Abstract
Dysfunction of the apoptotic pathway in prostate cancer cells confers apoptosis resistance towards various therapies. A novel strategy to overcome resistance is to directly target the apoptotic pathway in cancer cells. Apigenin, an anticancer agent, selectively toxic to cancer cells induces cell cycle arrest and apoptosis through mechanisms which are not fully explored. In the present study we provide novel insight into the mechanisms of apoptosis induction by apigenin. Treatment of androgen-refractory human prostate cancer PC-3 and DU145 cells with apigenin resulted in dose-dependent suppression of XIAP, c-IAP1, c-IAP2 and survivin protein levels. Apigenin treatment resulted in significant decrease in cell viability and apoptosis induction with the increase of cytochrome C in time-dependent manner. These effects of apigenin were accompanied by decrease in Bcl-xL and Bcl-2 and increase in the active form of Bax protein. The apigenin-mediated increase in Bax was due to dissociation of Bax from Ku70 which is essential for apoptotic activity of Bax. Apigenin treatment resulted in the inhibition of class I histone deacetylases and HDAC1 protein expression, thereby increasing the acetylation of Ku70 and the dissociation of Bax resulting in apoptosis of cancer cells. Furthermore, apigenin significantly reduced HDAC1 occupancy at the XIAP promoter, suggesting that histone deacetylation might be critical for XIAP downregulation. These results suggest that apigenin targets inhibitor of apoptosis proteins and Ku70–Bax interaction in the induction of apoptosis in prostate cancer cells and in athymic nude mouse xenograft model endorsing its in vivo efficacy.
Keywords: Prostate cancer, Apigenin, Inhibitor of apoptosis proteins, Apoptosis, Ku70, Histone deacetylase
Introduction
Prostate cancer is one of the most common malignancies in men and is the major cause of death in American men [1, 2]. Localized prostate cancer is eminently treatable; however, no curative therapy is available once the cancer becomes invasive and metastasizes [3, 4]. While prostate cancer initially responds well to androgen deprivation, this treatment results in the emergence of androgen-independent disease that is resistant to apoptosis [5]. A better understanding of this process could lead to the development of novel therapeutic strategies aimed at reducing mortality and morbidity associated with prostate cancer.
In recent years, many cellular factors involved in apoptosis have been identified and their roles in the apoptotic pathway have been elucidated [6, 7]. Initiation of apoptosis occurs through an extrinsic pathway by the interaction of cell death receptors with their ligands or an intrinsic pathway triggered by release of cytochrome C from mitochondria to the cytosol [6]. Both these pathways converge, which results in the activation of caspase proteolytic reaction. Apoptosis is a complex process regulated by several molecules that function as either promoters, including Bax, Bak, and caspases, or inhibitors of the cell death process such as Bcl-2, Bcl-xL, and the IAP family of proteins [7]. Over-expression of IAP proteins, including X-linked inhibitor of apoptosis (XIAP), IAP-1, IAP-2 and survivin, has been demonstrated in clinically derived prostate tumor specimens as well as patient derived cancer cell lines [8–10]. Several members of the IAP family have been shown to bind and directly suppress the activity of certain caspases, thereby suppressing apoptosis [11]. High XIAP expression has been shown to be a strong and independent predictor of prostate cancer recurrence and poor clinical outcome [12]. Similarly, increased survivin has been detected in human prostate cancer and its degree of expression correlates with tumor progression and increased invasiveness [13].
Another mechanism of apoptosis induction is activation of pro-apoptotic protein Bax [14]. In normal cells, Bax interaction with the C-terminus of the Ku70 protein has been shown to suppress apoptosis by sequestering it from the mitochondria [15, 16]. Ku70 is post-transcriptionally regulated by acetylation of the lysine residues in the C-terminal linker domain, resulting in release of Bax. Acetylation of Ku70 is regulated by histone acetyltransferase and histone deacetylase, the two opposing group of enzymes primarily involve in chromatin remodeling [17, 18]. Recent reports indicate a positive relationship between Ku70 and cancer progression and resistance to various therapies, highlighting Ku70–Bax interaction as an important therapeutic target [19].
Bioactive food components continue to gain attention for chemoprevention and/or therapy of prostate cancer [20–22]. Apigenin (4,5,7-trihydroxyflavone) is one such plant flavone under active investigation as an anticancer agent for prostate cancer as well as other type of malignancy, including cancers of colon, skin, lung and breast [23–26]. Mechanistic studies have suggested that apigenin exerts chemoprotective effects by blocking multiple signal transduction pathways such as NF-κB, IGF-I axis, PI3K-Akt, HIF-1α and β-catenin in various experimental models of prostate cancer [27–31]. Studies have shown that apigenin-induced prostate cancer cell death is initiated by generation of reactive oxygen species and p53 activation [32]. Apigenin has been shown to modulate MAPK, PI3K-Akt and loss of cyclin D1 associated with dephosphorylation of the tumor suppressor, retinoblastoma [33]. Apigenin inhibits focal adhesion kinase and Src kinase, resulting in decreased cell motility and cytoskeleton remodeling in invasive prostate cancer cells [34]. We have previously demonstrated that apigenin treatment of prostate cancer cells causes cell growth inhibition and arrest in the G0/G1 phase of the cell cycle, a function that was associated with increased levels of p21/waf1 and Bax proteins [27]. Additionally, we found evidence for the loss of Bcl-2 expression and increased multi-caspase activity, resulting in apoptosis [35]. More recently we have shown that apigenin inhibits class I HDACs in prostate cancer cells [36]. Based on these findings we sought to determine the role of IAP family proteins and Ku70–Bax interaction in apigenin-induced apoptosis using androgen-refractory human prostate cancer PC-3 and DU145 cells and PC-3 xenograft in athymic nude mice.
Materials and methods
Cell culture and reagents
Androgen refractory human prostate cancer cell lines PC-3 and DU145 were purchased from American Type Culture Collection. These cancer cell lines were cultured as monolayers in RPMI 1640 supplemented with 5 % heat-inactivated fetal bovine serum and 100 μg/ml streptomycin (Cellgro, VA) and maintained in an incubator with a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C. Apigenin (A.G. Scientific, Inc., CA), embelin (Biomol International, PA), trichostatin A (TSA) (Sigma-Aldrich, MO), pan-caspase Inhibitor; Z-VAD-FMK, caspase-9 Inhibitor; Z-LEHD-FMK, caspase-3 Inhibitor; Z-DEVD-FMK purchased from BioVision, CA were added to the complete cell culture medium (maximum concentration of DMSO, 0.1 % (v/v) in medium) before addition to subconfluent cells (60–70 % confluent). Cells treated only with DMSO served as a vehicle control. The following antibodies anti-XIAP (cat# 2045), anti-c-IAP1 (cat# 4952), anti-c-IAP2 (cat# 3130), anti-survivin (cat# 2808) anti-Bcl-XL (cat# 2762), anti-Bad (cat# 9292), anti-cleaved caspase-3 (cat# 9661), anti-caspase-9 (cat# 9501), anti-cleaved PARP (cat# 9544) were obtained from Cell Signaling, Beverly, MA whereas anti-BCl2 (cat# sc7382), anti-Bax (cat# sc493), anti-p21/waf1 (cat# sc817), anti-CBP (cat# sc583), anti-HDAC1 (cat# sc7872), anti-cytochrome-C (cat# sc13560) were procured from Santa Cruz Biotechnology, Santa Cruz, CA. Anti-Ku70 (H-308) (cat# sc9033) was a kind gift from Dr. Shigemi Matsuyama, Department of Pharmacology, Case Western Reserve University, Cleveland, OH.
