Abstract
Proper skin care is considered to be an important component of the total management plan for patients with acne vulgaris. A 28-day, open-label study provided both practical and scientific information on a designated skin care regimen in subjects with acne vulgaris. The cutaneous tolerability overall performance, and assessment of objective parameters evaluating the epidermal permeability barrier were documented with use of a specific foaming skin cleanser and a moisturizer with an SPF 30 broad spectrum rating in actively treated subjects with acne vulgaris. The results were favorable overall with the regimen shown to be nonirritating based on investigator and subject assessments, with high subject satisfaction and cosmetic acceptability ratings reported for both the foaming skin cleanser and the moisturizer with an SPF 30 broad spectrum rating. Objective instrumental testing of transepidermal water loss and epidermal hydration support that this skin care regimen assists in correcting epidermal permeability barrier dysfunctions that are innately present in acne vulgaris, worsened during a flare, and are known to be associated with many medications used to treat acne vulgaris. The recommendation of a specified skin care regimen incorporated into the overall management of acne vulgaris simplifies and standardizes the program for the patient, demonstrates a high level of interest by the clinician, and reduces the risk of the patient self-acquiring facial skin care products that may increase skin irritation.
Acne vulgaris (AV) is the most commonly encountered dermatological disorder in ambulatory dermatology practice in the United States regardless of ethnicity, gender, or skin color, comprising approximately 10 percent of office visits to dermatologists annually in 2009 based on data captured by the US federal government.1-3 A variety of medical therapies, both topical and systemic, are available for the treatment of AV, with selection of therapy for a given patient based primarily on the current severity at the time of presentation and history of previous treatments.4-6 One of the major limitations of topical therapies for AV, especially for facial AV, is the relatively high potential for tolerability reactions characterized by visible signs (i.e., erythema, scaling, peeling, edema, dryness, roughness) and/or symptoms (i.e., stinging, burning) of cutaneous irritation.4-10 These reactions can result from direct effects of active ingredient (i.e., retinoid, benzoyl peroxide [BP]) and/or the characteristics of the vehicle, with patients in some cases electing to discontinue treatment or use therapy intermittently, which usually results in less than optimal therapeutic outcomes.11-17 Sometimes, patients do not follow up with their dermatologist for further care after experiencing skin irritation from topical medications, which is unfortunate as the inflammation driving the disorder continues unchecked. Other times, patients treated with topical agents for AV try to work through the challenges of skin tolerabftity reactions, such as redness, peeling, and symptoms of skin irritation (i.e., stinging, burning, itching) by changing to “spot application” of medications to only acne lesions, using therapy less frequently and/or self-treating with moisturizer application to diminish the adverse visible signs and symptoms of irritated facial skin.18
Interestingly, many dermatologists do not regularly recommend pre-emptive adjunctive skin care from the start when initiating therapy for AV, which almost always includes one or more topical medications that are well-known to induce signs and symptoms of skin irritation in some patients, especially during the first 2 to 4 weeks of treatment.27 Additionally, published guidelines on the management of AV do not consistently address, especially in detail, the scientific and therapeutic principles that support rationally selected skin care, proper product selection, and the importance of dermatologist-directed skin care in the management of AV, likely due to the relative absence of clinical research that has strong evidence-based ranking.4-6 Such research is Hmited by the fact that the vast majority of skin care products, even those designated for use in patients with specific skin disorders (i.e., AV, eczema, psoriasis, rosacea), are obtained over-the-counter (OTC) without need for a prescription and are not subject to the requirements for extensive clinical study before being introduced into the marketplace. Publications on the “nuts and bolts” of how to most effectively integrate skin care into the therapeutic regimen for AV are also lacking, as are an adequate number of studies that incorporate established subjective and objective measures that are scientifically sound and well-recognized to demonstrate additive benefit that is clinically relevant. However, more data are progressively emerging, and the importance of gentle skin care and avoiding agents that can promote epidermal barrier impairment and exacerbate skin irritation are well-recognized in the dermatology literature and by an increasing number of clinicians in practice.11,12,22,28-32 Nevertheless, the concept of dermatologist-selected preemptive skin care in AV still requires more educational effort and additional clinical research. Interestingly, only six percent of 116 dermatology practitioners surveyed reported that they pre-emptively recommend the use of a moisturizer/barrier repair product when prescribing oral isotretinoin, approximately 30 percent stated that their approach is to wait for adverse cutaneous effects to emerge clinically before they address the problem, and approximately half (47.8%) reported that they do not recommend use of a moisturizer in oral isotretinoin-treated patients.27 The low percentage of dermatologists recommending adjunctive skin care in patients with AV undergoing oral isotretinoin therapy is especially surprising as it is well known that this agent induces xerotic skin changes that are usually clinically apparent.4-6,27 It is evident that more research and careful observation are needed regarding AV and the role of skin care, optimal formulation characteristics, and methods of integration with topical therapies to assist dinicians in this important area of clinical practice. Dermatology practitioners are commonly confronted with many patients with AV who have questions about which skin care products to use, and when not given specified information from the dermatology practice where they sought care, often seek advice from a variety of non-professional sources via retail stores, spas, skin care centers, and the internet.
