Abstract
Primary periodontal ligament stem cells (PDLSCs) are known to possess multidifferentiation potential and exhibit an immunophenotype similar to that described for bone-marrow-derived mesenchymal stem cells. In the present study, bromo-deoxyuridine (BrdU)–labeled ovine PDLSCs implanted into immunodeficient mice survived after 8 weeks post-transplantation and exhibited the capacity to form bone/cementum-like mineralized tissue, ligament structures similar to Sharpey's fibers with an associated vasculature. To evaluate self-renewal potential, PDLSCs were recovered from harvested primary transplants 8 weeks post-transplantation that exhibit an immunophenotype and multipotential capacity comparable to primary PDLSCs. The re-derived PDLSCs isolated from primary transplants were implanted into secondary ectopic xenogeneic transplants. Histomorphological analysis demonstrated that four out of six donor re-derived PDLSC populations displayed a capacity to survive and form fibrous ligament structures and mineralized tissues associated with vasculature in vivo, although at diminished levels in comparison to primary PDLSCs. Further, the capacity for long-term survival and the potential role of PDLSCs in dental tissue regeneration were determined using an ovine preclinical periodontal defect model. Autologous ex vivo–expanded PDLSCs that were prelabeled with BrdU were seeded onto Gelfoam® scaffolds and then transplanted into fenestration defects surgically created in the periodontium of the second premolars. Histological assessment at 8 weeks post-implantation revealed surviving BrdU-positive PDLSCs associated with regenerated periodontium-related tissues, including cementum and bone-like structures. This is the first report to demonstrate the self-renewal capacity of PDLSCs using serial xenogeneic transplants and provides evidence of the long-term survival and tissue contribution of autologous PDLSCs in a preclinical periodontal defect model.
Introduction
Previous studies have characterized progenitor cells residing in periodontal ligament (PDL) tissues, known as periodontal ligament stem cells (PDLSCs), which exhibit similar properties to those described for bone-marrow-derived mesenchymal stem cells (BMSCs) [1–3]. Further, PDLSCs possess unique properties compared with other MSC-like populations, including their high expression of the tendon-associated marker scleraxis and their ability to form multiple tissue types in vivo, including Sharpey's fibers, alveolar bone, and cementum [3,4]. Subsequent studies have shown that PDLSCs may play a potential role in regeneration of periodontal tissues, in small- and large-animal models (reviewed in [5]) and more recently, in two human clinical pilot studies, which reported increased function of damaged periodontal tissues in patients who received autologous ex vivo–expanded PDL-derived progenitor cells [6,7]. While assessment of tissue regeneration has been limited to indirect clinical observations, these preliminary findings illustrated long-term safety and some efficacy associated with implantation of PDLSCs in a clinical setting.
One important aspect in tissue regeneration is the survival of implanted stem cells and their capacity to undergo self-renewal. This has been extensively studied in non-MSC populations, including embryonic stem cells [8–10], hematopoietic stem cells, neural crest stem cells [11–13], and skin epithelial stem cells [14,15]. Self-renewal implies coordination of stem cell proliferation while maintaining their undifferentiated cell phenotype and multipotency, a process by which a single stem cell divides to generate an identical daughter cell and/or committed progenitor cell. The capacity of BMSCs to regenerate a bone and marrow organ was initially demonstrated in the rodents [16] and more recently in humans based on in vivo serial transplantation studies [17]. Similarly, the capacity to undergo self-renewal has also been investigated in MSC-like populations derived from adult dental pulp tissue, following serial ectopic transplantations into immunodeficient mice [18]. The process of self-renewal is thought to be governed by biological pathways that preserve the undifferentiated state of progenitor cells and is controlled by numerous intracellular effectors as well as a number of cytokines, growth factors, adhesion molecules, and extracellular matrix components present in the tissue microenvironment [19]. However, little is known about the mechanisms involved in the molecular regulation of self-renewal and long-term survival of different MSC-like populations [20–22]. In the present study we evaluated the survival, self-renewal capacity, and developmental potential of ovine-derived PDLSCs using a xenogeneic and an autologous transplantation model.
Materials and Methods
Establishment and expansion of PDLSC populations
Normally fully erupted premolars were extracted from mature Merino ewes under approved guidelines set by the University of Adelaide Human Research Ethics Committee (AEC No. 146/10). PDLSCs were isolated as previously described [4]. Briefly, PDL was scraped from the middle third root of the root surface. PDL tissues were digested in collagenase Type 1 (3 mg/mL; Worthington Biochemical Corporation) and dispase II (neutral protease; 4 mg/mL; Roche Diagnostics) for 2 h at 37°C to obtain single-cell suspensions of PDL cells. Primary cultures of PDLSCs were established and maintained in modified α-minimal essential medium (α-MEM; JRH Biosciences, Inc.) supplemented with 10% fetal calf serum, 2 mM l-glutamine (Sigma-Aldrich, Inc.), 100 μM l-ascorbate-2-phosphate (Wako Pure Chemical Industries, Ltd.), 1 mM sodium pyruvate (Sigma), 50 U/mL penicillin G (JRH Biosciences, Inc.), and 50 μg/mL streptomycin (JRH Biosciences, Inc.) in a humidified atmosphere of 37°C and 5% CO2.
Differentiation assays
Mineralization was induced as previously described [23,24]. Briefly, PDLSCs isolated from six different sheep, at passage 3, were seeded at 8×103 per cm2 and cultured in modified α-MEM supplemented with 10−7 M dexamethasone (Mayne Pharma) and 1.8 mM inorganic phosphate (KH2PO4; BDH Chemicals) for 28 days with media changes twice weekly. Mineral deposit formation was identified by Alizarin Red (Alizarin Red S; Sigma-Aldrich, Inc.) staining. Adipogenesis was induced as previously described [24–26]. Briefly, PDLSCs, at passage 3, were seeded at 8×103 per cm2 and cultured for 28 days in modified α-MEM supplemented with 10−7 M dexamethasone (Mayne Pharma) and 60 μM indomethacin (Sigma-Aldrich, Inc.) with media changes twice a week. Formation of lipid-laden cells was determined by Oil Red O (MP Biomedicals) staining. Chondrogenic cell pellets were prepared from 1×106 cells at passage 3, and cultured for 28 days in induction media±transforming growth factor β1 as previously described [20,24]. Pellet cultures were fixed with 4% paraformaldehyde, and sectioned for hematoxylin and eosin (H&E) staining and immunohistochemical staining with mouse anti-human collagen type II monoclonal antibody (MAB1330; Chemicon International).
