Abstract
Part 3 of this three-part review of atopic dermatitis and the stratum corneum barrier discerns how immune dysregulation, including upregulation of a TH2 inflammation pattern, augmented allergic sensitization, sustained wound healing inflammation, and impaired innate immunity, plays an integral role in the pathogenesis of atopic dermatitis. An increased understanding of the interdependence, polymorphisms, and dysregulations of epidermal barrier functions, including the stratum corneum permeability barrier, immune defense, and antimicrobial barriers, should provide further knowledge about the pathophysiological mechanisms that are clinically relevant and that contribute to the development of atopic dermatitis. Further understanding of these mechanisms should lead to newer therapies that target specific pathogenic components of atopic dermatitis.
Introduction
In Parts 1 and 2 of this three part review, the structural and functional abnormalities of the stratum corneum (SC) in atopic dermatitis (AD) and their pathophysiological and clinical implications were reviewed. These SC abnormalities do not exist independently with regard to AD pathogenesis. Rather, they are interrelated with altered immunologic and inflammatory responses associated with AD. As demonstrated in an experiment by Fallon et al1 with flaky mice, structural and functional defects of the SC permeabnity barrier alone are insufficient to induce or account for all of the abnormalities noted in AD. Dysregulation of immune response also appears to play a major integral role. Identified immune abnormalities in AD include increased TH2 inflammation,2,3 increased allergic sensitization,2,3 sustained wound healing inflammation,4 and impaired innate immunity5 Interestingly, the abnormalities in the immune system in AD may involve both augmented or suppressed immunologic detection and/or response, although the overall clinical effect often reflects hyper-reactive skin with induction of erythema, pruritus, eczematous, and sometimes urticarial skin changes.1-8 Although SC barrier integrity and function and abnormalities in immune response are discussed as separate entities in this three-part series, it is important to know that they are pathophysiologically interconnected.
TH2 Inflammation and Allergic Sensitization in Atopic Dermatitis
The heightened TH2 inflammatory response seen in AD results from a number of factors, with increased SP activity and sustained structural and functional SC barrier defects believed to be dominant contributors.2,3 As previously discussed in Part 1, increased SP activity has multiple physical effects on the SC. In addition, it induces significant perturbations in cutaneous immunological response. Increased SP activity mediates the conversion of interleukin (IL)-l alpha and beta from their pro-forms and stimulates their release from cytosol storage pools in corneocytes. These pro-inflammatory “jump start” cytokines then signal cascades of inflammation, which, in atopic patients, follows a TH2 response pattern.6
The depth and degree of inflammatory responses is also dysregulated in AD. When the SC permeability barrier is perturbed, the biosensor properties of the SC detect the increase in TEWL and decrease in SC water content. This detection initiates signaling cascades within the underlying epidermis, which are designed to self-repair SC structure and normalize SC function. This response results in a temporary increase in the biosynthesis of all major physiological lipids and lipid precursors in the epidermis. In healthy skin, the response to minor and temporary permeabnity barrier disruption usually remains localized to the epidermis; however, repeated or severe SC permeability barrier damage as seen in some skin diseases stimulates signaling cascades that not only engage the epidermal homeostatic response, but also initiate inflammatory responses involving the deeper layers of skin.7,8 Such persistent and unchecked SC barrier changes have been proposed to play a role in sustaining the dominant TH2 inflammatory pattern in AD.8,9