Despite the relative lack of clinical research in the area of skin care and AV, the importance of rationally selected adjunctive gentle skin care as an integral component of the overall management of several skin disorders (i.e., AV, rosacea, psoriasis, atopic dermatitis, other eczematous/xerotic skin disorders) has received much greater recognition in the dermatology literature and at major dermatology meetings over the past decade and continues to be an important subject to dinicians. This high level of current interest in proper skin care in patients with common skin disorders such as AV is based on both vigilant clinical observation and the steady increase in peer-reviewed publications and presentations that discuss both the practical implications and available scientific evidence.11-16,18-25,27-32
This article is Part 2 in a two-part series on the importance of proper skin care in the management of AV. The results of a 28-day, open-label, single-center clinical study evaluating the use of a designated skin care regimen in 91 patients who were already undergoing stabilized treatment for AV for at least 30 days are presented. The age range of patients included in the study was from 12 years to 45 years, allowing for inclusion of adolescents and adults who were actively affected by AV. This is relevant clinically as both populations are commonly encountered in clinical practice, with the recruitment design favoring a targeted number of adult women as this population has been identified as increasing in clinical practice.26
The designated skin care regimen defined in the study protocol included a specified brand foam wash (FW; Cetaphil® DermaControl™ Foam Wash, Galderma Laboratories, Fort Worth, Texas) used twice daily and a specified brand moisturizer with broad spectrum photoprotedion (M-SPF30; Cetaphil® DermaControl™ Moisturizer SPF 30, Galderma Laboratories) applied once daily in the morning. These skin care products are commercially available and were developed and formulated for use in acne-prone and acne-affected skin with the choice of ingredients chosen to address common skin-related needs of patients with AV. The development and formulation characteristics of both the FW and M-SPF30 are reviewed in more detail in Part 1 of this two-part series (DelRosso JQ. The Role of Skin Care as an Integral Component in the Management of Acne Vulgaris. Part 1: The Importance of Cleanser and Moisturizer Ingredients, Design, and Product Selection. J Clin Aesthet Dermatol. 2013;6[12]), and later in the discussion section of this article.
The major objectives of this study were to evaluate the cutaneous tolerability, overall performance, and cosmetic acceptability of the designated skin care regimen in patients who were already undergoing treatment for clinically evident AV. Other study parameters documented patient satisfaction outcomes (N=91) at the end of the study (Day 28) including the cosmetic acceptabuity and performance of the skin care regimen. In addition, objective instrumental testing was completed in a subset of study subjects (n=47) to determine the epidermal permeability barrier repair/maintenance properties (i.e., transepidermal water loss [TEWL], epidermal hydration by skin impedance [corneometry]) of the combined use of the FW and M-SPF30 in patients already using a stable regimen of topical acne medications for at least one month before enrollment in the study.
METHODS
Overall study design. General design. This was a single-center, open-label clinical trial designed primarily to assess the cutaneous tolerability, overall performance, and cosmetic acceptability of a designated regimen containing two skin care formulations, a facial skin cleanser (foam wash) and moisturizer with broad spectrum sunscreen activity (moisturizer SPF 30). The protocol requirement was that the study be completed in otherwise healthy subjects with AV who are actively undergoing therapy for AV with a stable regimen for at least one month. The study was conducted in compliance with the Declaration of Helsinki and current Good Clinical Practice guidelines. An institutional review board (IRB; IntegReview; Austin, Texas) approved the study protocols and related documents and forms. All potential subjects were informed regarding the study details with all informed consent information provided prior to deciding on their participation in the study. All enrolled subjects were given written informed consent materials, which were required from the subject for entry into the study and from a legal guardian if the patient was a minor.
Study products. All study subjects used Cetaphil® DermaControl™ Foam Wash (FW; Galderma Laboratories, Fort Worth, Texas) twice daily (morning and evening) and Cetaphil® DermaControl™ Moisturizer SPF 30 (M-SPF30; Galderma Laboratories) once daily (morning after cleansing) for 28 days. Both products were provided directly to each enrolled subject.
Study assessments. The study design purposefully enrolled two groups of subjects, each group completing different study courses with regard to follow up and certain study procedures. All subjects in both study groups underwent completion of subjective and objective assessments of specific parameters. With subjective assessments of facial skin tolerability, subjects were asked about the occurrence of facial skin symptoms occurring over the course of the study, specifically itching, burning, stinging, and skin tightness. Objective assessments of facial skin tolerability were completed by the investigator, who was a board-certified dermatologist, with evaluation of visible signs associated with facial skin irritation or intolerance, specifically erythema, edema, dryness, and roughness. All study subjects had facial photographs taken at the study center at baseline and at each follow-up visit through completion of the study using the second generation VISIA CR® system (Canfield Scientific, Fairfield, New Jersey).