Flow cytometric analysis
To characterize the immunophenotype of ex-vivo-expanded PDLSCs, flow cytometric analysis was used to measure the expression of mesenchymal- and non-mesenchymal stem cell–associated surface markers according to standard procedures. Briefly, cells, at passage 3, were incubated with primary monoclonal antibodies specific to MSC-associated markers (CD29, CD44, CD166, and STRO-4 or HSP90β), hematopoietic, non-MSC-associated markers (CD14, CD31, and CD45) and isotype-matched controls. Cell suspensions were then incubated with a secondary detection reagent, goat anti-mouse immunoglobulin G-Phycoerythrin (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). Analysis was performed on a fluorescence-activated cell sorter fitted with a 250 MW argon laser (Beckman Coulter Cytomics FC500, using CXP Cytometry List Mode Data Acquisition and Analysis Software version 2.2; Beckman Coulter).
Transplantation of ovine PDLSCs into immunocompromized mice
All procedures were conducted in accordance with guidelines of an approved small-animal protocol (SA Pathology AEC No. 139/09). Ovine PDLSCs, at passage 4–5, were labeled with cell-proliferation-labeling reagent bromo-deoxyuridine (BrdU) 48 and 24 h prior to transplantation. Approximately, 4×106 PDLSCs from each of the six donors were mixed with 40 mg hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder (Zimmer, Inc.), and then clotted with 20 μL mouse fibrinogen (30 mg/mL in phosphate-buffered saline) and 20 μL mouse thrombin (100 U/mL in 2% CaCl2) to form a plug and then subcutaneously transplanted into the dorsal surface of 8-week-old immunocompromized (NOD/SCID) mice as previously described [27]. HA/TCP scaffold transplants, without PDLSCs, were used as experimental controls. Two replicate transplants, for each donor, were performed.
All PDLSC transplants were recovered 8 weeks after transplantation. One half of every transplant was fixed in 4% paraformaldehyde, decalcified in 0.5 M ethylenediaminetetraaceticacid (EDTA), paraffin embedded, and sectioned. H&E staining and modified tetrachrome (MT) staining were performed to illustrate the formation of mineralized tissues within transplants and characterize their composition, respectively. MT staining utilized a series of stains, including Weigert's iron hematoxylin, 1% phosphotungstic acid solution, picro-orange, and Ponceau mixture, to effectively distinguish osteoid tissue (deep blue) from mature mineralized bone (bright red), connective tissue (pale blue), and cartilage (pale blue) [28]. Immunohistochemistry was performed using cementum and bone markers collagen type I (COL-I), bone sialoprotein (BSP), and osteopontin (OPN), and alpha smooth muscle actin (αSMA), a marker of vasculature as previously described [29,30]. The survival and localization of transplanted cells was investigated by immunohistochemistry, targeting CD44 (MSC marker) and BrdU-expressing cells within the implant. Detailed information on all the used antibodies is provided in Supplementary Table S1.
Isolation and characterization of transplanted ovine PDLSCs
The other half of every transplant was digested in equal volumes of collagenase (Type I, 3 mg/mL) and dispase (Type II, 4 mg/mL) and resuspended cells were cultured in modified α-MEM for three passages. Ovine PDLSCs were isolated from the expanded heterogeneous cell populations based on their high expression of HSP90β [31], and depleted of any host-derived Sca1 (Ly-6A)–expressing mouse MSCs, by fluorescence activated cell sorting (FACS) using an Epics Altra HyPer Sort FACS machine (Beckman Coulter). Sorted cells were further expanded for three passages and then used for functional characterization in vitro and transplantation experiments in vivo, as described earlier.
Transplantation of autologous PDLSCs into an ovine periodontal defect model
All procedures were performed in accordance with guidelines of an approved animal ethics protocol (SA Pathology AEC No. 146/10). Twenty-four hours prior to surgery, primary, culture-expanded, autologous PDLSCs, derived from seven donors, were treated with cell-proliferation-labeling reagent BrdU (GE Healthcare) for cell tracking purposes. Immediately prior to surgery, 2×106 in-vitro-expanded BrdU-labeled PDLSCs were detached from tissue culture flasks with 0.05% Trypsin/EDTA, neutralized with α-MEM+10% autologous serum. A 4.5×2.5×1.5 mm3 piece of Gelfoam scaffold (gelatin sponge; Pharmacia Corporation) was then added to PDLSCs and mixed together. Gelfoam scaffold controls (without PDLSCs) were spotted with 10 μL α-MEM+10% autologous serum. All implants were clotted with 10 μL fibrinogen (Sigma; 30 mg/mL) and 10 μL thrombin (Sigma; 100 U/mL) 10 min prior to implantation.
A standard periodontal defect was created using a 5-mm trephine on an exposed buccal plate of alveolar bone covering the mandibular first premolar of seven female sheep, on both the left and right sides. Exposed tooth root was scaled of PDL and cementum. Defects were then filled with either autologous PDLSCs attached to Gelfoam scaffold or Gelfoam scaffold alone. All defects were then covered with a Gore-Tex® resorbable barrier membrane (W.L. Gore and Associates, Inc.). Finally, operative sites were covered with the mucoperiosteal flap and sutured in place. Surgery was performed under general anesthesia and post-operative antibiotics and analgesia were administered.
All PDLSC transplants were recovered 6 weeks after transplantation, fixed in 4% paraformaldehyde, decalcified in 10% formic acid (pH 1.8), paraffin embedded, and sectioned for H&E and MT staining. Immunohistochemistry was performed using antibodies reactive to ovine cementum and bone markers, OPN, BSP, COL-I, and the blood vessel marker αSMA as previously described [29,30]. To demonstrate the contribution of implanted autologous PDLSCs toward periodontal tissue regeneration, survival and localization of transplanted cells within the defects were investigated using immunodetection of BrdU.
Cell imaging
Images were generated using the digital slide scanner NanoZoomer system (Hamamatsu Photonics).
