Abstract
Amatoxins, including α-amanitin, are bicyclic octapeptides found in mushrooms (Agaricomycetes, Agaricales) of certain species in the genera Amanita, Galerina, Lepiota, and Conocybe. Amatoxins and the chemically similar phallotoxins are synthesized on ribosomes in Amanita bisporigera, Amanita phalloides, and Amanita ocreata. In order to determine if amatoxins are synthesized by a similar mechanism in another, distantly related mushroom, we obtained genome survey sequence data from a monokaryotic isolate of Galerina marginata, which produces α-amanitin. The genome of G. marginata contains two copies of the α-amanitin gene (GmAMA1-1 and GmAMA1-2). The α-amanitin proprotein sequences of G. marginata (35 amino acids) are highly divergent from AMA1 of A. bisporigera except for the toxin region itself (IWGIGCNP in single-letter amino acid code) and the amino acids immediately upstream (N[A/S]TRLP). G. marginata does not contain any related toxin-encoding sequences besides GmAMA1-1 and GmAMA1-2. DNA from two other α-amanitin-producing isolates of Galerina (G. badipes and G. venenata) hybridized to GmAMA1, whereas DNA from the toxin non-producing species Galerina hybrida did not. Expression of the GmAMA1 genes was induced by growth on low carbon. RNASeq evidence indicates that both copies of GmAMA1 are expressed approximately equally. A prolyl oligopeptidase (POP) is strongly implicated in processing of the cyclic peptide toxins of A. bisporigera and Conocybe apala. G. marginata has two predicted POP genes; one, like AbPOPB of A. bisporigera, is present only in the toxin-producing isolates of Galerina and the other, like AbPOPA of A. bisporigera, is present in all species. Our results indicate that G. marginata biosynthesizes amatoxins on ribosomes by a pathway similar to Amanita species, involving a genetically encoded proprotein of 35 amino acids that is post-translationally processed by a POP. However, due to the high degree of divergence, the evolutionary relationship between AMA1 in the genera Amanita and Galerina is unclear.
Keywords: Amatoxin, Amanita, Cyclic peptide
1. Introduction
Ribosomally encoded cyclic peptides are known from a number of taxa including mammals, plants, bacteria, and fungi (Craik, 2006). Some, like the cyanobactins and cyclotides of symbiotic marine bacteria and plants, respectively, are cyclized head-to-tail (Donia and Schmidt, 2011; Oman and van der Donk, 2010; Walsh et al., 2010). Others, such as the conotoxins of marine snails, are cyclized by multiple disulfide bonds (Han et al., 2008). Collectively, ribosomally synthesized peptides are characterized by small size, high chemical stability, and a wide range of potent biological activities (Craik, 2006).
Most fatal mushroom poisonings are due to the amatoxins, such as α-amanitin, which are present at high concentrations in many species of Amanita sect. Phalloideae (Wieland, 1986). Amatoxins and the related phallotoxins are bicyclic peptides containing a unique Trp–Cys cross bridge (with the trivial name tryptathionine) and several hydroxylated amino acids (May and Perrin, 2007). In addition, the phallotoxins contain one D-amino acid. Amatoxins are defining inhibitors of eukaryotic RNA polymerase II (pol II) (Novello et al., 1970), whereas phallotoxins specifically bind and stabilize F-actin (Wehland et al., 1977).
Unlike other fungal cyclic peptides, the amatoxins and phallotoxins are synthesized on ribosomes (Hallen et al., 2007a). The gene for α-amanitin from Amanita bisporigera, AMA1, is predicted to encode a proprotein of 35 amino acids, with the amino acid sequence of the mature toxin (i.e., IWGIGCNP in single-letter amino acid code) flanked by conserved sequences. Genes encoding β-amanitin (IWGIGCDP), phallacidin (AWLVDCP), and phalloidin (AWLATCP) were also found in species of Amanita sect. Phalloideae (Hallen et al., 2007a). Amanita species are predicted to biosynthesize many more cyclic peptides than those currently known, because their genomes contain at least 30 sequences related to AMA1. Genes in this extended family are characterized by conserved sequences flanking a hypervariable “toxin” region of 7–10 amino acids (Hallen et al., 2007a; Luo et al., 2010).
In the amatoxin gene family from Amanita species, the amino acid immediately upstream of the toxin region is an invariant Pro, and Pro is always the last amino acid in the toxin itself. A member of the prolyl oligopeptidase (POP) family of proline-specific proteases is probably responsible for the initial post-translational cleavage of the proprotein at the carboxyl side of the two Pro residues to release the linear peptide corresponding to the mature toxin (Luo et al., 2009). Evidence in support of this conclusion includes purification and identification of a POP from Conocybe apala that can process a synthetic phalloidin precursor to release the linear peptide; identification of a POP gene (AbPOPB), that is restricted in taxonomic distribution to toxin-producing species of Amanita; and immunocytochemical co-localization of AbPOPB with α-amanitin in tissues of A. bisporigera (Luo et al., 2010).