Cell viability assay
The effect of apigenin on cell proliferation was determined by MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazoliumbromide] assay and growth inhibition was assessed as the percent viability where vehicle-treated cells were taken as 100 % viable as previously described [36].
HDAC activity assay
Histone deacetylases (HDACs) activity was measured in PC-3 whole cell lysate after apigenin treatment at various times, according to the vendor's protocol using colorimetric HDAC activity assay kit (cat # #K331-100) from BioVision, CA.
Immunoprecipitation
PC-3 cells, after treatment with apigenin and TSA for 24 h, were lysed in buffer containing 50 mM Hepes pH 7.4, 60 mM KCl, 15 mM NaCl, 0.65 mM spermidine, 0.34 M saccharose, 2 mM EDTA, 0.5 mM EGTA, 0.5 mM DTT, 0.05 % Triton-X and cocktail protease inhibitors (Roche USA, Madison, WI). Cells were incubated on ice for 15 min and then sonicated at 250 J. Preclearing of proteins were performed using 20 μl of AG-Plus agarose beads (Santa Cruz) with 200 μg of protein extracts. Immunoprecipiation was performed using 200 μg of protein extracts incubated for 6 h with 5 μg Ku70 antibody and AG-Plus Agarose. Further three times washing of this complex was performed with the same lysis buffer by spinning at 3,000 rpm for 10 min. Western blot of pelleted complex was performed and transferred nitrocellulose membrane was probed with acetylated lysine and Bax antibody to show the association between the Ku70 and Bax and the levels of acetylated lysine after apigenin treatment.
HDAC1 small interfering RNA transfection of PC-3 cells
Human-specific HDAC1 small interfering RNA (siRNA) was transfected into PC-3 cells using Fugene 6 Transfection Reagent Kit (Cat# 11814443001; Roche, Indianapolis, IN) according to the manufacturer's protocol. Briefly, 2 × 105 cells per well were seeded in a 6-well plate and allowed to grow until 70 % confluence. The HDAC1 siRNA mix with transfection reagents were overlaid on cells for 6 h at 37 °C and transferred into growth medium for 18–20 h. At 24 h post-transfection, fresh medium was added to the cells, and at 48 h post-transfection, cells were harvested and analyzed for viability using MTT assay. Knockdown of HDAC1 after transfection was confirmed using Western blot analysis.
Western blot analysis
PC-3 and DU145 cells were treated with or without apigenin for various doses (5–40 μM) and times, the cells were harvested, washed with cold PBS, and lysed with ice-cold lysis buffer supplemented with protease inhibitors as detailed previously [35, 36]. For immunoblotting, proteins (20–35 μg) were resolved on 4–20 % Trisglycine gels and transferred onto a nitrocellulose membrane. After blocking the nonspecific binding sites, the membrane was incubated overnight with the primary antibody at 4 °C overnight. The membrane was then incubated with the appropriate horse-radish peroxidase-conjugated secondary antibody for 2 h at room temperature and the immunoreactive bands were visualized using the enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ). Each membrane was then stripped and reprobed with anti-β-actin antibody to verify equal protein loading.
Chromatin Immunoprecipitation (ChIP) assay
PC-3 cells were treated with 20 μM concentration of apigenin for 48 h and then cells were incubated in media containing 1 % formaldehyde for 15 min at room temperature for cross-linking. The reaction was then terminated using a 0.125 M final concentration of glycine. After cross-linking, cells were lysed, chromatin was digested using monococcal nuclease enzyme and incubated with anti-acetylated histone H3 (Lys373), anti-CBP, anti-p300 and anti-HDAC1 antibodies overnight at 4 °C. After reversing the cross-linking by incubating the samples at 65 °C overnight, DNA was purified using phenol–chloroform-isoamyl reagent followed by ethanol precipitation. DNA was then dissolved in nuclease-free water. Immunoprecipitated DNAs, beads or input controls were subjected to PCR amplification for 30 cycles of the following cycling conditions: stage 1, 95 °C for 2 min (1 cycle); stage 2, 95 °C for 30 s and 60 °C for 30 s and 72 °C for 1 min (30 cycles); and stage 3, 72 °C for 3 min (1 cycle). Primers used were as follows: XIAP forward primers TTTTACTTTATGACTTGAATGATGTGG and XIAP reverse primers TTCCTTATTGATGTCTGCAGGT. PCR products were subjected to electrophoresis using a 2 % agarose gel.
Apoptosis by ELISA
Apoptosis was assessed by M30-ApoptosenseTM ELISA kit (Alexis Biochemicals, San Diego, CA) according to the manufacturer's protocol and color developed was read at 450-nm against the blank and values were plotted against standards provided and expressed as units per liter.
Tumor xenograft study
The animal experiment was conducted in accordance with the guidelines established by the University's Animal Research Committee and with the NIH Guidelines for the Care and Use of Laboratory Animals. Approximately 1 million PC-3 cells suspended in 0.05 ml medium and mixed with 0.05 ml Matrigel were subcutaneously injected in the left and right flank of each mouse to initiate tumor growth. After implantation, the animals were kept under supervision for growth of tumor. Two weeks after cell inoculation, animals were divided into three equal groups of six mice each. Apigenin (10 mg) was suspended in 1 ml vehicle material (0.5 % methyl cellulose and 0.025 % Tween 20) by sonication for 30 s at 4 °C and further diluted for appropriate concentration. Apigenin, 20 and 50 μg/mouse/day was administered by gavage in 0.2 ml of a vehicle, daily for 8 weeks throughout the study. These doses are comparable to the daily consumption of flavonoid in humans as reported in previously published studies [23–26, 37]. The first group received only 0.2 ml vehicle material by gavage daily and served as a control group. The second and third groups of animals received 20 and 50 μg/mouse/day doses of apigenin in vehicle for 8 weeks, respectively.
Statistical analysis
Changes in tumor volume and body weight during the course of the experiments were visualized by scatter plot. Differences in tumor volume (mm3) and body weight at the termination of the experiment among various groups were examined using analysis of variance (ANOVA) followed by Tukey's multiple comparison procedure. The statistical significance of differences between control and treatment group was determined by simple ANOVA followed by multiple comparison tests. All tests were two-tailed and P values <0.05 were considered to be statistically significant.