Study groups. The enrollment target was a total of 80 study subjects, 40 per group. Subjects enrolled into study group 1 (Gl) had subjective and objective assessments completed and facial photographs obtained at baseline, Day 14, and Day 28 (end of study). The subset of subjects enrolled into study group 2 (G2) underwent subjective and objective assessments, facial photography, and instrumental measurements to assess TEWL and epidermal hydration status of facial skin obtained at baseline, Day 14, and Day 28 (end of study). At Day 7, the subjects in G2 underwent all the same assessments except for the objective assessment of facial skin tolerability by the investigator. All study subjects (Gl and G2 groups) completed a cosmetic acceptability questionnaire at the end of the study (Day 28).
Instrumental measurements. The instrumental measurements completed in G2 were included to objectively and quantitatively document recognized parameters that assess epidermal (stratum corneum) permeability barrier function. TEWL was measured using a Tewameter® 300, and epidermal hydration was measured by skin impedance testing (corneometry) using the NOVA DPM 9003®.
Adverse events. Adverse events (AEs) were recorded throughout the study. A technician asked each subject at each visit about facial skin irritation and any other AEs and also confirmed any concomitant medications used during the study, including changes from visit to visit. Subjects were given diaries to record time of application, any product performance observations they observed, and any comments on skin tolerability and safety.
STUDY SUBJECTS
Enrollment considerations. The study protocol allowed for inclusion of males or females with currently active AV who were between the ages of 12 and 45 years. All enrolled subjects were required to be undergoing their current treatment for AV for at least one month before the start of the study. A dedicated effort was made to ensure that approximately 75 percent of the enrolled subjects were between the ages of 12 and 19 years and that the majority of the remaining adult subjects were female. Individuals with any visible dermatological disorder or abnormal skin pigmentation (including tattoos) that may have interfered with subjective or objective study assessments were excluded. Pregnant females were excluded from the study.
Subject disposition. A total of 97 subjects were enrolled with 91 subjects completing the 28-day study (Table 1). Reasons for lack of study completion in six enrolled subjects were noncompHance (n=4), lost to follow up (n=l), and protocol violation (n=l).
TABLE 1.
Patient demographics and disposition
| ENROLLED, N | 97 |
| COMPLETED STUDY, N | 91 (4 noncompliant, 1 lost to follow-up, 1 protocol violation) |
| MEAN AGE, YEARS | 18.4 years (SD+/-7.6 years) age range 12-45 years median age 16 years 71 subjects (78%) between ages 12 and 19 |
| GENDER, N (%) | |
| Male | 40 (44%) |
| Female | 51 (56%) |
| ETHNICITY, N (%) | |
| Caucasian | 36 (39.6%) |
| African American | 36 (39.6%) |
| Hispanic | 14 (15.4%) |
| Native American | 1 (1.1%) |
| Other | 4 (4.4%) |
Gl included 44 subjects and G2 included 47 subjects. Seventy-eight percent of subjects who completed the study were between the ages of 12 and 19 years.
CONCURRENT ACNE TREATMENT
The protocol inclusion criteria required that all subjects be currently undergoing stable acne treatment for at least one month prior to study entry and with no anticipated change in acne medication for the length of the study. Acne treatments were defined as either prescription (Rx) or over-the-counter (OTC) acne products. Rx acne treatments included topical and oral antibiotics, topical retinoids, topical combination products, oral isotretinoin, and oral contraceptives. OTC acne treatments included acne cleansers and moisturizers, benzoyl peroxide products, salicylic acid products, medicated wipes or pads, acne masks or scrubs, and acne medicated lotions.
Of the 97 subjects screened, 58 subjects (59.8%) reported only one concomitant medication, and 28 subjects (28.9%) reported two concomitant medications. Of note, some subjects reported they had used more than four concomitant acne treatments and more than 14 different OTC acne brands. Table 2 outlines the concomitant medications reported by the subjects.
TABLE 2.
Concurrent acne treatments used by study subjects (%)
| NUMBER OF CONCURRENT ACNE TREATMENTS [SUBJECTS, N (%)] | |
| 1 treatment | 58 (59.8) |
| 2 treatments | 28 (28.9) |
| 3 treatments | 6 (6.2) |
| 4 treatments | 4 (4.1) |
| 5 treatments | 1 (1.0) |
| TOTAL NUMBER OF OTC* OR RX** ACNE PRODUCTS# | |
| OTC | 104 |
| Rx | 49 |
| TYPES OF ACNE TREATMENT PRODUCTS | |
| OTC cleanser | 53 |
| Non-acne Rx | 16 |
| OTC spot treatment | 15 |
| OTC medicated pad/wipes | 15 |
| Rx combination topical | 15 |
| Oral contraceptives | 7 |
| Oral antibiotics | 5 |
| Topical antibiotics | 5 |
| OTC medicated lotion | 5 |
| OTC mask/scrub | 4 |
| OTC acne kit | 4 |
| OTC moisturizer | 4 |
| Oral isotretinoin | 3 |
| Rx topical retinoid | 2 |
OTC=over-the-counter;
Rx=presciption
Note that number of treatments is greater than 97 as subjects may have been on more than one acne treatment.
