Abstract
This study examines the role of protein kinase C (PKC) and AMP-activated kinase (AMPK) in acetaminophen (APAP) hepatotoxicity. Treatment of primary mouse hepatocytes with broad-spectrum PKC inhibitors (Ro-31-8245, Go6983), protected against APAP cytotoxicity despite sustained JNK activation. Broad-spectrum PKC inhibitor treatment enhanced p-AMPK levels and AMPK regulated survival-energy pathways including autophagy. AMPK inhibition by compound C or activation using an AMPK activator oppositely modulated APAP cytotoxicity, suggesting p-AMPK and AMPK regulated energy survival pathways particularly autophagy play a critical role in APAP cytotoxicity. Ro-31-8245 treatment in mice upregulated p-AMPK levels, increased autophagy (i.e. increased LC3-II formation, p62 degradation) and protected against APAP-induced liver injury, even in the presence of sustained JNK activation and translocation to mitochondria. In contrast, treatment of hepatocytes with classical PKC inhibitor (Go6976) protected against APAP by inhibiting JNK activation. Knockdown of PKC-α using antisense (ASO) in mice also protected against APAP-induced liver injury by inhibiting JNK activation. APAP treatment resulted in PKC-α translocation to mitochondria and phosphorylation of mitochondrial PKC substrates. JNK 1 and 2 silencing in vivo decreased APAP-induced PKC-α translocation to mitochondria, suggesting PKC-α and JNK interplay in a feed-forward mechanism to mediate APAP-induced liver injury. Conclusion: PKC-α and other PKC(s) regulate death (JNK) and survival (AMPK) proteins, to modulate APAP-induced liver injury.
Introduction
Acetaminophen (APAP) is the most common cause of acute liver failure in the United States, accounting for 46% of all cases (1). APAP hepatotoxicity involves the active participation of signal transduction pathways that activate JNK (2). Inhibition of JNK prevents APAP-induced liver injury even in the presence of extensive GSH depletion and covalent binding (3). We have proposed a two hit hypothesis to mitochondria as the central mechanism mediating APAP-induced liver injury. APAP is metabolized to NAPQI by CYP2e1, which depletes GSH and leading to covalent binding in cytoplasm and mitochondria (first hit). Mitochondrial GSH depletion and covalent binding increase the generation of mitochondrial reactive oxygen species (ROS) that activate JNK, through upstream MAP kinase pathways (4). Activated JNK translocates to mitochondria binding to Sab (second hit), an outer membrane protein, which is phosphorylated by JNK and is required for toxicity. JNK binding to Sab on mitochondria leads to further enhancement of ROS generation by a mechanism that is not yet understood; the enhanced ROS is important in sustaining JNK activation and inducing the mitochondrial permeability transition (MPT) to mediate hepatocyte necrosis (5). JNK signaling is essential for APAP-induced programmed necrosis, and other signaling proteins such as GSK-3β, MLK-3, and ASK-1 that mediate APAP-induced liver injury acting upstream to modulate JNK signaling (4, 6-8).
Aside from MAPK, other signaling pathways may be activated by ROS. Previously we have shown that hydrogen peroxide induced necrosis of primary mouse hepatocytes is modulated by activation of Protein Kinase C (PKC) and subsequent inactivation of AMP-activated kinase (AMPK). PKC inhibitors significantly protect against H2O2 induced hepatocyte necrosis through activation of an AMPK kinase survival pathway (9). There are at least 11 known isoforms of PKC, which are divided into 3 major classes: the classical group (α, βI, βII, and γ), which can be activated by diacylglycerol (DAG), calcium or phorbol esters; the novel group (δ, ε, η, and θ), which can be activated by DAG but is insensitive to calcium; and the atypical group (ζ and λ/ι), which are insensitive to calcium, DAG, and phorbol esters (10). Distribution of PKC isoforms in different organs and tissues is variable (11, 12). Five isoforms (α, βII, δ, ε, ζ) have been shown to be present in the liver (13). Whether PKC activation or inhibition protects or promotes cell death may depend on the model of injury, cell type and which isoforms are involved (14, 15). PKC also plays a role in energy homeostasis, insulin signaling and glucose metabolism. Inhibition of atypical PKC has been shown to cause activation of AMP-activated kinase (AMPK), a key regulator of energy homeostasis in cultured endothelial cells (16).
AMPK is another serine-threonine kinase with a heterotrimeric complex consisting of catalytic α subunit and two regulatory subunits (β and γ) and serves as an important energy sensor in cells responding to the AMP: ATP ratio (17, 18). Phosphorylation at Thr 172 site in α subunit is essential for AMPK activation. AMPK activation promotes ATP production by switching off anabolic processes and turning on catabolic pathways (17). AMPK not only regulates energy homeostasis but also has cytoprotective effects in hepatocytes by inhibition of apoptosis, regulation of mitochondrial biogenesis, protection against mitochondrial injury and activation of autophagy (19-25).
AMPK activates autophagy through inhibition of mammalian target of rapamycin complex 1 (mTORC1). It has also recently been shown that APAP treatment inhibits mTORC1 and leads to activation of autophagy (26). Induction of autophagy is presumed to protect against APAP hepatotoxicity by removal of injured mitochondria (26). Autophagy is regulated by the autophagy-related proteins (Atg), which form protein complexes during assembly, docking and degradation of the autophagosome. Recently, it has been shown that knockout of Atg7, a ubiquitin E1-like enzyme required for autophagosome formation, in mice increased susceptibility to APAP-induced liver injury (27).
The roles of PKC and AMPK in APAP hepatotoxicity have not been previously explored. In the present study, we explore how broad-spectrum PKC inhibitors and silencing of PKC-α modulate AMPK, the master energy regulator in hepatocytes, and JNK signaling to mediate APAP-induced liver injury.
Materials and Methods
Materials
All inhibitors (Ro-31-8425, Go6983, Go6976, Compound C) and the activator (AMPK activator III, DHPO) were purchased from Calbiochem (San Diego, CA).
Antisense oligonucleotide (ASO) targeting mouse PKC-α (Isis pharmaceuticals, Carlsbad, CA) and a chemical control oligonucleotide were synthesized as 20-nt uniform phosphorothioate chimeric oligonucleotide and purified. Oligonucleotides were chimeric oligonucleotides containing five nuclease resistant 2′-O-methoxyethylribose-modified phosphorothioate residues on the 5′ and 3′-ends, flanking a 2′-deoxyribonucleotide/phosphorothioate region that supports RNase H-based cleavage of the targeted mRNA. Mice were injected intraperitoneally with PKC-α ASO or control ASO (scrambled) six times (50 mg/kg every other day).
