Abstract
Introduction
A higher prevalence of individuals affected by Parkinsonism was found in Valcamonica, Italy. This may be related to ferro-alloy smelters in the area, releasing manganese (Mn) in the air, soil and water for about a century. There exists individual susceptibility for Mn neurotoxicity.
Aim
To analyse how polymorphism in genes regulating Mn metabolism and toxicity can modify neurophysiological effects of Mn exposure.
Materials and Methods
Elderly (N=255) and adolescents (N=311) from Northern Italy were examined for neuromotor and olfactory functions. Exposure to Mn was assessed in blood and urine by atomic absorption spectroscopy and in soil by a portable instrument based on X-Ray fluorescence technology. Polymorphisms in the Parkinson-related gene ATPase type 13A2 (ATP13A2, also called PARK9: rs3738815, rs2076602, rs4920608, rs2871776, rs2076600), and in the secretory pathway Ca2+/ Mn2+ ATPase isoform 1 gene (SPCA1: rs218498, rs3773814, rs2669858) were analysed by TaqMan probes.
Results
For both adolescents and elderly, negative correlations between Mn in soil and motor coordination (Rs=−0.20, p<0.001, Rs=−0.13, p=0.05 respectively) were demonstrated. Also among adolescents, negative correlations were seen between Mn in soil with odor identification (Rs=−0.17, p<0.01). No associations were seen for Mn in blood or urine. ATP13A2 polymorphisms rs4920608 and rs2871776 significantly modified the effects of Mn exposure on impaired motor coordination in elderly (p for interaction= 0.029, p= 0.041 respectively), also after adjustments for age and gender. The rs2871776 altered a binding site for transcription factor Insulinoma-associated 1.
Conclusions
ATP13A2 variation may be a risk marker for neurotoxic effects of Mn in humans.
Keywords: SPCA1, Parkinson, motor, odor, tremor, gene-environment, neurophysiological, ferro-manganese
1. Introduction
Although being an important essential element, manganese (Mn) is also a recognized neurotoxic metal. This has been shown for more than one century in the occupational literature starting from sir James Couper (1837) and is now assessed extensively also in the general population. Studies on adolescents exposed to high Mn in drinking water (Bouchard et al., 2011) and airborne particulate (Riojas-Rodríguez et al., 2010, Menezes-Filho et al., 2011) have shown impairment of cognitive functions. Adults with environmental exposure to Mn have shown motor impairment regarding position changes in hand movements (Rodríguez-Agudelo et al., 2006). Impairment of postural balance was also observed across different age groups of an environmentally exposed population (Standridge et al., 2008).
Similar to other elements, such as arsenic, lead and beryllium (Nordberg et al., 2007; Scinicariello et al., 2007; Engström, 2011) the genetic background of an individual probably determines Mn metabolism (Curran et al., 2009) and Mn toxicity, but so far the susceptibility genes for Mn are unknown. On the other hand, the genetics of juvenile Parkinson may provide information for Mn susceptibility. Genetic mutations play an important role in causing juvenile form of Parkinsonism, and one of the target genes is ATP13A2 (Myhre et al., 2008). ATP13A2 belongs to a large group of lysosomal transport proteins in the 5-P type ATPase family. The exact function of ATP13A2 is still unclear, but there is evidence that this protein is involved in the transport of multiple cations (Mn, nickel, cadmium and selenium) from the cytosol to the lysosomal lumen (Schmidt et al., 2009; Santoro et al., 2011). Dopaminergic loss caused by α-synuclein overexpression in animal and neuronal Parkinson disease model is avoided by co-expression of ATP13A2, and a yeast ortholog of ATP13A2 helps to protect cells from Mn toxicity (Gitler et al., 2009). These findings suggest that reduced function, such as caused by polymorphisms, of ATP13A2 is associated with more Mn toxicity and less protection against Parkinsonian changes.
Secretory pathway Ca2+/ Mn2+ ATPase isoform 1 (SPCA1: encoded by ATP2C1) has become known as a Ca2+/ Mn2+ transporter pump, and is localized in the membrane of the Golgi apparatus in mammalian cells (Murín et al., 2006). The Golgi apparatus participates in Ca2+ and Mn2+ transport from cytosol to the Golgi lumen (Sepúlveda et al., 2009). These cations are used for specific luminal processes such as protein sorting and endosome fusion (Dode et al., 2006). SPCA1 plays an important role in Mn2+ homeostasis and is the only known P-type ATPase, which with high affinities can transport Mn2+. Silencing of SPCA1 results in the deficiency of Ca and/or Mn uptake, which impairs Ca2+ homeostasis inside the Golgi stores and disturbs alteration of neuronal polarity (Sepúlveda et al., 2009).
