Abstract
Background
Effective integration of visual information is necessary to utilize abstract thinking, but patients with schizophrenia have slow eye movement and usually explore limited visual information. This study examines the relationship between abstract thinking ability and the pattern of eye gaze in patients with schizophrenia using a novel theme identification task.
Methods
Twenty patients with schizophrenia and 22 healthy controls completed the theme identification task, in which subjects selected which word, out of a set of provided words, best described the theme of a picture. Eye gaze while performing the task was recorded by the eye tracker.
Results
Patients exhibited a significantly lower correct rate for theme identification and lesser fixation and saccade counts than controls. The correct rate was significantly correlated with the fixation count in patients, but not in controls.
Conclusions
Patients with schizophrenia showed impaired abstract thinking and decreased quality of gaze, which were positively associated with each other. Theme identification and eye gaze appear to be useful as tools for the objective measurement of abstract thinking in patients with schizophrenia.
Keywords: Schizophrenia, Abstract thinking, Theme identification, Eye gaze
Background
Abstract thinking is defined as the ability to think beyond the immediate, specific stimulus situation and to think about situations in general, symbolic modes [1]. Abstract thinking is the opposite of concrete thinking, which is thinking of objects or ideas as specific items. Deficits in abstract thinking are prominent in patients with schizophrenia, particularly in chronic patients and patients with severe symptoms [1]. Deficits in abstract thinking can cause patients with schizophrenia to run into substantial difficulties in social interactions during daily life and work life [2,3].
Many studies have investigated abstract thinking in schizophrenia [1,3-7], but most have used neuropsychological tests, including the Wisconsin card-sorting test. This test evaluates abstract thinking ability, such as rule-based categorization [8], and is a measure for other aspects of the executive functions, including cognitive flexibility [9,10]. Some studies focused on abstract thinking using the Gorham proverb test or the Benjamin proverb test [1,4,5], but results from these tests can be influenced by education level and general frontal lobe functions such as working memory [11].
Measurement of eye gaze can be a powerful way to analyze the processing of visual information [12]. Eye gaze has an important role in cognition through attention, reading, and scene perception [13]. A “top-down” process makes an important contribution to the control of eye movement in that the cognitive and affective status of a person is significant in eye gaze [14]. Previous studies on eye gaze have demonstrated that patients with schizophrenia clearly show atypical eye gaze patterns or scan paths [15,16]. Restricted scanning in patients with schizophrenia has a close relationship with negative symptoms, including avolition and affective blunting, deficits in executive functions, and problems with social interaction [16,17].
It has been reported that healthy individuals direct their gaze according to their purpose and focus their gaze on the principal component, whereas patients with schizophrenia have slow eye movement and usually explore limited visual information [18]. These abnormal gaze patterns and limited visual searching may make it difficult for them to obtain significant information about objects and then integrate the information. In addition, effective integration of visual information is necessary to utilize abstract thinking [3]. In the draw-a-person technique [19], for example, abstract thinking was evaluated by checking for integrative information in the context of the proportion of each part of the whole person, integrity of the figure, and consistency of lines throughout the figure [20]. Taken together, this evidence suggests that abnormal eye gaze is related to impairment in abstract thinking. However, no one has yet studied the association between visual searching and abstract thinking in patients with schizophrenia.
The aim of this study was to elucidate the nature of the relationship between abstract thinking and visual searching in schizophrenia. To investigate our hypothesis that impairment in abstract thinking is associated with severity of abnormal eye gaze in patients with schizophrenia, we created a theme identification task, in which subjects judged a theme of presented pictures while their visual scan paths were concurrently monitored. We selected theme identification in this task in order to exclusively evaluate abstract thinking ability.
Method
Participants
Twenty patients with schizophrenia (11 males) and 22 healthy controls (12 males) participated in this study. All patients were recruited from outpatient clinic of Gangnam Severance Hospital, and healthy controls were recruited using open advertisement on the Internet. The exclusive diagnoses of schizophrenia in the patient group and the exclusions of psychiatric disorders in the control group were made based on the Structural and Clinical Interview for DSM-IV (SCID-IV) [21]. Exclusion criteria included the presence of mental retardation, significant neurological or medical illness, and severe ophthalmological diseases. All patients were taking atypical antipsychotics, and the mean chlorpromazine equivalent dose was 513.9 ± 590.8 mg. This study was approved by the institutional review board of Yonsei University Severance Hospital. All participants provided voluntary written, informed consent before the study began.