Animals
Male mice (C57 BL/6; 6-8 weeks of age) were obtained from Harlan Bioproducts (Indianapolis, IN). The animals were fasted overnight (water was available) prior to experiments. All the treatments were administered intraperitoneally; APAP (Sigma) was dissolved in warm PBS (55°C) and cooled to 37°C before injection into mice (300 mg/kg). In experiments with PKC inhibitor (Ro-31-8425), PKC inhibitor was dissolved in DMSO (8.3%, v/v) and PBS (1mg in 125 μl of DMSO diluted with 1375 μl of PBS). PKC inhibitor (10 mg/kg) was injected (intraperitoneally, 0.3 ml injection) into mice 1 h prior to APAP injection (500-800 mg/kg). Since DMSO reduces APAP-induced liver injury by inhibiting NAPQI production, studies with PKC inhibitors dissolved in PBS-DMSO require higher APAP doses (500-800 mg/kg APAP) to induce liver injury than studies using ASO (300 mg/kg APAP), where DMSO is not used.
Isolation of Liver Mitochondria and Cytoplasm
Mitochondria were isolated from the livers by differential centrifugation (28). Livers were excised and homogenized in H-medium (210 mM mannitol, 70 mM sucrose, 2 mM HEPES, 0.05% BSA (w/v), plus protease and phosphatase inhibitors). The homogenate was centrifuged at 800 × g for 10 min, the pellet removed, and the centrifugation process repeated. The resulting supernatant was centrifuged at 8,500 × g for 15 min. The supernatant (“cytoplasmic” post-mitochondrial S9 fraction) was collected and stored. The pellet (mitochondrial fraction) was washed with H-medium and the centrifugation repeated. The mitochondria were resuspended in H-medium before oxygen electrode and Western blot analysis.
Measurements of respiration in isolated mitochondria
Respiration was measured in freshly isolated mitochondria by monitoring oxygen consumption with a Clark-type electrode (Hanstech, UK) in respiration buffer (230 mM mannitol, 70 mM sucrose, 30 mM Tris-HCl, 5 mM KH2PO4, 1 mM EDTA, pH 7.4) (3). Isolated mitochondria (0.50-0.70 mg) were added to 1ml of respiration buffer and oxygen consumption (state III respiration) was monitored in the presence of mitochondrial substrates (succinate 7.5 mM –complex II substrate) with ADP (250 ⎧M).
Cell culture
Primary mouse hepatocytes were isolated as previously described from C57BL/6 mice (9). Cell culture experiments were performed in two different ways with inhibitors being added before or after APAP treatment: Pre- treatment or post-treatment. In pre-treatment, first hepatocytes were treated with various inhibitors. 1h later hepatocytes were treated with various doses of acetaminophen in medium in the presence of an inhibitor. After 2 h of exposure to APAP, the PKC inhibitors and APAP were removed and the culture media was exchanged. In the post-treatment protocol, hepatocytes were first exposed to different doses of APAP in media. After 2h exposure to APAP, the culture media was changed and the APAP was removed; then hepatocytes were treated overnight with various inhibitors, such as PKC inhibitors, compound C. Additional studies were done without removal of APAP in the presence or absence of PKC inhibitors.
Determination of apoptosis and necrosis
After 16-24 h of various treatments, cells were double stained with 8 μg/ml Hoechst 33258 and 1 μM Sytox green. Hepatocytes were treated for 15 minutes with Hoechst 33258, a nucleic acid stain that binds dsDNA of both live and dead cells in order to assess nuclear fragmentation. Sytox green, a nucleic acid dye that stains dead cells (cells with compromised membranes) was added just before analysis. After the cells were stained, culture dishes were observed under an OLYMPUS fluorescent microscope. Quantitation of total and necrotic cells (Sytox green positive) was performed as previously described by counting > 1,000 cells in 10 different fields (9). Very little apoptosis (nuclear fragmentation) was observed (< 5%) in hepatocytes with either APAP or PKC inhibitor treatments.
Immunoblot
AMPK-α, p-AMPK-α (thr172), JNK, p-JNK (p46 and p54), LC3A/B, LC3B, SQSTMI/P62, prohibitin1, and β-actin were obtained from Cell Signaling Technologies (Danvers, MD). PKC-α and PKC-ε antibodies were obtained from Santa Cruz (Santa Cruz, CA). PKC activity was assessed in hepatocyte lysate by Western blot using an antibody (Cell Signaling Technologies) that recognizes proteins phosphorylated by PKC (PKC recognition motif: serine residues surrounded by arginine or lysine at the –2 and +2 positions and a hydrophobic residue at the +1 position). The antiserum to the NAPQI protein adducts was provided by Drs. Jack A. Hinson and Laura James of University of Arkansas. Cyp2e1 antibody was obtained from Stressgen Biotechnologies (San Diego, CA). All gels shown were representative samples from three experiments. Densitometry was performed using the NIH Image J program.
Histological Analysis
Livers were removed, fixed with 10% buffered formalin, embedded in paraffin, and cut into 5 μm thick sections. All specimens were stained with hematoxylin/eosin and evaluated under a light microscope.
HPLC measurements for GSH and GSSG
GSH and GSSG were detected using reverse-phase HPLC and a Coulochem II electrochemical detector (ESA Laboratories, Chelmsford, MA) as previously described (3). At collection time points, samples were treated with 5% metaphosphoric acid to prevent GSH autoxidation.
Statistical Analysis
Statistical analyses were performed using paired Student's t test or ANOVA. p ≤ 0.05 was defined as statistically significant.
Results
PKC plays an important role in APAP-induced necrosis in primary cultured hepatocytes
Pre-treatment of primary cultured hepatocytes with two different broad-spectrum PKC inhibitors (Ro-31-8425, Go6983) and a classical PKC inhibitor (Go6976), significantly protected against APAP cytotoxicity (Figure 1A). To ensure that PKC inhibitors protect against APAP cytotoxicity without affecting APAP metabolism, in subsequent experiments PKC inhibitors were added 2 hours after APAP treatment, at the time the APAP was removed (post-treatment). Similar to pre-treatment experiments, post-treatment with PKC inhibitors significantly protected against APAP induced hepatocyte necrosis (Figure 1B). The post-treatment model required higher APAP concentrations to cause cytotoxicity, since APAP was removed after two hours to eliminate the possibility that PKC inhibitors could protect against APAP by modulating GSH depletion or covalent binding. As expected, post-treatment with PKC inhibitor did not affect covalent binding of NAPQI (Supplemental Figure 1A).
Figure 1. Pre- and post-treatment of PKC inhibitors protect primary cultured hepatocytes from APAP.