Results
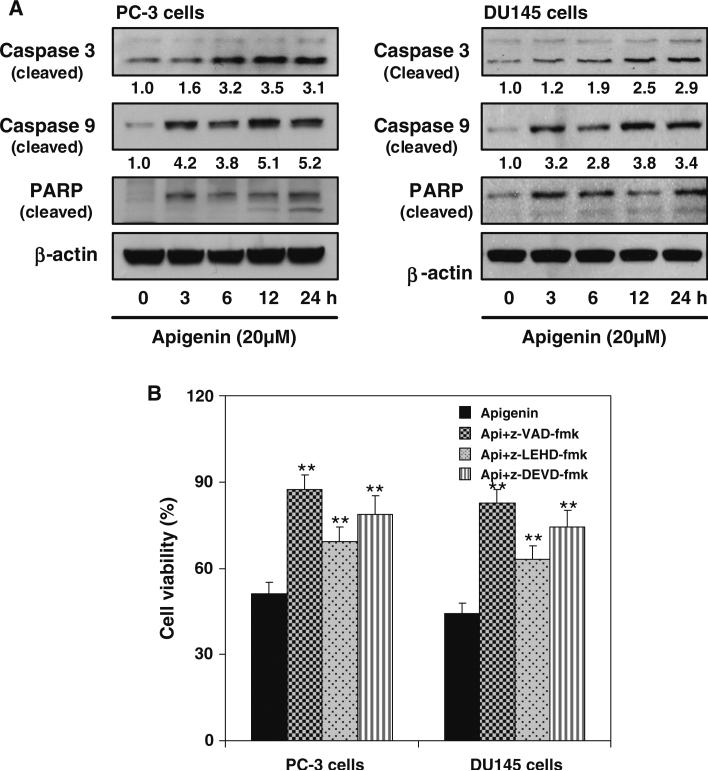
Initially, we determined dose–response and time course kinetic effect of apigenin on cell viability and apoptosis induction using androgen-refractory human prostate cancer PC-3 and DU145 cells. Apigenin treatment reduced viability of PC-3 and DU145 cells in a dose-dependent manner (data not shown). Treatment of PC-3 cells with 5–40 μM apigenin caused 18–51 % and DU145 cells resulted in 8–58 % decrease in cell viability. Both the cell lines were sensitive to apigenin-mediated reduction in cell survival. Next we determine the apoptotic effects of apigenin which may be through activation of a cascade of caspases. Because PARP-specific proteolytic cleavage by caspases is considered to be characteristic of apoptosis, the cleavage of caspase 9 and caspase 3 were evaluated. Treatment of PC-3 and DU145 cells with 20 μM apigenin caused activation of caspase 9 in time-dependent manner as indicated by increase in cleaved product of caspase 9. Activation of caspase 3 was detected after apigenin treatment as a double band representing the p19 proteolytic fragment and the active subunit p17, respectively. Similar results were observed in PARP cleavage, further indicating the specificity of the apoptotic effect of apigenin by caspase activation in prostate cancer cells (Fig. 1a).
Fig. 1.
Anti-proliferative and apoptotic effect of apigenin on human prostate cancer cells. a Effect of apigenin on protein expression of cleaved caspase 3/9 and PARP cleavage. The cells were treated with 20 μM apigenin for specified times and Western blotting was performed. Time-dependent increase in protein expression of cleaved caspase 3/9 and PARP cleavage was observed in both cell lines after apigenin treatment. β-actin was used as loading control. Numeric values represent the protein level normalized to the loading control (β-actin). b Effect of caspase inhibitor treatment on apigenin-mediated cell viability in prostate cancer cells. The cells were pretreated with 0.1 % DMSO, pan caspase inhibitor (z-VAD-fmk), caspase 9 inhibitor (z-LEHD-fmk) and caspase 3 inhibitor (z-DEVD-fmk) at 150 μM for 1 h followed by 20 μM apigenin treatment for 24 h. Cell viability was measured by MTT assay. The experiment was repeated thrice with similar results. Bars represent mean ± SE, **P < 0.001 versus control. Details are described in “Materials and methods” section
To confirm the mechanism responsible for apigenin-mediated apoptosis, prostate cancer cells were pretreated with broad spectrum caspase inhibitor (z-VAD-fmk), a caspase 9 specific inhibitor (z-LEHD-fmk) and a caspase 3 specific inhibitor (z-DEVD-fmk). Cell viability measurement was performed after 24 h incubation with apigenin with or without 1 h pretreatment with these caspase inhibitors at 150 μM dose. Pre-treatment of cells with all three caspase inhibitors significantly reduced the ability of apigenin to induce cell death in PC-3 and DU145 cells (Fig. 1b). The caspase inhibitor alone did not cause any significant change in cell viability (data not shown).
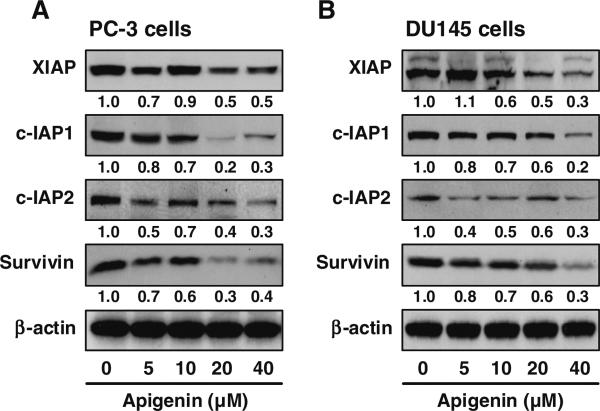
Members of the IAP family of protein, including XIAP, survivin, c-IAP1 and c-IAP2 have emerged as critical regulators of apoptotic cell death by various stimuli [8–11]. We sought to determine the possible role of these proteins in regulation of apigenin-induced apoptosis. As shown in Fig. 2, treatment of PC-3 and DU145 cells with apigenin resulted in a dose-dependent downregulation of XIAP protein expression. The effect of apigenin treatment on survivin was relatively less pronounced compared to XIAP. Nonetheless, apigenin-mediated down-regulation of survivin protein was clearly discernable after treatment of PC-3 and DU145 cells with 20 and 40 μM apigenin for 24 h. Moreover, expression of c-IAP1 and c-IAP2 protein was markedly reduced in both PC-3 and DU145 cells after apigenin treatment (Fig. 2).
Fig. 2.