Behavioral tasks
We developed a theme identification task to evaluate participants’ abstract thinking ability, and the stimuli used consisted of 20 emotional (10 positive and 10 negative) pictures from the International Affective Picture System (IAPS) [22]. Three words were presented in the bottom of the pictures, and participants were asked to indicate the main theme of the picture by selecting one of these words. The three words were chosen to indicate a main theme word (“theme word”), to be related to but more concrete than the theme word (“related word”), and to be present in the picture but have no direct relationship to the main theme (“unrelated word”). For example, as presented in Figure 1, wedding was the theme word (the correct answer), bride was the related word, and earring was the unrelated word. In order to determine the stimuli to be used in this study, 15 normal participants (8 males) participated in a pilot theme identification task, and only stimuli with correct rates of more than 60% were selected. There were 20 task trials, and each individual trial consisted of two phases that lasted for a total of 6 seconds. In the first 3-second phase, a stimulus picture was presented without words, and participants were asked to explore the picture and think about what the theme was (searching phase). In the next 3-second phase, three words were presented, and participants were instructed to select one of them (reaction phase).
Figure 1.
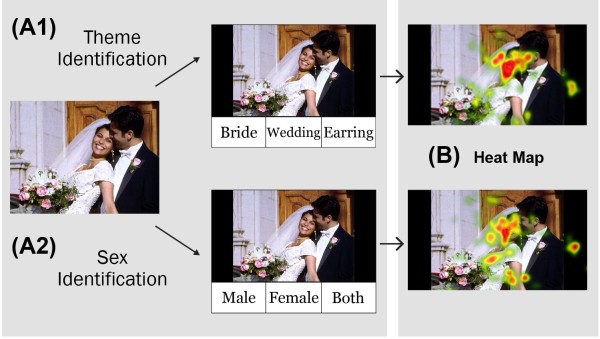
An example of the experimental tasks and analysis. The experiment consisted of theme or sex identification for consecutively presented pictures. During theme identification (A1), participants were asked to select the theme word. In this example, “Wedding” was the theme word, “Bride” was the related word, and “Earring” was the unrelated word. During sex identification (A2), participants were asked to select the sex of the people in the picture. In the analysis (B), a heat map was automatically revealed by the eye tracker, and the area of interest (AOI) was defined as the area that healthy controls had viewed for more than 50 ms (colored dark red on the heat map).
As a control task for theme identification, a sex identification task was employed to evaluate participants’ ability to think concretely. The same 20 pictures presented in the theme identification task were used for the sex identification task, and participants were asked to decide whether the picture included only a male, only a female, or both. The sex identification task also consisted of the searching phase for 3 seconds and the subsequent reaction phase for 3 seconds.
The correct rates and response times for the two tasks were examined using statistical analysis, as described below. In the theme identification task, the selection rates of the related and unrelated words as wrong answers were also calculated.
Eye movement recordings
Eye movement recording was performed using the SensoMotoric Instrument (SMI, Boston, USA) eye movement monitoring system, which included the Remote Eye Tracking Device 5 (RED5, infrared eye camera), Experiment Center 3.0 (used for stimuli presentation), and SMI iView (eye tracking software). The gaze data were analyzed using SMI BeGaze. In order to maintain a stable head position and reliable detection of gaze, the jaws of participants were fixed with a special device, and the eye position was always measured at a distance of approximately 70 cm from the computer monitor. Before the main experiment, participants were instructed to fix their gaze at several circles to calibrate and validate their eye movements. The sampling rate of eye gaze was 120 Hz; therefore, the maximum number of samples during the searching phase was 360. The numbers of samples, fixations, and saccades were measured. A fixation was counted when participants gazed at the monitor within an area of 100 pixels for more than 80 ms. A saccade was counted between fixation and fixation, between blink and blink, or between fixation and blink.
To compare between patients and controls with respect to the eye gaze patterns that fell within the core area gazed at by the controls, we set up the “area of interest” (AOI), which was defined as the area that controls looked at for more than 50 ms. The rate of fixation in the AOI during the searching phase was calculated for each subject and stimulus.