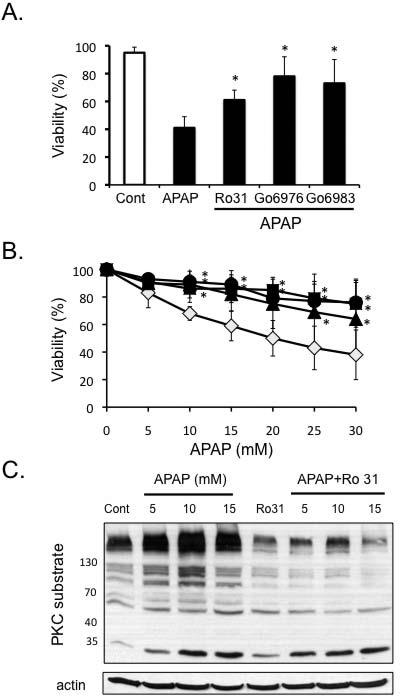
A) Pre-treatment of PKC inhibitors protects against APAP cytotoxicity. Hepatocytes were pre-treated 1 h with PKC inhibitors prior to the addition of APAP (5 mM). APAP was subsequently not removed and viability assessed at 18 hours. B) Post-treatment of hepatocytes with PKC inhibitors protects against APAP. Hepatocytes were treated with APAP for 2 h, then APAP was removed, and the cells were treated with different PKC inhibitors or DMSO for control [(broad-spectrum PKC inhibitors - Ro-31-8425 (5μM; ▲), Go6983 (10μM; ●) or classical PKC inhibitor - Go6976 (10μM; ■), or DMSO (control, ◇)]. Necrotic cells were determined using Sytox green 16-24 h after APAP treatment. Results are mean ± S.D. * p value ≤ 0.05 versus APAP treatment alone. C) APAP treatment of hepatocytes increases PKC activity (proteins phosphorylated by PKC), which is blocked by Ro-31-8425 treatment. Hepatocytes were treated with various doses of APAP with or without Ro-31-8425 (post-treatment). PKC activity was assessed in hepatocyte lysate by Western blot using an antibody that recognizes proteins phosphorylated by PKC (PKC recognition motif: serine residues surrounded by arginine or lysine at the – 2 and +2 positions and a hydrophobic residue at the +1 position).
PKC activation (proteins phosphorylated by PKC) increased in a dose-dependent manner following APAP treatment and was inhibited by broad-spectrum PKC inhibitor, Ro-31-8425 treatment (post-treatment; Figure 1C). Similar inhibition of APAP-induced PKC activation was observed with Go6983 and Go6976 treatment (supplemental Figure 1B). These findings suggest that PKC(s) are activated by APAP treatment in hepatocytes and play an important role in mediating necrotic cell death induced by APAP.
PKC inhibitors protect against APAP cytotoxicity through JNK dependent and independent pathways
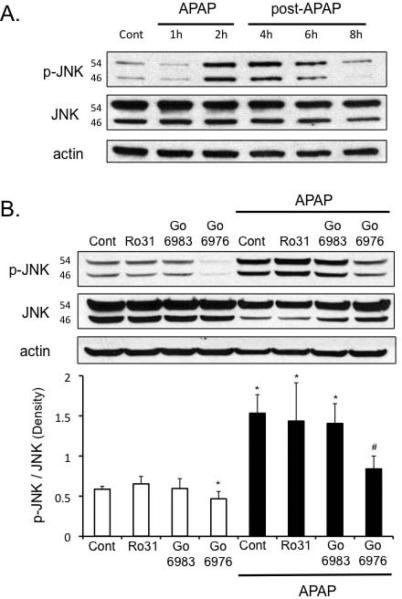
We next examined whether or not the protection of PKC inhibitors against APAP in cultured hepatocytes was mediated through an effect on JNK. As expected, APAP treatment in cultured hepatocytes activated JNK, starting around 2 hours (Figure 2A). Treatment of hepatocytes with broad-spectrum PKC inhibitors, Go6983 and Ro-31-8425 (post-treatment), did not affect the enhanced p-JNK levels induced by APAP treatment (Figure 2B). In contrast, the classical PKC inhibitor, Go6976, decreased p-JNK levels under basal conditions and in the presence of APAP. This suggests broad-spectrum PKC inhibitors protected against APAP through a JNK-independent pathway, while classical PKC inhibitor protected through a JNK-dependent pathway.
Figure 2. Different effects of broad-spectrum versus classical PKC inhibitors on p-JNK levels and p-AMPK levels following APAP treatment in primary cultured hepatocytes.
A) Time course of JNK phosphorylation following APAP treatment (2 h) in hepatocytes. Hepatocytes were treated with APAP for 2 hours, then APAP was removed and hepatocytes incubated in fresh media without APAP for another 2-6 hours (post-APAP). B) Effect of broad-spectrum PKC inhibitors - Ro-31-8425 (5 μM), Go6983 (10 μM) or classical PKC inhibitor - Go6976 (10 μM) on JNK phosphorylation in hepatocytes following APAP treatment. C) Time course of p-AMPK following APAP treatment in hepatocytes. Hepatocytes were treated with APAP for 2 hours, then APAP was removed and hepatocytes incubated in fresh media without APAP for another 2-4 hours (post-APAP). D) Effect of broad-spectrum PKC inhibitors - Ro-31-8425 (5 μM), Go6983 (10 μM) or classical PKC inhibitor - Go6976 (10 μM) on p-AMPK levels in hepatocytes following APAP treatment. In the time course experiments, hepatocytes were treated with APAP (25 mM) for two hours, APAP was removed, and hepatocytes were incubated in fresh media without APAP for the times indicated (post-APAP). In experiments involving PKC inhibitors, hepatocytes were treated with APAP (25 mM) for 2 h, then APAP was removed, and the cells were treated with different PKC inhibitors or DMSO for control for another 2 h. Western blot analyses were performed using antisera against p-JNK, JNK, p-AMPK (Thr 172), AMPK and β-Actin. Densitometry was determined using Image J. Results are mean ± S.D. * p value ≤ 0.05 versus control; # p value ≤ 0.05 versus APAP treatment alone.
Broad-spectrum PKC inhibitors modulate p-AMPK to protect hepatocytes from APAP cytotoxicity
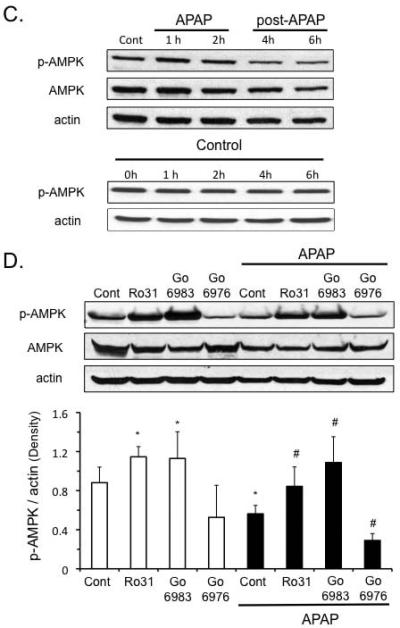
APAP treatment caused a decline in p-AMPK levels, which was due in part to an overall decline in AMPK levels in cultured hepatocytes (Figure 2C). p-AMPK levels were not altered with time in untreated, control samples (Figure 2C). Broad-spectrum PKC inhibitors increased the basal levels of p-AMPK and increased p-AMPK levels in the presence of APAP, even though total AMPK levels still remained suppressed (Figure 2D). In contrast, classical PKC inhibitor Go6976 caused a further decline in p-AMPK and AMPK levels triggered by APAP treatment. This difference between the effect of broad-spectrum and classical PKC inhibitors on p-AMPK levels suggests that APAP treatment possibly activates novel and/or atypical PKC(s) that have an inhibitory effect on p-AMPK, which is blocked by broad-spectrum PKC inhibitors, but not classical PKC inhibitors.
