Abstract
Adipose tissue modifies the development of cardiovascular disease in a complex manner: obesity is a major risk factor, but particularly when accompanied with a central fat distribution. For that reason the characteristics of visceral adipose tissue attracted the majority of research interest thus far and measurement of waist circumference is now recommended for everyday clinical practice. However, the direct, causative role of visceral fat in cardiometabolic disease remains to be established. Epidemiological and clinical studies show that accumulation of fat subcutaneously, in the gluteo-femoral area, is protective for cardiovascular disease, but the exact molecular mechanisms remain again unclear. In the last few years, imaging allowed the study of smaller fat depots that may interact locally with important tissues: epicardial fat with the myocardium, perivascular fat with the vessel wall and the developing atherosclerotic plaque, renal sinus fat with the renal artery. Unraveling the heterogeneous fat distribution and metabolic phenotypes in human obesity will facilitate optimal assessment of cardiovascular risk in overweight and obese individuals.
Keywords: visceral adipose tissue, subcutaneous adipose tissue, gluteo-femoral adipose tissue, epicardial adipose tissue, renal sinus adipose tissue
Introduction
The association between obesity and cardiovascular risk has been described in multiple epidemiological studies and is by now universally accepted [1]. However, this is far from a straightforward relationship; for any given body mass index (BMI) level, there is significant variability with some lean individuals developing disease and others remaining healthy despite severe obesity, the so-called metabolically healthy obese (MHO) [2]. A significant part of this variability can be attributed to variation in fat distribution and depot differences in adipose tissue function.
Adipose tissue is unique in that it is dispersed throughout the body in discreet depots, which are thought to constitute “separate mini-organs” [3]. Almost 90% of the adipose tissue is found subcutaneously (subcutaneous adipose tissue – SAT), mainly in the abdominal, gluteal and femoral areas. Visceral adipose tissue (VAT) and smaller depots in close proximity to the heart (epicardial adipose tissue – EAT), kidneys, joints, eyes, etc make up the remaining 10-20% [4]. The size of each depot and the balance between them is important for the cardiometabolic risk of the individual: a peripheral fat distribution (in the limbs) is favorable and a central one (truncal, both subcutaneous and visceral) detrimental as will be discussed in more detail below. The mechanisms linking fat distribution and cardiovascular risk are complex and involve changes in whole body glucose and lipid metabolism, effects on traditional and novel cardiovascular risk factors (e.g. hypertension, inflammation) and the systemic or local actions of adipokines secreted by the adipose tissue.
Epidemiological and imaging studies link adipose tissue depots and atherosclerosis risk, severity and progression
Central obesity and cardiovascular morbidity and mortality
The idea that central fat distribution is associated with higher disease risk is far from novel [5] and is supported by a wealth of epidemiological and clinical data (reviewed in [6]). In a meta-analysis of 58 prospective studies of >220,000 free of cardiovascular disease individuals, the hazard ratio (HR) for cardiovascular disease was 1.23 [95% confidence intervals (CI) 1.17–1.29] per 1 standard deviation higher baseline value of BMI, 1.27 (95% CI 1.20–1.33) with waist circumference and 1.25 (95% CI 1.19–1.31) with waist-to-hip ratio, after adjustment for age, sex, and smoking status. These associations remained significant, although attenuated, after further adjustment for systolic blood pressure, history of diabetes, and total and HDL cholesterol. Moreover, within each tertile of BMI, HRs increased log-linearly with waist circumference and waist-to-hip ratio [7]. Similar findings with hazard ratio (HR) for cardiovascular disease 1.15 [95% CI 1.04–1.27] per 1 standard deviation higher baseline value of waist circumference and 1.15 (95% CI 1.04–1.27) with waist-to-hip ratio were reported in a meta-analysis of nine British studies comprising 82,864 subjects [8].
These data illustrate the notion that measures of central fat distribution, such as waist circumference and waist-to-hip ratio, can improve risk stratification in conjunction with obesity indices. On the other hand, it should also be kept in mind that all the correlations between BMI, waist circumference and fat mass are very high (Pearson's r > 0.90), and all three show comparable correlations with VAT mass (r ≈ 0.75). Therefore, to examine the association between waist circumference and cardiovascular risk [9] and draw conclusions about its specific effect on disease risk, it is essential to adjust for BMI, which is not done in many studies.
Among individuals with established vascular disease, high BMI is sometimes associated with better prognosis, the so-called “obesity paradox” [10]. Central obesity though remains detrimental and associated with increased mortality: among 15,923 subjects with coronary artery disease (CAD) followed up for 2.3 years, HR for death was 1.70 (95% CI 1.58-1.83) after adjustment for age, sex, smoking, diabetes, hypertension, heart failure, and BMI [11]. Interestingly, when patients with CAD were categorized into four groups based on fat distribution and BMI (normal BMI/peripheral fat distribution, normal BMI/central fat distribution, obese/peripheral fat distribution, obese/central fat distribution) those with central obesity and normal BMI showed the poorest survival compared with all other groups [12].