Amatoxins and the chemically related phallotoxins are also found in certain mushroom species in the genera Galerina, Lepiota, and Conocybe, which are phylogenetically not closely related to Amanita (Bresinsky and Besl, 1990; Hallen et al., 2003; Matheny et al., 2006). Galerina is a genus of cosmopolitan wood-rotting mushrooms that can degrade both hardwoods and softwoods (Enjalbert et al., 2004; Gulden et al., 2001, 2005). Species of Galerina sect. Naucoriopsis can contain levels of α-amanitin higher than Amanita phalloides, the most common cause of fatal human mushroom poisoning (Enjalbert et al., 2002). Although species of Galerina are relatively small and obscure, they occasionally cause human and animal poisonings worldwide (Enjalbert et al., 2004; Kaneko et al., 2001; Muraoka et al., 1999).
The presence of the same complex metabolite in phylogenetically widely divergent species of fungi raises the question of how this situation evolved, i.e., whether it was by descent from a common ancestor, convergent evolution, or horizontal gene transfer. To address these possibilities, we here identify and characterize the genes for α-amanitin in Galerina marginata. The results also give support to the utility of G. marginata as an experimental system to elucidate and manipulate the full biosynthetic pathway of α-amanitin.
2. Materials and methods
2.1. Biological materials
Four species of Galerina were obtained from Centraalbureau voor Schimmelcultures (CBS), Utrecht, Netherlands, including G. marginata (CBS 339.88), Galerina badipes (CBS 268.50), Galerina venenata (CBS 924.72), and Galerina hybrida (CBS 335.88). G. marginata CBS 339.88 is monokaryotic and was confirmed to make α-amanitin. G. venenata is considered synonymous with G. marginata (Gulden et al., 2001, 2005). The cultures were maintained on potato dextrose agar. For DNA isolation, the isolates were cultured in liquid medium for 15–30 d with rotary shaking at 120 rpm at 23 °C. The medium was HSV-2C, which contains (per liter) 1 g yeast extract, 2 g glucose, 0.1 g NH4Cl, 0.1 g CaSO4·5 H2O, 1 mg thi amine·HCl, and 0.1 mg biotin, pH 5.2 (Muraoka and Shinozawa, 2000). For induction experiments, the media had the same formulation, except that high carbon (HSV-5C) and low carbon (HSV-1C) media contained 5 g glucose and 1 g glucose, respectively (Muraoka and Shinozawa, 2000).
2.2. Nucleic acid isolation and genome sequencing
Lyophilized fungal mycelia were ground in liquid nitrogen with a mortar and pestle. High molecular weight DNA was isolated using genomic-tip 100/G (Qiagen, Germantown, MD; catalog #10,243) and RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA), following the manufacturers’ protocols.
Genomic DNA was sequenced by 454 pyrosequencing at the Research Technology Support Facility (RTSF) at Michigan State University. A general library was constructed using standard protocols and sequenced on a 454 GSFLX Titanium Sequencer. Raw reads were assembled with Newbler and assembled into a searchable database.
2.3. Cloning and gene characterization
AMA1 and PHA1 are the designations for the α-amanitin- and phallacidin-encoding genes of A. bisporigera, respectively; the prefix Ab is used to designate other genes from A. bisporigera. The prefix Gm is used to designate all genes from G. marginata. Using AMA1 as the search query, two homologs were identified in the partial genome survey sequence of G. marginata and designated as GmAMA1-1 and GmAMA1-2. PCR primers for each gene were designed based on the partial sequences and used to amplify full length copies. The amplicons were cloned into Escherichia coli DH5α and sequenced. Homologs of the two A. bisporigera prolyl oligopeptidases (AbPOPA and AbPOPB) were found in the G. marginata genome sequence (Luo et al., 2010). The G. marginata genes with highest identity to AbPOPA and AbPOPB were designated as GmPOPA and GmPOPB, respectively.
PCR primers unique to GmAMA1-1 and GmAMA1-2 were designed and used for isolation of genomic clones of each gene. For GmAMA1-1, the unique primers were 5′-CTCCAATCCCCCAACCACAAA-3′ (forward) and 5′-GTCGAACACGGCAACAACAG-3′ (reverse). For GmAMA1-2, the primers were: 5′-GAAAACCGAATCTCCAATCCTC-3′ (forward), and 5′-AGCTCACTCGTTGCCACTAA-3′ (reverse). For cloning full-length genomic clones of GmPOPA and GmPOPB, PCR primers corresponding to the amino and carboxyl termini of both genes (which were on four different contigs) were designed from the genome survey sequence. The forward primers were 5′-TTTAGGGCAGTGATTTCGTGACA-3′ and 5′-AACAGGGAGGCGATTATTCAAC-3′, and the reverse primers were 5′-GAACAATCGAACCCATGACAAGAA-3′ and 5′-CCCCCATTGATTGTTACCTTGTC-3′. The primer pairs were used in both combinations and successful amplification indicated the correct pairing of 5′ and 3′ primers. The resulting amplicons were cloned into E. coli DH5α and sequenced.
The genomic DNA sequences were used for primer design to obtain full-length cDNAs by Rapid Amplification of cDNA Ends (RACE) using the SMART RACE kit (Clontech, Mountain View, CA). A cDNA copy of GmAMA1-1 was obtained using primers 5′-CCAACGACAGGCGGGACACG-3′ (5′-RACE) and 5′-GACCTTTTTGCTTTAACATCTACA-3′ (3′-RACE), and of GmAMA1-2 with primers 5′-GTCAACAAGTCCAGGAGACATTCAAC-3′ (5′-RACE) and 5′-ACCGAATCTCCAATCCTCCAACCA-3′ (3′-RACE). The RACE primers for GmPOPA were 5′-CGGCGTTCCAAGGCGATGATAATA-3′ (5′-RACE) and 5′-CATCTCCATCGACCCCTTTTTCAGC-3′ (3′-RACE), and for GmPOPB 5′-AGTCTGCCGTCCGTGCCTTGG-3′ (5′-RACE) and 5′ CGGTACGACTTCACGGCTCCAGA-3′ (3′-RACE). Sequences generated from the RACE reactions were used to assemble full-length cDNAs of all four genes.