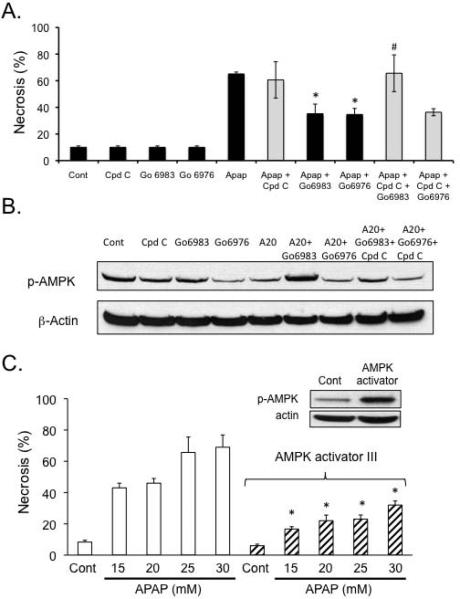
To confirm the importance of p-AMPK in APAP cytotoxicity, we modulated AMPK activity using an inhibitor and an activator. The AMPK inhibitor, compound C (Cpd C), prevented p-AMPK upregulation induced by the broad-spectrum PKC inhibitor Go6983 and reversed its protective effect against APAP cytotoxicity (Figure 3A and B). On the other hand, in hepatocytes treated with Go6976 and APAP, Cpd C treatment did not reverse the protective effect against APAP cytotoxicity presumably because p-AMPK levels were already markedly decreased. These data support the interpretation that broad-spectrum PKC inhibitors protect through an AMPK-dependent but JNK-independent pathway, while the classical PKC inhibitor Go6976 protects through an AMPK-independent but JNK-dependent pathway. To further confirm that activation of AMPK is important in APAP cytotoxicity, hepatocytes were treated with an AMPK activator (AMPK activator III, Calbiochem). AMPK activator significantly protected against APAP cytotoxicity in primary cultured hepatocytes in the post-treatment protocol (Figure 3C).
Figure 3. p-AMPK plays a key role in APAP cytotoxicity in cultured hepatocytes.
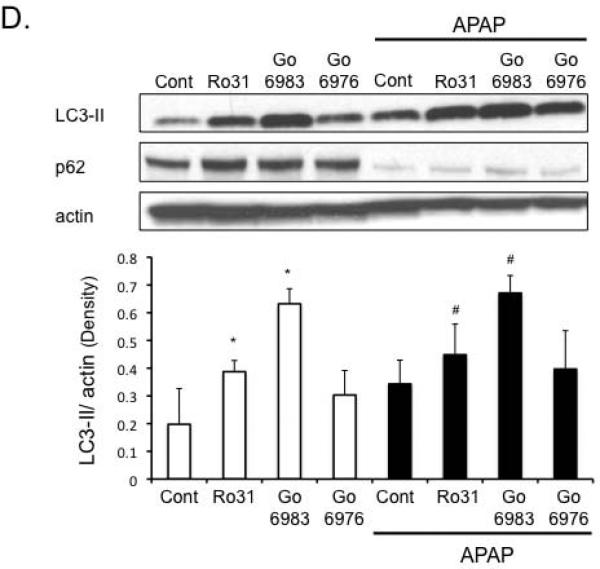
A) Compound C, an AMPK inhibitor, negates the protective effects of broad-spectrum PKC inhibitor Go6983 but not classical PKC inhibitor Go6976 against APAP cytotoxicity. Hepatocytes were treated with PKC inhibitor Go6983 or Go6976 (2 h post APAP 25 mM treatment) in the presence or absence of compound C (Cpd C; 10μM). *p ≤ 0.05 compared to APAP treatment; #p ≤ 0.05 compared to Go6983 plus APAP (without compound C). B) Effect of Compound C on p-AMPK levels in cultured hepatocytes. C) AMPK activator treatment protects hepatocytes from APAP cytotoxicity. After 2 h of APAP treatment, APAP was removed and hepatocytes were treated with AMPK activator III (50 μM) or DMSO for controls. Western blot (inset) was performed on hepatocyte extracts treated with DMSO and AMPK activator III to confirm activation of AMPK by the small molecule activator. D) Effect of PKC inhibitors on autophagy markers (p62 degradation, LC3-II formation) following APAP treatment. *p ≤ 0.05 compared to control; #p ≤ 0.05 compared to APAP treatment.
p-AMPK regulates survival pathways, including autophagy, that are important in regulating APAP cytotoxicity
We next examined possible downstream targets of p-AMPK that may be involved in protecting hepatocytes against APAP. P-AMPK is known to activate autophagy through inhibition of mTORC1, which has been suggested to be protective against APAP cytotoxicity (24, 26). APAP treatment alone to cultured hepatocytes appears to enhance autophagy as indicated by key marker of autophagy, nucleoporin 62 (p62) degradation (Figure 3D). Another marker of autophagy, microtubule-associated protein light chain 3-II (LC3-II) formation also appeared to increase with APAP treatment, although the increase was not statistically significant. LC3-II formation (Figure 3D). Go6983, which protected against APAP cytotoxicity more effectively than Ro-31-8425, was more effective than Ro-31-8425 in enhancing LC3-II formation. P62 levels were already maximally depleted with APAP treatment, and thus could not be used to assess differences with PKC inhibitors. Classical PKC inhibitor, Go6976, showed a trend of increasing LC3-II formation, but this was not statistically significant. Taken together, the data suggests broad-spectrum PKC inhibitor treatment may be protecting against APAP, even when JNK activity is sustained, at least to some extent through an AMPK-dependent alone or in the presence of APAP increase of autophagy.