Results
PDLSCs exhibit the capacity to survive and form multiple tissue types in vivo
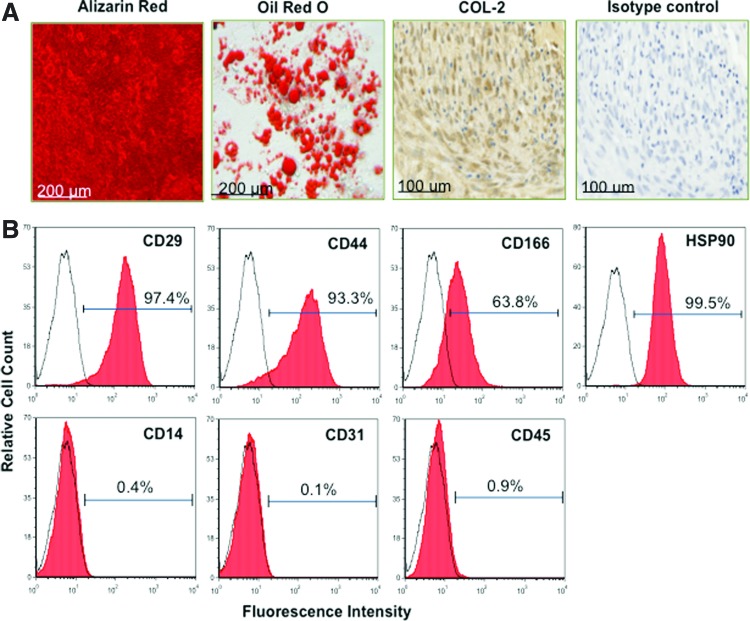
Primary, culture-expanded ovine PDLSCs exhibited the capacity to differentiate into cells of osteogenic/cementogenic, adipogenic, and chondrogenic lineages, as evidenced by the formation of Alizarin Red-positive mineralized nodules, lipid-containing adipocytes positive for Oil Red O, and collagen II-expressing chondrocyte pellets, respectively (Fig. 1A). Further, immunophenotypic profiling demonstrated that PDLSCs expressed high cell surface levels of CD29, CD44, CD166, and STRO-4 (HSP90) and lacked the expression of hematopoietic markers CD14, CD31, and CD45 (Fig. 1B), as previously shown for other MSC-like populations.
FIG. 1.
In vitro characterization of primary-ovine-derived periodontal ligament stem cells (PDLSCs). (A) Ovine PDLSCs displayed the capacity to differentiate into cells of osteogenic, adipogenic, and chondrogenic lineages. This was illustrated by their ability to form Alizarin-Red-stained mineral nodules, Oil Red O–stained lipid lobules, and collagen II (COL-2)–expressing pellet cultures, respectively. (B) Immunophenotypic profiling confirmed high levels of expression of CD29, CD44, CD166, and HSP90 [mesenchymal stem cell (MSC)–associated markers] and negligible levels of the hematopoietic cell markers CD14, CD31, and CD45. Color images available online at www.liebertpub.com/scd
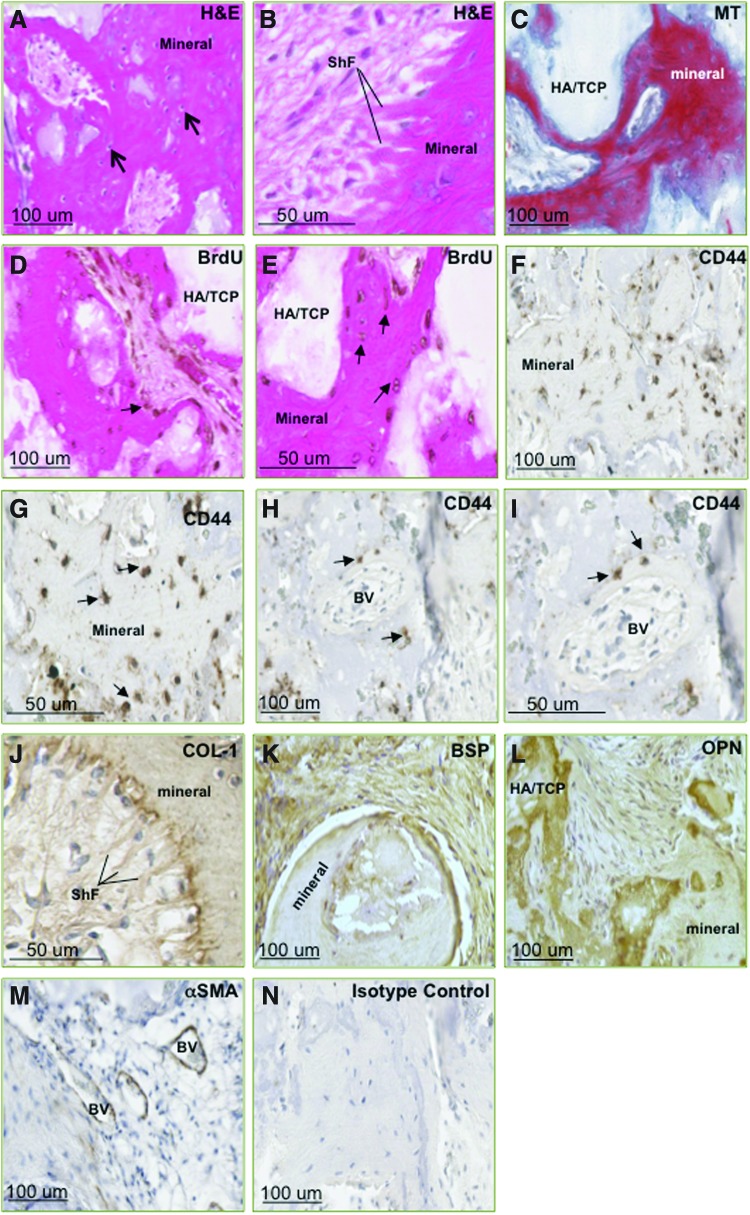
To assess the developmental potential of PDLSCs in this study, BrdU-labeled cells were mixed with HA/TCP particles and then subcutaneously transplanted into the dorsal surface of NOD/SCID mice. The transplanted PDLSCs from six donor populations exhibited the capacity to form mineralized and fibrous tissues, including the associated vasculature, in vivo (Fig. 2). Newly generated bone/cementum-like tissue was observed throughout the implants, lined with osteoblasts/cementoblasts with the presence of osteo/cementocyte-like cells residing in the lacunae, embedded within the tissue matrix, as depicted in images of H&E-stained sections (Fig. 2A, B). Bright red staining displayed in MT-stained sections confirmed the presence of mature lamellar bone (Fig. 2C). Assessment of cellular expression of BrdU and the MSC-associated marker CD44 within the implants was used to track the localization of donor PDLSCs in order to assess cell survival and contribution to the formation of new tissues (Fig. 2D–I). Expression of CD44 was noted in cells lining the periphery of mineralized structures and blood vessels, suggesting that the PDLSCs were associated with the formation of these tissues. Similarly, BrdU expression was observed in cells embedded within and lining the mineralized structures and blood vessel walls, indicating that the donor PDLSCs possessed the capacity for long-term survival and to contribute to the generation of multiple tissue types in vivo. Expression of COL-I, BSP, and OPN within the newly generated tissues identified the cementum/bone-like tissue structures and the surrounding osteoblast/cementoblast-like cells (Fig. 2J–L). Formation of collagen bundles, resembling Sharpey's fibers and inserting into the mineralized tissue, was evident in H&E images and further illustrated in COL-I-stained sections. Additionally, cells integrated within the newly formed vasculature expressed smooth muscle marker αSMA (Fig. 2M).