Peripheral fat accumulation is protective
Recent systematic reviews identified and summarized data on 37,725 individuals followed-up on average for 11.2 years in nine prospective cohort studies that examine the relationship between hip circumference and cardiovascular disease risk (three studies), cardiovascular mortality (four) or total mortality (two studies) [13, 14]. All, but one, report an inverse association between hip circumference and the respective outcome in both male and female subjects. This association became apparent only after adjustment for waist circumference and/or BMI. Due to the strong bivariate associations between BMI and waist and hip circumference, as they all reflect total adiposity, it is important to adjust for BMI and waist circumference in any model to reveal the independent effects of hip circumference on cardiovascular morbidity and mortality [13, 14]. In addition, peripheral fat distribution is associated with traditional risk factors, lower blood pressure, improved insulin sensitivity and a beneficial lipid profile, as reviewed before [15].
Peripheral fat distribution is also an integral part of the MHO phenotype [16-18]. The incidence of cardiovascular disease among these individuals is in some studies comparable to the normal-weight population [17-21], and in other studies intermediate between normal-weight and metabolically unhealthy obese subjects [22], but altogether significantly lower than what would be expected solely on the basis of BMI.
Anthropometric indices for clinical predictions
The question that arises from the epidemiological studies briefly discussed above is whether measurements of fat distribution should become standard part of the every day assessment of cardiovascular risk. This question was formally addressed in the Emerging Risk Factors Collaboration study which showed that addition of BMI, waist circumference, waist-to-hip ratio or of their combination does not improve cardiovascular risk prediction over and above the one based on systolic blood pressure, history of diabetes and lipids [7]. Nevertheless, the American Heart Association in its most recent scientific statement on the assessment of adiposity recommends measurement of both BMI and waist circumference in everyday clinical practice. It also emphasizes the need for sex-, age-, race- and BMI-specific cut-off values of waist circumference [23]. In a population study, the optimal BMI and waist circumference thresholds for the identification of increased cardiovascular risk appear to be comparable in white and African American men (29.1 versus 30.4 kg/m2, and 99.1 versus 99.4 cm, respectively), but significantly lower in white compared to African American women (30.0 versus 32.9 kg/m2, and 91.9 versus 96.8 cm) [24]. When VAT area was assessed by computed tomography (CT) though, the threshold for increased cardiovascular risk was higher in both white men and women compared to the African American ones (140 versus 82 cm2 in men 141 versus 97 cm2 in women) [25]. In addition, a number of new indices have been proposed as superior to the use of BMI or of circumference measurements (summarized in Table 1).
Table 1.
New anthropometric indices.
| Author | Index | Formula | Population | Predictive of | Related citations |
|---|---|---|---|---|---|
| Amato et al [26] | Visceral Adiposity Index | Males: Females: |
N=1,498 primary care subjects | • Cardiovascular events •Cerebrovascular events |
Knowles et al [27] Mohammadreza et al [28] Al-Daghri et al [29] Zhang et al [30] |
| Arsenault et al [31] | Hypertriglyceridemic waist | Males : WC ≥ 90 cm and TG ≥ 2.0 mmol/L Females: WC ≥ 85 cm and TG ≥ 1.5 mmol/L |
N=21,787 Age 45-79 y Follow-up 9.8 y |
• Coronary artery disease | De Graaf et al [32] Blackburn et al, [33] Zhang et al [30] |
| Bergman et al [34] | Body Adiposity Index | N=1,733 Mexican Americans Age 35 (18-67) y |
• % body adiposity | Melmer et al [35] Moliner-Urdiales et al [36] |
|
| Ashwell et al [37] | Waist-to-Height Ratio | Waist/Height | N=305,851 (meta-analysis) | • Cardiovascular risk factors • Myocardial infarction Cardiovascular mortality |
Sluik et al [38] |
| Trefethen LN [39] | “New BMI” | No data available |
http://people.maths.ox.ac.uk/trefethen/bmi.html (accessed 03/25/13) |
||
BMI: Body Mass Index; HDL: High Density Lipoprotein; TG: Triglycerides; WC: Waist Circumference.
Insights from imaging studies
From a different point view, it can be argued that anthropometric indices are only indirect measures and possibly poor approximations of the respective adipose tissue depots. Waist circumference reflects VAT as well as abdominal SAT, while hip circumference depends on the amount of gluteal fat, but also on muscle mass. With the advent of detailed imaging techniques, it is possible to obtain more accurate measurements of the above depots, together with smaller, so far overlooked, depots.
CT and magnetic resonance imaging (MRI) studies can examine the independent associations of abdominal SAT and VAT with risk factors and disease. Both earlier [40] and more recent studies indicate that VAT [41, 42] and the ratio between VAT/SAT [43] show the closest correlations to cardiometabolic risk. In addition, increased VAT for comparable BMI levels appears to mediate the adverse metabolic profile of South and East Asian populations [44, 45].
Epicardial and perivascular adipose tissues in relation to atherosclerosis
Given their anatomical proximity to the heart, the coronaries, or other susceptible to atherosclerosis arteries, the epicardial, pericardial and perivascular adipose tissue depots have attracted considerable interest over the last few years. In cross-sectional studies, higher amounts of epicardial/pericardial adipose tissue are associated with higher prevalence of cardiovascular disease; this relationship is seen in both sexes and is attenuated after adjustment for BMI and other risk factors [46-48]. Interestingly, increased pericardial and peri-aortic depots do not always coexist and significant discordance is noted in more than 20% of individuals [49]. The thoracic peri-aortic adipose tissue is more closely associated to central obesity and metabolic syndrome, but its associations with cardiovascular risk or disease are less significant and become apparent only after adjustment for VAT [48-50].