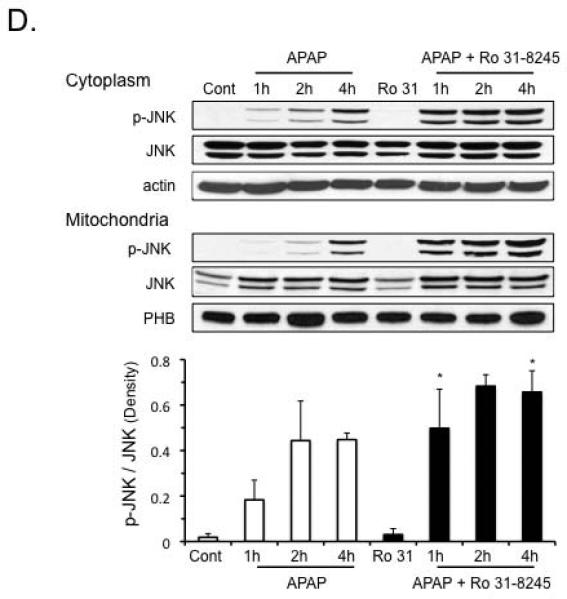
PKC inhibitor Ro-31-8425 protects against APAP-induced liver injury in vivo despite sustained JNK activation
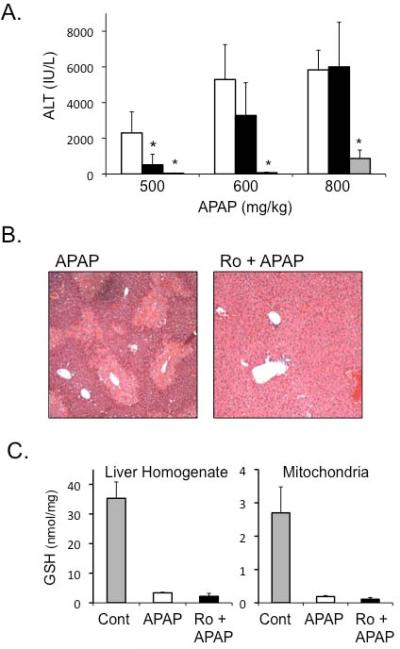
We next determined if PKC inhibitor, Ro-31-8425 could protect against APAP in vivo. Other inhibitors could not be studied due to solubility issues requiring excessive DMSO. Mice were pre-treated with Ro-31-8425 (10mg/kg dissolved in DMSO and PBS) 1 h prior to treatment with various doses of APAP. Ro-31-8425 treatment significantly protected against an APAP dose of 500mg/kg, but not at higher doses of APAP, as observed with JNK inhibitor treatment (Figure 4A). Centrilobular necrosis caused by APAP was markedly reduced with Ro-31-8425Ro-31-8425 did not affect GSH depletion caused by APAP (Figure 4C). In addition, Ro-31-8425 pre-treatment significantly enhanced JNK phosphorylation and translocation to mitochondria caused by APAP treatment (Figure 4D). Thus as observed in vitro, Ro-31-8425 could protect against APAP hepatotoxicity even though JNK activity was sustained, or even elevated in vivo, with Ro-31-8425 treatment.
Figure 4. Broad-spectrum PKC inhibitor protects against APAP-induced liver injury in vivo through a JNK-independent pathway.
A) Effect of broad-spectrum PKC inhibitor or JNK inhibitor on serum ALT levels following APAP treatment. Mice were pre-treated with Ro-31-8452 (10 mg/kg in DMSO (8.3%) and PBS; black bars), JNK inhibitor (SP600125; 10 mg/kg in DMSO (8.3%); gray bars) or PBS with equivalent amounts of DMSO for control, 1 h prior to APAP treatment (open bars). B) H&E histology of mice treated with APAP 500 mg/kg with or without Ro-31-8425. C) Effect of Ro-31-8425 treatment on GSH levels in liver homogenate and isolated mitochondria following APAP treatment (2 h). GSH was measured using HPLC with electrochemical detection. D) Effect of Ro-31-8425 treatment on JNK activation and translocation to mitochondria following APAP treatment in vivo. PHB1 = Prohibitin1, a mitochondrial loading control. * p value ≤ 0.05 versus APAP treatment at equivalent time points or dose. N = 4–10 mice per group.
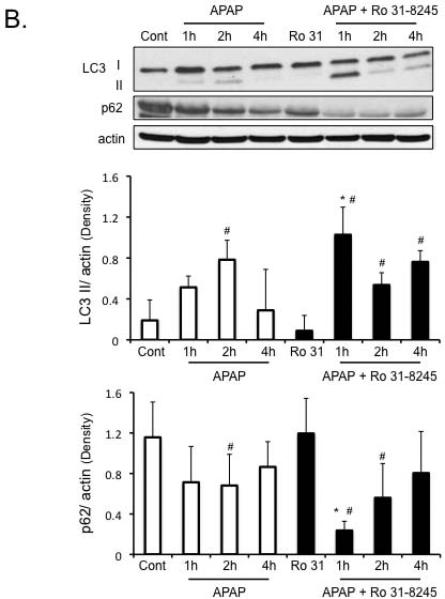
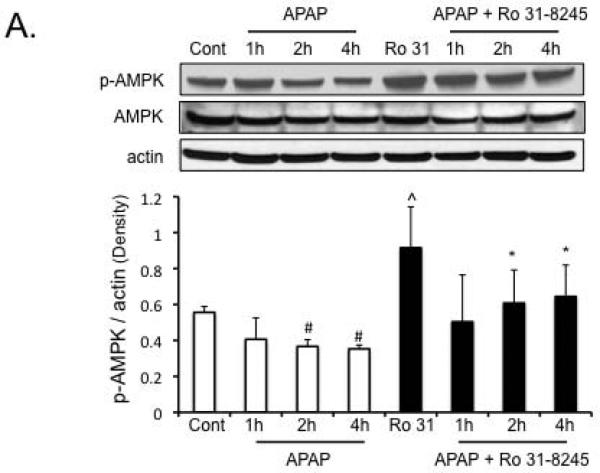
Protection against APAP-induced liver injury by Ro-31-8425 treatment is associated with enhanced p-AMPK levels and enhanced autophagy
As seen in vitro, APAP treatment caused a significant decline in p-AMPK levels in the liver. Ro-31-8425 treatment enhanced p-AMPK levels, alone and in the presence of APAP, despite some decline in total AMPK levels (Figure 5A). The enhanced p-AMPK levels found with Ro-31-8425 pre-treatment in vivo were associated with an enhanced autophagy in the liver. APAP treatment alone, enhanced LC3-II formation and p62 degradation significantly at 2 hours in the liver, suggesting an induction of autophagy by APAP treatment (Figure 5B). Ro-31-8425 pre-treatment, on the other hand, significantly enhanced LC3-II formation at all time points examined following APAP treatment and increased p62 degradation at 1 and 2 hours following APAP treatment. The enhancement of autophagy by Ro-31-8425 treatment was most prominent at the 1 hour APAP treatment time point; where a significant LC3-I to LC3-II conversion and increase in p62 degradation were observed compared to APAP treatment alone. These data suggest Ro-31-8425 treatment in vivo enhances autophagy (especially at the 1 hour following APAP), which has been suggested to protect against APAP (26).