Alignments of genomic and cDNA copies was done with Spidey (http://www.ncbi.nlm.nih.gov/spidey/) and Splign (http://www.ncbi.nlm.nih.gov/sutils/splign/splign.cgi).
2.4. DNA and RNA blotting
DNA for Southern blotting was digested with PstI and electrophoresed in 0.7% agarose. Probe labeling, blotting, and filter hybridization followed standard protocols (Scott-Craig et al., 1990). Hybridizations were performed for 15 h at 65 °C as described (Luo et al., 2010). Roughly 2 μg of DNA were loaded per lane. Probes were made by labeling genomic DNA of GmAMA1-1, GmPOPA, and GmPOPB with [32P]dCTP.
For the GmAMA1 induction experiment, G. marginata was cultured in HSV-5C media for 30 d and then transferred to HSV-5C or HSV-1C and grown for an additional 10 d. The resulting mycelia were lyophilized and stored at −80 °C prior to RNA extraction (Hallen et al., 2007b). Full-length cDNA was prepared using the GeneRacer RACE kit, following the manufacturer’s protocols. Hybridization probes were amplified from the GmAMA1 cDNA using a specific 5′ primer (5′-ATGTTCGACACCAACTCCACT-3′) and GeneRacer 3′ nested primer (5′-CGCTACGTAACGGCATGACAGTG-3′). Probe labeling, RNA gel electrophoresis, and blotting followed standard protocols (Scott-Craig et al., 1990). Each lane was loaded with 15 μg total RNA.
2.5. Nucleotide sequence accession numbers
Sequence data from this article can be found in the GenBank/EMBL database under the following accession numbers: JN827311 for GmAMA1-1, JN827312 for GmAMA1-2, JN827313 for GmPOPA, and JN827314 for GmPOPB.
2.6. Amanitin extraction and analysis
G. marginata was cultured in HSV-5C media for 30 d and then transferred to fresh HSV-1C medium for an additional 10 d. After harvest, the mycelium was lyophilized and stored in at −80 °C. A portion of dried mycelium (0.2 g) was ground in liquid nitrogen and mixed with 2 ml methanol:water:0.01 M HCl (5:4:1) (Enjalbert et al., 1992; Hallen et al., 2003). The suspension was incubated at 22 °C for 30 min and then centrifuged at 10,200 × g for 10 min at 4 °C. The supernatant was collected and filtered through a 0.22 μm filter. Chromatographic separation was done on a C18 column (Vydac 218TP54) attached to an Agilent Model 1100 HPLC with detection at 230, 290, and 305 nm. Elution solution A was water + 0.1% trifluoroacetic acid, and solution B was acetonitrile + 0.075% trifluoroacetic acid. The flow rate was 1 ml/min with a gradient from 100% A to 100% B in 30 min. An α-amanitin standard (Sigma A2263) was dissolved in water at a concentration of 100 μg/ml. Loadings were 40 μl unknown or 20 μl standard.
3. Results
3.1. Identification of the α-amanitin genes in G. marginata
One plate of 454 pyrosequencing was performed on G. marginata genomic DNA and assembled into contigs totaling approximately 73 MB. This is approximately two times the expected size of the genome based on the average of known basidiomycetes. The sequences were put into a database and searched using AMA1, PHA1, AbPOPA, and AbPOPB. The contigs defined in these searches were used to design PCR primers for amplification of full length sequences of each gene.
Two homologs of AMA1 were found in the genome of G. marginata and named GmAMA1-1 and GmAMA1-2. GmAMA1-1 contains three introns and GmAMA1-2 contains two (Fig. 1 and Supplementary Fig. S1). The three introns of GmAMA1-1 are 53, 60, and 60 nt in length in the identical locations as the three introns of AMA1 (Hallen et al., 2007a). The first intron in both GmAMA1-1 and GmAMA1-2 interrupts the third codon before the stop codon. GmAMA1-1 and GmAMA1-2 differ at eight nucleotides out of 108 nucleotides in the coding region (i.e., from the ATG through the TGA stop codon), two of which result in amino acid changes and six of which are silent (Fig. 2). There are numerous nucleotide differences between GmAMA1-1 and GmAMA1-2 in the 5′ and 3′ untranscribed regions but also large stretches of identity. The major difference between GmAMA1-1 and GmAMA1-2 is that the latter gene is 104 bp shorter. The indel spans the second intron of GmAMA1-1 in the 3′ UTR (Fig. 1) and accounts for the presence of only two introns in GmAMA1-2 (Fig. 1 and Fig. S1).
Fig. 1.