STATISTICAL ANALYSIS
Subjective and objective tolerability scores were compared to baseline scores using a Wilcoxon signed-rank test. Bioinstrumentation data were compared to baseline values using a paired t-test. A binomial test with a priori 50/50 distribution assumption was used to determine significance to questionnaire responses. Subjects who agreed with the product attribute were placed into the “success” category and those who did not agree with the attribute were placed into the “failure” category. Subjects who did not agree or disagree were pooled with the negative responses. All data were analyzed at the 95% confidence level. Only subjects with at least one post-baseline time point were included in the statistical analysis.
RESULTS
Subject distribution. Ninety-seven subjects were enrolled, and 91 completed the study (Table 1). The mean age among study subjects who completed the 28-day study was 18.4 years and the median age was 16.0 years. Females comprised 56 percent (n=51) of those who completed the study. The ethnicity disposition was 39.6 percent Caucasian (n=36), 39.6 percent African American (n=36), and 15.4 percent Hispanic (n=14) (Table 1). Additionally, 78 percent (n=71) were between the ages of 12 and 19 years. Female subjects comprised 60 percent of the remaining study population over the age of 19 years (n=12).
Subjective assessments. There were no significant differences in assessments of facial skin tolerability by the study subjects for stinging, tightness, itching, and burning at any time point during the study (Table 3).
TABLE 3.
Mean facial skin tolerability scores by subjective assessment (study subjects)
| MEAN SCORE# | ||||
|---|---|---|---|---|
| Baseline (n=94) | Day 7(n=48) | Day 14 (n=91) | Day 28 (n=91) | |
| Itching | 0.02 | 0.02 | 0.00 | 0.00 |
| P value* | – | 1.000 | 1.000 | 1.000 |
| Burning | 0.00 | 0.00 | 0.00 | 0.00 |
| P value* | – | N/C | N/C | N/C |
| Stinging | 0.02 | 0.00 | 0.00 | 0.00 |
| P value* | – | 1.000 | 1.000 | 1.000 |
| Tightness | 0.03 | 0.06 | 0.01 | 0.02 |
| P value* | – | 1.000 | .750 | 1.000 |
P value relative to baseline;
4-point rating scale (0-3): 0=none, 1 =mild, 2=moderate, 3=severe
N/C=not calculated due to zero values.
Objective assessments. Objective facial skin tolerability assessments determined by the investigator showed no significant changes in mean scores relative to baseline for erythema, edema, and roughness at any time point during the study (Table 4). A slight but significant increase in the mean score for facial skin dryness was observed at Day 14 (P=0.001) and Day 28 (P=0.002) relative to baseline (Table 4).
TABLE 4.
Mean facial skin tolerability scores by objective assessment (investigator)
| MEAN SCORE# | |||
|---|---|---|---|
| Baseline (n=94) | Day14(n=91) | Day 28 (n=91) | |
| Erythema | 0.22 | 0.33 | 0.20 |
| P value* | — | 0.059 | .754 |
| Edema | 0.01 | 0.00 | 0.00 |
| P value* | — | 1.000 | 1.000 |
| Dryness | 0.00 | 0.12 | 0.11 |
| P value* | — | 0.001 | 0.002 |
| Roughness | 0.01 | 0.00 | 0.07 |
| P value* | — | 1.000 | 0.125 |
P value relative to baseline;
4-point rating scale (0-3): 0=none, 1 =mild, 2=moderate, 3=severe
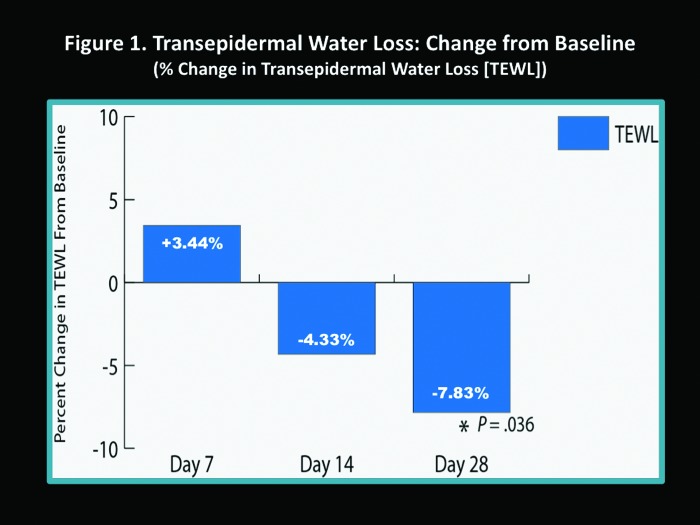
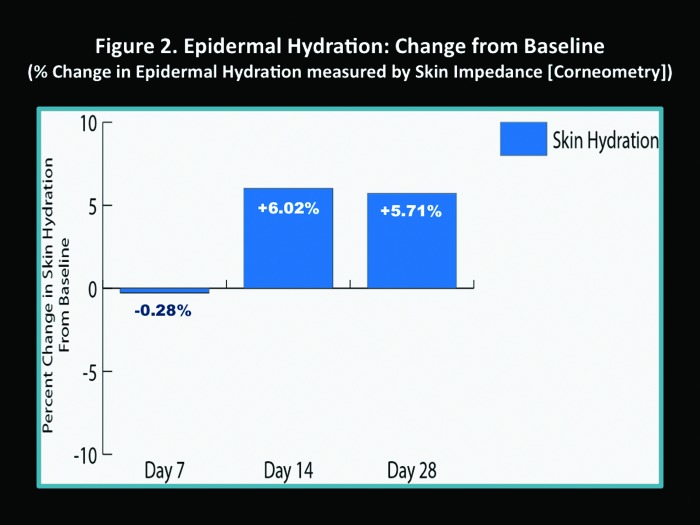
Instrumental measurements. The percent change in TEWL scores increased at Day 7 compared to baseline (3.44%); however, this was not statistically significant (Figure 1). At Day 14, TEWL had decreased compared to baseline (-4.33%), and by Day 28, TEWL had significantly decreased compared to baseline (-7.83%, P=0.036) (Figure 1). Epidermal hydration slightly decreased at Day 7 (-0.28%), but increased by Day 14 (6.02%) and Day 28 (5.71%), although comparison to baseline was not statistically significant (Figure 2).
Figure 1.
Transepidermal water loss (TEWL): Change from baseline (% change in TEWL)
Figure 2.
Epidermal hydration: Change from baseline (% change in epidermal hydration measured by skin impedance [Corneometry])