FIG. 2.
Primary PDLSCs exhibit the capacity to survive and form multiple tissue types in vivo. Following ectopic transplantation and 8 weeks of incubation in vivo, hematoxylin and eosin (H&E) staining of retrieved implants illustrated formation of mineralized tissue [modified tetrachrome (MT)] along hydroxyapatite/tricalcium phosphate (HA/TCP) carrier material, with numerous osteocyte/cementocyte-like cells embedded in the mineralized matrix [arrows (A)] and presence of Sharpey's-like fibers [ShF (B)]. Mineralized structures appear bright red following MT staining (C), representative of mature lamellar bone. Implanted PDLSCs, localized by targeting bromo-deoxyuridine (BrdU) (D, E) and CD44 (F–I) expression, were found lining and embedded in the mineralized matrix (arrows), surrounding fibrous tissue and blood vessel (BV) walls. Expression of an array of bone/cementum markers (J–L), including collagen type I (COL-I), bone sialoprotein (BSP), and osteopontin (OPN), demonstrated differentiation of implanted PDLSCs into cells of osteogenic/cementoblastic lineage. Cells associated with newly generated BV expressed alpha smooth muscle actin (αSMA) (M). There was no detectable non-specific labeling present in control sections stained with isotype-matched antibodies (N). Color images available online at www.liebertpub.com/scd
Re-derived PDLSCs exhibit the capacity to survive and form multiple tissue types in secondary transplants
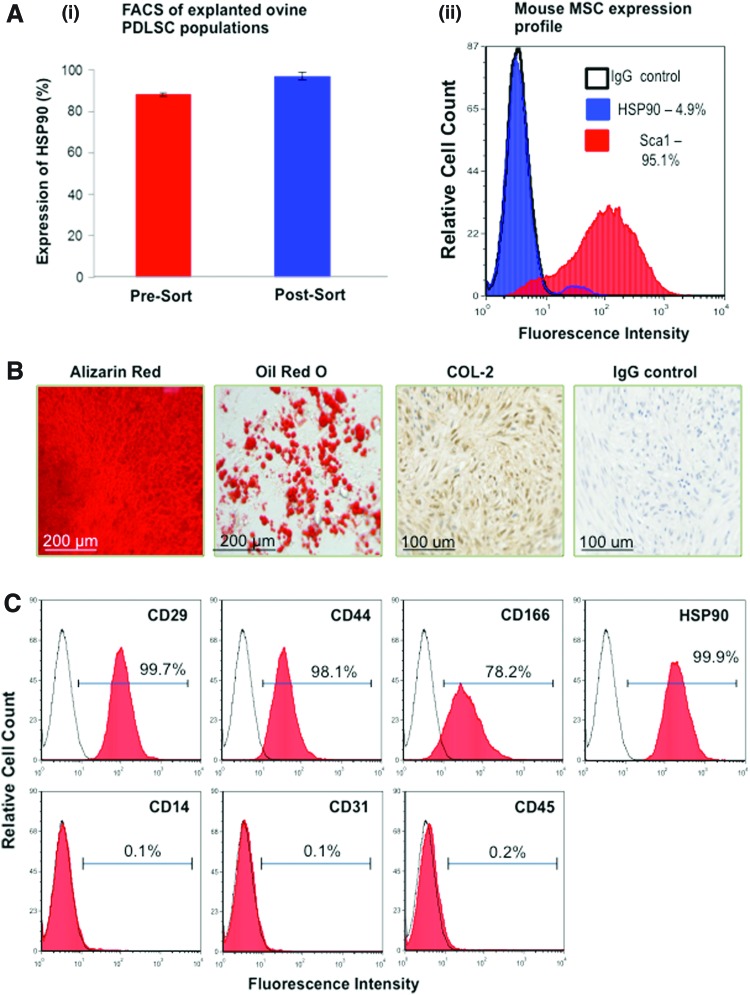
To evaluate the self-renewal potential of PDLSCs, re-derived PDLSCs were recovered from harvested primary transplants 8 weeks post-surgery. High levels of HSP90 expression were detected in unsorted MSC population cultured from recovered explants [Fig. 3A(i)—red bar]. This was indicative of the survival and maintenance of initially implanted ovine PDLSCs within the explanted tissues. Re-derived PDLSCs were then selected, based on their expression of STRO-4 (HSP90β) [31], and this highly purified population [Fig. 3A(i)—blue bar] was expanded in vitro under normal growth conditions. To address potential host-MSC contamination, during FACS, ovine PDLSCs were depleted of Sca1-expressing mouse MSCs. Immunophenotypic profiling of mouse MSCs illustrated their lack of HSP90 expression [Fig. 3A(ii)].
FIG. 3.
In vitro characterization of re-derived PDLSCs. Heterogeneous cell populations derived from mouse explants expressed high levels of HSP90 prior to sorting, n=6 [A(i) red bar]. Purified populations of ovine PDLSCs, enriched for HSP90-expressing cells and depleted of Sca1-expressing cells, were generated by fluorescence activated cell sorting [A(i) blue bar]. Immunophenotypic profiling of mouse MSCs demonstrated high levels of Sca1 and lack of HSP90 expression, representative of n=3 [A(ii)]. (B) Expanded ovine PDLSCs re-derived from primary transplants retained PDLSC-like properties and exhibited a comparable potential to undergo osteogenesis, adipogenesis, and chondrogenesis in vitro. (C) Under inductive conditions, re-derived PDLSCs formed mineralized matrix and lipid-laden adipocytes, and underwent chondrogenic differentiation as illustrated by Alizarin Red, Oil Red O, and collagen II (COL-2) staining, respectively. Re-derived PDLSCs expressed high levels of CD29, CD44, CD166, and HSP90 (MSC-associated markers) and lacked the expression of the hematopoietic cell markers CD14, CD34, and CD45. Color images available online at www.liebertpub.com/scd
Functional characterization studies demonstrated that the re-derived PDLSC populations from the primary transplants maintained their capacity to form mineralized nodules, lipid-laden lobules, and undergo chondrogenesis, under appropriate inductive conditions in vitro (Fig. 3B). Further, high expression levels of the cell surface markers CD29, CD44, CD166, and STRO-4 and lack of CD14, CD31, and CD45 expression were consistent with the immunophenotypic profile observed in the primary PDLSC populations (Fig. 3C).