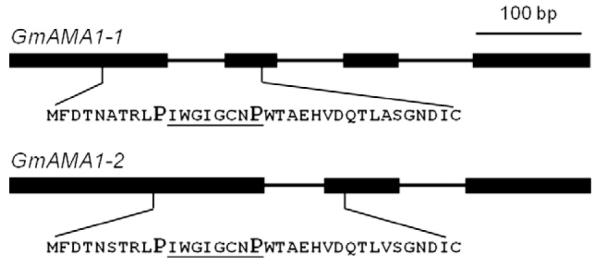
Gene structure of the two α-amanitin genes (GmAMA1-1 and GmAMA1-2) in Galerina marginata. Exons are indicated by heavy lines and introns by thin lines. The predicted proprotein sequences and their locations are indicated below.
Fig. 2.
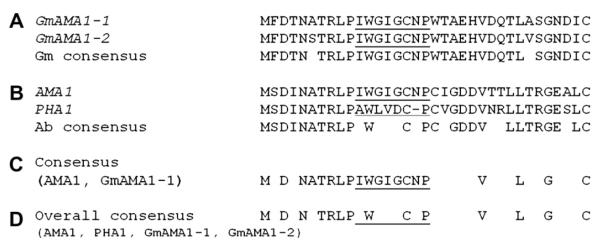
Alignments of the predicted amino acid sequences of the proproteins of α-amanitin-encoding genes in G. marginata and A. bisporigera. (A) Alignment of the two copies of the α-amanitin-encoding genes in G. marginata (GmAMA1-1 and GmAMA1-2). (B) Alignment of AMA1 (encoding α-amanitin) and PHA1 (encoding phallacidin) from A. bisporigera (Ab) (from Hallen et al., 2007a). A gap was introduced in the sequence of PHA1 because phallacidin has one fewer amino acid than α-amanitin. (C) Consensus between AMA1, the α-amanitin-encoding gene of A. bisporigera, and copy 1 (GmAMA1-1) of the α-amanitin-encoding gene of G. marginata. (D) Consensus among AMA1, PHA1, GmAMA1-1, and GmAMA1-2.
The translational start site of a gene is generally assumed to be the first in-frame ATG codon after the transcriptional start site. For GmAMA1-1, this indicates a start site that is analogous to AMA1 of A. bisporigera. For GmAMA1-2, however, there is an in-frame ATG that is 78 nucleotides upstream of the ATG indicated in Fig. 2, which would result in a proprotein of 61 amino acids instead of 35 as predicted for AMA1 and GmAMA1-1. We cannot exclude that this is the actual translational start of GmAMA1-2. However, AMA1, PHA1, and GmAMA1-1 all lack any in-frame ATG codons between their transcriptional starts and the indicated ATG, and therefore it is unlikely that GmAMA1-2 translation starts at the upstream ATG. Another argument against the first ATG being the actual translational start is that POPs preferentially act on peptides shorter than 40 amino acids (Szeltner and Polgár, 2008).
Assuming that translation of GmAMA1-2 begins at the second ATG, GmAMA1-1 and GmAMA1-2 both encode 35-amino acid proproteins, the same size as the proprotein of AMA1 in A. bisporigera (Hallen et al., 2007a). The toxin-encoding region (IWGIGCNP) is in the same relative position as it is in AMA1. There are 31 nucleotide differences between GmAMA1-1 and AMA1 in the coding region of 108 nucleotides (from the ATG through the stop codon). This results in a low level of amino acid conservation except for the toxin region and the amino acids immediately upstream of the toxin region (N[A/S]TRLP) (Fig. 2).
The proproteins of almost all of the members of the large family of genes related to AMA1 and PHA1 in A. bisporigera, A. phalloides, and Amanita ocreata start with a conserved five amino acid motif, MSDIN (Hallen et al., 2007a; Luo et al., 2009). The first five amino acids of the two G. marginata α-amanitin genes are MFDTN. To test if the G. marginata toxin-encoding genes were part of a family as was found in A. bisporigera, the G. marginata database was searched with the upstream and downstream amino acid sequences of GmAMA1-1 and GmAMA1-2 and no additional related sequences were identified. In addition, searching the A. bisporigera database with the conserved regions of GmAMA1-1 and GmAMA1-2 did not reveal any related sequences in the A. bisporigera genome beyond the known MSDIN family members previously described (Hallen et al., 2007a; Luo et al., 2009).
3.2. Distribution of α-amanitin genes in the genus Galerina
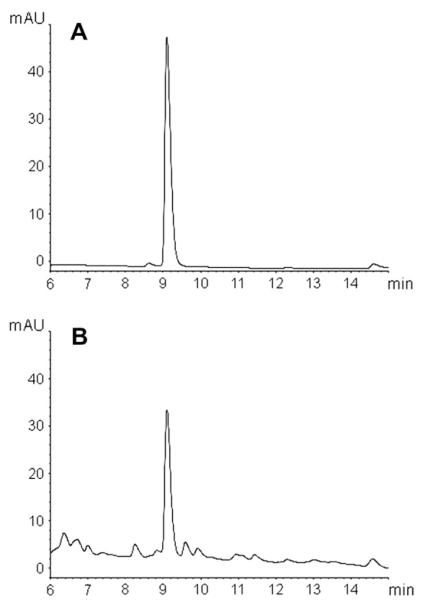
Within the genus Amanita, AMA1 and PHA1 are present only in section Phalloideae, which contains all of the known amatoxinand phallotoxin-producing species in this genus (Hallen et al., 2007a). To explore the distribution of the α-amanitin genes in relation to toxin production in Galerina, we compared four species of Galerina by DNA and RNA blotting. Recent taxonomic revision of this genus indicates that G. marginata and G. venenata are synonyms, whereas G. hybrida and G. badipes are still considered to be separate species (Enjalbert et al., 2004; Gulden et al., 2001, 2005). GmAMA1-1 hybridized to all three α-amanitin producers (G. marginata, G. badipes, and G. venenata) but not to the toxin nonproducer, G. hybrida (Fig. 3). In contrast to Amanita species, which exhibit multiple hybridizing bands when probed with AMA1 or PHA1, the pattern in Galerina was less complex. Only two bands were present, indicating that GmAMA1 is not part of an extended gene family in G. marginata. Genomic DNA digested with additional restriction enzymes also produced a similar pattern of only two hybridizing bands (data not shown). This pattern of hybridization is consistent with the genome survey sequence, which indicates that G. marginata has two and only two sequences related to GmAMA1-1. The genome survey sequence and cDNA analysis indicate that both genes encode α-amanitin (Fig. 2), and that our isolate of G. marginata does not make other compounds related to α-amanitin. In particular, the genome survey sequence did not contain a DNA sequence that could encode β-amanitin, which differs from α-amanitin by one amino acid (Asp in place of Asn). HPLC analysis of G. marginata CBS 339.88 indicated that it does not produce β-amanitin in detectable quantities (Fig. 4). The G. marginata sample contained ~0.4 mg α-amanitin/g dry weight.