More recently, prospective data have started accumulating: the volume of epicardial adipose tissue [51] and its expansion [52] were both associated with an increase of coronary artery calcification over time. In patients with established coronary artery disease (n=194, age 59.4 years, 80% men, BMI 28.7 ± 4.6 kg/m2), EAT measured by echocardiography was not predictive of new major cardiovascular events over a follow-up period of 3.6 years [53]. In the general population though, EAT is predictive of future coronary events: among 4,093 participants in the Heinz Nixdorf Recall cohort study (age 59.4 years, 47% men, follow-up 8.0 years), doubling of EAT was associated with a 1.5-fold risk of coronary events after adjustment for cardiovascular risk factors [HR 1.54 (95% CI 1.09 – 2.19)][54].
Other small depots: kidney and breast
Renal sinus fat, a small depot (volume of 4.6±3.2 cm3 in MRI) is of interest because it surrounds directly the renal artery and vein. Increased renal sinus fat, also called “fatty kidney”, was associated with the presence of hypertension in two middle-aged populations and this relationship remained significant after accounting for BMI and/or VAT [55, 56]. The breast adipose tissue has been studied in MRI studies of healthy, premenopausal women with a wide range of adiposity and its volume correlated positively with central obesity and VAT, and negatively with leg adipose tissue. No associations were found with cardiovascular risk factors in either of these studies [57, 58].
Pathophysiological mechanisms that link specific depots to cardiometabolic disease
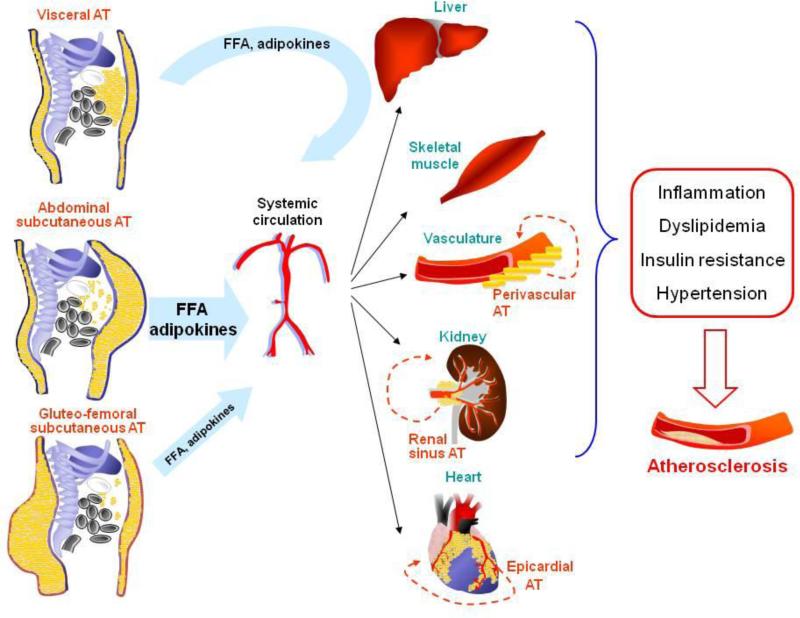
Elucidating the mechanisms that differentiate the significance of each depot is a challenging scientific question that is potentially important for disease prevention and treatment. Factors like the cellular characteristics, the location and the function of different adipose tissue deposits have all been linked to their effects in three major hypotheses, as discussed in the following paragraphs (see also Figure 1).
Figure 1. Adipose tissue depots influence the development of atherosclerosis through multiple direct and indirect mechanisms.
Subcutaneous adipose tissue depots (abdominal, gluteal and femoral) release in the systemic circulation free fatty acids (FFA) and numerous adipokines/cytokines that affect the function of critical tissues (liver, skeletal muscle, kidney, heart, vessel wall) and have detrimental effects on traditional (dyslipidemia, hypertension, diabetes) or novel (inflammation) cardiovascular risk factors. Visceral adipose tissue products are drained through the portal vein directly to the liver, affect hepatic function and a percentage of them enters the systemic circulation and has systemic effects. Smaller depots may have local effects: the perivascular fat can interact with the vessel wall, the epicardial fat with the myocardium and the coronary arteries and the renal sinus fat with the renal artery/vein.
A. The subcutaneous sink and the toxicity of ectopic adipose tissue
The subcutaneous depots are often considered as the main adipose sites that ensure effective energy deposition under conditions of caloric excess and release under conditions of negative energy balance. According to this hypothesis, accumulation of fat as VAT and in the smaller internal depots reflects the inability of SAT for further triglyceride storage and has detrimental effects on the surrounding tissues, particularly liver, skeletal muscle and the heart [59]. This is evident in the case of lipodystrophies [60] and in the use of thiazolidinediones (rosiglitazone or pioglitazone): administration of pioglitazone to overweight/obese, insulin resistant but non-diabetic subjects induced adipogenesis and expansion of SAT, reduction of VAT and improved insulin sensitivity [61].