The purpose of this study was to analyse, in a population of adolescents and elderly from Northern Italy, how genetic variation in ATP13A2 and SPCA1 modifies neurotoxic effects of environmental exposure to Mn.
2. Materials and methods
2.1 Study population
This cross-sectional study comprises a population of 311 adolescent boys and girls (11–14 years), and 255 elderly men and women (63–80 years) from Italy with varying Mn exposure through ferroalloy foundries in the area. We considered here adolescents and elderly as potentially susceptible population strata to Mn exposure. Two different areas of the province of Brescia in Italy have been studied: the Valcamonica valley as exposed area, and the Garda Lake as reference area. Three ferroalloy plants have been operating in Valcamonica for a century until 2001. The emission in the atmosphere, soil and water of this area has caused contamination with various metals including Mn, lead, iron, chromium, zinc, and copper that has overlapped the naturally occurring geological composition. Comprehensive assessment of environmental exposure has been conducted on deposited dust (Zacco et al., 2009) and airborne particles that are still present due to re-suspension related to car traffic and construction work (Borgese et al., 2011). Further description of the methodology concerning neuropsychological testing and exposure assessment will be available in a separate publication (Lucchini et al., 2011).
All subjects underwent an extended assessment of neurological testing on motor, cognitive sensory and behavioral functions. None of the subjects was a confirmed patient or had a medical-proved diagnosis of Parkinson’s disease-like syndromes. Data were collected also for alcohol-drinking (estimated g per week) and smoking habit (number of cigarettes per day). All blood samples were collected in EDTA vacutainers (Sarstedt monovette) during the same week of the neurological testing and stored at −20°C for subsequent DNA extraction and analysis.
2.2 Exposure assessment
Mn was measured in blood and urine by atomic absorption spectroscopy at the Laboratory of Industrial Toxicology of the University of Brescia. Due to the strong homeostatic regulations of Mn in the body, it is difficult to assess the exposure to Mn with human biomarkers. Soil is considered as a potential indicator of cumulative exposure to heavy metals and has been observed as predictive of neurological effects in children (Liu et al., 2010). Thus, environmental exposure to manganese in these individuals was also assessed by the concentration of Mn in surface soil. Concentrations were analyzed with a portable instrument based on X-Ray Fluorescence (XRF) (Thermo Scientific Niton, model XLt) equipped with GPS geo-referencing capability. Kringing GIS interpolation was used to estimate the soil levels for the houses without family gardens.
2.3 Neuropsychological testing
Motor coordination, odor identification, tremor, and sway intensity was examined in the study populations. Motor coordination was explored with five subtests of the Luria Nebraska Motor Battery (Golden et al., 1980): dominant hand clench, non dominant hand clench, alternative hand clench, finger-thumb touching with dominant hand, finger-thumb touching with non dominant hand. In the first set, the subject had to close and open, as fast as possible, the dominant and non-dominant hand and also the two hands alternatively. In the second set, the subject had to touch thumb (of dominant and non-dominant hand) with each finger as fast as possible (each exercise took 10 seconds). We used the Sniffin’ Sticks-Olfactory test-Screening 12 to assess olfactory identification. The subject smell the tip of 12 fiber sticks filled with different scents and must identify the odors (e.g. coffee, orange) from a list (Hummel et al., 2001). Tremor was assessed as the subject held a stylus for about 10 seconds. The hand vibrations were recorded and displaced in a time axis plot on the computer screen (Tremor 7.0 DPD, Denmark). The accelerations were analyzed by methods drawn from vibrations measurements. Body sway (motor-balance) was recorded by a balance plate, producing signals from three sensors to map the position of the force centre during the test period. This centre is defined as the center of equilibrium of the three vertical forces, recorded at the three supports of the sway plate. During the test, subject stands erect on the sway plate, which is visualized on the computer screen, where the journey of the force centre can be observed in an X-Y coordinate system.
2.4 Genotype analyses
TagSNPs that carry the information about the genetic variation in a larger segment of the gene were selected from ATP13A2 and SPCA1, using the CEPH population as a reference and the Hapmap data (www.hapmap.org). The selected polymorphisms in ATP13A2 were rs3738815 (C_2190590_20: assay number for polymorphism in the TaqMan® SNP Genotyping Assays of Applied Biosystems, Foster city, CA, USA), rs2076602 (C_15866733_10), rs4920608 (C_2190586_10), rs2871776 (C_2190583_10) and rs2076600 (C_15866734_10). The three SNPs studied in SPCA1 were rs218498 (C_2966605_10), rs3773814 (C_27482612_10), and rs2669858 (C_16051815_10).