We further assessed the tissue regeneration capacity of re-derived PDLSCs isolated from primary transplants, by implanting these populations into secondary ectopic transplants. Histomorphological analysis of 8-week-old secondary ectopic transplants demonstrated that four out of six donor PDLSC populations displayed a capacity to form fibrous and mineralized tissues with associated vasculature in vivo, as observed in H&E- and MT-stained tissue sections although at diminished levels in comparison to the primary transplants (Fig. 4A–E). Positive cellular staining for CD44 and BrdU indicated that a minor proportion of the implanted re-derived PDLSCs survived 8 weeks post-implantation, and directly contributed to the formation of fibrous and mineralized structures, as indicated by the integration of positively stained cells within and surrounding newly generated structures (Fig. 4F, G). Donor cells lining along or embedded within the mineralized tissue expressed the bone-related markers COL-I, BSP, and OPN (Fig. 4H–J). Donor cells present within the newly generated vasculature also expressed αSMA (Fig. 4K).
FIG. 4.
Transplanted, re-derived ovine PDLSCs exhibit the capacity to survive and form multiple tissue types in vivo. Formation of bone/cementum-like tissues was evident by eosinophillic and bright red staining illustrated in H&E- (A–D) and MT- (E) stained sections, respectively. Presence of organized collagen bundles, resembling ShF, was noted throughout the transplants. BrdU and CD44 expression (arrows) confirms that implanted PDLSCs survived and contributed to the formation of fibrous and mineralized structures (F, G). Positive staining of cells lining and embedded in the mineralized tissue matrix for COL-I (H), BSP (I), and OPN (J) demonstrated their cementoblastic/osteogenic lineage commitment. Newly formed vasculature was assessed by αSMA expression (K). There was no detectable non-specific labeling present in control sections stained with isotype-matched antibodies (L). Color images available online at www.liebertpub.com/scd
Autologous transplantation of ovine PDLSCs leads to long-term survival and contribution to periodontal tissue regeneration
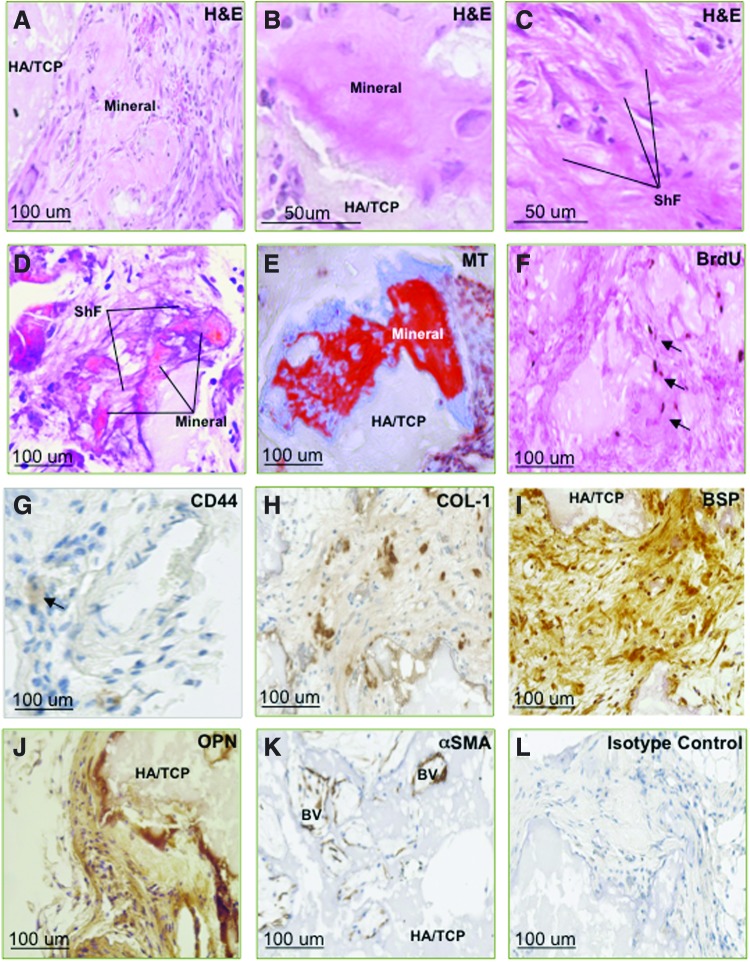
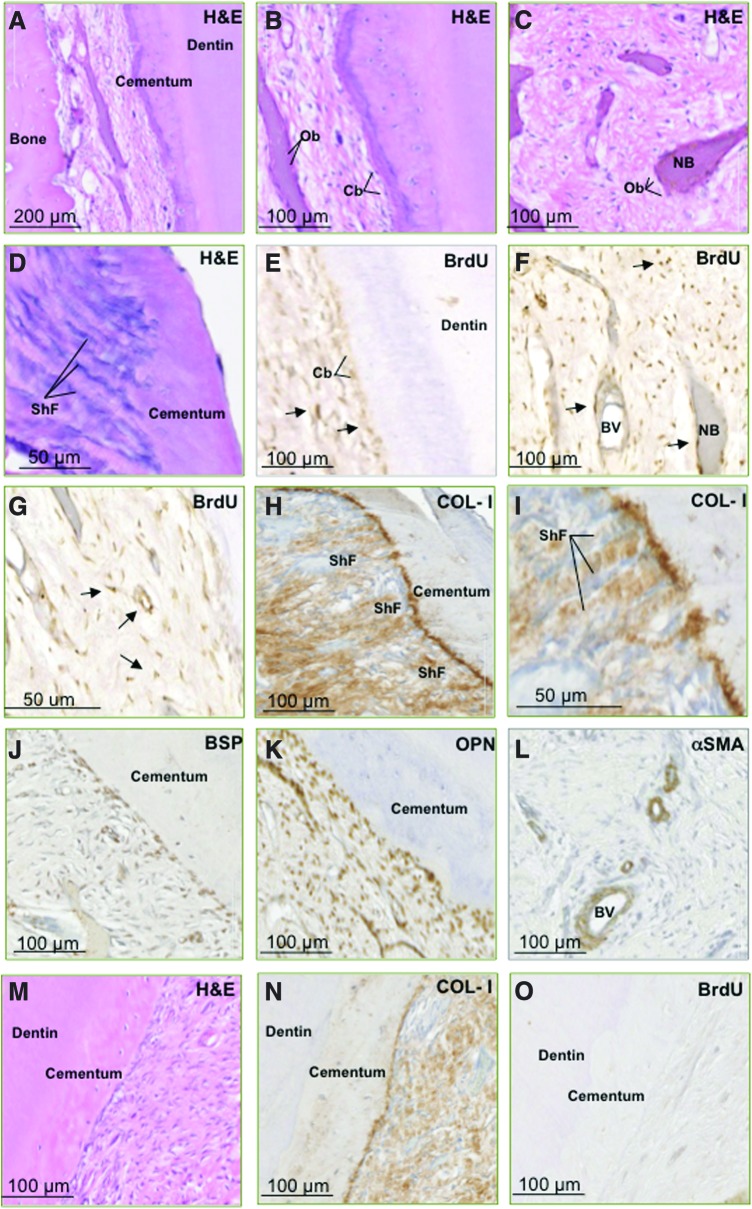
We next assessed the capacity for long-term survival and the potential role of PDLSCs in tissue regeneration of dental structures in an ovine preclinical periodontal defect model. Autologous ex vivo–expanded PDLSCs were prelabeled with BrdU and then seeded onto non-osteoconductive Gelfoam scaffolds before being transplanted into fenestration defects surgically created in the periodontium of the second premolars. Histological assessment at 8 weeks post-implantation revealed the regeneration of periodontium-related tissues, including cementum and bone-like structures (Fig. 5). Newly generated cementum was lined by cementoblast-like cells and formed alongside pre-existing cementum housing numerous cementocyte-like cells in the matrix-embedded lacunae (Fig. 5A–D). Further, numerous organized bundles of collagen fibers inserting into the cementum resembling Sharpey's fibers were noted, expressing high levels of COL-I (Fig. 5H, I).
FIG. 5.
Autologous transplantation of ovine PDLSCs contributes to periodontal tissue regeneration. BrdU-labeled autologous PDLSCs that were transplanted into surgically created periodontal defects exhibited the capacity to regenerate ovine periodontium. PDLSCs contributed to regeneration of bone, cementum, and collagen fibers (A–D). BrdU expression (arrows) was localized in osteoblast-like cells (Ob), cementoblast-like cells (Cb), and cells lining the BV walls (E–G). Expression of COL-I and distribution of the staining clearly demonstrated the presence and functional arrangement of regenerated ShF (H, I). Cells within the regenerated periodontium tissue expressed BSP and OPN, markers of bone/cementum (J, K). Newly generated vasculature (BV) was distinguished by the expression of αSMA (L). Control defects, treated with Gelfoam® scaffolds alone stained with H&E (M), exhibited lower levels of COL-1 staining (N) and lacked BrdU expression (O). Color images available online at www.liebertpub.com/scd