Cosmetic acceptability. At the end of the study (Day 28), a significant majority of subjects favorably rated FW and M-SPF30 in all responses to the cosmetic acceptability questionnaire. In particular, subjects felt that FW and M-SPF30 were easily incorporated into their daily skin routine (FW=96.7%; M-SPF30=95.6%), were nonirritating (FW=94.5%; M-SPF30=96.7%), liked the texture (feel) of the products (FW=95.6%; M-SPF30=92.3%), would recommend them to family and friends (FW=96.7%; M-SPF30=95.6%), and had a positive overall impression of the skin care products (FW=93.4%; M-SPF30=91.2%). To add, more than 85 percent of the 91 subjects completing the study believed the M-SPF30 helped improve the facial skin tolerability of the medications they were using for treatment of AV.
Facial skin tolerability. There were no statistically significant increases in erythema, edema, or roughness as compared to baseline after 14 days and 28 days of use of the FW and M-SPF30 products. The investigator assessment of increased mean score for facial skin dryness at both Day 14 (p=0.001) and Day 28 (p=0.002) did not appear to be clinically relevant when compared to patient-assessed dryness as only one patient reported moderate dryness during the study and continued to use the FW and M-SPF30 products throughout the entire duration of the study. There were no discontinuations from the study related to adverse events including skin tolerability reactions.
DISCUSSION
This clinical trial brings forth several important practical considerations related to the use of designated skin care as a component of the therapeutic regimen used for management of AV. First, the recommendation of a specific facial cleanser (FW) that achieved high satisfaction ratings from study subjects and was very well tolerated, coupled with directions on how and when to use it, simplifies the treatment program for the patient, and obviates the need for the patient to select a product on their own among the myriad of cleansers for AV that are available in the marketplace. The subject assessments of the FW captured in the survey completed at the end of the study were highly favorable with the following percent of study subjects noting specific observations about the FW:
96.7 percent—easy to apply
92.5 percent—left skin feeling smooth and soft
94.5 percent—not irritating
97.8 percent—did not cause stinging or burning of facial skin
81.6 percent—easily removed makeup
96.7 percent—easy to incorporate into their daily routine
81.3 percent—easier to use than current cleanser due to foaming properties
62.6 percent—preferred over currently used skin cleanser.
Simplification for the patient is also true with the recommendation of a designated moisturizer (M-SPF30), which, in addition to providing skin hydration and aiding in repair of the epidermal permeability barrier, also provides ultraviolet B (UVB) and UVA photoprotection rated as SPF 30. The M-SPF30 was evaluated for both UVA and UVB photoprotection using the most recently required testing methods and successfully achieved the broad spectrum designation and a sun protection factor (SPF) rating of 30.33,34 The “built in” photoprotection component of M-SPF30 is especially important for patients with AV, as the recommendation to avoid sun exposure and use sunscreen is included in the product information and approved package inserts of many topical and oral medications used to treat AV, such as those containing a topical retinoid, benzoyl peroxide, or a tetracydine class antibiotic.35-40 The M-SPF30 was also rated high in many patient satisfaction and cosmetic acceptability parameters in this current study, with the following percent of patients noting specific observations about M-SPF30:
87.9 percent—improved skin tolerability with use of acne medications
86.8 percent—allowed them to not miss applications of acne medications
85.7 percent—helped overall treatment.