Impaired SC barrier function, in addition to increasing TH2 inflammation directly as described above, leads to increased antigen exposure, with continued and/or worsening SC dehydration causing further stimulation of TH2 inflammation.2 The increased antigen exposure and the TH2 predominant inflammation in turn stimulates increased allergic sensitization and immunoglobulin E (IgE) levels in AD.2,3 AD patients may be more susceptible to allergic sensitization from water-soluble allergens such as nickel, as least partially secondary to the decrease in lipids and/or ceramides in AD skin.10
An increase in TH2 inflammation causes a wide range of changes in the skin. These changes (summarized in Figure 1) include increases in IL-4 and IL-13 and decreases in antimicrobial peptides (AMPs), the latter predisposing the staphylococcal colonization, which in some cases may contribute to initiation and/or prolongation of flares of AD.3,5 Increased IL-4 stimulates the production of IgE and IgGl from B cells and increases the secretion of the chemokine CCL18 from antigen presenting cells and eosinophils.11 Functionally, CCL18 is thought to attract naive CD4 and CD8 T cells, B lymphocytes, and immature dendritic cells further perpetuating and amplifying the immune response.11,12 It is therefore not surprising that increased serum levels of CCL18 in AD have been shown to correlate with an increase in clinical severity scores, serum eosinophils, and serum IgE levels. In addition, this chemokine is also found in lung tissue and may partially explain the link between asthma and eczematous dermatitis in atopic individuals.11,12
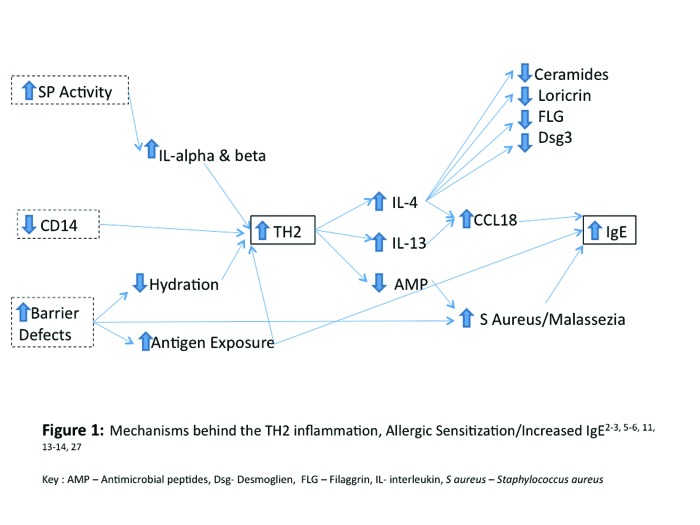
Figure 1.
Mechanisms behind the TH2 inflammation, allergic sensitization/increased IgE2,3,5,6,11,13,14,27
AMP=antimicrobial peptides; Dsg=desmoglien; FLG=filaggrin; IL=interleukin; S aureus=Staphylococcus aureus
IL-4 has significant effects on almost every component of the SC barrier. It adversely influences barrier function by 1) decreasing ceramide production (ceramides are key constituents of the intercellular lipid membrane); 2) reducing loricrin synthesis (loricin is crucial to the formation of the cornified envelope); 3) downregulating desmoglein-3 (Dsg-3) expression (Dsg-3 is required for the normal attachment of neighboring corneocytes); and 4) decreasing filaggrin expression (filaggrin influences SC structure and is the precursor of natural moisturizing factors [NMFs]).13,14
Increased IL-13 also plays a role in stimulating IgE and CCL18 production; however supporting evidence for the nature and impact of its effects is not as strong as the data for IL-4.2,11
TH2 inflammation decreases the production of several antimicrobial peptides (AMPs) including human cathelicidin product (hCAP), LL-37 (major cathelicidin peptide), and human beta defensins 2 and 3 (hBD 2 and hBD 3).5,15,16 It is important to note that decreases in AMPs along with the SC barrier defects seen in AD contribute to the increased colonization of pathogens, such as Staphylococcus aureus and Malassezia furfur in AD skin.5 The increased colonization of atopic skin with S. aureus not only increases the risk of infections in AD patients, but also perpetuates the elevation of IgE and allergic sensitization, and certain strains of S. aureus produce exotoxins and other proteins that can act as superantigens that participate in the elicitation and/or prolongation of AD flares.17,18 It has been documented that the natural diversity of organisms that occurs on the surface of the skin (microbiome) decreases during AD flares, with an increase in staphylococcal species. This linkage between inflammatory disease and microbial skin colonization patterns sheds light on the role that the microbial organisms play in immune response both overall and in AD.19 Figure 1 summarizes causes and effects of increased TH2 immune responses and IgE in AD. As depicted in this figure, decreased CD14 also contributes to the increase in TH2 inflammation in AD and will be discussed in later sections.