B. Adipokines and endocrine effects
By synthesizing and releasing a number of cytokines and other proteins, adipose tissue exerts endocrine effects on a number of tissues, the vasculature itself and others that contribute indirectly to cardiovascular disease, like liver and skeletal muscle. Adipokines like, leptin, interleukin-6 and -8, monocyte chemoattractant protein 1, serum amyloid A, plasminogen activator inhibitor 1, angiotensinogen and an array of others, are upregulated in obesity and can affect all stages of atherosclerosis from monocyte recruitment to foam cell formation, smooth muscle cell proliferation, plaque destabilization and thrombosis (reviewed in [62, 63]).
Although a detailed description of their individual effects is outside of the scope of this review, specific mention is required of adiponectin, a highly abundant circulating hormone. In contrast to other adipokines that are increased in obesity, adiponectin is reduced in obese individuals. In addition to BMI, adiponectin levels are influenced by sex (higher in women compared to men), ethnicity (higher in Caucasians compared to Hispanics and African Americans) and fat distribution (positively correlated with lower body fat and negatively with truncal fat) [64]. Circulating adiponectin levels are associated with cardiovascular disease, but in a complex way: in the general population, adiponectin is inversely related to traditional risk factors and future events; in older individuals, this association is U-shaped with a protective effect for concentrations up to 12.4 mg/L [65]. On the contrary, in populations with pre-existing cardiovascular disease, higher adiponectin levels are associated with poorer prognosis [65-67]. In agreement with epidemiological data, recent in vitro studies suggest that under certain experimental conditions adiponectin can have adverse effects on macrophages, T cells [68], in addition to its better established atheroprotective effects (reviewed in [69, 70]). Thus, the exact role of adiponectin in chronic inflammatory states remains for now poorly understood [71].
C. The portal hypothesis and other local/direct effects of smaller depots
The portal hypothesis posits that, when enlarged, the lipolytically active VAT releases significant amounts of free fatty acids directly to the portal circulation and consequently to the liver [72]. These in turn will be transported to the systemic circulation in very-low-density lipoproteins, will stimulate hepatic glucose production and suppress hepatic insulin clearance, leading to dyslipidemia, hyperinsulinemia and insulin resistance [72]. Indeed, it was later shown that with visceral obesity the contribution of VAT to the free fatty acids that reach the liver increases from <10% to over 30-40% [73]. However, increased concentration of free fatty acids in the portal compared to the systemic circulation has not been documented in humans (reviewed in [74]) and the majority of free fatty acids that reach the liver is derived from SAT, and particularly the upper body SAT even in obese individuals [73]. In addition to free fatty acids, VAT releases interleukin-6 to the portal vein, which in turn induces synthesis of C-reactive protein in the liver and contributes to systemic inflammation [75].
Smaller adipose tissue depots, like the epicardial one, are probably devoid of systemic effects. On the other hand, they may have pathophysiological significance because of their effects on the heart and the coronary arteries. In in vitro studies, adipokines produced by EAT can induce expression of adhesion molecules in human coronary artery endothelial cells and migration of monocytes [76] and affect the contractile function and insulin responses of cardiomyocytes [77], as well as fibrosis of the atrial myocardium [78]. Perivascular adipose tissue can regulate vascular tone and thus blood pressure control through the release of a relaxing factor, recently identified as methyl-palmitate [79]. Finally, obesity is accompanied by reduced angiogenesis and capillarization within the adipose tissue itself, which may be linked to the concomitant inflammation and insulin resistance [80, 81].
Experimental studies to firmly establish the independent roles of separate depots
It has to be noted that although new data accumulate steadily on the characteristics of each adipose tissue depot and on their relationship to metabolic and cardiovascular diseases, studies that will establish a direct, causal relationship are extremely difficult. They require experimental manipulations that will target a specific depot and alter its function without affecting the rest of the depots or total body adiposity. Very few studies provide such information, but there are some important insights from some clinical studies and animal models, as described below.
A mouse model without perivascular adipose tissue
Murine perivascular adipose tissue has features of brown adipose tissue and as a result, it can generate heat to preserve intravascular temperature upon cold exposure [82]. A mouse model in which the adipogenic transcription factor PPARγ was deleted specifically in smooth muscle cells (SMPG KO mice) was characterized by complete lack of perivascular adipose tissue with normal subcutaneous and gonadal depots. Studies of this model show that activation of perivascular fat by cold exposure can attenuate high-fat diet induced atherosclerosis, at least partly through enhanced lipid clearance and prostacyclin release [82]. Although the human relevance of these findings is unclear, the study demonstrates the potential of this depot to modulate vascular damage in vivo.
Omental adipose tissue in humans: how important is it?
As already discussed, the links between VAT and cardiometabolic disease have attracted extensive interest. However, VAT correlates closely with intrahepatic TG content and it has been proposed that the former represents mostly a marker of the latter, rather than a hazardous tissue per se. Thus obese, middle-aged individuals with high intrahepatic TG content have reduced insulin sensitivity in comparison to others with equal VAT but low intrahepatic TG. On the contrary, individuals with high VAT were as insulin sensitive as those with low VAT provided that they were matched on intrahepatic TG content [83]. These findings are in accordance with interventional studies that involve removal of the omental depot in obese individuals. In eight out of nine published studies, addition of omentectomy to gastric bypass or sleeve gastrectomy did not augment weight loss or the improvement in insulin sensitivity and other metabolic indices achieved by the bariatric intervention itself (Table 2) [84-91]. Although longer-term follow-up and the incidence of cardiovascular events can be assessed in future studies, these data question the causative role of omental adipose tissue in metabolic dysfunction.