Clinical and cognitive measures
Another measure of abstract thinking ability was the Similarities subtest (range: 0–28) in the Korean version of the Wechsler Adult Intelligence Scale [23]. Intelligence was measured using the Raven’s standard progressive matrices (RPM) [24]. Anhedonia was measured using the Physical and Social Anhedonia Scale [25]. To identify clinical symptoms and antipsychotics-induced Parkinsonian symptoms of patients, the Positive and Negative Syndrome Scale (PANSS) [26] and Simpson-Angus Scale (SAS) [27] were employed.
Statistical analysis
Demographic and clinical data and rates of correct responses were compared between the two groups using independent-sample t-tests, except for sex, which was analyzed using the Chi-square test. Gaze data of sample, fixation, and saccade counts were used as the dependent variables in repeated measures analysis of variance (ANOVA), with the group as a between-subjects factor and the task type (theme identification versus sex identification) as a within-subjects factor. In each group, correlation analyses were performed on clinical and demographic data, the rates of correct responses, and eye gaze data. Linear regression analysis was used to determine the interaction effects of group and quality of gaze with respect to the rate of correct responses for each task.
Results
Demographic and clinical characteristics
The demographic and clinical data are listed in Table 1. There were no significant differences between patients and controls for age and sex, but education years were significantly fewer (t = 2.46, df = 30.5, d = 0.77, p = 0.02) in patients. The between-group difference in raw scores of the RPM was not statistically significant (t = 1.75, df = 28.9, d = 0.55, p = 0.091), but raw scores of the Similarities subtest were significantly lower in patients than in controls (t = 3.12, df = 40, d = 0.96, p = 0.003). Scores of the Physical and Social Anhedonia Scale were significantly higher in patients (physical, t = 2.12, df = 40, d = 0.67, p = 0.04; social, t = 3.09, df = 40, d = 0.95, p = 0.004).
Table 1.
Clinical characteristics of the subjects
| Control (N = 22) | Schizophrenia (N = 20) | p-value* | |
|---|---|---|---|
| Male/Female |
12/10 |
11/9 |
0.610 |
| Age (years) |
30.2 ± 11.7 |
31.4 ± 9.3 |
0.720 |
| Education (years) |
15.7 ± 1.4 |
14.3 ± 2.3 |
0.020 |
| RPM |
50.6 ± 8.3 |
43.9 ± 15.2 |
0.091 |
| Similarity |
18.7 ± 3.9 |
14.7 ± 4.5 |
0.003 |
| Physical Anhedonia |
12.2 ± 8.1 |
18.4 ± 10.1 |
0.035 |
| Social Anhedonia |
10.1 ± 6.8 |
16.6 ± 6.7 |
0.004 |
| Simpson-Angus Scale |
|
1.0 ± 1.4 |
|
| PANSS_positive |
|
13.5 ± 5.3 |
|
| PANSS_negative |
|
17.3 ± 5.8 |
|
| PANSS_general |
|
33.4 ± 10.1 |
|
| Duration of illness (years) |
|
4.5 ± 5.8 |
|
| Chlorpromazine equivalent dose (mg) | 513.9 ± 590.8 |
*by chi-square for categorical variable and independent sample t-test for continuous variable. RPM: raw score of Raven’s standard Progressive Matrices, Similarity: Score of the Similarity test, PANSS: Positive and Negative Syndrome Scale.
Tasks performance
As shown in Table 2, the rates of correct theme identification were significantly lower in patients (81.0 ± 17.0%) than controls (91.1 ± 9.9%) (t = 2.39, df = 40, d = 0.73, p = 0.02). This group difference was significant during the negative emotional condition (t = 2.30, df = 30.7, d = 0.72, p = 0.03), but not during the positive condition. However, the rates of correct sex identification showed no difference between patients (90.1 ± 9.7%) and controls (90.7 ± 8.6%). In addition, as shown in Figure 2, there was a significant difference between the two groups in the selection rate of related words among incorrect responses during the theme identification task (control, 97.7 ± 6.8%; schizophrenia, 84.1 ± 21.0%; t = 2.44, df = 32, d = 0.87, p = 0.02). In other words, the proportion of unrelated words among the wrong answers was significantly higher in patients with schizophrenia than controls. There was no group difference in the response time for either the theme or the sex identification tasks.
Table 2.