Sustained Wound Healing Inflammation in Atopic Dermatitis
The wound-healing response in skin is a highly regulated series of interdependent events that restores tissue homeostasis following an injury. One of the initial responses following trauma to the skin is inflammatory, in which immune cells infiltrate the skin in order to ward off invading microbes. This is followed by the proliferation of progenitor cells, which subsequently differentiate and replace damaged tissue. Finally, there is remodeling of tissue architecture to integrate the new structures into the preexisting tissue.20,21 In AD, a sustained wound healing type inflammatory response is evident. This wound signature of AD demonstrates inflammation, proliferation of progenitor cells leading to epidermal hyperplasia, and tissue remodeling resulting in the development of spongiosis.20,21
Caspase 8 is a cysteine-aspartic acid protease (caspase) protein, encoded by the CASP8 gene. Induced by Fas and various apoptotic stimmi, the sequential activation of caspase 8 plays a central role in cell apoptosis.22,23 A simplistic way of viewing its role is that of “quenching” or shutting down the wound healing response. The absence of caspase-8 has been shown to promote the inflammatory and proliferative phases of the wound healing response. Given the similarities between the wound signature in AD and the wound healing response seen in capase 8 deficiency, Li et al4 set out to test whether the genetic ablation of caspase-8 in a knock out mouse model could be used as a system to understand the wound signature seen in AD. Li et al4 found that the conditional deletion of epidermal caspase-8 in the knock out mouse model recapitulates many of the clinical hallmarks of AD, such as epidermal thickening (acanthosis), scaling, elevated serum immunoglobulins, a biphasic T-helper cell response, mast cell infiltration, and spongiosis.4,24 However, one notable difference between the caspase-8 model and AD is the late onset of spongiosis in the mouse model. Spongiosis is one of the first histological changes in AD.24 The investigators attribute this difference to delay in the activation of matrix metalloproteinase-2 although this theory has not been substantiated.24
Li et al4 postulate that the chronic repression of caspase-8 in AD may result from a gain of function mutation in the transcriptional repressor of caspase-8, which renders it constitutively active and therefore continually represses the activity of caspase 8. Alternatively, the authors speculate that an unknown factor that normally would induce the expression of epidermal caspase-8 following wound closure may be malfunctioning in AD skin resulting in the sustained repression of caspase 8.4 Whatever the explanation for the decreased caspase-8 activity in human AD skin, an important question is whether this down-regulation of caspase-8 is a cause or a consequence of AD. Based on the fact that simply removing epidermal caspase-8 via the knockout mouse model induces many of the cardinal features of AD, Li et al4 believe that the loss of caspase-8 contributes directly to the pathophysiology of AD.4
Impaired Innate Immunity in Atopic Dermatitis
Innate immunity, in contrast to the adaptive immune response of T and B lymphocytes, is a rapid response system that does not specify its response toward pathogens and is not capable of immunologic memory. The innate immune system recognizes regular patterns in microbial structures that are essential for the survival of the organism, such as the bacterial cell wall. These microbial patterns are called pathogen-associated molecular patterns (PAMPs), and the molecules of the innate immune system that recognizes these patterns are called pattern recognition receptors (PRR).25-27
PRRs can bind to pathogens at extracellular sites and mediate their elimination through recruitment of antimicrobial factors, such as complement, or PRRs can stimulate microbicidal host responses through intracellular signaling events, such as IL-1 and tumor necrosis factor (TNF).28 Examples of PRR that stimulate microbicidal host responses through intracellular signaling include toll-like receptors (TLRs), CD14 receptors, and nucleotide-binding oligomerization domain-containing receptors (NOD).28-31
