HHMI; Foundation Fighting Blindness; Stephen A. Wynn Foundation; Grousbeck Family Foundation; Leo, Jacques & Marion Hauser Family Vision Restoration Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- Armani D, Liu C, Aluru N. Re-configurable fluid circuits by PDMS elastomer micromachining. Micro Electro Mechanical Systems, 1999. MEMS ’99. Twelfth IEEE International Conference on.1999. pp. 222–227. [Google Scholar]
- Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AC, Hippert C, Duran Y, West EL, Bainbridge JWB, Warre-Cornish K, Luhmann UFO, Lakowski J, Sowden JC, Ali RR, Pearson RA. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:354–359. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinger BC, Rowley AP, Chen K, Humayun MS, Weiland JD. Finite element modeling of retinal prosthesis mechanics. J. Neural Eng. 2009;6:055006. doi: 10.1088/1741-2560/6/5/055006. [DOI] [PubMed] [Google Scholar]
- Checa-Casalengua P, Jiang C, Bravo-Osuna I, Tucker BA, Molina-Martinez IT, Young MJ, Herrero-Vanrell R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Controlled Release. 2011;156:92–100. doi: 10.1016/j.jconrel.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Chen K, Rowley AP, Weiland JD. Elastic properties of porcine ocular posterior soft tissues. J. Biomed. Mater. Res. Part A. 2010;93A:634–645. doi: 10.1002/jbm.a.32571. [DOI] [PubMed] [Google Scholar]
- Chen K, Weiland JD. Anisotropic and inhomogeneous mechanical characteristics of the retina. J. Biomech. 2010;43:1417–1421. doi: 10.1016/j.jbiomech.2009.09.056. [DOI] [PubMed] [Google Scholar]
- Chen K, Weiland JD. Mechanical Characteristics of the Porcine Retina in Low Temperatures. Retin. -J. Retin. Vitr. Dis. 2012;32:844–847. doi: 10.1097/IAE.0b013e318225d0c9. [DOI] [PubMed] [Google Scholar]
- Chen Q, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Clapper JD, Guymon CA. Physical behavior of cross-linked PEG hydrogels photopolymerized within nanostructured lyotropic liquid crystalline templates. Macromolecules. 2007;40:1101–1107. [Google Scholar]
- da Silva GR, Fialho SL, Siqueira RC, Jorge R, Cunha Junior A.d.S. Implants as drug delivery devices for the treatment of eye diseases. Brazilian Journal of Pharmaceutical Sciences. 2010;46:585–595. [Google Scholar]
- Del Priore L, Tezel T, Kaplan H. Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Invest. Ophthalmol. Vis. Sci. 2004;45:985–992. doi: 10.1167/iovs.03-0662. [DOI] [PubMed] [Google Scholar]
- Diniz B, Thomas P, Thomas B, Ribeiro R, Hu Y, Brant R, Ahuja A, Zhu D, Liu L, Koss M, Maia M, Chader G, Hinton DR, Humayun MS. Subretinal Implantation of Retinal Pigment Epithelial Cells Derived From Human Embryonic Stem Cells: Improved Survival When Implanted as a Monolayer. Invest. Ophthalmol. Vis. Sci. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJI, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JWB, Sowden JC, Ali RR. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 2013;31:741. doi: 10.1038/nbt.2643. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette MD, Park H, Hsiao JC, Jain SR, Larson BL, Langer R, Freed LE. Combined Technologies for Microfabricating Elastomeric Cardiac Tissue Engineering Scaffolds. Macromolecular Bioscience. 2010;10:1330–1337. doi: 10.1002/mabi.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust J, Reh TA. Adult Donor Rod Photoreceptors Integrate into the Mature Mouse Retina. Invest. Ophthalmol. Vis. Sci. 2011;52:5266–5272. doi: 10.1167/iovs.10-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liu L, Lu B, Zhu D, Ribeiro R, Diniz B, Thomas PB, Ahuja AK, Hinton DR, Tai Y, Hikita ST, Johnson LV, Clegg DO, Thomas BB, Humayun MS. A Novel Approach for Subretinal Implantation of Ultrathin Substrates Containing Stem Cell-Derived Retinal Pigment Epithelium Monolayer. Ophthalmic Res. 2012;48:186–191. doi: 10.1159/000338749. [DOI] [PubMed] [Google Scholar]
- Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opinion on Drug Delivery. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Molecular Vision. 2007;13:1783–1792. [PubMed] [Google Scholar]