Behavioral responses in the theme and sex identification tasks
| Control (N = 22) | Schizophrenia (N = 20) | p-value* | |
|---|---|---|---|
|
Theme identification |
|
|
|
| Correct rate (%) |
|
|
|
| Positive |
91.7 ± 10.5 |
84.9 ± 19.9 |
0.167 |
| Negative |
89.9 ± 12.3 |
77.8 ± 20.4 |
0.029 |
| Overall |
91.1 ± 9.9 |
81.0 ± 17.0 |
0.022 |
| Response time (msec) |
|
|
|
| Positive |
1629.3 ± 254.9 |
1534.0 ± 391.3 |
0.351 |
| Negative |
1704.5 ± 263.7 |
1682.5 ± 405.6 |
0.835 |
| Overall |
1666.9 ± 242.6 |
1608.3 ± 377.3 |
0.549 |
|
Sex identification |
|
|
|
| Correct rate (%) |
|
|
|
| Positive |
88.2 ± 11.0 |
87.2 ± 12.6 |
0.794 |
| Negative |
93.2 ± 8.4 |
94.0 ± 8.5 |
0.747 |
| Overall |
90.7 ± 8.6 |
90.1 ± 9.7 |
0.846 |
| Response time (msec) |
|
|
|
| Positive |
1060.4 ± 274.6 |
1122.8 ± 526.7 |
0.639 |
| Negative |
1030.5 ± 477.3 |
1032.2 ± 528.6 |
0.991 |
| Overall | 1045.4 ± 339.1 | 1077.5 ± 518.6 | 0.812 |
*by independent sample t-test.
Figure 2.
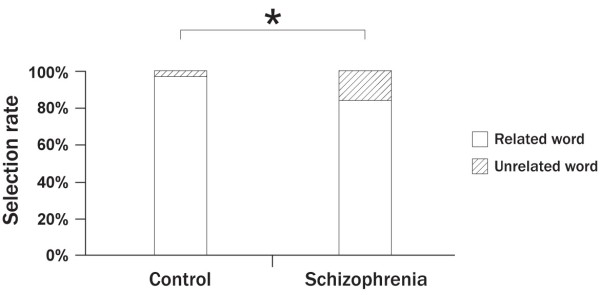
Comparison of the patient and control groups for the selection rates of related versus unrelated words in the trials with wrong selections in the theme identification task (* P < 0.05).
Eye gaze results
For the fixation and saccade counts, there was no significant main effect of the task type and no significant group × task-type interaction. However, there were significant main effects of group on fixation [F (1,40) = 6.78, partial η 2 = 0.145, p = 0.01] and saccade [F (1,40) = 6.54, partial η 2 = 0.141, p = 0.01] (Table 3). Patients showed lower fixation and saccade counts than controls during both tasks [theme (fixation: t = 2.24, df = 38.3, d = 0.69, p = 0.031; saccade: t = 2.12, df = 37.2, d = 0.66, p = 0.041), sex (fixation: t = 2.84, df = 40, d = 0.88, p = 0.007; saccade: t = 2.86, df = 40, d = 0.87, p = 0.007)]. For the fixation rate in the AOI and the sample count, there were no significant main effects of the group or task type and no significant group × task-type interaction.
Table 3.
Eye gaze results during the theme and sex identification tasks
| Control (N = 22) | Schizophrenia (N = 20) | p-value* | |
|---|---|---|---|
|
General eye gaze |
|
|
|
| Abstract or Theme |
|
|
|
| Sample count |
338.7 ± 8.6 |
323.7 ± 41.2 |
0.102 |
| Fixation count |
17.4 ± 7.3 |
12.1 ± 8.1 |
0.031 |
| Saccade count |
19.1 ± 6.9 |
14.1 ± 8.2 |
0.041 |
| Concrete or Sex |
|
|
|
| Sample count |
335.7 ± 12.6 |
319.7 ± 53.9 |
0.184 |
| Fixation count |
17.2 ± 7.3 |
10.4 ± 8.1 |
0.007 |
| Saccade count |
18.5 ± 6.1 |
12.2 ± 8.2 |
0.007 |
|
AOI gaze |
|
|
|
| Abstract or Theme |
|
|
|
| AOI fixation/Total fixation (%) |
7.5 ± 6.7 |
5.5 ± 5.2 |
0.300 |
| Concrete or Sex |
|
|
|
| AOI fixation/Total fixation (%) | 7.3 ± 5.7 | 7.4 ± 6.5 | 0.955 |
*by repeated measures analysis of variance and post hoc t-test.