DNA was extracted from whole blood by QIAmp DNA mini kit (Qiagen, Heidelberg) according to the instructions of the manufacturer. SNPs for ATP13A2 and SPCA1 were analysed by TaqMan® allelic discrimination assay (ABI 7900 instrument, Applied Biosystems, Foster City, CA, USA). The reaction volume was 10 μL, and the reaction consisted of 1X TaqMan® Universal Master Mix (Applied Biosystems), 1 X TaqMan® SNP Genotyping Assay Mix, and 8 ng DNA). In each run, negative controls were included. Genotyping analysis was repeated for 5 % of individuals with 100% concordance.
2.5 Statistics
Hardy-Weinberg equilibrium (HWE) analysis was undertaken using the χ2-test. In order to evaluate the associations between Mn exposure markers and various neurobehavioral functions, we performed a Spearman r correlation. We chose associations between Mn exposure markers and outcome that were strongest for further evaluation of genetic effect modification by multivariate linear regression analysis. The multivariate model was run with a cross product interaction term between Mn in soil and genotype. If no effect modification was to be seen in the interaction model, the model was performed without the interaction term in order to assess main effects. When the number of variant homozygotes was very few this group was combined with the heterozygous carriers. All statistical analyses were performed using the software PASW (Statistical Packages for Social Sciences, version 18; SPSS, Chicago, IL USA). A p-value <0.05 denotes statistical significance. Bioinformatic analysis was performed for potential disruption of transcription factor binding sites by the programme from Genomatix (www.genomatix.de).
3. Results
Demographic information of the study population and exposure markers are presented in Table 1. The exposure markers in blood and urine showed that Mn in blood was similar, but Mn in urine was higher in elderly compared to the adolescents. For the elderly, the exposure marker Mn in soil (MnS) was negatively associated with Mn in blood (MnB; Spearman r correlation Rs=−0.136, P = 0.036), but no significant associations were found for MnS with MnU (Rs =−0.033, P = 0.61), or MnB with MnU (Rs=0.12, P = 0.076). Among adolescents no significant associations were found between the exposure markers (MnS with MnB Rs=0.009, P = 0.88; MnS with MnU Rs=0.094, P = 0.10; MnB with MnU Rs=0.094, P = 0.10).
Table 1.
Descriptive characteristics and exposure data for the two study populations a) elderly and b) adolescents.1
| Variable | Distribution | Rs2 Motor coordination | Rs2 Odor | Rs2 Sway Velocity CE | Rs2 Tremor Intensity L/R | Rs with Tremor Centre frequency L/R |
|---|---|---|---|---|---|---|
| a) Elderly | ||||||
| Age3 (Y) | 69 (63–80) | −0.11 | −0.17* | 0.22** | −0.018 / −0.079 | −0.084 / −0.15* |
| Women/ Men (N) | 140/115 | 0.14* | −0.17* | 0.34** | 0.30** / 0.36** | 0.27** / 0.26** |
| Smoking (N:Never/Yes /Ex) | 172/20/63 | 0.091 | −0.078 | 0.098 | 0.12 / 0.064 | 0.02 / 0.046 |
| Alcohol intake3 (g/day) | 12.8 (0–205) | 0.014 | −0.075 | 0.21* | 0.15* / 0.18* | 0.11 / 0.11 |
| MnS3 (ppm) | 786 (313–1724) | −0.13* | −0.093 | −0.048 | −0.11 / −0.035 | −0.027 / 0.026 |
| MnB3 (μg/L) | 9 (3.6–22) | −0.002 | −0.027 | 0.028 | −0.086 / −0.047 | 0.030 / −0.11 |
| MnU3 (μg/L) | 0.14 (0.06–12) | −0.048 | −0.017 | 0.022 | −0.080 / −0.15* | −0.12 / −0.089 |
| Motor coordination3 (N of correct tasks) | 11 (5–18) | 0.17* | −0.037 | 0.048 / 0.062 | 0.056 / 0.072 | |
| Odor identification3 (N of correct recognitions) | 9 (0–12) | −0.11 | −0.027 / −0.017 | 0.002 / −0.030 | ||
| Sway Velocity CE3 | 15 (3–74) | 0.27** / 0.35** | 0.12 / 0.18* | |||
| b) Adolescents | ||||||
| Age3 (Y) | 12 (11–14) | 0.11* | 0.20** | −0.083 | −0.17* / −0.14* | 0.11 / 0.095 |
| Girls/ Boys (N) | 153/158 | −0.048 | −0.16* | 0.22** | 0.26** / 0.29** | 0.11 / 0.16* |
| MnS3 (ppm) | 529 (160–1730) | −0.20** | −0.17* | 0.065 | −0.066 / −0.027 | −0.021 / 0.027 |
| MnB3 (μg/L) | 11 (4–24) | 0.020 | 0.082 | −0.071 | 0.062 / 0.065 | −0.057 / −0.010 |
| MnU3 (μg/L) | 0.08 (0.05 – 5.4) | −0.10 | −0.022 | −0.075 | −0.098 / −0.083 | −0.095 / −0.018 |
| Smoking (N) (Yes/No) | 3/3014 | |||||
| Alcohol intake (N) (Yes/No) | 9/295 | 0.006 | −0.098 | 0.001 | −0.038 / −0.053 | 0.13* / 0.074 |
| Motor coordination3 (N of correct tasks) | 13 (6–21) | 0.080 | −0.010 | −0.069 / 0.009 | 0.11 / −0.014 | |
| Odor identification1 (N of correct recognitions) | 10 (4–12) | −0.032 | −0.048 / −0.074 | 0.068 / 0.093 | ||
| Sway Velocity CE3 | 14 (2–43) | 0.27** / 0.24 | −0.051 / −0.051 | |||
Abbreviations: MnS; manganese in soil, MnB; Mn in blood, MnU; Mn in urine adjusted for mean density 1.020, CE; Closed Eyes.