Immunohistochemical analysis was performed to assess the expression of BrdU, and bone/cementum-related markers within the newly formed tissues. BrdU-positive cells were found lining the newly formed layers of cementum or embedded within the mineralized structures and surrounding fibrous tissue and vasculature (Fig. 5E–G). This indicated that the implanted PDLSCs contributed to the generation of multiple tissue types. Additionally, cells lining the cementum and osteoid-like tissue expressed the mineral-associated markers COL-I, BSP, and OPN (Fig. 5J, K) consistent with a cementoblastic/osteoblastic lineage, while cells associated with the vasculature expressed αSMA, a marker associated with immature MSC populations, smooth muscle cells, and pericytes (Fig. 5L).
Defects treated with Gelfoam scaffold alone exhibited a decreased level of COL-I expression and presented a less-organized formation of collagen bundles representative of Sharpey fibers (Fig. 5M, N). Lack of BrdU expression in control sections (Fig. 5O) demonstrated the specificity of BrdU detection in PDLSC-treated defects and confirms the integration and contribution of implanted cells to periodontal tissue regeneration.
Discussion
The capacity of MSCs for long-term survival and participate in tissue regeneration following transplantation in vivo is dependent on a number of factors including their ability to undergo self-renewal. The capacity to reconstitute the same compartment of phenotypically defined progenitor cells has been demonstrated for bone-marrow-derived skeletal progenitors, with the ability to establish a hematopoietic bone marrow microenvironment over serial ectopic transplants in vivo [17]. Further, dental pulp stem cells have also been shown to exhibit a capacity to undergo self-renewal as assessed by their ability to regenerate dentin-pulp-like complexes over serial transplantations in vivo [18]. In the present study, we demonstrate, for the first time, that PDLSCs exhibit a self-renewal capacity in a serial xenogeneic transplantation model and contribute to long-term periodontal tissue regeneration following implantation of autologous PDLSCs into a periodontal defect model.
Following harvest of the primary ectopic xenogeneic transplants, ovine PDLSCs were isolated by FACS from a heterogeneous population of mature stromal cells and contaminating host mouse cells, based on their ability to maintain high expression levels of the MSC-associated marker STRO-4 (HSP90β) [31]. Comparative in vitro characterization of primary PDLSCs and re-derived PDLSCs isolated from primary ectopic transplants illustrated equivalent differentiation capacities and immunophenotypic characteristics. Immunohistological assessment of both the primary and the secondary transplants determined that the implanted primary and re-derived PDLSCs were able to survive 8 weeks post-transplantation without undergoing extensive proliferation, as determined by BrdU labeling. Further, antibodies specific to ovine antigens recognizing the MSC-associated marker CD44; bone/cementum makers COL-1, BSP, and OPN; and vascular marker αSMA demonstrated multitissue contribution. These findings suggest that a proportion of PDLSCs retain their MSC properties following extensive expansion ex vivo and long-term transplantation in vivo, as they contributed to the formation of mineralized structures, fibrous tissue, and differentiated into perivascular cells within the implants. The results imply that a subset of implanted PDLSCs terminally differentiated into functional fibroblasts, pericytes, and cells of osteogenic lineage, while a subset remained in the undifferentiated, progenitor state, maintaining an immature MSC phenotype and giving rise to a population of MSC daughter cells, indicative of a hierarchical compartment of progenitor populations.