Table 2.
Omentectomy, weight loss and metabolic parameters.
| Author | N | Subject characteristics | Treatment | Follow-up | Outcome |
|---|---|---|---|---|---|
| Fabbrini et al [84] | 22 | Obese men and women | Roux-en-Y gastric bypass ± omentectomy | 12 months | Weight loss, lipids, FPG, insulin sensitivity: no difference between groups |
| Fabbrini et al [84] | 7 | Obese men and women with T2DM | Omentectomy | 3 months | Insulin sensitivity, diabetes medications: no change compared to baseline |
| Herrera et al [85] | 22 | Obese men and women | Roux-en-Y gastric bypass ± omentectomy | 1, 3, 6, 12 months | Weight loss, lipids, FPG, insulin, adipokines: no difference between groups |
| Dillard et al [86] | 28 | Obese men and women | Roux-en-Y gastric bypass ± omentectomy | 3 months | Weight loss: no difference between groups FPG, total- and VLDL-cholesterol: reduction only in the omentectomy group |
| Tamboli et al [87] | 21 | Obese men and women | Roux-en-Y gastric bypass ± omentectomy | 6, 12 months | Skeletal muscle gene expression: greater reduction of inflammatory genes in the omentectomy group |
| Wu et al [88] | 40 | Obese men and women | Sleeve gastrectomy ± partial enterectomy and omentectomy | 1, 3, 6, 12 months | Weight loss: no difference between groups |
| Dunn et al [89] | 40 | Obese men and women | Roux-en-Y gastric bypass ± omentectomy | 1 month | Weight loss, insulin sensitivity, hepatic glucose production, hepatic insulin sensitivity index : no difference between groups |
| Lima et al [90] | 20 | Obese premenopausal women with MS | Roux-en-Y gastric bypass ± omentectomy | 1, 6, 12 months | Weight loss and reduction in CRP: greater in omentectomy group. Insulin sensitivity: no difference between groups |
| Sdralis et al [91] | 21 | Obese men and women | Sleeve gastrectomy ± omentectomy | 7 days, 1, 3, 12 months | Weight loss, lipids, FPG, insulin, CRP, adipokines: no difference between groups |
CRP: C-reactive protein; FPG: fasting plasma glucose; T2DM: type 2 diabetes mellitus; VLDL: very low density lipoprotein
Diet, exercise and visceral adiposity
Identification of dietary patterns associated preferentially with central obesity and high cardiovascular risk can be of major public health significance. In a cross-sectional assessment of diet of 497,308 individuals (age 25-70 years, 70.7% women) from 10 European countries, adherence to a Mediterranean diet, enriched in vegetables, fruits, legumes and unsaturated fatty acids, low in meat and dairy products, was associated with significantly lower waist circumference in both men and women, but not with BMI [92]. A similar dietary pattern, high in fruits and low in processed meat, margarine, soft drinks and white bread was related to smaller increases in waist circumference during 5.5 years of follow-up in the 48,631 participants of the European Prospective Investigation into Cancer and Nutrition study [93]. These observational data support the findings of a recent randomized trial: a study comparing two Mediterranean diets, supplemented with olive oil or mixed nuts, with a control, low fat, diet was terminated prematurely due to lower incidence of major cardiovascular events in the Mediterranean diet groups (multivariable adjusted hazard ratio 0.70 (95% CI 0.54 – 0.92) [94].
Caloric restriction induces weight loss and women appear to lose more VAT as percent of total body fat loss compared to men [95]. However, there is no specific diet that preferentially targets VAT: diets with 25% versus 15% protein, 40% versus 20% fat, 65% versus 35% carbohydrates, all resulted in similar reductions in total body fat, lean mass, abdominal SAT and VAT [95].
Exercise is the second feature of healthy lifestyle. In overweight and obese adults, aerobic exercise alone, without concomitant caloric restriction, can lead to significant reduction of VAT after 12 weeks; on the contrary, resistance training has no significant effects on VAT [96, 97]. In addition, some studies report that exercise can facilitate VAT reductions during diet-induced weight loss [98] or prevent VAT regain after successful dieting and weight loss [99] (and reviewed in [6]).
Brown adipose tissue in humans: it exists – how important is it?
The “rediscovery” of brown adipose tissue (BAT) in adult humans (100) has created a lot of scientific interest on the molecular and physiological properties of this depot. Based on its ability to dissipate energy as heat by uncoupling oxidative phosphorylation and ATP synthesis (a function of uncoupling protein-1, UCP-1), BAT is seen as a potential new therapeutic tools to combat obesity. Although outside of the scope of this review, there is now evidence that UCP-1 expressing adipocytes are present in humans in small depots with the characteristics of classical BAT (101), but also interspersed in the WAT depots, so-called “brite” or “beige” adipocytes (102). The amount of BAT depends on several factors including sex, age and BMI (103) and is increased after surgical weight loss (104). In animal models, multiple hormones, molecular pathways and pharmacological agents (from catecholamines, natriuretic peptides, the retinoic acid pathway to irisin and thiazolidinediones, reviewed in 105) can expand and activate brown/brite-beige fat. Translational studies will be needed to clarify whether these tissues affect whole body metabolism in humans and to identify efficient tools to employ them towards cardiometabolic health.