AOI, area of interest.
Correlations
The rates of correct theme identification were significantly correlated with the scores of the Similarities subtest (r = 0.55, p = 0.01) and scores on the Social Anhedonia Scale (r = −0.58, p = 0.01) in patients, but not in controls. There was no other significant correlation between task performance and clinical variables including years of education and general intelligence in either group.
Fixation count (theme: r = 0.48, p = 0.03; sex: r = 0.57, p = 0.01) and saccade count (theme: r = 0.59, p = 0.01; sex: r = 0.65, p = 0.002) in both tasks were correlated with the intelligence score in patients, while only fixation count (theme: r = 0.47, p = 0.03, sex: r = 0.44, p = 0.04) in both tasks was correlated with the intelligence score in controls. Eye gaze results in both tasks did not show any significant correlation with scores of the SAS and doses of antipsychotics in patients.
Linear regression analysis showed a significant interaction effect (p = 0.009) between group and fixation count with respect to the rate of correct theme identification (equation for controls: correct rate = 93.463 - 0.135 × fixation count; equation for patients: correct rate = 65.978 + 1.236 × fixation count), but no significant interaction effect was found with respect to sex identification. There was a significant correlation between the rates of correct theme identification and fixation counts in patients (r = 0.59, p = 0.006), but not in controls. There was also no significant correlation between the rate of correct sex identification and fixation in controls or patients (Figure 3).
Figure 3.
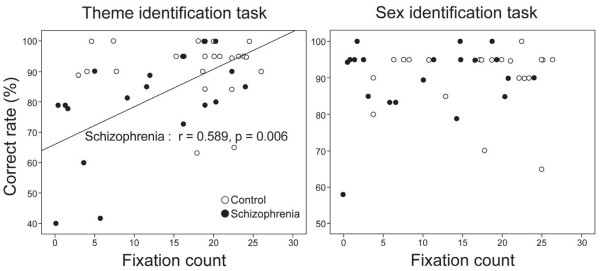
Relationship between the eye fixation counts and rates of correct selections in each group. A significant correlation was found only during the theme identification task in the patient group.
Discussion
In this study, we developed a new theme identification task to exclusively assess abstract thinking ability. As expected, patients scored worse for theme identification and for reasoning similarities than controls, and they showed a significant correlation between scores of the Similarities subtest and rates of correct theme identification. These results are consistent with previous findings of deficits in abstract thinking in schizophrenia [1,3-7], suggesting that our task may be an effective alternative measurement. Another major result of this study is that patients selected unrelated words more often as wrong answers during theme identification than controls. Because the unrelated words were the most concrete words for the picture, these results suggest that patients tend to think more concretely than controls. Given that the selection rate of related words as wrong answers could detect subtle differences in abstract thinking, our task may be an alternative or supplementary tool for the evaluation of abstract thinking.
In contrast with earlier findings of abnormal results on the proverb test only in older people [11,28], our younger patients showed abnormalities in identifying the theme, suggesting that detection of deficits in abstract thinking at a younger age may be an advantage of our task. Another advantage of our task is to provide a measure of performance that is unaffected by education or general knowledge unlike the proverb test, and to reflect judgments in some situations similar to real life. However, given that patients with severe symptoms exhibited increased impairment in abstract thinking in a previous report [1], our finding indicates that the poor performance by patients with schizophrenia can be affected by the course of the illness. Meanwhile, it should be noted that healthy controls showed no significant correlation between scores of the Similarities subtest and rates of correct theme identification. This may be attributed to the ceiling effect by consistently high performance because our task was relatively easy for them. In other words, their relatively high correct rates and small variance (see Table 1) could mask the correlation. Therefore, the advantages of our new task may be limited to patients with difficulties in abstract thinking.
Patients showed decreased eye fixation and saccadic eye movement compared with controls during both tasks, suggesting that patients used more inactive and ineffective searching to get information from stimuli. This is consistent with a previous finding that patients had decreased eye fixation and saccades [29]. In addition, deficits in eye gaze were not more remarkable during the abstract thinking task than the concrete thinking task, that is, deficits were found for both tasks. However, the rate of correct selections in patients was lower only in the abstract thinking task. These results may have occurred because the minimal demand of visual searching is higher for abstract thinking than concrete thinking. It is noteworthy that the rate of correct theme identification was correlated with fixation count in patients, but not in controls. This finding may be due to the ceiling effect, which implies that controls had already searched the stimuli with sufficient eye fixations. In contrast, patients had not usually explored the stimuli with sufficient eye fixations, and thus, differences in eye fixations may connect to changes in abstract thinking ability.