Spearman r correlations (Rs) between characteristic variables and Mn measurements on the one hand and neurophysiological testing on the other:
denotes p<0.05;
p<0.001.
Median and range are given.
too few smokers to calculate correlation
Median scores and range of motor coordination test, sway, tremor and odor identification of the two study groups are shown in the Table 1. Age influenced negatively the olfactory function and sway in the elderly, whereas motor and olfactory functions improved with age among the adolescents. A gender influence was found in both age groups, with males showing higher tremor intensity, centre frequency and body sway, and decreased odor identification compared to females. Elderly men had better motor coordination than women. Smoking habits did not influence the health outcomes in elderly, whereas alcohol intake increased tremor intensity and sway among the elderly and centre frequency of the left hand among adolescents. There were too few smokers among the adolescents to calculate correlation coefficients. Excluding those adolescents that had not clearly indicated that they neither consumed alcohol nor tobacco (N=18) did not change our findings.
The relationship between Mn in urine, blood and soil and neurophysiological outcomes was investigated using Spearman r correlation coefficient (Table 1). There was a negative correlation between Mn in soil and motor coordination for both adolescents and elderly and odor identification for adolescents. Mn in soil demonstrated a stronger correlation with the neurological outcomes compared with the measured data of blood and urine. Thus, the further analysis on genetic effect modification focused on Mn in soil as an exposure marker for Mn exposure.
The genotype frequencies for ATP13A2, and SPCA1 were all in Hardy Weinberg equilibrium (data not shown). The allelic frequencies were almost the same in elderly and adolescents (Table 2). The allele frequencies were very similar to those in a European population (CEU population: from http://www.ncbi.nlm.nih.gov/SNP) (< 17 % difference).
Table 2.
Allelic frequencies for ATP13A2 and SPCA1 among adolescents and elderly in Northern Italy, as compared to European populations.1
| ATP13A2 | Adolescents % | Elderly % | European population % |
|---|---|---|---|
| rs3738815 | |||
| A | 26 | 25 | 14 |
| G | 74 | 75 | 86 |
| rs2076602 | |||
| A | 74 | 75 | 86 |
| T | 26 | 25 | 14 |
| rs4920608 | |||
| C | 44 | 42 | 33 |
| T | 56 | 58 | 67 |
| rs2871776 | |||
| A | 47 | 49 | 50 |
| G | 53 | 51 | 50 |
| rs2076600 | |||
| C | 65 | 66 | 82 |
| T | 35 | 34 | 18 |
| SPCA1 | |||
| rs218498 | |||
| A | 63 | 63 | 63 |
| G | 37 | 37 | 37 |
| rs3773814 | |||
| A | 85 | 86 | 85 |
| C | 15 | 14 | 15 |
| rs2669858 | |||
| C | 16 | 18 | 16 |
| T | 84 | 82 | 84 |
Compared to the CEU + TSI population: from http://www.ncbi.nlm.nih.gov/SNP.