Conclusions
Obesity is now recognized as a major public health issue and a driver of a number of comorbidities, including cardiovascular disease. A more recent notion is that obesity is a heterogeneous condition. Body fat distribution, which reflects the size of the larger fat depots, provides an additional level of risk classification for obese individuals and is now recommended part of every day clinical practice. Smaller depots, like the epicardial, when located in critical areas, may interact directly with important tissues, like the vessel wall, the myocardium and the renal vessels, but their in vivo significance remains unknown. Although epidemiological studies have provided and continue to provide ample data, unraveling the effects of each depot in experimental studies remains a big challenge. As a result, therapeutic approaches that will target specific fat deposits are currently lacking and maintaining a healthy body weight through lifestyle measures is still the cornerstone of cardiometabolic health.
Acknowledgements
This work was supported by NIH DK080448, P30 DK046200 (to S.K.F.) and the Evans Center for Interdisciplinary Biomedical Research Affinity Research Collaborative on Sex Differences in Adipose Tissue at Boston University School of Medicine.
Footnotes
Conflict of Interest
Kalypso Karastergiou and Susan K. Fried declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have beenhighlighted as:
• Of importance
•• Of major importance
- 1.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samocha-Bonet D, Chisholm DJ, Tonks K, Campbell LV, Greenfield JR. Insulin-sensitive obesity in humans - a ‘favorable fat’ phenotype? Trends Endocrinol Metab. 2012;23:116–124. doi: 10.1016/j.tem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: mplication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 6.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 7.Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12:680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes (Lond) 2007;31:1552–1553. doi: 10.1038/sj.ijo.0803653. [DOI] [PubMed] [Google Scholar]
- 10.Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens. 2013;7:85–94. doi: 10.1016/j.jash.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 11*.Coutinho T, Goel K, Corrêa de Sá D, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [This study shows that there is no “central obesity paradox”, i.e. central fat distribution is associated with increased morbidity/mortality in subjects with established cardiovascular disease.] [DOI] [PubMed] [Google Scholar]
- 12.Coutinho T, Goel K, Corrêa de Sá D, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol. 2013;61:553–560. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 13*.Cameron AJ, Magliano DJ, Söderberg S. A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obes Rev. 2013;14:86–94. doi: 10.1111/j.1467-789X.2012.01051.x. [Recent meta-analysis illustrating the independent relationship between peripheral fat distribution and morbidity/mortality.] [DOI] [PubMed] [Google Scholar]
- 14.Heitmann BL, Lissner L. Hip Hip Hurrah! Hip size inversely related to heart disease and total mortality. Obes Rev. 2011;12:478–481. doi: 10.1111/j.1467-789X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 15.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 16.Calori G, Lattuada G, Piemonti L, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–215. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appleton SL, Seaborn CJ, Visvanathan R, et al. on behalf of the North West Adelaide Health Study Team: Diabetes and Cardiovascular Disease Outcomes in the Metabolically Healthy Obese Phenotype: A cohort study. Diabetes Care. 2013 doi: 10.2337/dc12-1971. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega GL, Adams-Huet B, Peshock R, et al. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 19.Ogorodnikova AD, Kim M, McGinn AP, et al. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2012;20:651–659. doi: 10.1038/oby.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97:2482–2488. doi: 10.1210/jc.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega FB, Lee DC, Katzmarzyk PT, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–397. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 23**.Cornier MA, Després JP, Davis N, et al. American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council: Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [Recent review of the epidemiology of obesity and its related comorbidities, together with issues and methods available for assessing adiposity in adults. It concludes with practical recommendations for the clinician in practice towards identifying more at-risk overweight/obese individuals.] [DOI] [PubMed] [Google Scholar]
- 24.Katzmarzyk PT, Bray GA, Greenway FL, et al. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring) 2011;19:1272–1278. doi: 10.1038/oby.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr. 2013;97:480–486. doi: 10.3945/ajcn.112.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowles KM, Paiva LL, Sanchez SE, et al. Waist Circumference, Body Mass Index, and Other Measures of Adiposity in Predicting Cardiovascular Disease Risk Factors among Peruvian Adults. Int J Hypertens. 2011;2011:931402. doi: 10.4061/2011/931402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadreza B, Farzad H, Davoud K, Fereidoun Prof AF. Prognostic significance of the complex “Visceral Adiposity Index” vs. simple anthropometric measures: Tehran lipid and glucose study. Cardiovasc Diabetol. Mar 7. 2012;11:20. doi: 10.1186/1475-2840-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Daghri NM, Al-Attas OS, Alokail MS, et al. Visceral adiposity index is highly associated with adiponectin values and glycaemic disturbances. Eur J Clin Invest. 2013;43:183–189. doi: 10.1111/eci.12030. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Shu XO, Li H, et al. Visceral adiposity and risk of coronary heart disease in relatively lean Chinese adults. Int J Cardiol. 2013 Mar 1; doi: 10.1016/j.ijcard.2013.01.275. pii: S0167-5273(13)00354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arsenault BJ, Lemieux I, Després JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182:1427–1432. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Graaf FR, Schuijf JD, Scholte AJ, et al. Usefulness of hypertriglyceridemic waist phenotype in type 2 diabetes mellitus to predict the presence of coronary artery disease as assessed by computed tomographic coronary angiography. Am J Cardiol. 2010;106:1747–1753. doi: 10.1016/j.amjcard.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Blackburn P, Lemieux I, Lamarche B, et al. Hypertriglyceridemic waist: a simple clinical phenotype associated with coronary artery disease in women. Metabolism. 2012;61:56–64. doi: 10.1016/j.metabol.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring) 2011;19:1083–1089. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melmer A, Lamina C, Tschoner A, et al. Body Adiposity Index and Other Indexes of Body Composition in the SAPHIR Study: Association With Cardiovascular Risk Factors. Obesity (Silver Spring) 2012 Jun 22; doi: 10.1002/oby.20289. doi: 10.1038/oby.2012.160. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Moliner-Urdiales D, Artero EG, Lee DC, et al. Body adiposity index and all-cause and cardiovascular disease mortality in men. Obesity (Silver Spring) 2013 Mar 20; doi: 10.1002/oby.20399. doi: 10.1002/oby.20399. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 38.Sluik D, Boeing H, Montonen J, et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol. 2011;174:22–34. doi: 10.1093/aje/kwr048. [DOI] [PubMed] [Google Scholar]
- 39.Trefethen LN. [April 4 2013]; http://people.maths.ox.ac.uk/trefethen/bmi.html.
- 40.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 41.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JD, Borel AL, Nazare JA, et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012;97:1517–1525. doi: 10.1210/jc.2011-2550. [DOI] [PubMed] [Google Scholar]
- 43.Kaess BM, Pedley A, Massaro JM, et al. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nazare JA, Smith JD, Borel AL, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- 45.Lear SA, Chockalingam A, Kohli S, Richardson CG, Humphries KH. Elevation in cardiovascular disease risk in South Asians is mediated by differences in visceral adipose tissue. Obesity (Silver Spring) 2012;20:1293–1300. doi: 10.1038/oby.2011.395. [DOI] [PubMed] [Google Scholar]
- 46.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang G, Wang D, Zeb I, et al. Intra-thoracic fat, cardiometabolic risk factors, and subclinical cardiovascular disease in healthy, recently menopausal women screened for the Kronos Early Estrogen Prevention Study (KEEPS). Atherosclerosis. 2012;221:198–205. doi: 10.1016/j.atherosclerosis.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thanassoulis G, Massaro JM, Hoffmann U, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Britton KA, Pedley A, Massaro JM, et al. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the framingham heart study. J Am Heart Assoc. 2012;1:e004200. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yerramasu A, Dey D, Venuraju S, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 52.Nakanishi R, Rajani R, Cheng VY, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis. 2011;218:363–368. doi: 10.1016/j.atherosclerosis.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 53.Albuquerque FN, Somers VK, Blume G, et al. Usefulness of epicardial adipose tissue as predictor of cardiovascular events in patients with coronary artery disease. Am J Cardiol. 2012;110:1100–1105. doi: 10.1016/j.amjcard.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 54**.Mahabadi AA, Berg MH, Lehmann N, et al. Association of Epicardial Fat With Cardiovascular Risk Factors and Incident Myocardial Infarction in the General Population: The Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [This prospective study shows that epicardial adipose tissue volume is associated with fatal and nonfatal coronary events in the general population independent of traditional cardiovascular risk factors.] [DOI] [PubMed] [Google Scholar]
- 55.Chughtai HL, Morgan TM, Rocco M, et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster MC, Hwang SJ, Porter SA, et al. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janiszewski PM, Saunders TJ, Ross R. Breast volume is an independent predictor of visceral and ectopic fat in premenopausal women. Obesity (Silver Spring) 2010;18:1183–1187. doi: 10.1038/oby.2009.336. [DOI] [PubMed] [Google Scholar]
- 58.Schautz B, Later W, Heller M, Müller MJ. Bosy-Westphal A: Associations between breast adipose tissue, body fat distribution and cardiometabolic risk in women: cross-sectional data and weight-loss intervention. Eur J Clin Nutr. 2011;65:784–790. doi: 10.1038/ejcn.2011.35. [DOI] [PubMed] [Google Scholar]
- 59.Carobbio S, Rodriguez-Cuenca S, Vidal-Puig A. Origins of metabolic complications in obesity: ectopic fat accumulation. The importance of the qualitative aspect of lipotoxicity. Curr Opin Clin Nutr Metab Care. 2011;14:520–526. doi: 10.1097/MCO.0b013e32834ad966. [DOI] [PubMed] [Google Scholar]
- 60.Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3125. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLaughlin TM, Liu T, Yee G, et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 2010;18:926–931. doi: 10.1038/oby.2009.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 63.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–T36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 64.Turer AT, Khera A, Ayers CR, et al. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kizer JR, Benkeser D, Arnold AM, et al. Associations of total and high-molecular- weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126:2951–2961. doi: 10.1161/CIRCULATIONAHA.112.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson SR, Sabatine MS, Wiviott SD, et al. TIMI Study Group: Assessment of adiponectin and the risk of recurrent cardiovascular events in patients presenting with an acute coronary syndrome: observations from the Pravastatin Or atorVastatin Evaluation and Infection Trial-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22). Am Heart J. 2011;161:1147–1155. doi: 10.1016/j.ahj.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Hascoet S, Elbaz M, Bongard V, et al. Adiponectin and long-term mortality in coronary artery disease participants and controls. Arterioscler Thromb Vasc Biol. 2013;33:e19–29. doi: 10.1161/ATVBAHA.112.300079. [DOI] [PubMed] [Google Scholar]