Fig. 3.
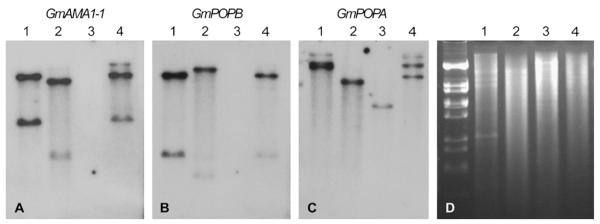
DNA blot of Galerina species. Lane 1, G. marginata; lane 2, G. badipes; lane 3, G. hybrida; lane 4, G. venenata. Panel A Probed with GmAMA1-1; panel B probed with GmPOPB; panel C, probed with GmPOPA; panel D, gel stained with ethidium bromide.
Fig. 4.
Reverse-phase HPLC analysis of amatoxins in Galerina marginata strain CBS 339.88 grown on a medium containing low carbon (see Materials and Methods). (A) α-amanitin standard. (B) extract of G. marginata. Elution was monitored at 305 nm.
3.3. Regulation of GmAMA1 by low carbon
Successful amplification of GmAMA1-1 and GmAMA1-2 by reverse transcriptase PCR with gene-specific primers indicated that both genes are transcribed in culture. This conclusion was supported by RNASeq performed at the DOE Joint Genome Institute (unpublished results). More than 50 million expression sequences were obtained from a culture of G. marginata grown on low carbon. Approximately equal numbers of gene-specific sequence reads were obtained from GmAMA1-1 and GmAMA1-2.
Expression was also studied by RNA blotting. Muraoka and Shinozawa (2000) showed that α-amanitin production in Galerina fasciculata is up-regulated on low glucose medium (carbon starvation). Expression of GmAMA1-1 and/or GmAMA1-2 were also up-regulated by carbon starvation in G. marginata and G. badipes (Fig. 5). Due to their high nucleotide similarity, this experiment could not distinguish between expression of GmAMA1-1 and GmAMA1-2. As expected from the DNA blot results, RNA from the toxin nonproducer, G. hybrida, gave no signal when the fungus was grown in either high or low carbon (Fig. 5).
Fig. 5.
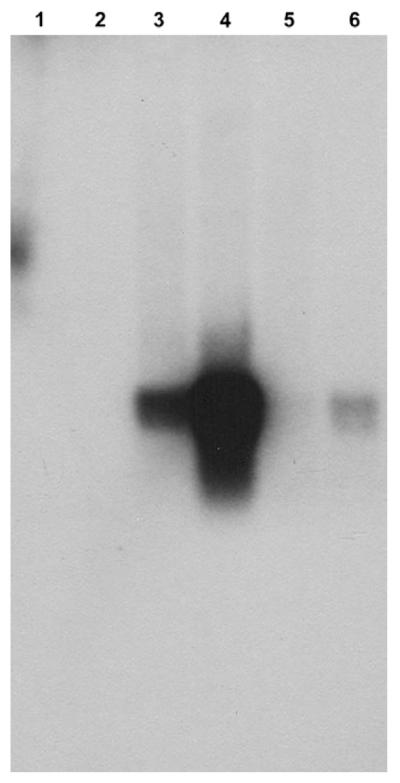
RNA blotting of Galerina strains under different growth conditions. The probe was GmAMA1-1. Lane 1: G. hybrida grown on high carbon. Lane 2: G. hybrida, low carbon. Lane 3: G. marginata, high carbon. Lane 4: G. marginata, low carbon. Lane 5: G. badipes, high carbon. Lane 6: G. badipes, low carbon. Each lane was loaded with 15 μg total RNA. The major band in lanes 3, 4 and 6 is ~300 bp. The higher molecular weight signal in lane 1 is spurious.