Effect of apigenin on the protein expression of XIAP, c-IAP1, c-IAP2 and survivin in PC-3 and DU145 cells. The cells were treated with specified dose of apigenin for 24 h and Western blotting was performed. Dose-dependent decrease in protein expression was observed in both cell lines. β-actin was used as loading control. Numeric values represent the protein level normalized to the loading control (β-actin). Details are described in “Materials and methods” section
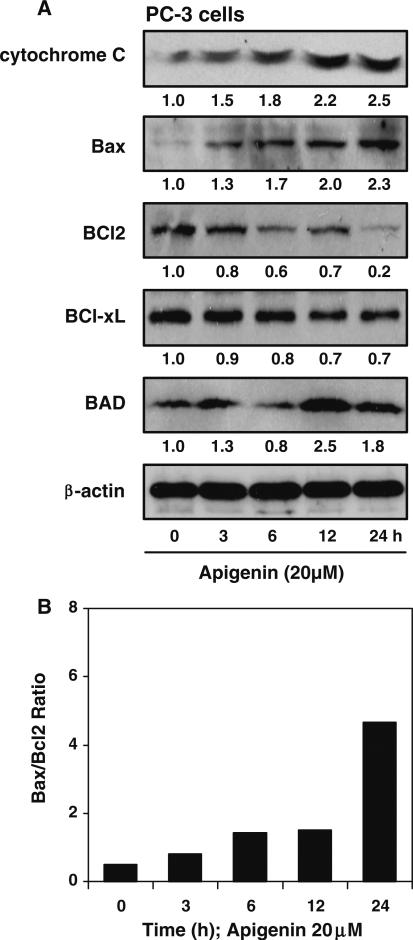
The cleavage of caspase 9/3 by apigenin suggests that its apoptotic effect involves the mitochondrial apoptotic pathway. As shown in Fig. 3a, treatment of PC-3 cells with 20 μM apigenin was followed by an obvious increase in cytochrome C in time-dependent manner. Specifically the upregulation of cytochrome C by apigenin was increased at 3 h and sustained until 24 h. Since Bcl-2 family protein influences cellular apoptosis via regulation of the cytochrome C release, which then mediates caspase activation, the effects of apigenin on the protein expression of anti-apoptotic Bcl-2, Bcl-xL and pro-apoptotic protein Bad and Bax was evaluated. Treatment of PC-3 cells with 20 μM apigenin from 3 to 24 h inhibited protein expression of Bcl-2 and Bcl-xL in a time-dependent manner. Furthermore, apigenin treatment resulted in increased protein expression of Bad and Bax in a time-dependent manner. Increased Bax expression was observed as early as 3 h of apigenin treatment with further increase up to 24 h. A simultaneous decrease in Bcl-2 tilted the Bax/Bcl-2 ratio in favor of apoptosis (Fig. 3b). Since Bax is mutated in DU145 cells, we performed further studies on PC-3 cells. Taken together, these data suggest that cytochrome C activation and Bcl-2 family regulation in response to apigenin play a role in induction of apoptosis.
Fig. 3.
Pro-apoptotic effect of apigenin on human prostate cancer cells. a Effect of apigenin on the protein expression of cytochrome C, Bax, Bcl2, Bcl-xL and Bad in PC-3 cells. The cells were treated with 20 μM apigenin for specified times and Western blotting was performed. Time-dependent increase in cytochrome C, Bax and Bad and a simultaneous decrease in Bcl2 and Bcl-xL protein expression was observed after apigenin treatment. β-actin was used as loading control. Numeric values represent the protein level normalized to the loading control (β-actin). b Bax/Bcl2 ratio was increased in time-dependent manner after 20 μM apigenin treatment favoring apoptosis. Details are described in “Materials and methods” section
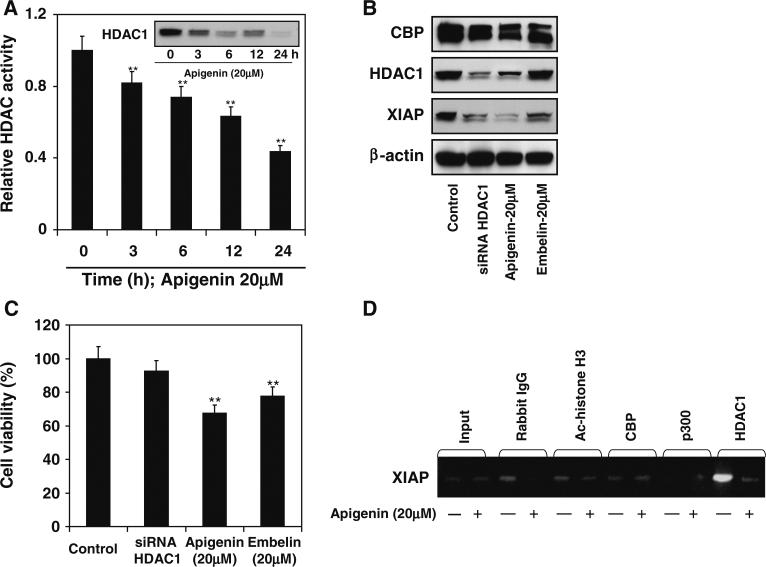
To understand the mechanism of apigenin-mediated apoptosis of prostate cancer cells we focused our attention on histone modifying enzymes. Studies have demonstrated that histone deacetylase inhibitor trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA) induce apoptosis in cancer cells by downregulation of Bcl-2 and XIAP [38]. We evaluated HDAC activity and protein expression after apigenin treatment. As shown in Fig. 4a, exposure of PC-3 cells to 20 μM apigenin resulted in significantly decreased total class I HDAC activity. Apigenin-mediated decrease in HDAC activity was initiated at 3 h and sustained through 24 h of exposure. A significant decrease in HDAC1 protein expression was observed after apigenin treatment to these cells (Fig. 4a).
Fig. 4.
Effect of apigenin-mediated inhibition of HDAC activity on XIAP expression in human prostate cancer PC-3 cells. a Effect of apigenin on class I HDAC activity. The cells were treated with 20 μM apigenin for specified times and HDAC enzyme assay was performed. Time-dependent decrease in HDAC activity was observed in PC-3 cells after apigenin treatment. The experiment was repeated twice with similar results. Bars represent mean ± SE, **P < 0.001 versus control. Inset figure shows protein expression for HDAC1 after 20 μM apigenin treatment at specified times. Time-dependent decrease in HDAC1 expression was observed after apigenin treatment. b Effect of HDAC1 knockdown and treatment with apigenin and embelin on HDAC1, CBP and XIAP protein expression. The cells were treated with 20 μM apigenin, 20 μM embelin for 24 h and transient knockdown of HDAC1 was achieved by siRNA treatment and Western blotting was performed. HDAC1 knockdown resulted in decrease in XIAP, whereas apigenin treatment caused decrease in protein expression of HDAC1 and XIAP and embelin treatment resulted in inhibition of XIAP protein expression. β-actin was used as loading control. c Cell viability was performed by MTT assay. Apigenin and embelin treatment resulted in decrease cell survival whereas HDAC1 knockdown did not result in appreciable decrease in cell viability. The experiments were repeated three times with similar results. Bars represent mean ± SE, **P < 0.001 versus control. d Occupancy of CBP/p300, acetylated histone H3, and HDAC1 at the XIAP promoter in PC-3 cells. Apigenin (20 μM) treatment caused decrease occupancy of HDAC1 on the XIAP promoter as shown by ChIP assay. Details are described in “Materials and methods” section
To determine the relationship between HDAC1 and XIAP, in the next experiment PC-3 cells were used to knockdown HDAC1 by siRNA interference, treated with 20 μM apigenin and 20 μM embelin, a known inhibitor of XIAP for 24 h. Knockdown of HDAC1 resulted in significant decrease in XIAP protein expression. Treatment of cells with apigenin resulted in marked decrease in HDAC1 and XIAP protein, whereas embelin treatment resulted in downregulation of XIAP protein. This correlated with simultaneously reduced viability of PC-3 cells, which was highest after apigenin treatment. No significant changes in the CBP protein level was noted after these treatments (Fig. 4b, c).