- 68.Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem. 2012;287:36896–36904. doi: 10.1074/jbc.M112.409516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32:S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 70.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 71.Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflam. 2011;2011:376909. doi: 10.4061/2011/376909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- 73.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frayn KN. Visceral fat and insulin resistance--causative or correlative? Br J Nutr. 2000 Mar;83(Suppl 1):S71–7. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- 75.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 76.Karastergiou K, Evans I, Ogston N, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 77.Greulich S, Maxhera B, Vandenplas G, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. 2012;126:2324–2334. doi: 10.1161/CIRCULATIONAHA.111.039586. [DOI] [PubMed] [Google Scholar]
- 78.Venteclef N, Guglielmi V, Balse E, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2013 Mar 22; doi: 10.1093/eurheartj/eht099. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 79.Lee YC, Chang HH, Chiang CL, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–1171. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 80.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 81.Gealekman O, Guseva N, Hartigan C, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Chang L, Villacorta L, Li R, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [This study shows that murine perivascular adipose tissue has features of brown fat and that loss of this depot results in impaired vascular thermoregulation and promotes atherosclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84**.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [In this randomized-controlled trial, a decrease in VAT through omentectomy, alone or in combination with RYGB surgery, did not improve insulin sensitivity in obese patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrera MF, Pantoja JP, Velázquez-Fernández D, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 2010;33:1413–1418. doi: 10.2337/dc09-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dillard TH, Purnell JQ, Smith MD, et al. Omentectomy added to Roux-en-Y gastric bypass surgery: a randomized, controlled trial. Surg Obes Relat Dis. 2011 Oct 19; doi: 10.1016/j.soard.2011.09.027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamboli RA, Hajri T, Jiang A, et al. Reduction in inflammatory gene expression in skeletal muscle from Roux-en-Y gastric bypass patients randomized to omentectomy. PLoS One. 2011;6:e28577. doi: 10.1371/journal.pone.0028577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J, Ye H, Wang Y, et al. Comparative study of laparoscopic sleeve gastrectomy with and without partial enterectomy and omentectomy. Surg Obes Relat Dis. 2012;8:275–280. doi: 10.1016/j.soard.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–142. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lima MM, Pareja JC, Alegre SM, et al. Visceral fat resection in humans: Effect on insulin sensitivity, beta-cell function, adipokines and inflammatory markers. Obesity (Silver Spring) 2012 Sep 14; doi: 10.1002/oby.20030. doi: 10.1002/oby.20030 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Sdralis E, Argentou M, Mead N, et al. A Prospective Randomized Study Comparing Patients with Morbid Obesity Submitted to Sleeve Gastrectomy With or Without Omentectomy. Obes Surg. 2013 Mar 23; doi: 10.1007/s11695-013-0925-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 92.Romaguera D, Norat T, Mouw T, et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J Nutr. 2009;139:1728–1737. doi: 10.3945/jn.109.108902. [DOI] [PubMed] [Google Scholar]
- 93.Romaguera D, Ängquist L, Du H, et al. Food composition of the diet in relation to changes in waist circumference adjusted for body mass index. PLoS One. 2011;6:e23384. doi: 10.1371/journal.pone.0023384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94*.Estruch R, Ros E, Salas-Salvadó J, et al. the PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med. 2013;368:1279–1290. [This is a randomized-controlled trial with 7,447 participants at high risk for cardiovascular disease assigned to two Mediterranean diets or a control diet. The study was terminated prematurely due to the protective effect of the Mediterranean diet on major cardiovascular events.] [Google Scholar]
- 95.de Souza RJ, Bray GA, Carey VJ, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr. 2012;95:614–625. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta- analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68–91. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 97.Vissers D, Hens W, Taeymans J, et al. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8:e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy JC, McDaniel JL, Mora K, et al. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol. 2012;112:79–85. doi: 10.1152/japplphysiol.00355.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hunter GR, Brock DW, Byrne NM, et al. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity (Silver Spring) 2010;18:690–695. doi: 10.1038/oby.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007 Aug;293(2):E444–52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 101.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren PO, Mori MA, Molla M, Tseng YH. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013 May;19(5):635–9. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012 Jul 20;150(2):366–76. doi: 10.1016/j.cell.2012.05.016. doi: 10.1016/j.cell.2012.05.016. Epub 2012 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F FDG-detected BAT in humans. J Clin Endocrinol Metab. Jan. 2011;96(1):192–9. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 104.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–E1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 105.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. Feb 1. 2013;27(3):234–50. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]