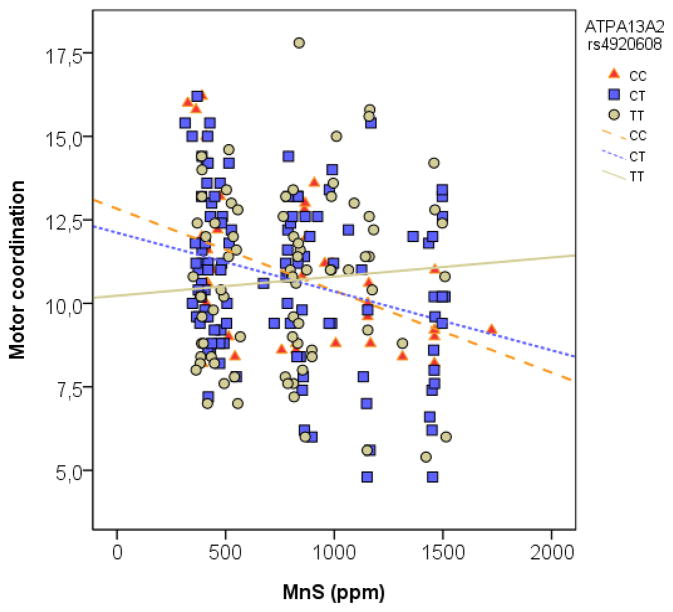
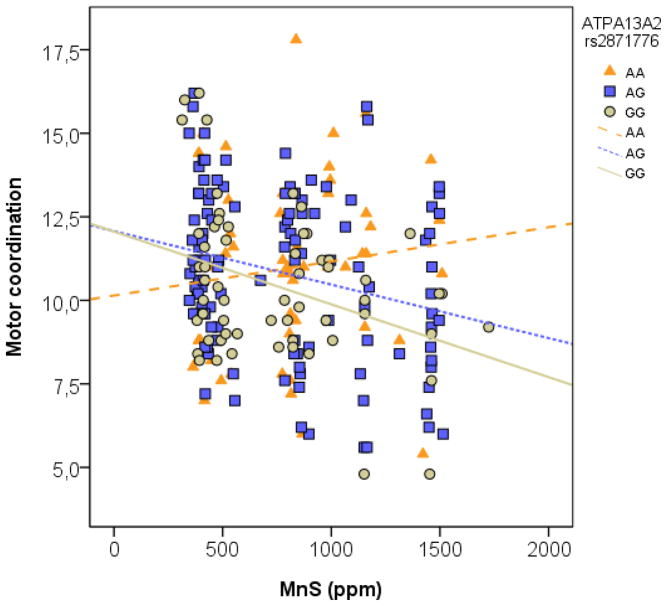
Polymorphisms in rs4920608 and rs2871776 in ATP13A2 significantly modified the association between Mn in soil and motor coordination in elderly (p=0.029, p=0.041 respectively; Table 3, Figures 1 & 2). This was still significant when adjusted for age and gender (p=0.032, p=0.044). Smoking and alcohol consumption did not contribute significantly to the adjusted model and were therefore excluded. None of the polymorphisms alone had a significant influence on motor coordination (Supplemental Material, Table 1) only in combination with Mn exposure as shown in Table 3. Figure 1 shows that with increasing Mn exposure, the rs4920608 CT and CC carriers had a decreased motor coordination, compared with TT carriers. In Figure 2, motor coordination was reduced for rs2871776 GG and GA carriers, compared to the AA individuals with increasing Mn exposure. There was modest linkage disequilibrium between the two polymorphism [r2=0.48 in the CEU (Utah residents with Northern and Western European ancestry from the CEPH collection) + TSI (Tuscan in Italy) populations from the Hapmap database (www.hapmap.org)].
Table 3.
Effect modification of ATP13A2 genotype on the association between manganese (Mn) in soil and motor function (Luria Nebraska Test).1
| Gene/ ATP13A2 | Coefficient2 (95% CI) | P-value interaction | Coefficient3 (95% CI) | P-value interaction |
|---|---|---|---|---|
| Elderly
| ||||
| rs4920608 | 0.029 | 0.032 | ||
| TT | −0.00057 (−0.0012; 0.0023) | 0.00044 (−0.0014; 0.0022) | ||
| CT | −0.0018 (−0.0029; −0.00061) | −0.0020 (−0.0032; −0.00085) | ||
| CC | −0.0025 (−0.0042; −0.00076) | −0.0024 (−0.0040; −0.00077) | ||
| rs2871776 | 0.041 | 0.046 | ||
| AA | 0.0010 (−0.0012; 0.0033) | 0.00073 (−0.0013; 0.0028) | ||
| AG | −0.0016 (−0.0027; −0.00047) | −0.0019 (−0.0030; −0.00071) | ||
| GG | −0.0022 (−0.0037; −0.00068) | −0.0022 (−0.0037; −0.00070) | ||
|
| ||||
| Adolescents
| ||||
| rs4920608 | 0.69 | 0.60 | ||
| TT | −0.0021 (−0.0036; 0.00060) | −0.0023 (−0.0038; −0.00073) | ||
| CT | −0.0013 (−0.0025; −0.000081) | −0.0012 (−0.0024; 0.000034) | ||
| CC | −0.0016 (−0.0039; 0.00071) | −0.0015 (−0.0038; 0.00080) | ||
| rs2871776 | 0.20 | 0.17 | ||
| AA | −0.0030 (−0.0048; −0.0012) | −0.0031 (−0.0049; −0.0013) | ||
| AG | −0.0011 (−0.0022; 0.00080) | −0.0011 (−0.0023; 0.00001) | ||
| GG | −0.0011 (−0.0030; 0.00070) | −0.00088 (−0.0029; 0.0011) | ||
The model was run for Motor function as outcome and with manganese in soil, genotype and a cross-product term for Mn in soil and genotype. The p-value for the cross term product is shown. In order to obtain effect estimates (beta-coefficients) for the effect of the genotypes, the analysis with motor function as outcome and Mn in soil as dependent variable (beta coefficient given) was run but stratified for the different genotypes.