The investigator assessments of facial skin tolerability did not demonstrate any concerning signals with use of the combined skin care regimen (FW and M-SPF30). Dryness was perceived by the investigator as a mean change among the subjects as compared to baseline; however, it was not determined to be of the magnitude to suggest any major clinical relevance. To add, the change in assessment of dryness perceived by the investigator did not correlate with the study patient assessments. The patients in the study did not identify facial skin dryness as a major concern with the exception of one patient who reported moderate facial dryness and did not discontinue use of FW or M-SPF30 during the study.
The results obtained from measurements of TEWL and epidermal hydration that were completed in 47 of the 91 enrolled subjects who completed the study may be misleading if one views the data in a cursory manner. The data are interpreted more accurately if one considers that these results reflect the reparative effects of the skin care regimen (FW and M-SPF30) on epidermal barrier function during acne treatment. The reparative properties of the skin care regimen offset the ongoing effects of concurrently used topical and/or oral acne therapies, which are known to induce impairment of the epidermal permeability barrier by increasing TEWL.11-16,27 In other words, the ultimate outcome measures of decreased TEWL and increased epidermal hydration represent the ability of the skin care regimen (FW and M-SPF30) to achieve the following two main objectives that support the use of a designated skin care regimen in patients with acne: (1) help repair and maintain epidermal permeability barrier function when used concurrently with topical and/or oral acne medications known to impair the permeabnity barrier and (2) help to offset the epidermal permeability barrier impairment associated with AV both inherently and secondary to inflammation that occurs during an acne flare.11,13,14 The TEWL and epidermal hydration results demonstrate that the skin care regimen (FW and M-SPF30) when used concomitantly with AV medications contributes to the improvement of epidermal permeability barrier function after 14 days and 28 days. This further exemplifies the importance of pre-emptive gentle skin care in patients who are undergoing medical treatment for AV.
What are the characteristics of the FW and the M-SPF30 that suggest specific adaptability for patients with acne-prone skin and acne-affected skin, including those undergoing treatment for AV? The development and formulation characteristics of both the FW and M-SPF30 are available from the medical services department of the manufacturer and have been reviewed in Part 1 of this two-part article series; however, a summary is provided below.33,41
The development of a facial wash that is adaptable for use on acne-prone skin, acne-affected skin, and acne-treated skin warrants a product that can lather enough to remove sebum and other unwanted material present on the skin surface (i.e., dirt, makeup, desquamated corneocytes), as many patients with AV have increased facial sebum production.42 The cleanser must be easy to apply, cosmetically pleasing, and have minimal potential for inducing skin irritation. The FW used in the study achieved high subject satisfaction and cosmetic acceptability ratings for all of these characteristics. Important excipients in the FW are glycerin (humectant), dipotassium glycyrrhizate (anti-inflammatory effects), and polyethylene glycol congeners (lubricant and dispersant with low irritation potential), all agents included to minimize cutaneous irritation and to impart a smooth skin texture.33,41 The major advance included in the FW is the novel surfactant, zinc coceth sulfate, which exhibits effective detergent qualities to cleanse skin, produces enough lather to be perceived as effective by those using it, has a low irritation potential, and exerts the ability to maintain function at lower ranges 33,41,43
As with the FW, the M-SPF30 achieved very high subject satisfaction and cosmetic acceptability ratings in this study of actively treated AV. The M-SPF30 moisturizer formulation includes several ingredients that were incorporated to address specific challenges that occur in acne-prone skin, acne-affected skin, and acne-treated skin. Ingredients included in the M-SPF30 to assist with epidermal permeability barrier repair and maintenance are a ceramide precursor (pseudoceramide 5), glycerin (humectant), and dimethicone (occlusive emollient).33 Other excipients included to aid in reducing the potential for skin irritation and also reported to exhibit antiinflammatory effects are allantoin, panthenol, and glycerretinic acid.44,45 Silica microbeads and corn starch are included as sebum absorbants adaptable for patients with oily skin to reduce “facial shine” and skin oiliness/greasiness, but are not problematic for patients with normal or dry skin.33,41 A matte-effect powder is also included to produce a smooth, non-shiny overall appearance to facial skin.33,41 Broad spectrum SPF 30 photoprotection is provided through the inclusion of avobenzone 3%, octisalate 5%, and octocrylene 7%, which are partitioned in plant-derived, lipid-based, lamellar spheres called oleosomes.46,47 The partitioning of the individual sunscreen components into the oleosomes increases their stability by preventing their physical interaction prior to application, allowing for a total sunscreen ingredient concentration of 15% with an SPF 30 rating.33,41 This concentration is markedly lower than what is typically needed to achieve an SPF 30 based on comparisons with other commercially available daily facial moisturizer SPF 30 products, with the lower sunscreen concentration more likely to reduce the risk of cutaneous irritation.33
This 28-day, open label clinical study provided practical and scientific information on designated skin care in subjects undergoing treatment for AV. In addition, the cutaneous tolerability and overall performance of a protocol-specified skin care regimen composed of a foaming skin cleanser (FW) and a moisturizer with SPF 30 broad spectrum rating (M-SPF30) were documented in actively treated subjects with AV. The results were favorable overall with the regimen shown to be nonirritating based on investigator and subject assessments, with high subject satisfaction and cosmetic acceptability ratings reported for both the FW and the M-SPF30. Objective instrumental testing of TEWL and epidermal hydration suggest that this skin care regimen helps to offset the impairments of the epidermal permeability barrier that are innately present in AV and worsened during a flare and are also known to be induced by many of the medications used to treat AV. The recommendation of a specified skin care regimen incorporated into the overall management of AV simpLfies and standardizes the program for the patient, demonstrates an important level of interest on the part of the dinician, and reduces the risk of the patient acquiring facial skin care products on their own that may be detrimental rather than helpful.