3.4. Prolyl oligopeptidase genes in G. marginata
Several lines of evidence implicate a prolyl oligopeptidase (POP) in the processing of the proproteins of amatoxins and phallotoxins (Luo et al., 2009, 2010). The G. marginata partial genome survey contained two homologs of the POP genes of A. bisporigera. Genomic PCR, reverse transcriptase PCR, and RACE were used to obtain full length copies of the two genes and determine their intron/exon structures (Fig. 6). GmPOPA has 18 introns, which is the same number as AbPOPA, and GmPOPB has 17 introns, one fewer than AbPOPB (Luo et al., 2010). The amino acid sequences of the predicted translational products of GmPOPA (738 amino acids) and GmPOPB (730 amino acids) are 57% identical to each other. The GmPOPA protein is 65% identical to AbPOPA and 58% identical to AbPOPB, and GmPOPB is 57% identical to AbPOPA and 75% identical to AbPOPB.
Fig. 6.
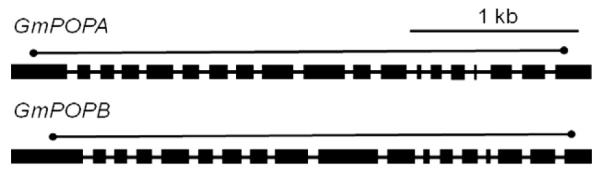
Structures of GmPOPA and GmPOPB encoding putative prolyl oligopeptidases from G. marginata. Thick bars indicate exons and thin bars indicate introns. The lines above the gene models indicate the positions of the coding regions.
Sequences hybridizing to AbPOPA are present in amatoxin and phallotoxin-producing and non-producing species of Amanita, whereas AbPOPB is present only in the amatoxin-producing species (Luo et al., 2010). Similarly, by DNA blotting GmPOPA was found to be present in all four specimens of Galerina, but GmPOPB was not present in the amanitin non-producing species G. hybrida (Fig. 3). The high similarity of the hybridization patterns when genomic DNA of G. venenata and G. marginata were probed with GmAMA1, GmPOPA, and GmPOPB is consistent with them being the same species (Gulden et al., 2001). The association of POPB with amanitin production in both A. bisporigera and G. marginata, and the higher amino acid identity of GmPOPA to AbPOPA and of GmPOPB to AmPOPB are consistent with a role for POPB in amanitin biosynthesis in the two species. All other basidiomycetes in GenBank and at the DOE Joint Genome Institute (JGI) have single POP genes, which are probably functional homologs of POPA.
4. Discussion
Amatoxins and phallotoxins are the first ribosomally encoded cyclic peptides described in the kingdom Mycota, a taxonomic group that contains an abundance of nonribosomal peptide synthetases (Walton et al., 2004). Like species of Amanita, G. marginata also synthesizes α-amanitin on ribosomes. The α-amanitin genes in G. marginata show significant resemblance to their homologs in Amanita mushrooms, including intron/exon structure, proprotein length, and probable post-translational processing by a dedicated prolyl oligopeptidase (POP). The G. marginata genes differ from those of A. bisporigera in several ways. First, the proproteins share little amino acid identity except in the toxin region itself (IWGIGCNP) and in the amino acid motif (N[A/S]TRLP) found immediately upstream of the toxin region. One of these amino acids is a completely conserved Pro residue, which would be necessary for recognition by the processing POP. It is possible that the other upstream conserved amino acids (i.e., N[A/S]TRL) are important for recognition of the proproteins by POPB or for cyclization, if this step occurs before proteolytic processing. Second, G. marginata contains two nearly identical copies of the α-amanitin gene, whereas A. bisporigera has only one. Conversely, A. bisporigera has two copies of the gene for phallacidin (PHA1), whereas G. marginata does not biosynthesize phallotoxins nor have any genes that could encode these cyclic heptapeptides (Hallen et al., 2007a). Third, the genome of G. marginata contains only two sequences related to the α-amanitin genes, whereas. A. bisporigera has a large family of related sequences (>30 members), which encode predicted, but chemically unknown, cyclic peptides. These predicted peptides contain 7–10 amino acids and most of them lack Trp and Cys and therefore cannot form tryptathionine, which is characteristic of the amatoxins and phallotoxins.
G. marginata and other species of Galerina are well-known to make α-amanitin (Enjalbert et al., 2004; Muraoka et al., 1999; Muraoka and Shinozawa, 2000). No species of Galerina has been reported to produce phallotoxins, but some have been reported to make β-amanitin, which differs from α-amanitin in having Asp in place of Asn. The difference between these two forms of amanitin is probably genetically encoded and not catalyzed by, e.g., a transamidase, because the genome of A. phalloides contains a gene that could encode β-amanitin (Hallen et al., 2007a). Our isolate of G. marginata does not synthesize β-amanitin and the genome lacks a gene for β-amanitin. G. marginata produces, at most, traces of β-amanitin in culture (Benedict et al., 1966; Benedict and Brady, 1967). We have not detected β-amanitin in several North American specimens of Galerina (unpublished results). Therefore, it appears that some species or isolates of Galerina do make β-amanitin and others do not. Other forms of amanitin, such as γ-amanitin and ε-amanitin, differ from α-amanitin and β-amanitin in their pattern of hydroxylation, which is not expected to be genetically encoded directly.