Figure 5. Protection against APAP-induced liver injury by Ro-31-8425 treatment is associated with enhanced p-AMPK levels and enhanced autophagy.
A) Effect of APAP and Ro-31-8425 pre-treatment on p-AMPK levels in vivo. B) Changes in autophagy markers (p62 degradation, LC3-II formation) following APAP treatment with or without Ro-31-8425 pre-treatment in vivo. * p value ≤ 0.05 versus APAP treatment at equivalent time points. ^ p value ≤ 0.05 versus control. # p value ≤ 0.05 versus respective controls (control or Ro-31-8425 without APAP treatment). N = 4–10 mice per group.
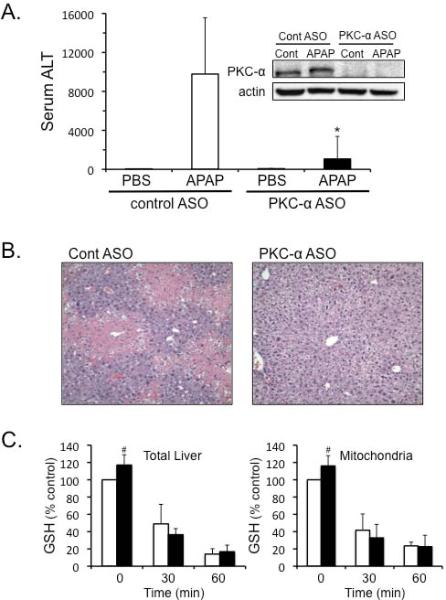
Silencing PKC-α using antisense protects against APAP-induced liver injury through a JNK-dependent pathway in vivo
We next silenced PKC-α expression using antisense (ASO) to determine if PKC-α was responsible for the JNK-dependent protection observed with classical PKC inhibitor Go6976 treatment in cultured hepatocytes. Silencing PKC-α using ASO protected against APAP-induced liver injury in mice, as seen by significant decline in the ALT levels and decreased centrilobular necrosis (Figure 6A and B). Silencing PKC-α caused a slight increase in basal GSH levels in the liver and liver mitochondria (Figure 6C), while also increasing Cyp2e1 levels in the liver (Supplemental Figure 2A). However, silencing PKC-α did not affect the rate of GSH depletion caused by APAP (Figure 6C). As observed with Go6976 treatment in vitro, silencing PKC-α significantly inhibited JNK phosphorylation and translocation to mitochondria (Figure 6D). This suggests that PKC-α plays a key role in activating and/or sustaining JNK during APAP hepatotoxicity.
Figure 6. Silencing PKC-α protects against APAP-induced liver injury through a JNK-dependent pathway in vivo.
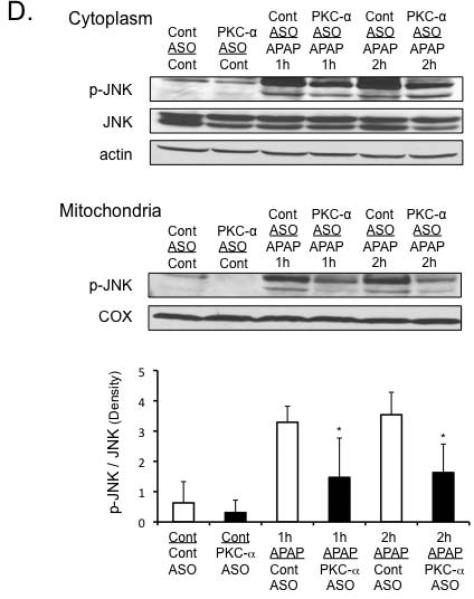
Mice were treated with PKC-α or control ASO for 2 weeks, and then treated with APAP (300 mg/kg). A) Serum ALT levels. Western blots (insets) were performed on liver samples from treated mice to confirm the silencing of PKC-α. B) H&E histology C) GSH measurement in liver and isolated mitochondria. D) JNK activation and translocation to mitochondria.N = 5 mice per group. * p value ≤ 0.05 versus APAP treatment in control ASO; # p value ≤ 0.05 versus the control from the control ASO treated mice.
We previously observed that silencing PKC-α also affects PKC-ε levels, possibly due to regulation of PKC-ε by PKC-α (9). To confirm that the protective effects of PKC-α ASO against APAP were due to PKC-α and not PKC-ε, APAP hepatotoxicity was tested in PKC-ε knockout mice. PKC-ε knockout mice were not protected against APAP-induced liver injury (Supplemental Figure 2B), suggesting PKC-ε is not involved in APAP hepatotoxicity.
PKC-α translocates to mitochondria and inhibits mitochondrial respiration during APAP-induced liver injury
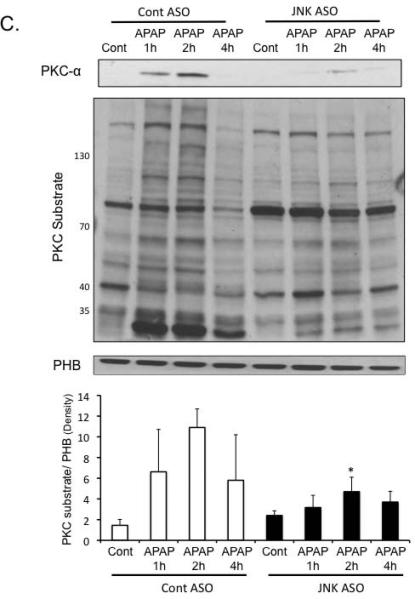
PKC-α, like JNK, has been reported to translocate to mitochondria to decrease respiration and enhance ROS generation (29). We observed that APAP treatment caused PKC-α translocation to mitochondria, which was associated with increased phosphorylation of mitochondrial proteins by PKC (Figure 7A). Silencing of PKC-α with ASO decreased the level of mitochondrial proteins phosphorylated by PKC. APAP-induced mitochondrial dysfunction was also reduced in PKC-α silenced mice (Figure 7B), suggesting that PKC-α translocation and phosphorylation may play a role in mitochondrial dysfunction during APAP hepatotoxicity.
Figure 7. PKC-α translocates to mitochondria and phosphorylates mitochondrial proteins during APAP hepatotoxicity.