As hypothesized, our results showed a significant correlation between abstract thinking and eye fixation in patients. The eye is the most effective sensory organ for gathering information, and eye gaze may be an important factor for cognition [30]. A person who cannot integrate information from gaze may get some troubles in abstract thinking [31,32]. Because visual searching seems to be influenced by a “top-down process” [14], patients with schizophrenia who have executive dysfunctions may have ineffective searching strategies when abstract thinking is required [33]. It is unclear what mechanism is underlying this relationship between defects in abstract thinking and abnormalities in oculomotor movement or visual searching behaviors. The frontal eye field has been postulated to represent the region of oculomotor control [34], and the dorsolateral prefrontal cortex (DLPFC) plays a role in abstract thinking [35,36]. The DLPFC has direct connections with the cortical ocular motor areas including the frontal eye field and may be involved in the decisional processes in ocular motor behavior [37]. Furthermore, patients with schizophrenia showed functional abnormalities in the frontal eye field [38] and DLPFC [39] during eye movement. Taken together, this evidence suggests that disruptions in functional circuitry between the cortical oculomotor area and the DLPFC may be a reason for both deficits in abstract thinking and eye gaze of patients with schizophrenia. Future neuroimaging studies will be needed to verify our speculation.
In terms of emotion, patients with schizophrenia showed lower rates of correct theme identification than controls, particularly during the negative emotional condition. According to previous studies, normal people showed better task performance when the emotional valence and arousal were prominent [40], while patients with schizophrenia exhibited low task performance during a negative emotional situation [41,42]. Furthermore, patients with schizophrenia also had reduced prefrontal activity while negative emotional stimuli were presented [43]. As mentioned above, the DLPFC is important to thinking abstractly. Thus, our finding of more deficits in abstract thinking during negative emotional conditions may also reflect prefrontal dysfunction.
Moreover, we found a negative correlation between abstract thinking ability and severity of social anhedonia. This is in line with a previous finding of the significant relationship between abstract thinking and negative symptoms in schizophrenia [44]. Considering that deficits in abstract thinking may make it difficult to perform social behaviors [2,45] and that problems in social behavior may be a stressful condition triggering the emergence of anhedonia [46], it makes sense that impaired abstract thinking can contribute to social anhedonia as well. On the other hand, social anhedonia may cause social dysfunctions [45], and social dysfunctions may limit the development of mental ability including abstract thinking [47]. Therefore, social anhedonia seems to have some effects on abstract thinking as well. Accordingly, we can only explain that abstract thinking is closely correlated with social anhedonia. A future longitudinal study is needed to characterize the causal relationship between abstract thinking and social anhedonia in schizophrenia.
There are some limitations in this study. Our patients were taking antipsychotic medications. Several antipsychotics can create extrapyramidal symptoms such as muscle rigidity, and they could impair eye movements in patients. It should be noted, however, that there were no significant correlations between scores of the SAS, doses of antipsychotics, and gaze results. There was a significant group difference in years of education, although intelligence was not significantly different between patients and controls. The order of the tasks was not counterbalanced across subjects to avoid the exposure to the visual stimuli before theme identification. Because the sex identification task was always performed later, the lack of group difference in the performance of sex identification might be due to the greater effect of practice and familiarity with the stimuli among patients. This study had a relatively small sample size, and it was not clear in our design whether the relationship between eye gaze and abstract thinking remained stable over time.
Conclusions
Our results suggest that patients with schizophrenia exhibit impaired abstract thinking and decreased quality of gaze. This study provides evidence that deficits in eye gaze may be associated with impaired abstract thinking in schizophrenia. Theme identification and eye gaze tasks may be a useful tool for objective measurement of abstract thinking in patients with schizophrenia. Future studies are needed to clarify the mechanism of the link between abstract thinking and eye gaze in schizophrenia.
Abbreviations
IAPS: International affective picture system; AOI: Area of interest; RPM: Raven’s standard progressive matrices; PANSS: Positive and negative syndrome scale; SAS: Simpson-angus scale; ANOVA: Analysis of variance.