Unadjusted analysis.
Analysis adjusted for age and gender.
Figure 1.
Motor coordination as a function of manganese in soil (MnS). The simple regression was made to illustrate the slope of the different genotypes.
Figure 2.
Motor coordination as a function of manganese in soil (MnS). The simple regression was made to illustrate the slope of the different genotypes.
There was no genetic effect modification among adolescents for ATP13A2. Also, there was no effect of SPCA1 in neither group on the association between Mn and neurophysiological outcomes.
Bioinformatic analysis revealed that the ATP13A2 rs2871776 G allele deleted a binding site for the transcription factor Insm1 (Insulinoma-associated 1). No binding site was modified by the different alleles of the rs4920608 SNP.
4. Discussion
This study reveals impairment of motor function in individuals living in the vicinity of ferroalloy emissions and associated with the Mn content in deposited dust, which is particularly relevant in an area were previous research has shown a high prevalence of Parkinsonism, (Lucchini et al., 2007). In addition to exposure-related changes of neurological functions, this is the first study to show that genetic predisposition can further amplify these effects in susceptible individuals. Common genotypes of two ATP13A2 polymorphisms were associated with reduced motor coordination of Mn, but only among the elderly. Hypothetically, the genetic profile causes increased risk only after long-term exposure, such as in elderly long-term residents from this region.
There are some limitations in this study. One concern is the marker of Mn exposure. We here analysed the interrelation between Mn exposure and motor-sensory functions with different exposure biomarkers for Mn, with the strongest effect seen for soil Mn and motor coordination, as assessed with the Luria Nebraska motor test, and odor identification. Soil can also be considered as a suitable indicator of lifetime and cumulative exposure to metals that are stable and long-lived in the environment, and accumulate in soils over time (Aelion et al., 2009). This is particularly important for Mn that causes long-term effects on the nervous system with a cumulative mechanism of toxicity (Lucchini and Zimmerman, 2009). Few studies have considered soil in the assessment of health effects due to the environment. Liu et al. (2010) have found an association between arsenic in soil and mental retardation in adolescents. Soil may be able to integrate different exposure sources (airborne particles, water, foods) and absorption routes in a longitudinal fashion. This may lead to the assessment of the total body load of chemicals like metals that can accumulate in the organism and especially in the brain, causing long-term effects. However, limited information is available in the literature on soil background levels of heavy metals specific to these study regions. It is likely that there are no associations between B-Mn or U-Mn and neuromotor functions as Mn is also an essential element and thus, there are strict homeostatic mechanisms for the regulation of the levels of Mn in blood and in urine. We assume that the exposure route to the central nervous system is from inhaled dust through absorption and translocation in the olfactory nerve (Lucchini et al., 2011), which may explain the positive finding between Mn in soil and motor coordination.
A further concern is that multiple comparisons were made in this study; some of the reported associations may be false positive and the results of ATP13A2 need to be verified. Also, other genes involved in Mn metabolism and toxicity are probably important. For example, there is in vitro evidence that the wildtype of Parkinson-associated mutation G2019S of leucine-rich repeat kinase 2 (PARK8) has a dramatically reduced catalytic activity in the presence of Mn while the catalytic activity of the variant is unchanged (Lovitt et al., 2010; Covy and Giasson, 2011; Covy and Giasson, 2010).
The protective role of ATP13A2 of the toxicity induced by alpha-synuclein in animal models of Parkinson disease, as well as the toxic effects of Mn exposure (Gitler et al., 2009) suggest that polymorphisms in this gene may render susceptibility to Parkinson disease and Mn toxicity. Our study lends some support to this notion. Both rs4920608 and rs2871776 SNPs are located in intron regions of ATP13A2: rs2871776 is located in the upper half of the gene and rs4920608 towards the end of the gene, but the functional impacts of both SNPs are still unknown. The SNPs included in this study were selected because they are tagging genetic variation for larger segments of the ATP13A2 gene. Their role in parkinsonism is still unknown apart from rs3738815, which was not associated with Parkinson disease (Vilarino-Guell et al., 2009), in a candidate gene study of ATP13A2. The SNPs in our study have not been been identified as parkinsonism-susceptibility markers in genome-wide associations studies (Fung et al., 2006, Maraganore et al., 2005).