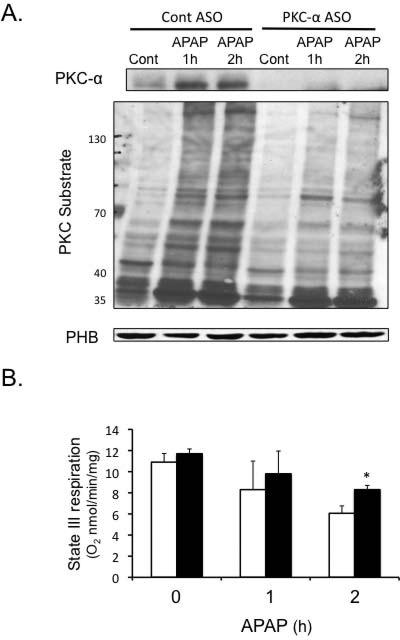
PKC-α ASO or control ASO mice were treated with APAP (300 mg/kg). At various times liver mitochondria were isolated using differential centrifugation. APKC-α translocation to mitochondria and phosphorylation by mitochondrial proteins. B) PKC-α translocation to mitochondria corresponded to a decline in mitochondrial respiration (Empty bar = control ASO; black bars = PKC-α ASO). Mitochondrial respiration was measured in the presence of succinate plus ADP (state III respiration). C) Silencing JNK 1 and 2 decreased PKC-α translocation to mitochondria and decreased levels of mitochondrial proteins phosphorylated by PKC. Mitochondria were isolated from JNK ASO or control ASO treated mice at various times following APAP treatment. Mitochondria proteins phosphorylated by PKC were assessed in isolated mitochondria by immunoblotting using an antibody that recognizes proteins phosphorylated by PKC (PKC recognition motif: serine residues surrounded by arginine or lysine at the –2 and +2 positions and a hydrophobic residue at the +1 position). Densitometry of mitochondrial proteins were determined using Image J. * p value ≤ 0.05 versus mice treated Control ASO. N = 4.)
The fact that silencing PKC-α reduced JNK activation and translocation to mitochondria suggested that JNK may be downstream of PKC-α. To confirm the sequence of signaling events, we silenced JNK 1 and 2 using ASO and examined the effect on PKC-α translocation to mitochondria. Surprisingly, silencing JNK reduced PKC-α translocation to mitochondria and reduced the level of mitochondrial proteins phosphorylated by PKC (Figure 7C). Although some variability and aberrant changes in a few phosphorylated PKC substrate proteins were observed, overall there was a dramatic decrease in proteins phosphorylated after APAP treatment in the JNK ASO treated mice. This suggests that PKC-α and JNK participate jointly through a feed-forward interconnected mechanism to mediate APAP-induced liver injury.
Discussion
PKC plays an important role in APAP hepatotoxicity
APAP hepatotoxicity is mediated by a signal transduction pathway involving JNK activation and translocation to mitochondria (3). In this study using a wide variety of PKC inhibitors and silencing of PKC-α, we observed that the PKC family members also play an important role in APAP hepatotoxicity. The mechanism of protection by PKC inhibitors differed markedly, with broad-spectrum PKC inhibitors (Go6983 or Ro-31-8425) protecting through a JNK-independent, AMPK-dependent pathway, and the classical PKC inhibitor (Go6976) or silencing PKC-α protecting through a JNK-dependent, AMPK-independent pathway.
Broad-spectrum PKC inhibitors protect against APAP hepatotoxicity through an AMPK-dependent, JNK-independent pathway
Our findings with broad-spectrum PKC inhibitors suggest that APAP activates various PKC isoforms that modulate p-AMPK. However, although chemically distinct, these small molecule broad-spectrum PKC inhibitors could have off-target effects and other proteins besides PKC might be inhibited (30). Regardless, our work with PKC inhibitors uncovered the importance of p-AMPK in modulating APAP hepatotoxicity. APAP treatment decreased the level of p-AMPK in hepatocytes and the liver, which was prevented by broad-spectrum PKC inhibitors. The loss of p-AMPK may therefore be an important contributing mechanism in mediating APAP hepatotoxicity.
AMPK is known to promote autophagy, a pro-survival pathway, through inhibition of mTORC1. APAP treatment alone increased LC3-II formation and p62 degradation both in vitro and in vivo, suggesting APAP treatment promotes autophagy, as previously reported (26). Enhancement of AMPK by broad-spectrum PKC inhibitors was associated with an enhancement of autophagy markers both in vitro (increase LC3-II formation) and in vivo (increase LC3-II formation, p62 degradation). Broad spectrum PKC inhibitor may be protecting against APAP both in vitro and in vivo by enhancing autophagy, a protective mechanism against APAP (26). However, autophagy is difficult to accurately measure, particularly in vivo, and more work is needed to definitively assert that broad-spectrum PKC inhibitors enhance autophagy through an AMPK-dependent pathway.
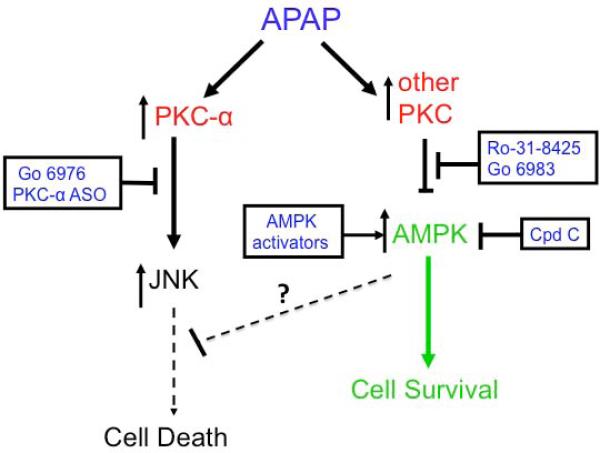
Overall, our work demonstrates for the first time that protection against APAP hepatotoxicity can occur despite sustained JNK activation. Many signaling pathways have been shown to modulate APAP hepatotoxicity, but they do so by modulating JNK signaling. Thus it was surprising that Ro-31-8425 protected against APAP hepatotoxicity both in cultured hepatocytes and in vivo despite enhanced JNK phosphorylation and translocation to mitochondria. Although APAP hepatotoxicity requires activation of JNK-mediated cell death pathways, AMPK may concomitantly contribute by suppressing or counteracting the downstream effects of p-JNK binding to mitochondrial Sab (Figure 8). Thus, broad-spectrum PKC inhibitors upregulate AMPK, which promotes survival pathways (i.e. autophagy) in parallel with JNK death pathways, resetting the threshold for cell death.
Figure 8. APAP hepatotoxicity involves activation of JNK and downregulation of p-AMPK.
APAP hepatotoxicity not only requires JNK activation that activates cell death pathways, but also requires downregulation of p-AMPK mediated by PKC. Broad-spectrum PKC inhibitor treatment prevents the downregulation of AMPK that normally occurs during APAP hepatotoxicity. AMPK mediates important energy generation and survival pathways that can protect against APAP, even when JNK remains active.