Several lines of evidence implicate a prolyl oligopeptidase (POP) in the initial processing of the proproteins of amatoxins and phallotoxins. First, in the extended MSDIN family of Amanita, and as we have now shown for the α-amanitin genes of G. marginata, the flanking Pro residues are completely conserved. During posttranslational processing, one Pro remains in the mature toxin and the other is removed with the flanking peptide. Second, an enzyme that proteolytically cleaves a synthetic phalloidin proprotein, isolated from the phalloidin-producing fungus C. apala, was identified as a POP (Luo et al., 2009). The same enzyme cleaves at both Pro residues to release the mature linear peptide (AWLATCP in the case of phalloidin). Third, toxin-producing species of Amanita have two POP genes, whereas all other sequenced basidiomycetes have only one. One of the Amanita POP genes, AbPOPB, is taxonomically restricted to toxin-producing species, like AMA1 and PHA1 themselves (Luo et al., 2010). Fourth, the distribution of AbPOPB and α-amanitin overlap in mushroom tissues (Luo et al., 2010), indicating a cytological connection between α-amanitin biosynthesis and accumulation. The results in the current paper indicate that G. marginata also has two POP genes, like A. bisporigera but unlike other, toxin non-producing species of mushrooms. GmPOPB is absent from the toxin nonproducing species G. hybrida, and therefore it cannot have an essential housekeeping function. We hypothesize that AbPOPB and GmPOPB are dedicated to biosynthesis of the amatoxins and/or phallotoxins in their respective species.
Galerina and Amanita are in different families of the Agaricales (Strophariaceae and Amanitaceae, respectively) (Kirk et al., 2008). This raises the question of the evolutionary origins of the capacity to biosynthesize the same complex secondary metabolite, which requires multiple coordinated biosynthetic activities and encoding genes. Our results indicate that the fundamental biosynthetic pathway is the same in both taxa, probably including proprotein processing by a POP. However, it remains unclear from our studies if this situation is the result of horizontal gene transfer, descent from a common ancestor, or convergent evolution. The fact that the extended MSDIN family members of Amanita are closer in sequence to each other than are the α-amanitin genes of Amanita and Galerina suggests that, if α-amanitin biosynthesis in the two genera has a common evolutionary origin, expansion of the Amanita MSDIN family occurred after the split. Identification of the biosynthetic genes for amatoxins and phallotoxins in other genera, such as Lepiota and Conocybe, might shed light on these alternative evolutionary scenarios.
G. marginata has several advantages as an experimental system for further work on the elucidation and manipulation of the amatoxin biosynthetic pathway. First, G. marginata can be cultured under laboratory conditions, unlike most species of Amanita (Benedict et al., 1966; Benedict and Brady, 1967; Muraoka and Shinozawa, 2000; Zhang et al., 2005). Second, G. marginata produces α-amanitin in culture and production can be induced by carbon starvation. Third, genomic sequencing and genetic studies should be facilitated by the availability of a toxin-producing monokaryotic strain of G. marginata. Fourth, the panoply of toxin genes is reduced from >30 members to only two in G. marginata, simplifying the analysis of novel compounds that might be produced by genetic manipulations. Fifth, G. marginata clearly has the enzymes necessary for the post-translational processing of the proprotein of GmAMA1 into mature α-amanitin, which must include cyclization, formation of the Trp-Cys cross-bridge of tryptathionine, and several hydroxylations. Therefore, it is reasonable to expect that G. marginata will correctly catalyze at least some of the required biosynthetic steps with non-natural peptides, such as cyclization and tryptathionine formation, leading to novel cyclic peptides based on the α-amanitin scaffold.
Supplementary Material
Acknowledgments
We thank Jeff Landgraf, Shari Tjugum-Holland, Christi Hemming, and Kevin Carr of the MSU Research Technology Support Facility (RTSF) for the 454 sequencing and bioinformatics assistance. This research was supported by Grant DE-FG02-91ER20021 to the Plant Research Laboratory from the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences, Office of Science, US Department of Energy, and by Grant 1R01-GM088274 from the National Institutes of Health General Medical Sciences.
Footnotes
Appendix A. Supplementary material Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.fgb.2011.12.005.
References
- Benedict RG, Tyler VE, Brady LR, Weber LJ. Fermentative production of Amanita toxins by a strain of Galerina marginata. J. Bacteriol. 1966;91:1380–1381. doi: 10.1128/jb.91.3.1380-1381.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RG, Brady LR. Further studies on fermentative production of toxic cyclopeptides by Galerina marginata (Fr Kühn) Lloydia. 1967;30:372–378. [Google Scholar]
- Bresinsky A, Besl H. A colour atlas of poisonous fungi: a handbook for pharmacists, doctors and biologists. Wolfe, Würzburg; Germany: 1990. [Google Scholar]
- Craik DJ. Seamless proteins tie up their loose ends. Science. 2006;311:1563–1564. doi: 10.1126/science.1125248. [DOI] [PubMed] [Google Scholar]