Despite their comparable multipotency in vitro, a reduction in the number of CD44- and BrdU-positive PDLSCs was evident in the secondary transplants. This finding suggests that long-term ex vivo expansion of PDLSCs had an effect on their survival and self-renewal capacities, which subsequently altered the developmental potential of the remaining progeny. Previous studies have reported that downregulation of telomerase activity in culture-expanded MSC-like populations leads to eventual cellular senescence [32,33]. Other studies have also reported that MSC self-renewal and lineage specification are regulated by multiple interacting pathways, based on global gene expression profiling of BMSC survival during differentiation and dedifferentiation in vitro [19]. Numerous molecules have been implied in the maintenance of stemness in MSCs, including cytokines and growth factors fibroblast growth factor (FGF)2, platelet-derived growth factor-BB (PDGF-BB), epidermal growth factor [34–40], and Wnt family members [41–43]. Involvement of Wnt signaling has been implicated in the control of MSC fate determination and identified as a potential mediator of self-renewal in MSCs [43–46]. The role of PDGF signaling in homeostasis, maintenance, and recruitment of MSCs has also been investigated [38,47–49]. Further, platelet-derived growth factor receptor β signaling promotes self-renewal of BMSCs, via activation of AKT and ERK transduction pathways, to stimulate proliferation and inhibit differentiation, respectively [50]. Similarly, FGF2 plays a role in self-renewal of adipose-derived MSCs through FGF2 activation of the ERK1/2 pathway [39]. However, one of the major challenges and limiting factors associated with many of these studies is the heterogeneity of isolated MSC populations. To circumvent such issues, comparative genomic and proteomic profiling of well-characterized self-renewing clonal subsets of MSCs could be performed to identify potential key regulators and signaling pathways involved in self-renewal. Previous studies have demonstrated that PDLSCs contain highly proliferative multipotent clonal subsets [20], which present a powerful model for elucidating the underlying processes involved in stem cell self-renewal and lineage commitment and identifying master transcriptional regulatory switches and interaction between signaling pathways. Using this approach, two molecules that were found to be highly expressed by long-lived multipotential MSC populations, the basic-helix-loop-helix transcription factor Twist-1 and the histone 3 lysine 27 methyltransferase Ezh2, have subsequently been demonstrated to be involved in the maintenance and growth of immature MSC populations [21,22]. These studies highlight the complex transcriptional and epigenetic changes involved in MSC self-renewal and cell commitment, and the issues of identifying immature and committed populations during ex vivo expansion.
The regenerative potential of PDLSCs has previously been shown in preclinical large-animal studies and human clinical pilot trials for periodontal regeneration [6,7,51–54]. However, most studies have failed to identify the cell populations post-transplantation and their direct contribution to tissue regeneration. To further characterize the long-term survival and regenerative potential of PDLSCs, we implanted ex-vivo-expanded autologous PDLSCs into an ovine periodontal defect model, to mimic a naturally inductive environment, representative of a periodontal disease state, where complete functional and sustainable periodontal regeneration is difficult to achieve due to the complexity of the structure and the integration of multiple tissue types that comprise the periodontium. These studies found that autologous PDLSCs exhibited a capacity to regenerate complex tissue structures within the periodontium in vivo. In this study, BrdU-labeled, autologous ovine PDLSCs transplanted into a surgically created periodontal defect demonstrated contribution to the regeneration of multiple tissues, including alveolar bone, cementum, fibrous tissue, Sharpey's fibers, and blood vessels. Further, the autologous PDLSCs demonstrated the ability to differentiate into matrix-synthesizing osteoblasts and cementum-forming cementoblasts. The regeneration of COL-I-positive Sharpey's fibers is of considerable importance for periodontal regeneration through the integration of regenerated tissues into a functional structure and confirms restoration of the lost tissue as well as its pivotal role in tooth support. Importantly, surviving PDLSCs lacked a high turnover in vivo as demonstrated by their retention of BrdU following 8 weeks post-transplantation and may be able to contribute to the long-term tissue maintenance and homeostasis.
In conclusion, the present study has demonstrated that PDLSCs are a highly potent population of progenitor cells, with the ability to differentiate into specialized lineages and hence regenerate functional tissues as well as the potential to retain their stem-cell-like properties. Combined, these findings provide a greater insight into the current knowledge bases on PDLSCs and illustrate their ability to regenerate complex structures of the periodontium that may be utilized for future clinical applications.
Supplementary Material
Acknowledgments
This study was supported by NH&MRC Project Grant no. APP1043994.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gronthos S, Mankani M, Brahim J, Robey PG. and Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG. and Shi S. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY. and Shi S. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155 [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Mrozik K, Shi S. and Bartold PM. (2006). Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int 79:310–317 [DOI] [PubMed] [Google Scholar]
- 5.Hynes K, Menicanin D, Gronthos S. and Bartold PM. (2012). Clinical utility of stem cells for periodontal regeneration. Periodontol 2000 59:203–227 [DOI] [PubMed] [Google Scholar]
- 6.Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S. and Shi S. (2010). Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis 16:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gault P, Black A, Romette JL, Fuente F, Schroeder K, Thillou F, Brune T, Berdal A. and Wurtz T. (2010). Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol 37:750–758 [DOI] [PubMed] [Google Scholar]
- 8.Golan-Mashiach M, Dazard JE, Gerecht-Nir S, Amariglio N, Fisher T, Jacob-Hirsch J, Bielorai B, Osenberg S, Barad O, et al. (2005). Design principle of gene expression used by human stem cells: implication for pluripotency. FASEB J 19:147–149 [DOI] [PubMed] [Google Scholar]
- 9.Wei CL, Miura T, Robson P, Lim SK, Xu XQ, Lee MY, Gupta S, Stanton L, Luo Y, et al. (2005). Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells 23:166–185 [DOI] [PubMed] [Google Scholar]
- 10.Stewart R, Stojkovic M. and Lako M. (2006). Mechanisms of self-renewal in human embryonic stem cells. Eur J Cancer 42:1257–1272 [DOI] [PubMed] [Google Scholar]
- 11.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA. and Lemischka IR. (2002). A stem cell molecular signature. Science 298:601–604 [DOI] [PubMed] [Google Scholar]
- 12.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC. and Melton. DA. (2002). “Stemness”.: transcriptional profiling of embryonic and adult stem cells. Science 298:597–600 [DOI] [PubMed] [Google Scholar]