Footnotes
DISCLOSURE:Dr. Del Rosso has served as a consultant, advisory board participant, clinical investigator, and/or speaker for Allergan, Bayer Healthcare (Dermatology), Eisai, Galderma, Medicis (a division of Valeant), Obagi Medical Products, Onset Dermatologies, PharmaDerm, Primus, Promius, Quinnova, Fianbaxy, Taro Pharmaceuticals, TriaBeauty, Unilever, Valeant, and Warner-Chilcott. Dr. Del Rosso has served as a consultant for Galderma Laboratories on the subjects of acne, acne treatments, skin care, and skin care products including the products discussed in this article. He has received compensation for these services including his participation with this article (Part 2). This article was written by the authors and submitted to the journal by the lead author. Internal review by Galderma was completed to evaluate for accuracy of content prior to submission to the journal. After journal submission, the lead author served as the single point of contact with the journal and completed finalization of the article including any response to queries that arose during peer review and/or editorial review by journal staff. Ms. Brandt is an employee of Galderma Laboratories, L.P.
REFERENCES
- 1.Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11(4):466–473. [PubMed] [Google Scholar]
- 2.Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African-American women. J Eur Acad Dermatol Venereol. 2011;25(9):1054–1060. doi: 10.1111/j.1468-3083.2010.03919.x. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock MA, Boyle MM. Statistics of interest to the dermatologist. In: Del Rosso JQ, editor. Yearbook of Dermatology and Dermatologic Surgery. Vol. 67 Philadelphia: Elsevier-Mosby; 2012. [Google Scholar]
- 4.Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the global alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49:S1–S37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 5.Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the global alliance to imoprove outcomes in acne group. J Am Acad Dermatol. 2009;60:S1–350. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663. doi: 10.1016/j.jaad.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Thielitz A, Gollnick H. Topical retinoids in acne vulgaris: update on efficacy and safety. Am J Clin Dermatol. 2008;9(6):369–381. doi: 10.2165/0128071-200809060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Leyden JJ, Tanghetti EA, Miller B, et al. Once daily tazarotene 0.1% gel versus once daily tretinoin 0.1% microsponge gel for the treatment of facial acne vulgaris: a double blind randomized trial. Cutis. 2002;70(5):295–298. [PubMed] [Google Scholar]
- 9.Galvin SA, Gilbert R, Baker M, et al. Comparative tolerance of adapalene 0.1% and six different tretinoin formulations. Br J Dermatol. 1998;139(Suppl 52):34–40. doi: 10.1046/j.1365-2133.1998.1390s2034.x. [DOI] [PubMed] [Google Scholar]
- 10.Gold LS, Tan J, Cruz-Santana A, et al. Adapalene-BPO study group: a North American study of adapalene-benzoyl peroxide combination gel in the treatment of acne. Cutis. 2009;84(2):110–116. [PubMed] [Google Scholar]
- 11.Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? Do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aestfuet Dermatol. 2013;6(2):18–24. [PMC free article] [PubMed] [Google Scholar]
- 12.Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aestfuet Dermatol. 2011;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275–281. [PubMed] [Google Scholar]
- 14.Weber SU, Thiele JJ, Han N, et al. Topical tocotrienol supplementation inhibits lipid peroxidation but fails to mitigate increased transepidermal water loss after benzoyl peroxide treatment of human skin. Free Radix; Biol Med. 2003;34:170–176. doi: 10.1016/s0891-5849(02)01187-5. [DOI] [PubMed] [Google Scholar]
- 15.Elias PM. Epidermal effects of retinoids: supramolecular observations and clinical implications. J Am Acad Dermatol. 1986;15(4 Pt 2):797–809. doi: 10.1016/s0190-9622(86)70236-3. [DOI] [PubMed] [Google Scholar]
- 16.Elias PM, Fritsch PO, Lampev M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44(6):531–540. [PubMed] [Google Scholar]