Based on the ability of apigenin to inhibit HDAC1, we anticipated that downregulation of HDAC1 is critical for XIAP suppression. To further confirm our findings, we examined the recruitment of CBP/p300 and HDAC1 to the XIAP promoter after apigenin treatment. As shown in Fig. 4d, neither CBP/p300 was associated with XIAP promoter; however, apigenin significantly reduced HDAC1 occupancy at the XIAP promoter, suggesting that histone decaetylation may be critical for XIAP downregulation.
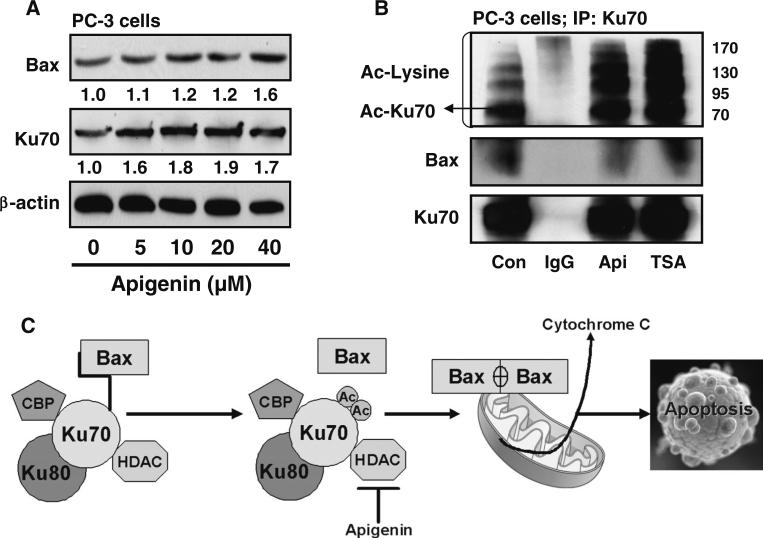
Our earlier experiments have shown that apigenin inhibits class I HDAC activity and expression in prostate cancer cells [36]. This might affect the dynamic balance between deacetylation and acetylation processes affecting gene expression. Since Ku70–Bax interaction regulates Bax-mediated mitochondrial apoptosis [15, 16], we studied the effect of apigenin on Bax and Ku70 expression. As shown in Fig. 5a, treatment of PC-3 cells with apigenin resulted in dose-dependent increase in Bax expression, whereas a modest increase was observed in Ku70 protein expression. Studies have shown that HDAC inhibitors increase acetylation of Ku70, facilitating Bax release from Ku70 and in turn inducing apoptosis [17]. Next we immunoprecipitated Ku70, followed by probing of the immunocomplex with an antibody against acetylated lysine. As shown in Fig. 5b, treatment with 20 μM apigenin resulted in increased acetylation of Ku70, and its interaction with Bax was decreased after apigenin treatment. Similar results were noted after treatment of cells with 80 nM trichostatin A, a HDAC inhibitor, which also resulted in increased Ku70 acetylation and its decreased association with Bax, initiating Bax-mediated apoptosis (Fig. 5c).
Fig. 5.
Effect of apigenin on Ku70–Bax interaction in human prostate cancer cells. a Effect of apigenin on the protein expression of Bax and Ku70 in PC-3 cells. The cells were treated with specified dose of apigenin for 24 h and Western blotting was performed. Dose-dependent increase in protein expression of Bax and Ku70 was observed in these cells. β-actin was used as loading control. Numeric values represent the protein level normalized to the loading control (β-actin). b Effect of apigenin on Ku70 acetylation and release of Bax from Ku70–Bax complex in PC-3 cells. The cells were treated with IgG, 20 μM apigenin and 80nM HDAC inhibitor, trichostatin A (TSA) for 24 h and subjected to immunoprecipitation using Ku70 antibody. Western blotting was performed with acetylated lysine and Bax. A significant increase in Ku70 acetylation was observed after apigenin and TSA treatment with simultaneous decrease in Bax from the Ku70–Bax complex. c Schematic presentation of the effect of apigenin on Ku70 acetylation and disruption of Ku70–Bax complex releasing Bax to initiate apoptosis in PC-3 cells. Details are described in “Materials and methods” section
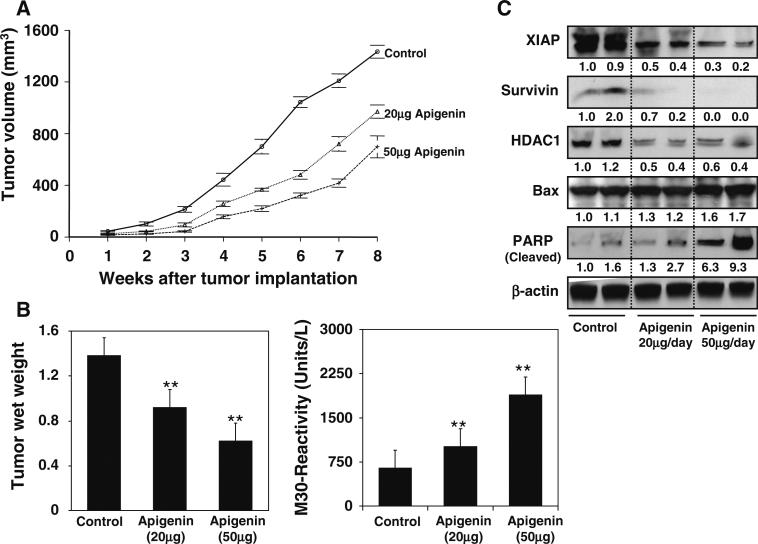
Our previous experiments have demonstrated the effectiveness of apigenin in inducing apoptosis in prostate cancer cells by downregulation of the IAP family of proteins. We extended these studies to determine whether these events occur in vivo using a PC-3 tumor xenograft model. We designed a protocol that simulates a therapeutic regimen, wherein apigenin was provided at 20 and 50 μg/mouse/day through gavage after 2 weeks of cell inoculation and continued for 8 weeks. In this experimental protocol, intake of apigenin inhibited the growth of tumor xenograft at both test doses. As shown in Fig. 6a and b, tumor volume was inhibited by 32.5 and 51.3 % (P < 0.001) and the wet weight of tumor was decreased by 33.3 and 55.1 % (P < 0.001), respectively, at the termination of the experiment. There were no adverse effects of apigenin treatment on animal health, food intake or body weight (data not shown). The average daily intake of food did not differ between the control and treated groups. Moreover, body weight was not significantly different throughout the duration of the study, suggesting that apigenin was essentially non-toxic at the dietary concentrations used in the study.
Fig. 6.