- 13.De Filippis L. and Binda E. (2012). Concise review: self-renewal in the central nervous system: neural stem cells from embryo to adult. Stem Cells Transl Med 1:298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M. and Fuchs E. (2004). Defining the epithelial stem cell niche in skin. Science 303:359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanpain C, Lowry WE, Geoghegan A, Polak L. and Fuchs E. (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118:635–648 [DOI] [PubMed] [Google Scholar]
- 16.Friedenstein AJ. (1980). Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus 25:19–29 [DOI] [PubMed] [Google Scholar]
- 17.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M. and Bianco P. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324–336 [DOI] [PubMed] [Google Scholar]
- 18.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG. and Shi S. (2002). Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535 [DOI] [PubMed] [Google Scholar]
- 19.Song L, Webb NE, Song Y. and Tuan RS. (2006). Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells 24:1707–1718 [DOI] [PubMed] [Google Scholar]
- 20.Menicanin D, Bartold PM, Zannettino AC. and Gronthos S. (2010). Identification of a common gene expression signature associated with immature clonal mesenchymal cell populations derived from bone marrow and dental tissues. Stem Cells Dev 19:1501–1510 [DOI] [PubMed] [Google Scholar]
- 21.Cakouros D, Isenmann S, Cooper L, Zannettino A, Anderson P, Glackin C. and Gronthos S. (2012). Twist-1 induces Ezh2 recruitment regulating histone methylation along the Ink4A/Arf locus in mesenchymal stem cells. Mol Cell Biol 32:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenmann S, Arthur A, Zannettino AC, Turner JL, Shi S, Glackin CA. and Gronthos S. (2009). TWIST family of basic helix-loop-helix transcription factors mediate human mesenchymal stromal/stem cell growth and commitment. Stem Cells 27:2457–2468 [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S, Graves SE, Ohta S. and Simmons PJ. (1994). The STRO-1+fraction of adult human bone marrow contains the osteogenic precursors. Blood 84:4164–4173 [PubMed] [Google Scholar]
- 24.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A. and Simmons PJ. (2003). Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116:1827–1835 [DOI] [PubMed] [Google Scholar]
- 25.Gimble JM. (1998). Marrow stromal adipocytes. In: Marrow Stromal Cell Culture. Beresford JN. and Owen M, eds. Cambridge University Press, Cambridge, pp. 67–87 [Google Scholar]
- 26.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 27.Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D. and Robey PG. (1997). Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12:1335–1347 [DOI] [PubMed] [Google Scholar]
- 28.Ralis ZA. and Watkins G. (1992). Modified tetrachrome method for osteoid and defectively mineralized bone in paraffin sections. Biotech Histochem 67:339–345 [DOI] [PubMed] [Google Scholar]
- 29.Chen SC, Marino V, Gronthos S. and Bartold PM. (2006). Location of putative stem cells in human periodontal ligament. J Periodontal Res 41:547–553 [DOI] [PubMed] [Google Scholar]
- 30.Psaltis PJ, Carbone A, Nelson AJ, Lau DH, Jantzen T, Manavis J, Williams K, Itescu S, Sanders P, et al. (2010). Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc Interv 3:974–983 [DOI] [PubMed] [Google Scholar]
- 31.Gronthos S, McCarty R, Mrozik K, Fitter S, Paton S, Menicanin D, Itescu S, Bartold PM, Xian C. and Zannettino AC. (2009). Heat shock protein-90 beta is expressed at the surface of multipotential mesenchymal precursor cells: generation of a novel monoclonal antibody, STRO-4, with specificity for mesenchymal precursor cells from human and ovine tissues. Stem Cells Dev 18:1253–1262 [DOI] [PubMed] [Google Scholar]
- 32.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG. and Wang CY. (2002). Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol 20:587–591 [DOI] [PubMed] [Google Scholar]
- 33.Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG. and Kassem M. (2002). Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol 20:592–596 [DOI] [PubMed] [Google Scholar]
- 34.Gronthos S. and Simmons PJ. (1995). The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood 85:929–940 [PubMed] [Google Scholar]
- 35.Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K. and Kato Y. (2001). Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 288:413–419 [DOI] [PubMed] [Google Scholar]
- 36.Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R. and Quarto R. (2003). Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res 287:98–105 [DOI] [PubMed] [Google Scholar]
- 37.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI. and Welter JF. (2005). FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol 203:398–409 [DOI] [PubMed] [Google Scholar]
- 38.Kang YJ, Jeon ES, Song HY, Woo JS, Jung JS, Kim YK. and Kim JH. (2005). Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 95:1135–1145 [DOI] [PubMed] [Google Scholar]
- 39.Zaragosi LE, Ailhaud G. and Dani C. (2006). Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells 24:2412–2419 [DOI] [PubMed] [Google Scholar]
- 40.Tamama K, Fan VH, Griffith LG, Blair HC. and Wells A. (2006). Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 24:686–695 [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49 [DOI] [PubMed] [Google Scholar]
- 42.Kleber M. and Sommer L. (2004). Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol 16:681–687 [DOI] [PubMed] [Google Scholar]
- 43.Boland GM, Perkins G, Hall DJ. and Tuan RS. (2004). Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93:1210–1230 [DOI] [PubMed] [Google Scholar]
- 44.Etheridge SL, Spencer GJ, Heath DJ. and Genever PG. (2004). Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 22:849–860 [DOI] [PubMed] [Google Scholar]
- 45.De Boer J, Wang HJ. and Van Blitterswijk C. (2004). Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 10:393–401 [DOI] [PubMed] [Google Scholar]
- 46.Ling L, Nurcombe V. and Cool SM. (2009). Wnt signaling controls the fate of mesenchymal stem cells. Gene 433:1–7 [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C, Ishizawa S, Fujimori T, Nabeshima Y, Umezawa A, et al. (2008). PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res 23:1519–1528 [DOI] [PubMed] [Google Scholar]
- 48.Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, Jelinek DF. and Kay NE. (2010). Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood 116:2984–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veevers-Lowe J, Ball SG, Shuttleworth A. and Kielty CM. (2011). Mesenchymal stem cell migration is regulated by fibronectin through alpha5beta1-integrin-mediated activation of PDGFR-beta and potentiation of growth factor signals. J Cell Sci 124:1288–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gharibi B, Ghuman MS. and Hughes FJ. (2012). Akt- and Erk-mediated regulation of proliferation and differentiation during PDGFRbeta-induced MSC self-renewal. J Cell Mol Med 16:2789–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S. and Wang S. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S. and Wang S. (2008). Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26:1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JY, Jeon SH. and Choung PH. (2011). Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant 20:271–285 [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Kim KH, Seo BM, Koo KT, Kim TI, Seol YJ, Ku Y, Rhyu IC, Chung CP. and Lee YM. (2009). Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol 80:1815–1823 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.