- 17.Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17–24. doi: 10.1016/j.det.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SR, Chen DM. How patients experience and manage dryness and irritation from acne treatment. J Drugs Dermatol. 2011;10(6):605–608. [PubMed] [Google Scholar]
- 19.Del Rosso JQ. The use of moisturizers as an integral component of topical therapy for rosacea: clinical results based on the assessment of skin characteristics study. Cutis. 2009;84:72–76. [PubMed] [Google Scholar]
- 20.Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31–49. [PMC free article] [PubMed] [Google Scholar]
- 21.Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771–778. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 22.Del Rosso JQ. Moisturizers: function, formulation, and clinical applications. In: Draelos ZD, editor. Cosmeceuticals. Philadelphia: Saunders Elsevier; 2009. pp. 97–102. [Google Scholar]
- 23.Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatol Ther. 2004;17:49–56. doi: 10.1111/j.1396-0296.2004.04s1006.x. [DOI] [PubMed] [Google Scholar]
- 24.Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 25.Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol. 1997;14:321–324. doi: 10.1111/j.1525-1470.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 26.Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Del Rosso JQ. Clinical relevance of skin barrier changes associated with the use of oral isotretinoin: the importance of barrier repair therapy in patient management. J Drugs Dermatol. 2013;12(6):626–631. [PubMed] [Google Scholar]
- 28.Lebwohl M, Herrmann LG. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis. 2005;76(6 Suppl):7–12. [PubMed] [Google Scholar]
- 29.Del Rosso JQ. Understanding skin cleansers and moisturizers: the correlation of formulation science with the art of clinical use. Cosmetic Dermatohgy. 2003;16:19–31. [Google Scholar]
- 30.Johnson AW. Cosmeceuticals: function and the skin barrier. In: Draelos ZD, editor. Cosmeceuticals. Philadelphia: Saunders-Elsevier; 2009. pp. 7–14. [Google Scholar]
- 31.Goodman G. Cleansing and moisturizing in acne patients. Am J Clin Dermatol. 2009;10(Suppl 1):1–6. doi: 10.2165/0128071-200910001-00001. [DOI] [PubMed] [Google Scholar]
- 32.Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17:26–34. doi: 10.1111/j.1396-0296.2004.04s1003.x. [DOI] [PubMed] [Google Scholar]
- 33. Data on file. Fort Worth, Texas: Galderma Laboratories; 2013.
- 34.Sambandan DR, Ratner D. Sunscreens: an overview and update. J Am Acad Dermatol. 2011;64:748–758. doi: 10.1016/j.jaad.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 35. Retin A Micro 0.04%, 0.1% (microshere gel) [package insert]. Bridgewater, New Jersey: Valeant Pharmaceuticals; 2013.
- 36. Epiduo Gel [package insert]. Fort Worth, Texas: Galderma Laboratories; 2013.
- 37. Acanya Gel [package insert]. Bridgewater, New Jersey: Valeant Laboratories; 2013.
- 38. Tazorac Cream 0.1%, 0.05% [package insert]. Irvine, California: Allergan Pharmaceuticals; 2013.
- 39. Doryx Delayed Release (enteric coated) Tablets [package insert]. Rockaway, New Jersey: Warner Chilcott; 2013.
- 40. Solodyn Extended Release Tablets [package insert]. Bridgewater, New Jersey: Valeant Pharmaceuticals; 2013.
- 41.Del Rosso JQ. The role of skin care as an integral component in the management of acne vulgaris: part 1: importance of cleanser and moisturizer ingredients, design, and product selection. J Clin Aesthet Dermatol. 2013;6(12) [PMC free article] [PubMed] [Google Scholar]
- 42.James WD. Acne. N Engl J Med. 2005;352:1463–1472. doi: 10.1056/NEJMcp033487. [DOI] [PubMed] [Google Scholar]
- 43.Rigano L, Merlo E, Guala F, Villa G. Stabilized solutions of zinc coceth sulfate for cleansing and skin care. Cosmetics & Toiletries Magazine. 2005 [Google Scholar]
- 44.Int J Toxicol. 2007;26(Suppl2):79–112. Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. [Google Scholar]
- 45.Dohil M, et al. Atopic dermatitis and other inflammatory skin disease: natural ingredients for skin care and treatment. J Drugs Dermatol. 2011;10(9):S10–S14. [PubMed] [Google Scholar]
- 46.Beisson F, Ferté N, Bruley S, et al. Oil-bodies as substrates for lipolytic enzymes. Biochim Biophys Acta. 2001;1531(1-2):47–58. doi: 10.1016/s1388-1981(01)00086-5. [DOI] [PubMed] [Google Scholar]
- 47. [September 15, 2011]. http://www.botaneco.ca Oleosome Sunscreen Update Q4 2009 v2.