Effect of apigenin administration on PC-3 tumor growth and apoptosis in athymic nude mice. a Tumor volume after apigenin administration. Approximately 1 million cells were injected into both flanks of each mouse to initiate ectopic tumors, and apigenin was provided to the animals 2 weeks after tumor implantation mimicking therapeutic regimen. Mice were fed ad libitum with Teklad 8760 autoclaved high-protein diet. Apigenin was provided with 0.5 % methyl cellulose and 0.025 % Tween 20 as vehicle to these animals per-oral on a daily basis. Group I, control, received 0.2 ml vehicle only, Group II received 20 μg/day apigenin per mouse in 0.2 ml vehicle and Group III received 50 μg/day apigenin per mouse in 0.2 ml vehicle daily for 8 weeks. Tumor size was measured weekly in two dimensions throughout the study. b Wet weight of tumors is represented as the mean of 6–8 tumors from each group, quantitative measurement of apoptosis as demonstrated by M30 reactivity. The experiment was repeated twice in duplicate with similar results. Bars represent mean ± SD **P < 0.001 versus control. c Western blotting for XIAP, surviving, HDAC1, Bax and PARP cleavage in tumor lysates after apigenin administration at the indicated doses. β-actin was used as loading control. Numeric values represent protein levels normalized to the loading control (β-actin). The details are provided in “Materials and methods” section
At the termination of the study, xenografts were examined for the extent of tumor cell apoptosis. Compared to the untreated controls, induction of apoptosis was significantly increased in tumor xenografts in the same mice (P < 0.001) (Fig. 6b). We also measured the protein expression of XIAP and survivin as an effect of apigenin feeding. As shown in Fig. 6c, oral intake of apigenin at doses of 20 and 50 μg/mouse/day resulted in marked reduction in the protein expression of XIAP and survivin in PC-3 tumor xenografts. A dose-dependent decrease in HDAC1 expression, increase in Bax and PARP cleavage was observed in apigenin-administered mice.
Discussion
The present study reveals for the first time that apigenin treatment causes inhibition of XIAP, survivin, c-IAP1 and c-IAP2 in androgen-refractory PC-3 and DU145 prostate cancer cells. Furthermore, apigenin treatment led to decreased HDAC1, causing increased acetylation and disruption of Ku70–Bax interaction, thereby releasing Bax in the cytosol. Apigenin-mediated increase in Bax levels promotes apoptosis in prostate cancer cells.
The inhibitor of apoptosis protein is a family of structurally homologous cell survival molecules characterized by the presence of one to three baculoviral (BIR) IAP repeats in the BIR domain [39, 40]. The human IAP family consists of eight protein including NAIP (BIRC1), cIAP-1 (BIRC2), c-IAP2 (BIRC3), XIAP (BIRC4), survivin (BIRC5), Apollon/Bruce (BIRC6), ML-IAP (BIRC7 or livin) and ILP-2 (BIRC8), respectively. IAPs have been shown to protect cells from a wide range of apoptotic triggers, including FAS ligation, Bax, activated caspases, cytochrome C, TNFα as well as from stress including therapeutic agents, infection and radiation [40]. Studies demonstrate poor correlation between XIAP, c-IAP1, c-IAP2 protein levels with mRNA levels, consistent with emerging knowledge that expression of many IAPs is regulated predominantly at the level of protein stability [8–10, 41]. Previous studies have demonstrated that androgen-independent prostate cancer cells have elevated levels of a range of anti-apoptotic proteins, including IAPs and Bcl-2 family members [8–10], which forms the rationale for targeting these proteins. Current efforts are focused on the development of new therapeutic agents derived from natural sources.
Of the eight IAP protein members identified to date, XIAP is the best characterized and most potent inhibitor of apoptosis [8–10, 12]. Transient transfection of prostate cancer cells with XIAP targeting siRNA produced a prominent downregulation of XIAP that resulted in apoptosis and significantly increased sensitivity to chemotherapeutic drugs [42]. Higher XIAP expression has been demonstrated in high-grade PIN lesions, and its aberrant expression correlates with prostate cancer progression and poor prognosis [12]. The XIAP protein is structurally characterized by 3 BIR domains (BIR1, BIR2, and BIR3) and a ring-finger domain. Although BIR2 and BIR3 domains are necessary for XIAP to bind and inhibit caspase, the BIR1 of XIAP has been shown to interact with TAK1 binding protein, (TAB 1), an interaction that is essential for the recruitment of TAK1 and subsequent activation of NF-κB [43]. Apigenin-induced cell death is due to downregulation of XIAP and release of caspase 9 followed by activation of caspase 3 leads to apoptosis. Cells exposed to apigenin prior to treatment with caspase9/3 inhibitor, and general caspase inhibitor exhibit reduced cell death. These data indicate that apigenin-induced cell death is mediated by caspase activity. Another important role for the IAPs is in their regulation of NF-κB. Activation of NF-κB in tumor cells leads to increased transcription of the anti-apoptotic and pro-survival genes in these cells [39, 40]. Our previous studies have shown that apigenin-mediated apoptosis occurs through inhibition of NF-κB activation, which may occur through inhibition of IAP levels [27]. It would be interesting to explore the precise involvement of NF-κB and IAPs in apigenin-induced apoptosis of prostate cancer cells.
Survivin is another important member of the IAP family, implicated in control of apoptosis [8, 9]. Functional studies have demonstrated that suppression of survivin causes spindle defects and promotes apoptosis [44]. Survivin is strongly expressed in embryonic and fetal organs, but is undetectable in most terminally differentiated tissues. Survivin is over-expressed in most common forms of human cancer including prostate cancer [10, 13]. Survivin has been associated with established features of biologically aggressive prostate cancer, such as higher Gleason score and metastasis to regional lymph nodes, and consequently it is considered as a promising target for cancer therapy [13]. Our present study demonstrates that apigenin-induced apoptosis is mediated by downregulation of survivin both in cell culture and in an in vivo model of prostate cancer. Other family members of IAPs, including c-IAP1 and c-IAP2, are often simultaneously higher in clinical specimens and patient derived prostate cancer cell lines [8–10, 41]. Expression of c-IAP1 and c-IAP2 is cell cycle dependent, peaking at the G2/M phase of the cell cycle and contributing to survival during mitotic arrest. An integrative oncogenomic approach classifies c-IAP1 and c-IAP2 as possessing oncogenic properties. Over-expression of c-IAP1 in DU145 cells has been shown to trigger polyploidy. Knockdown of c-IAP1 protein increases spontaneous apoptosis in prostate cancer cells [45]. In the present study, apigenin treatment to PC-3 and DU145 cells exhibit inhibition of c-IAP1 protein during apoptosis. Studies have demonstrated upegulation of c-IAP1 and c-IAP2 levels in tumor cells after knockdown of XIAP by siRNA [46]. This may be a reason for failure of targeting individual IAP in cancer and advisable that simultaneous knockdown of all over-expressed IAPs may increase basal apoptosis levels. Our study demonstrates that apigenin has the potential to simultaneously target all IAPs including XIAP, survivin, c-IAP1 and c-IAP2, which are over-expressed in androgen-refractory prostate cancer cells. A similar study has demonstrated the importance of targeting multiple IAPs in enhancing the sensitivity of prostate cancer cells to apoptosis-inducing agents [46]. It will be worthwhile to study the effect of apigenin in sensitizing highly aggressive prostate cancer cells to chemotherapeutic agents.