Competing interests
All authors declare that they have no conflicts of interest, including no financial, personal or other relationships with other people or organizations.
Authors’ contributions
JO collected the data, analyzed and interpreted data, drafted the manuscript. JJK conceived the idea, designed the study, and participated in writing up and revising the manuscript. JWC and JSL collected the data and assisted data analysis and paper writing. All authors read and approved the final manuscript.
Contributor Information
Jooyoung Oh, Email: ojuojuoju@naver.com.
Ji-Won Chun, Email: cuneus@naver.com.
Jung Suk Lee, Email: thanato96@naver.com.
Jae-Jin Kim, Email: jaejkim@yonsei.ac.kr.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. NRF-2013R1A2A2A03068342).
References
- Harrow M, Adler D, Hanf E. Abstract and concrete thinking in schizophrenia during the prechronic phases. Arch Gen Psychiatry. 1974;10:27–33. doi: 10.1001/archpsyc.1974.01760130013002. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Abstract thinking and social behavior in schizophrenia. J Abnorm Psychol. 1956;10:208–211. doi: 10.1037/h0048126. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Bryson GJ, Davis LW, Bell MD. Relationship of impaired processing speed and flexibility of abstract thought to improvements in work performance over time in schizophrenia. Schizophr Res. 2005;10:211–218. doi: 10.1016/j.schres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Gorham DR. Use of the proverbs test for differentiating schizophrenics from normals. J Consult Psychol. 1956;10:435–440. doi: 10.1037/h0042949. [DOI] [PubMed] [Google Scholar]
- Nahor AB, Vannicelli M. The influence of instructional set on schizophrenic vs. organic concreteness. Confin Psychiatr. 1976;10:89–95. [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10:205–210. doi: 10.1016/S0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;10:25–40. doi: 10.1016/S0193-953X(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Schmittmann VD, Visser I, Raijmakers MEJ. Multiple learning modes in the development of performance on a rule-based category-learning task. Neuropsychologia. 2006;10:2079–2091. doi: 10.1016/j.neuropsychologia.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;10:54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Han K, Young Kim I, Kim JJ. Assessment of cognitive flexibility in real life using virtual reality: A comparison of healthy individuals and schizophrenia patients. Comput Biol Med. 2012;10:841–847. doi: 10.1016/j.compbiomed.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 2007;10:653–663. doi: 10.1017/S1355617707070816. [DOI] [PubMed] [Google Scholar]
- Mele M, Federici S. Gaze and eye-tracking solutions for psychological research. Cogn Process. 2012;10:261–265. doi: 10.1007/s10339-012-0499-z. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements and attention in reading, scene perception, and visual search. Q J Exp Psychol. 2009;10:1457–1506. doi: 10.1080/17470210902816461. [DOI] [PubMed] [Google Scholar]
- Niu Y, Todd RM, Anderson AK. Affective salience can reverse the effects of stimulus-driven salience on eye movements in complex scenes. Front Psychol. 2012;10:336. doi: 10.3389/fpsyg.2012.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedie SA, Clair DMS, Benson PJ. Atypical scanpaths in schizophrenia: evidence of a trait- or state-dependent phenomenon? J Psychiatry Neurosci. 2011;10:150–164. doi: 10.1503/jpn.090169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-H, Ku J, Han K, Kim E, Kim SI, Park J, Kim JJ. Deficits in eye gaze during negative social interactions in patients with schizophrenia. J Nerv Ment Dis. 2010;10:829–835. doi: 10.1097/NMD.0b013e3181f97c0d. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsushima E, Ohta K, Toru M, Han YH, Shen YC, Moussaoui D, David I, Sato K, Yamashita I, Kathmann N, Hippius H, Thavundayil JX, Lal S, Vasavan Nair NP, Potkin SG, Prilipko L. Stability of exploratory eye movements as a marker of schizophrenia — a WHO multi-center study. Schizophr Res. 2001;10:203–213. doi: 10.1016/S0920-9964(00)00181-X. [DOI] [PubMed] [Google Scholar]
- Delerue C, Boucart M. The relationship between visual object exploration and action processing in schizophrenia. Cogn Neuropsychiatry. 2012;10:334–350. doi: 10.1080/13546805.2011.646886. [DOI] [PubMed] [Google Scholar]
- Ries HA, Johnson MH, Armstrong HE Jr, Holmes DS. The draw-a-person test and process-reactive schizophrenia. J Proj Tech Pers Assess. 1966;10:184–186. doi: 10.1080/0091651X.1966.10120289. [DOI] [PubMed] [Google Scholar]