The rs2871776 G allele that was associated with adverse effect of manganese on motor coordination was associated with destroying a binding site for the transcription factor INSM1 that plays an important role in the developing central nervous system as shown in mouse (Farkas et al., 2008; Rosenbaum et al., 2011) and human embryos (Duggan et al., 2008). In adult tissues the expression seems to be limited to areas of neurogenesis (the hippocampus, rostral migratory pathway and the olfactory bulb) (Duggan et al., 2008). Absence of the binding site for this transcription factor on the ATP13A2 gene hypothetically might explain why adult carriers in our population perform more poorly in the motor coordination test upon Mn exposure.
In conclusion, this study showed early effects (i.e. below the threshold for the definition of clinical effects) on motor coordination and odor identification associated with lifetime environmental exposure to Mn. Further progression of reduced motor coordination(Almeida et al., 2002), and olfactory impairment (Haehner et al., 2009) are seen in Parkinson disease. The effect of Mn exposure on the nervous system was, in the elderly, influenced by genetic polymorphism of PARK9, a susceptibility gene for Parkinson disease. Further studies are warranted for elucidating mechanisms for susceptibility to Mn exposure.
Supplementary Material
Acknowledgments
The authors would like to thank Mrs. Karin Paulsson for help with genotyping. This study was supported by funding from the European Union through its Sixth Framework Programme for RTD (contract no FOOD-CT-2006-016253). It reflects only the authors’ views, and the European Commission is not liable for any use that may be made of the information contained therein. The project described was supported also by Award Number R01ES019222 from the National Institute Of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Conflict of interest: none declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aelion CM, Davis HT, McDermott S, Lawson AB. Soil metal concentrations and toxicity: associations with distances to industrial facilities and implications for human health. Sci Total Environ. 2009;407:2216–23. doi: 10.1016/j.scitotenv.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida QJ, Wishart LR, Lee TD. Bimanual coordination deficits with Parkinson’s disease: the influence of movement speed and external cueing. Mov Disord. 2002;17:30–7. doi: 10.1002/mds.10030. [DOI] [PubMed] [Google Scholar]
- Borgese L, Zacco A, Pal S, Bontempi E, Lucchini RG, Zimmerman NJ, Depero LE. A new non-destructive method for chemical analysis of particulate matter filters: The case of manganese air pollution in Vallecamonica (Italy) Talanta. 2011;84:192–198. doi: 10.1016/j.talanta.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–43. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. British Annals of Medicine and Pharmacology. 1837;1:41–42. [Google Scholar]
- Covy JP, Giasson BI. Alpha-Synuclein, leucine-rich repeat kinase-2, and manganese in the pathogenesis of parkinson disease. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covy JP, Giasson BI. The G2019S pathogenic mutation disrupts sensitivity of leucine-rich repeat kinase 2 to manganese kinase inhibition. J Neurochem. 2010;115:36–46. doi: 10.1111/j.1471-4159.2010.06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CP, Park RM, Ho S-M, Haynes EN. Incorporating genetics and genomics in risk assessment for inhaled manganese: from data to policy. NeuroToxicol. 2009;30:754–760. doi: 10.1016/j.neuro.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode L, Andersen JP, Vanoevelen J, Raeymaekers L, Missiaen L, Vilsen B, et al. Dissection of the Functional Differences between Human Secretory Pathway Ca2+/Mn-ATPase (SPCA) 1 and 2 Isoenzymes by Steady-state and Transient Kinetic Analyses. J Biol Chem. 2006;281:3182–3189. doi: 10.1074/jbc.M511547200. [DOI] [PubMed] [Google Scholar]
- Duggan A, Madathany T, de Castro SC, Gerrelli D, Guddati K, Garcia-Anoveros J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J Comp Neurol. 2008;507:1497–1520. doi: 10.1002/cne.21629. [DOI] [PubMed] [Google Scholar]
- Engström K, Vahter M, Mlakar SJ, Concha G, Nermell B, Raqib R, et al. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ Health Perspect. 2011;119:182–8. doi: 10.1289/ehp.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas LM, Haffner C, Giger T, Khaitovich P, Nowick K, Birchmeier C, et al. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron. 2008;60:40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Fung HC, Scholz S, Matarin M, Simon-Sanchez J, Hernandez D, Britton A, et al. Genome-wide genotyping in Parkinson’s disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 2006;5:911–6. doi: 10.1016/S1474-4422(06)70578-6. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, et al. α-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, Hammeke T, Purish A. Manual for the Luria Nebraska Neuropsychological battery. Western Psychological Services; Los Angeles: 1980. [Google Scholar]
- Haehner A, Hummel T, Reichmann H. Olfactory dysfunction as a diagnostic marker for Parkinson’s disease. Expert Rev Neurother. 2009;9:1773–9. doi: 10.1586/ern.09.115. [DOI] [PubMed] [Google Scholar]
- HapMap – International Human HapMap Project. Available: www.hapmap.org.
- Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function using a 4 minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110:976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- Liu Y, McDermott S, Lawson A, Aelion M. The relationship between mental retardation and developmental delays in adolescents and the levels of arsenic, mercury and lead in soil samples taken near their mother’s residence during pregnancy. Int J Hyg Environ Health. 2010;213:116–123. doi: 10.1016/j.ijheh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovitt B, Vanderporten EC, Sheng Z, Zhu H, Drummond J, Liu Y. Differential effects of divalent manganese and magnesium on the kinase activity of leucine-rich repeat kinase 2 (LRRK2) Biochemistry. 2010;49:3092–3100. doi: 10.1021/bi901726c. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, Bontempi E, Zimmerman NJ, Donald R, Smith DR. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicol. doi: 10.1016/j.neuro.2012.01.005. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara E, et al. High prevalence of parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Zimmerman N. Lifetime cumulative exposure as a threat for neurodegeneration: need for prevention strategies on a global scale. NeuroToxicology. 2009;30:1144–1148. doi: 10.1016/j.neuro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, et al. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77:685–93. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111:156–6. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murín R, Verleysdonk S, Raeymaekers L, Kaplán P, Lehotský J. Distribution of Secretory Pathway Ca2+ ATPase (SPCA1) in Neuronal and Glial Cell Cultures. Cell Mol Neurobiol. 2006;26:1355–1365. doi: 10.1007/s10571-006-9042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre R, Steinkjer S, Stormyr A, Nilsen GL, Zayyad HA, Horany K, Nusier MK, Klungland H. Significance of the parkin and PINK1 gene in Jordanian families with incidences of young-onset and juvenile parkinsonism. BMC Neurol. 2008;8:47. doi: 10.1186/1471-2377-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg GF, Gerhardsson L, Broberg K, Mumtaz M, Ruiz P, Fowler BA. Interactions in Metal Toxicology. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. 3. Academic Press; 2007. pp. 117–145. [Google Scholar]
- Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, Rodríguez-Agudelo Y. Intellectual function in Mexican adolescents living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118:1465–70. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Agudelo Y, Riojas-Rodríguez H, Ríos C, Rosas I, Sabido Pedraza E, Miranda J, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368:542–56. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JN, Duggan A, Garcia-Anoveros J. Insm1 promotes the transition of olfactory progenitors from apical and proliferative to basal, terminally dividing and neuronogenic. Neural Dev. 2011;6:6. doi: 10.1186/1749-8104-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro L, Breedveld GJ, Manganelli F, Iodice R, Pisciotta C, Nolano M, et al. Novel ATP13A2 (PARK9) homozygous mutation in a family with marked phenotype variability. Neurogenetics. 2011;12:33–39. doi: 10.1007/s10048-010-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Wolfe DM, Stiller B, Pearce DA. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem Biophys Res Commun. 2009;383:198–202. doi: 10.1016/j.bbrc.2009.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, Murray HE, Moffett DB, Abadin HG, Sexton MJ, Fowler BA. Lead and delta-aminolevulinic acid dehydratase polymorphism: where does it lead? A meta-analysis. Environ Health Perspect. 2007;115:35–41. doi: 10.1289/ehp.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda RM, Vanoevelen J, Raeymaekers L, Mata AM, Wuytack F. Silencing the SPCA1 (Secretory Pathway Ca2+-ATPase isoform 1) Impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J Neurosci. 2009;29:12174–12182. doi: 10.1523/JNEUROSCI.2014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNP database of National Center of Biotechnology Information. Available: www.ncbi.nlm.nih.gov/snp/
- Standridge JS, Bhattacharya A, Succop P, Cox C, Haynes E. Effect of chronic low level manganese exposure on postural balance: a pilot study of residents in southern Ohio. J Occup Environ Med. 2008;50:1421–9. doi: 10.1097/JOM.0b013e3181896936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Soto AI, Lincoln SJ, Ben Yahmed S, Kefi M, Heckman MG, et al. ATP13A2 variability in Parkinson disease. Hum Mutat. 2009;30:406–10. doi: 10.1002/humu.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacco A, Resola S, Lucchini RG, Albini E, Zimmerman N, Guazzetti S, Bontempi E. Analysis of settled dust with X-ray Fluorescence for exposure assessment of metals in the province of Brescia, Italy. J Environ Monit. 2009;11:1579–85. doi: 10.1039/b906430c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.