- Khodair M, Zarbin M, Townes-Anderson E. Synaptic plasticity in mammalian photoreceptors prepared as sheets for retinal transplantation. Invest. Ophthalmol. Vis. Sci. 2003;44:4976–4988. doi: 10.1167/iovs.03-0036. [DOI] [PubMed] [Google Scholar]
- Kinneberg KRC, Galloway MT, Butler DL, Shearn JT. Effect of Implanting a Soft Tissue Autograft in a Central-Third Patellar Tendon Defect: Biomechanical and Histological Comparisons. Journal of Biomechanical Engineering-Transactions of the Asme. 2011;133:091002. doi: 10.1115/1.4004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog. Retin. Eye Res. 2004;23:149–181. doi: 10.1016/j.preteyeres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- La Torre A, Lamba DA, Jayabalu A, Reh TA. Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol. Biol. 2012;884:229–246. doi: 10.1007/978-1-61779-848-1_16. [DOI] [PubMed] [Google Scholar]
- Lakowski J, Han Y-, Pearson RA, Gonzalez-Cordero A, West EL, Gualdoni S, Barber AC, Hubank M, Ali RR, Sowden JC. Effective Transplantation of Photoreceptor Precursor Cells Selected Via Cell Surface Antigen Expression. Stem Cells. 2011;29:1391–1404. doi: 10.1002/stem.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA. Transplantation of Human Embryonic Stem Cell-Derived Photoreceptors Restores Some Visual Function in Crx-Deficient Mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang P, Russell D, Reh TA. Generation, Purification and Transplantation of Photoreceptors Derived from Human Induced Pluripotent Stem Cells. Plos One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34:8140–8148. doi: 10.1016/j.biomaterials.2013.07.074. [DOI] [PubMed] [Google Scholar]
- Lim J, Byun S, Chung S, Park T, Seo J, Joo C, Chung H, Cho D. Retinal pigment epithelial cell behavior is modulated by alterations in focal cell-substrate contacts. Invest. Ophthalmol. Vis. Sci. 2004;45:4210–4216. doi: 10.1167/iovs.03-1036. [DOI] [PubMed] [Google Scholar]
- Little CJ, Bawolin NK, Chen X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Engineering Part B-Reviews. 2011;17:213–227. doi: 10.1089/ten.TEB.2010.0572. [DOI] [PubMed] [Google Scholar]
- Lu B, Zhu D, Hinton D, Humayun MS, Tai Y. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed. Microdevices. 2012;14:659–667. doi: 10.1007/s10544-012-9645-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Yaszemski M, Mikos A. Retinal pigment epithelium engineering using synthetic biodegradable polymers. Biomaterials. 2001;22:3345–3355. doi: 10.1016/s0142-9612(01)00172-7. [DOI] [PubMed] [Google Scholar]
- Ma J, Kabiel M, Tucker BA, Ge J, Young MJ. Combining chondroitinase ABC and growth factors promotes the integration of murine retinal progenitor cells transplanted into Rho(−/−) mice. Molecular Vision. 2011;17:1759–1770. [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Moore DB, Harris A, Siesky B. Republished review: The world through a lens: the vision of Sir Harold Ridley. Postgrad. Med. J. 2011;87:307–310. doi: 10.1136/pgmj.2009.163956rep. [DOI] [PubMed] [Google Scholar]
- Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, Simon CG., Jr. Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 2011;32:2256–2264. doi: 10.1016/j.biomaterials.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CD, Arner KM, Langer RS, Ghosh FK. Retinal transplantation using surface modified poly(glycerol-co-sebacic acid) membranes. Biomaterials. 2010;31:7978–7984. doi: 10.1016/j.biomaterials.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RM, Oregon A, Diniz B, Fernandes RB, Koss MJ, Charafeddin W, Hu Y, Thomas P, Thomas BB, Maia M, Chader GJ, Hinton DR, Humayun MS. In Vivo Detection of hESC-RPE Cells via Confocal Near-Infrared Fundus Reflectance. Ophthalmic Surgery Lasers & Imaging. 2013;44:380–384. doi: 10.3928/23258160-20130715-09. [DOI] [PubMed] [Google Scholar]
- Singh S, Woerly S, Mclaughlin B. Natural and artificial substrates for retinal pigment epithelial monolayer transplantation. Biomaterials. 2001;22:3337–3343. doi: 10.1016/s0142-9612(01)00171-5. [DOI] [PubMed] [Google Scholar]
- Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young M. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23:1579–1588. doi: 10.1634/stemcells.2005-0111. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, Riker MJ, Drack AV, Braun TA, Stone EM. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. ELife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Park I, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. Transplantation of Adult Mouse iPS Cell-Derived Photoreceptor Precursors Restores Retinal Structure and Function in Degenerative Mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Redenti SM, Jiang C, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ. The use of progenitor cell/biodegradable MMP2–PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010;31:9–19. doi: 10.1016/j.biomaterials.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Wang H, Tibbitt MW, Langer SJ, Leinwand LA, Anseth KS. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated PI3K/AKT pathway. Proceedings of the National Academy of Sciences. 2013;110:19336–19341. doi: 10.1073/pnas.1306369110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Gonzalez-Cordero A, Hippert C, Osakada F, Martinez-Barbera JP, Pearson RA, Sowden JC, Takahashi M, Ali RR. Defining the Integration Capacity of Embryonic Stem Cell-Derived Photoreceptor Precursors. Stem Cells. 2012;30:1424–1435. doi: 10.1002/stem.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JO, Gokhale M, Jensen RJ, Cogan SF, Rizzo Joseph F., III Tissue engineering applied to the retinal prosthesis: Neurotrophin-eluting polymeric hydrogel coatings. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2008;28:448–453. doi: 10.1016/j.msec.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwatwongwana F, Klunathon Y, Rangsri W, Promma N, Pattana S. Identification of Shear Modulus of Gelatin Blended with Carboxymethylcellulose Scaffolds Using Curve Fitting Method from Compressive Test. Journal of Materials Science Research, J Mater Sci Res. 2012;1:106-107–113. [Google Scholar]
- Wollensak G, Spoerl E. Biomechanical characteristics of retina. Retin. -J. Retin. Vitr. Dis. 2004;24:967–970. doi: 10.1097/00006982-200412000-00021. [DOI] [PubMed] [Google Scholar]
- Wu W, Peters W, Hammer M. Basic Mechanical-Properties of Retina in Simple Elongation. J. Biomech. Eng. -Trans. ASME. 1987;109:65–67. doi: 10.1115/1.3138644. [DOI] [PubMed] [Google Scholar]
- Yao J, Tucker BA, Zhang X, Checa-Casalengua P, Herrero-Vanrell R, Young MJ. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials. 2011;32:1041–1050. doi: 10.1016/j.biomaterials.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Yao S, Liu X, Wang X, Merolli A, Chen X, Cui F. Directing neural stem cell fate with biomaterial parameters for injured brain regeneration. Progress in Natural Science: Materials International. 2013;23:103–112. [Google Scholar]
- Zoldan J, Karagiannis ED, Lee CY, Anderson DG, Langer R, Levenberg S. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials. 2011;32:9612–9621. doi: 10.1016/j.biomaterials.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representative compressive stress/strain profile of the retina including a schematic of modulus calculation. As modulus is defined as the ratio of stress increase to strain increase in the elastic deformation region, values of stress at strain values between 0% and 10% deformation were plotted and the slope estimated using a trend line function.
Supplemental Figure 2. Measured and predicted moduli (in kPa) of fully hydrated crosslinked PEG as a function of sample composition. The effect of varying saline (5 - 80 wt%), PEGMA (10 - 85 wt%), and PEGDMA (10 – 85 wt%) content on the compressive modulus of the resulting polymer was predicted based on a mixture design model created using the square root of measured design points shown with either 3 or 6 replicates at each point, as indicated in the image.
Supplemental Figure 3. Measured and predicted moduli (in kPa) of semi-hydrated crosslinked PEG as a function of sample composition. The effect of varying saline (5 - 80 wt%), PEGMA (10 - 85 wt%), and PEGDMA (10 – 85 wt%) content on the compressive modulus of the resulting polymer was predicted based on a mixture design model created using the measured design points shown with either 3 or 6 replicates at each point, as indicated in the image.