Another surprising finding in this study was that Ro-31-8425 treatment further enhanced APAP-induced JNK phosphorylation in vivo. This finding would suggest that a PKC isoform is involved in suppressing JNK phosphorylation following APAP treatment. APAP treatment leads to activation of ASK, MLK3, and GSK-3β, which are upstream of JNK activation, but APAP may simultaneously activate a PKC isoform or other protein (inhibitable by Ro-31-8425) that partially dampens JNK activation. In RALA and other cell lines oxidative stress has been shown to activate PKC-n (inhibited by Ro-31-8425), which suppresses JNK activity through a PKD-dependent mechanism (31, 32). However, the existence of PKC-n and PKD in hepatocytes has not been determined. Whether PKC-n and PKD, or other proteins that can be inhibited by Ro-31-8425 negatively regulate JNK in the liver will require further work.
PKC-α protects against APAP hepatotoxicity through a JNK dependent pathway
Classical PKC inhibitor Go6976 protected against APAP, through an AMPK-independent (not inhibited by compound C) and JNK-dependent pathway. We found that PKC-α plays an important role in activating and/or sustaining JNK to mediate APAP hepatotoxicity. This presents an apparent contradiction with the observation that Ro-31-8425, a strong PKC-α inhibitor, did not inhibit JNK activation caused by APAP (Figure 4D). However, as previously mentioned, the broad-spectrum PKC inhibitors may be targeting atypical or unconventional PKCs, which inhibit pathways that downregulate JNK, resulting in enhanced p-JNK levels while at the same time activating AMPK survival mechanisms that are either downstream, or completely separate from JNK. APAP treatment caused PKC-α translocation to mitochondria and concomitantly increased PKC-dependent phosphorylation of mitochondrial proteins, which was accompanied by mitochondrial dysfunction. Previous studies have also suggested that PKC-α translocates to mitochondria, inhibiting mitochondrial respiration (29). Silencing of PKC-α expression reduced mitochondrial dysfunction caused by APAP suggesting that PKC-α was involved in disrupting mitochondrial respiration. However, whether mitochondria dysfunction was due directly to PKC-α or due to JNK translocation to mitochondria or both will require further investigation. Thus both JNK and PKC-α translocation to mitochondria may be required for maximal inhibition of mitochondrial respiration, and consequently increased ROS generation important in sustaining JNK activity during APAP hepatotoxicity.
In summary, using a wide variety of PKC inhibitors and silencing of PKC-α, we observed that PKC family members play an important role in APAP hepatotoxicity. The mechanism of protection by PKC inhibitors differed markedly. Both inhibition of classical PKCs by an inhibitor and silencing PKC-α protected through a JNK-dependent, AMPK-independent pathway. In contrast, two different broad-spectrum PKC inhibitors protected through a JNK-independent, AMPK-dependent pathway, which likely involves enhanced survival-energy pathways that exert a pro-survival effect despite the unique enhancement of sustained JNK activation.
Supplementary Material
Acknowledgements
This work was supported by NIH grant DK067215 (NK), AA016911 (DH) and the USC Research Center for Liver Diseases (P30 DK048522) cell culture, cell and tissue imaging (S10 RR022508) and analytical/metabolic/ instrumentation cores. PKC-ε knockout mice were kindly provided by Dr. Joel S. Karliner's lab at UCSF.
References
- 1.Lee WM, Squires RH, Jr., Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010:267–310. doi: 10.1007/978-3-642-00663-0_10. [DOI] [PubMed] [Google Scholar]
- 5.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–8255. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saberi B, Shinohara M, Ybanez MD, Hanawa N, Gaarde WA, Kaplowitz N, Han D. Regulation of H(2)O(2)-induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. American Journal of Physiology-Cell Physiology. 2008;295:C50–C63. doi: 10.1152/ajpcell.90654.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 11.Bareggi R, Grill V, Zweyer M, Narducci P, Martelli AM. Distribution of the extended family of protein kinase C isoenzymes in fetal organs of mice: an immunohistochemical study. Cell Tissue Res. 1995;280:617–625. doi: 10.1007/BF00318364. [DOI] [PubMed] [Google Scholar]
- 12.Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, et al. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croquet F, Brehier A, Gil S, Davy J, Feger J. Five isoenzymes of protein kinase C are expressed in normal and STZ-diabetic rat hepatocytes: effect of phorbol 12-myristate 13-acetate. Biochim Biophys Acta. 1996;1315:163–168. doi: 10.1016/0925-4439(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 14.Domenicotti C, Paola D, Vitali A, Nitti M, d'Abramo C, Cottalasso D, Maloberti G, et al. Glutathione depletion induces apoptosis of rat hepatocytes through activation of protein kinase C novel isoforms and dependent increase in AP-1 nuclear binding. Free Radic Biol Med. 2000;29:1280–1290. doi: 10.1016/s0891-5849(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 15.Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001;21:2929–2938. doi: 10.1523/JNEUROSCI.21-09-02929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 17.Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol. 2010;16:3731–3742. doi: 10.3748/wjg.v16.i30.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 19.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, et al. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- 21.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 22.Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol. 2010;79:1352–1362. doi: 10.1016/j.bcp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Samari HR, Seglen PO. Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and N6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J Biol Chem. 1998;273:23758–23763. doi: 10.1074/jbc.273.37.23758. [DOI] [PubMed] [Google Scholar]
- 24.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3:381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 25.Kewalramani G, Puthanveetil P, Wang F, Kim MS, Deppe S, Abrahani A, Luciani DS, et al. AMP-activated protein kinase confers protection against TNF-{alpha}-induced cardiac cell death. Cardiovasc Res. 2009;84:42–53. doi: 10.1093/cvr/cvp166. [DOI] [PubMed] [Google Scholar]
- 26.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igusa Y, Yamashina S, Izumi K, Inami Y, Fukada H, Komatsu M, Tanaka K, et al. Loss of autophagy promotes murine acetaminophen hepatotoxicity. J Gastroenterol. 2012;47:433–443. doi: 10.1007/s00535-011-0500-0. [DOI] [PubMed] [Google Scholar]
- 28.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Biswas G, Prabu SK, Avadhani NG. Modulation of mitochondrial metabolic function by phorbol 12-myristate 13-acetate through increased mitochondrial translocation of protein kinase Calpha in C2C12 myocytes. Biochem Pharmacol. 2006;72:881–892. doi: 10.1016/j.bcp.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem. 2004;279:31089–31097. doi: 10.1074/jbc.M404170200. [DOI] [PubMed] [Google Scholar]
- 32.Brändlin I, Hübner S, Eiseler T, Martinez-Moya M, Horschinek A, Hausser A, Link G, et al. Protein kinase C (PKC)eta-mediated PKC mu activation modulates ERK and JNK signal pathways. J Biol Chem. 2002;277:6490–6496. doi: 10.1074/jbc.M106083200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.