- Gustafson JL, Waehler CA. Assessing concrete and abstract thinking with the Draw-a-Person technique. J Pers Assess. 1992;10:439–447. doi: 10.1207/s15327752jpa5903_2. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute Biometric Research; 1996. [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville: University of Florida, Center for Research in Psychophysiology; 1995. [Google Scholar]
- Yeom TH, Park YS, Oh KJ, Lee YH. Korean Wechsler Adult Intelligence Scale (K-WAIS) Manual. Seoul: Hankook Guidance; 1992. [Google Scholar]
- Raven J. The Raven’s progressive matrices: change and stability over culture and time. Cogn Psychol. 2000;10:1–48. doi: 10.1006/cogp.1999.0735. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;10:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;10:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Janno S, Holi M, Tuisku K, Wahlbeck K. Validity of Simpson-Angus Scale (SAS) in a naturalistic schizophrenia population. BMC Neurol. 2005;10:5. doi: 10.1186/1471-2377-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Wolfe J, Lafleche G. Differences in abstraction ability with age. Psychol Aging. 1990;10:94–100. doi: 10.1037//0882-7974.5.1.94. [DOI] [PubMed] [Google Scholar]
- Bestelmeyer PEG, Tatler BW, Phillips LH, Fraser G, Benson PJ, St. Clair D. Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr Res. 2006;10:212–222. doi: 10.1016/j.schres.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neurosci Biobehav Rev. 2009;10:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde M, von Cramon DY, Schubotz RI. Differential role of anterior prefrontal and premotor cortex in the processing of relational information. Neuroimage. 2010;10:2890–2900. doi: 10.1016/j.neuroimage.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Ann N Y Acad Sci. 2007;10:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- Liu KCM, Chan RCK, Chan KKS, Tang JY, Chiu CP, Lam MM, Chan SK, Wong GH, Hui CL, Chen EY. Executive function in first-episode schizophrenia: a three-year longitudinal study of an ecologically valid test. Schizophr Res. 2011;10:87–92. doi: 10.1016/j.schres.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;10:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Berman K, Illowsky BP, Weinberger DR. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: Iv. further evidence for regional and behavioral specificity. Arch Gen Psychiatry. 1988;10:616–622. doi: 10.1001/archpsyc.1988.01800310020002. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman K, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: Iii. a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;10:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny CH, MÜRi RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann N Y Acad Sci. 2005;10:239–251. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Lee AKC, Agam Y, Vangel M, Goff DC, Barton JJS, Manoach DS. Abnormally persistent fMRI activation during antisaccades in schizophrenia: a neural correlate of perseveration? Schizophr Res. 2011;10:62–68. doi: 10.1016/j.schres.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res Neuroim. 2006;10:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Citron FMM, Weekes BS, Ferstl EC. Effects of valence and arousal on written word recognition: Time course and ERP correlates. Neurosci Lett. 2013;10:90–95. doi: 10.1016/j.neulet.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;10:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Quintana J, Lee J, Marcus M, Kee K, Wong T, Yerevanian A. Brain dysfunctions during facial discrimination in schizophrenia: selective association to affect decoding. Psychiatry Res Neuroim. 2011;10:44–50. doi: 10.1016/j.pscychresns.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Morris R, Green MJ, Lenroot R, Kulkarni J, Carr VJ, Weickert CS, Weickert TW. Reduced neural activity of the prefrontal cognitive control circuitry during response inhibition to negative words in people with schizophrenia. J Psychiatry Neurosci. 2012;10:379–388. doi: 10.1503/jpn.110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandina G, Bilder R, Turkoz I, Alphs L. Identification of clinically meaningful relationships among cognition, functionality, and symptoms in subjects with schizophrenia or schizoaffective disorder. Schizophr Res. 2013;10:312–318. doi: 10.1016/j.schres.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull. 1998;10:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6 J mice. Psychoneuroendocrinology. 2012;10:762–772. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Gottlieb G, Blair C. How early experience matters in intellectual development in the case of poverty. Prev Sci. 2004;10:245–252. doi: 10.1023/b:prev.0000045358.12782.6b. [DOI] [PubMed] [Google Scholar]