Apoptosis is a key tumor suppressor mechanism and can be initiated by activation of the pro-apoptotic factor Bax. Overall Bax activation seems to play a central role in mitochondria-dependent apoptosis [14–16]. The BH3-only proteins, including Bad, Bim/Bod, Bmf, Bik/Npk, BNIP3/NIX, BIK, NIP3, Noxa and Puma are immediate upstream triggers for Bax activation, either directly or indirectly by sequestration of anti-apoptotic protein Bcl-2 and Bcl-xL proteins [14]. In addition, Bax activation is regulated by p53 via transcriptional dependent and/or independent mechanism [6]. We showed that apigenin treatment significantly increased apoptotic effects in human prostate cancer cells, which do not possess wild-type p53, suggesting that apigenin-mediated increase in Bax is p53 independent. Further studies are needed to identify the mechanism of Bax increase in prostate cancer cells after apigenin treatment.
Recently it has been reported that changes in histone deacetylase (HDAC) expression in prostate cancer cells may be involved in mechanisms of abnormal cellular proliferation and may operate through chromatin-independent pathways [47]. Class I HDAC (HDAC1, 2, 3 and 8) are over-expressed in prostate cancer [6, 48]. HDAC1 and HDAC3 are highly expressed in prostate cancer and HDAC2 has been shown to be associated with shorter PSA relapse time [48]. HDAC1 plays a critical role in the transition of prostate cancer cells from androgen dependence to androgen independence [49]. Studies have shown that treatment of prostate cancer cells with HDAC inhibitors results in XIAP inhibition and increased apoptosis [42]. Our studies demonstrate that apigenin treatment of prostate cancer cells causes a rapid decrease in HDAC1 and loss of HDAC1 recruitment leads to histone deacetylation at the XIAP promoter. Additional studies of the decrease of HDAC1 and its effect on IAPs by apigenin are needed.
Histone modifying enzymes, histone deacetylases and histone acetyltransferase, the two opposing group of enzymes affect gene transcription through acetylation/deacetylation of histone and non-histone proteins, including p53, HSP90, Ku70, RelA/p65 and STATs, which play important roles in cell proliferation, survival and apoptosis [50]. Ku70, a DNA repair protein, has recently been shown to suppress apoptosis by sequestering Bax from mitochondria. Several studies have demonstrated the involvement of Ku70 and Bax inactivation in various human cancers, genotoxic and chemotherapeutic resistance [19]. Another study has shown that Ku70 is a target of class I/III HDAC and its deacetylation suppresses release of Bax from K70/80 complex in the nucleus and in turn results in Bax-induced apoptosis [16]. Acetylation of Ku70 by acetyltransferase such as CBP/p300 and PCAF in the C-terminal domain of Ku70 releases Bax from the Ku70/Ku80 complex [15, 16]. In our studies, apigenin-mediated decrease in HDAC1 shifts the acetylation/deacetylation equilibrium in favor of acetylation, causing increased acetylation of the lysine residues of Ku70, thereby releasing Bax from the complex and inducing apoptosis of prostate cancer cells (Fig. 5c). It would be interesting to determine the involvement of p53, RelA/p65 acetylation in prostate cancer cells by apigenin and their role in cell death.
In our cell culture studies, we observed induction of apoptosis, as well as impairment in cell survival, by exposure of prostate cancer cells to apigenin at 20 and 40 μM concentrations. Although these findings provide mechanistic insights, demonstration that these effects are also operative in vivo is required to establish a potential for clinical development. Our in vivo studies using 20 and 50 μg/day apigenin administration to mice with prostate cancer xenografts confirmed that apigenin intake significantly inhibited tumor growth, without any apparent signs of toxicity. Consistent with the findings in cell culture, apigenin intake resulted in downregulation of XIAP, survivin, HDAC1 protein expression, and increase in the levels of Bax favoring apoptosis, compared with control group. The dose of apigenin which is effective in vivo corresponds to an intake of 3.4 and 8.5 mg/day by an adult healthy 70 kg individual. These results indicate that apigenin is bio-available at these concentrations and has a therapeutic effect [31].
In summary, some critical conclusions drawn from the study are that apigenin has the ability to target the IAP family of proteins in prostate cancer cells and their inhibition renders cancer cells susceptible to apoptotic cell death. Furthermore, the IAP family of proteins may serve as surrogate biomarkers to assess apigenin response in clinical investigations. In conclusion, apigenin alone or in combination with other therapeutic modalities such as radiation and chemotherapy appears to have potentially beneficial activity in the management of highly aggressive prostate cancer.
Acknowledgments
This work was supported by grants from United States Public Health Services RO1CA108512, RO1AT002709 to SG and RO3CA1376676 to SS.
Abbreviations
- IAP
Inhibitor of apoptosis protein
- XIAP
X-linked inhibitor of apoptosis
- HDAC
Histone deacetylases
- NF-κB
Nuclear factor kappa-B
- IGF
Insulin-like growth factor
- PI3K
Phosphoinositide 3-kinase
- HIF
Hypoxia-inducible factor
- MAPK
Mitogen-activated protein kinases
- SAHA
Suberoylanilide hydroxamic acid
- TSA
Trichostatin A
Footnotes
Conflict of interest The authors have no competing interest.
Contributor Information
Sanjeev Shukla, Department of Urology, Case Western Reserve University & The Urology Institute, University Hospitals Case Medical Center, 10900 Euclid Avenue, Cleveland, OH 44106, USA.
Pingfu Fu, Department of Epidemiology & Biostatistics, Case Western Reserve University, Cleveland, OH 44106, USA.
Sanjay Gupta, Department of Urology, Case Western Reserve University & The Urology Institute, University Hospitals Case Medical Center, 10900 Euclid Avenue, Cleveland, OH 44106, USA; Department of Nutrition, Case Western Reserve University, Cleveland, OH 44106, USA; Divsion of General Medical Sciences, Case Comprehensive Cancer Center, Cleveland, OH 44106, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Prostate cancer facts and statistics on the American Cancer Society. website at: http://www.cancer.org/cancer/prostatecancer/index.