- Donia MS, Schmidt EW. Linking chemistry and genetics in the growing cyanobactin natural products family. Chem. Biol. 2011;22:508–519. doi: 10.1016/j.chembiol.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert F, Gallion C, Jehl F, Monteil H, Faulstich H. Simultaneous assay for amatoxins and phallotoxins in Amanita phalloides Fr By high-performance liquid chromatography. J. Chromatogr. 1992;598:227–236. doi: 10.1016/0021-9673(92)85052-u. [DOI] [PubMed] [Google Scholar]
- Enjalbert F, Rapior S, Nouguier-Soulé J, Guillon S, Amouroux N, Cabot C. Treatment of amatoxin poisoning: 20-year retrospective analysis. J. Toxicol. Clin. Toxicol. 2002;40:715–757. doi: 10.1081/clt-120014646. [DOI] [PubMed] [Google Scholar]
- Enjalbert F, Cassanas G, Rapior S, Renault C, Chaumont JP. Amatoxins in wood-rotting Galerina marginata. Mycologia. 2004;96:720–729. doi: 10.1080/15572536.2005.11832920. [DOI] [PubMed] [Google Scholar]
- Gulden G, Dunham S, Stockman J. DNA studies in the Galerina marginata complex. Mycol. Res. 2001;105:432–440. [Google Scholar]
- Gulden G, Stensrud Ø, Shalchian-Tabrizi K, Kauserud H. Galerina Earle: a polyphyletic genus in the consortium of dark-spored agarics. Mycologia. 2005;97:823–837. doi: 10.3852/mycologia.97.4.823. [DOI] [PubMed] [Google Scholar]
- Hallen HE, Watling R, Adams GC. Taxonomy and toxicity of Conocybe lactea and related species. Mycol. Res. 2003;107:969–979. doi: 10.1017/s0953756203008190. [DOI] [PubMed] [Google Scholar]
- Hallen HE, Luo H, Scott-Craig JS, Walton JD. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA. 2007a;104:19097–19101. doi: 10.1073/pnas.0707340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen HE, Huebner M, Shiu SH, Güldener U, Trail F. Gene expression shifts during perithecium development in Gibberella zeae (anamorph Fusarium graminearum), with particular emphasis on ion transport proteins. Fungal Genet. Biol. 2007b;44:1146–1156. doi: 10.1016/j.fgb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Han TS, Teichert RW, Olivera BM, Bulaj G. Conus venoms – a rich source of peptide-based therapeutics. Curr. Pharm. Des. 2008;14:2462–2479. doi: 10.2174/138161208785777469. [DOI] [PubMed] [Google Scholar]
- Kaneko H, et al. Amatoxin poisoning from ingestion of Japanese Galerina mushrooms. J. Toxicol. Clin. Toxicol. 2001;39:413–416. doi: 10.1081/clt-100105164. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA, editors. CAB International. United Kingdom; Wallingford, Oxon: 2008. [Google Scholar]
- Luo H, Hallen-Adams HE, Walton JD. Processing of the phalloidin proprotein by prolyl oligopeptidase from the mushroom Conocybe albipes. J. Biol. Chem. 2009;284:18070–18077. doi: 10.1074/jbc.M109.006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Hallen-Adams HE, Scott-Craig JS, Walton JD. Colocalization of amanitin and a candidate toxin-processing prolyl oligopeptidase in Amanita basidiocarps. Eukaryot. Cell. 2010;9:1891–1900. doi: 10.1128/EC.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny PB, et al. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia. 2006;98:982–995. doi: 10.3852/mycologia.98.6.982. [DOI] [PubMed] [Google Scholar]
- May JP, Perrin DM. Tryptathionine bridges in peptide synthesis. Pept. Sci. 2007;88:714–724. doi: 10.1002/bip.20807. [DOI] [PubMed] [Google Scholar]
- Muraoka S, Fukamachi N, Mizumoto K, Shinozawa T. Detection and identification of amanitins in the wood-rotting fungi Galerina fasciculata and Galerina helvoliceps. Appl. Environ. Microbiol. 1999;65:4207–4210. doi: 10.1128/aem.65.9.4207-4210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka S, Shinozawa T. Effective production of amanitins by two-step cultivation of the basidiomycete, Galerina fasciculata GF-060. J. Biosci. Bioeng. 2000;89:73–76. doi: 10.1016/s1389-1723(00)88053-6. [DOI] [PubMed] [Google Scholar]
- Novello F, Fiume L, Stirpe F. Inhibition by α-amanitin of ribonucleic acid polymerase solubilized from rat liver nuclei. Biochem. J. 1970;116:177–180. doi: 10.1042/bj1160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oman TJ, van der Donk WA. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 2010;6:9–18. doi: 10.1038/nchembio.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Craig JS, Panaccione DG, Cervone F, Walton JD. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell. 1990;2:1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeltner Z, Polgár L. Structure, function and biological relevance of prolyl oligopeptidase. Curr. Protein Pept. Sci. 2008;9:96–107. doi: 10.2174/138920308783565723. [DOI] [PubMed] [Google Scholar]
- Walsh CT, Acker MG, Bowers AA. Thiazolyl peptide antibiotic biosynthesis: a cascade of post-translational modifications on ribosomal nascent proteins. J. Biol. Chem. 2010;285:27525–27531. doi: 10.1074/jbc.R110.135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JD, Panaccione DG, Hallen HE. Peptide synthesis without ribosomes. In: Tkacz J, Lange L, editors. Advances in fungal biotechnology for industry, agriculture and medicine. Kluwer Academic; New York: 2004. pp. 127–162. [Google Scholar]
- Wehland J, Osborn M, Weber K. Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth. Proc. Natl. Acad. Sci. USA. 1977;74:5613–5617. doi: 10.1073/pnas.74.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T. Peptides of Poisonous Amanita Mushrooms. Springer; New York: 1986. [Google Scholar]
- Zhang P, Chen Z, Hu J, Ei B, Zhang Z, Hu W. Production and characterization of amanitin toxins from a pure culture of Amanita exitialis. FEMS Microbiol. Lett. 2005;252:223–228. doi: 10.1016/j.femsle.2005.08.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.