- 3.Moul JW, Mouraviev V, Sun L, Schroeck FR, Polascik TJ. Prostate cancer: the new landscape. Curr Opin Urol. 2009;19:154–160. doi: 10.1097/mou.0b013e328323f5d6. [DOI] [PubMed] [Google Scholar]
- 4.Punnen S, Cooperberg MR, D'Amico AV, Karakiewicz PI, Moul JW, Scher HI, Schlomm T, Freedland SJ. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.05.025. doi: 10.1016/j.eururo.2013.05.025. [Epub ahead of print] PubMed PMID: 23721958. [DOI] [PubMed] [Google Scholar]
- 5.Tsai HT, Penson DF, Makambi KH, Lynch JH, Van Den Eeden SK, Potosky AL. Efficacy of intermittent androgen deprivation therapy vs. conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82(2):327–333. doi: 10.1016/j.urology.2013.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta K, Thakur VS, Bhaskaran N, Nawab A, Babcook MA, Jackson MW, Gupta S. Green tea polyphenols induce p53-dependent and p53-independent apoptosis in prostate cancer cells through two distinct mechanisms. PLoS ONE. 2012;7:e52572. doi: 10.1371/journal.pone.0052572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deveraux QL, Schendel SL, Reed JC. Antiapoptotic proteins. The bcl-2 and inhibitor of apoptosis protein families. Cardiol Clin. 2001;19:57–74. doi: 10.1016/s0733-8651(05)70195-8. [DOI] [PubMed] [Google Scholar]
- 8.McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002;51:133–140. doi: 10.1002/pros.10061. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 10.Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A, Peehl D, Gao GJ, Reed JC. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9:4914–4925. [PubMed] [Google Scholar]
- 11.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 12.Seligson DB, Hongo F, Huerta-Yepez S, Mizutani Y, Miki T, Yu H, Horvath S, Chia D, Goodglick L, Bonavida B. Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin Cancer Res. 2007;13:6056–6063. doi: 10.1158/1078-0432.CCR-07-0960. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM, Ashfaq R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100:751–757. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 14.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 15.Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S. Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol. 2003;5:320–329. doi: 10.1038/ncb950. [DOI] [PubMed] [Google Scholar]
- 16.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen CS, Wang YC, Yang HC, Huang PH, Kulp SK, Yang CC, Lu YS, Matsuyama S, Chen CY, Chen CS. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res. 2007;67:5318–5327. doi: 10.1158/0008-5472.CAN-06-3996. [DOI] [PubMed] [Google Scholar]
- 18.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 19.Gullo C, Au M, Feng G, Teoh G. The biology of Ku and its potential oncogenic role in cancer. Biochim Biophys Acta. 2006;1765:223–2234. doi: 10.1016/j.bbcan.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010;17:R39–R52. doi: 10.1677/ERC-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Bommareddy A, Singh SV. Garlic constituent diallyl trisulfide suppresses x-linked inhibitor of apoptosis protein in prostate cancer cells in culture and in vivo. Cancer Prev Res (Phila) 2011;4:897–906. doi: 10.1158/1940-6207.CAPR-10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakao K, Desineni S, Hahm ER, Singh SV. Phenethyl isothiocyanate suppresses inhibitor of apoptosis family protein expression in prostate cancer cells in culture and in vivo. Prostate. 2012;72:1104–1116. doi: 10.1002/pros.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyle JA, Sharp L, Little J, Duthie GG, McNeill G. Dietary flavonoid intake and colorectal cancer: a case–control study. Br J Nutr. 2010;103:429–436. doi: 10.1017/S0007114509991784. [DOI] [PubMed] [Google Scholar]
- 25.Tang NP, Zhou B, Wang B, Yu RB, Ma J. Flavonoids intake and risk of lung cancer: a meta-analysis. Jpn J Clin Oncol. 2009;39:352–359. doi: 10.1093/jjco/hyp028. [DOI] [PubMed] [Google Scholar]
- 26.Peterson J, Lagiou P, Samoli E, Lagiou A, Katsouyanni K, La Vecchia C, Dwyer J, Trichopoulos D. Flavonoid intake and breast cancer risk: a case–control study in Greece. Br J Cancer. 2003;89:1255–1259. doi: 10.1038/sj.bjc.6601271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 28.Shukla S, MacLennan GT, Fu P, Gupta S. Apigenin attenuates insulin-like growth factor-I signaling in an autochthonous mouse prostate cancer model. Pharm Res. 2012;29:1506–1517. doi: 10.1007/s11095-011-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcino-genesis. 2013 doi: 10.1093/carcin/bgt316. [Epub ahead of print] PubMed PMID: 24067903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirzoeva S, Kim ND, Chiu K, Franzen CA, Bergan RC, Pelling JC. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 31.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 32.Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008;44:1833–1845. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6:1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 34.Franzen CA, Amargo E, Todorović V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/ Src signaling mechanism. Cancer Prev Res (Phila) 2009;2:830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 35.Shukla S, Gupta S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol Carcinog. 2004;39:114–126. doi: 10.1002/mc.10168. [DOI] [PubMed] [Google Scholar]
- 36.Pandey M, Kaur P, Shukla S, Abbas A, Fu P, Gupta S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: in vitro and in vivo study. Mol Carcinog. 2012;51:952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 38.Devi GR. XIAP as target for therapeutic apoptosis in prostate cancer. Drug News Perspect. 2004;17:127–1234. doi: 10.1358/dnp.2004.17.2.829046. [DOI] [PubMed] [Google Scholar]
- 39.de Almagro MC, Vucic D. The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol. 2012;34:200–211. [PubMed] [Google Scholar]
- 40.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Berriguete G, Fraile B, de Bethencourt FR, Prieto-Folgado A, Bartolome N, Nuñez C, Prati B, Martínez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. Role of IAPs in prostate cancer progression: immunohistochemical study in normal and pathological (benign hyperplastic, prostatic intraepithelial neoplasia and cancer) human prostate. BMC Cancer. 2010;10:18. doi: 10.1186/1471-2407-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano Y, Bilim V, Yuuki K, Muto A, Kato T, Nagaoka A, Tomita Y. Molecular targeting of Bcl-2 overcomes prostate cancer cell adaptation to XIAP gene downregulation. Prostate Cancer Prostatic Dis. 2009;12:34–40. doi: 10.1038/pcan.2008.27. [DOI] [PubMed] [Google Scholar]
- 43.Lu M, Lin SC, Huang Y, Kang YJ, Rich R, Lo YC, Myszka D, Han J, Wu H. XIAP induces NF-kappaB activation via the BIR1/TAB 1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho CY, Wong CH, Li HY. Perturbation of the chromosomal binding of RCC1, Mad2 and survivin causes spindle assembly defects and mitotic catastrophe. J Cell Biochem. 2008;105:835–846. doi: 10.1002/jcb.21879. [DOI] [PubMed] [Google Scholar]
- 45.McEleny K, Coffey R, Morrissey C, Williamson K, Zangemeister-Wittke U, Fitzpatrick JM, Watson RW. An antisense oligonucleotide to cIAP-1 sensitizes prostate cancer cells to Fas and TNFalpha mediated apoptosis. Prostate. 2004;59:419–425. doi: 10.1002/pros.10371. [DOI] [PubMed] [Google Scholar]
- 46.Gill C, Dowling C, O'Neill AJ, Watson RW. Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferation. Mol Cancer. 2009;8:39. doi: 10.1186/1476-4598-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B, Gold DL, Sekido Y, Huang TH, Issa JP. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 48.Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 50.Gabrielli B, Brown M. Histone deacetylase inhibitors disrupt the mitotic spindle assembly checkpoint by targeting histone and nonhistone proteins. Adv Cancer Res. 2012;116:1–37. doi: 10.1016/B978-0-12-394387-3.00001-X. [DOI] [PubMed] [Google Scholar]