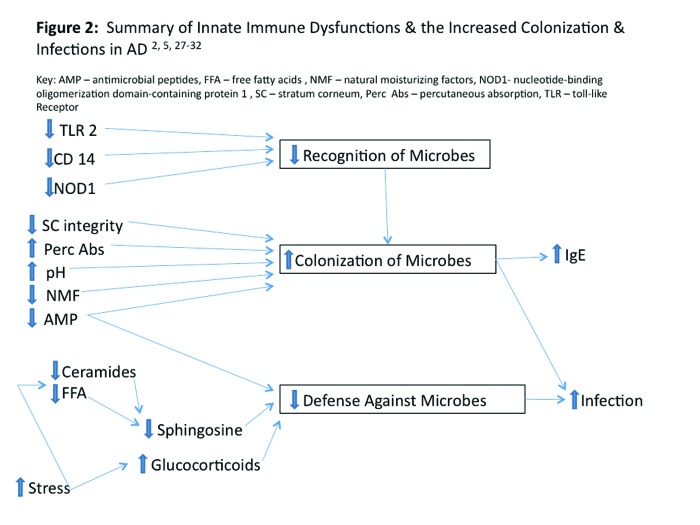
A normally functioning innate immune response system provides the first line of immunologic defense against microbial invasion by recognizing pathogens and coordinating an early rapid response to prevent and/or contain infection. In AD, there are abnormalities of the innate immune system, which lead to impaired microbial recognition, increased colonization with certain pathogenic microbial organisms, and decreased defense against infections. Polymorphisms in TLR-2, CD14, and NODI PRRs and deficiencies in AMPs are thought to be the major innate immune abnormalities responsible for the diminished capacity of innate immunologic response in AD.5,28-36 Reduction in AMPs accounts for the increased susceptibility to staphylococcal pyoderma and eczema herpeticum in patients with AD.5,37,38 Figure 2 summaries the innate immune abnormalities in AD.
Figure 2.
Summary of innate immune dysfunctions and the increased colonization and infections in atopic dermatitis2,5,27-32
AMP=antimicrobial peptides; FFA=free fatty acids; NMF=natural moisturizing factors;
NOD1 =nucleotide-binding oligomerization domain-containing protein 1; SC=stratum corneum; Perc
Abs=percutaneous absorption; TLR=toll-like receptor
TLR-2 is a PRR involved in the recognition of bacterial and fungal components. Examples of such components include LPS, peptidoglycan, and lipoteichoic acid. Defects in TLR-2 impair the ability of skin to recognize pathogens and signal an immune response. In AD, it has been shown that there are several specific polymorphisms in TLR-2 that are associated with higher rates of infection, including S. aureus infection and a more severe AD phenotype.30,31
In a study involving a total of 78 adult patients with mild-to-severe AD and 39 healthy age-matched control subjects, the investigators found that the TLR-2 R753Q allele was associated with variations in the intracellular portion of the receptor and was most closely associated with AD compared to the other polymorphisms studied. Of the 79 patients with AD, 11.5 percent had a detectable TLR-2 R753Q gene mutation, while 2.5 percent of healthy controls carried the mutation. Also, the AD patients carrying the TLR-2 R753Q allele variant exhibited a more severe form of AD based on scoring atopic dermatitis (SCORAD) scores as compared to those AD patients not carrying the allele. None of the patients with the detectable TLR-2 R753Q gene mutations had a SCORAD score of less than 30 points. In addition, the AD patients with TLR-2 R753Q showed higher levels of total serum IgE and superantigen-specific IgE, therefore demonstrating, indirectly, the association between this allele, a more severe phenotype of AD, and an increased susceptibility to colonization, infection, and allergic sensitization.30 In summary, AD patients with the TLR-2 R753Q polymorphism exhibit a more severe phenotype of AD detectable by markedly elevated IgE antibody levels; increased SCORAD scores30; and increased susceptibility to colonization, infection and allergic sensitization. This is further evidenced by increased superantigen-specific IgE and increased rates of detectability of S. aureus.30,37,38
CD 14 is a PRR that is important in the development and maturation of the immune system.28,32,33 There are two types of CD 14, the membrane bound CD14 (CD14) and the soluble CD14 (sCD14). Membrane-bound CD 14 is expressed primarily on macrophages and B cells and is part of the Hpopolysaccharide (LPS)-recognition complex that binds microbial and other bacterial wall components while soluble CD14 facilitates the interaction of membrane-bound CD14 cell populations with LPS. When activated in the presence of LPS, normal levels of CD14 initiate a signal transduction via TLR 4, resulting in high secretion of IL-12. Increased secretion of IL-12 promotes a TH1 dominant immune response and simultaneously downregulates the TH2 immune response.28,32,33 Thus, in infants, the normal exposure of skin to flora (and LPS) upregulates TH1 immunity via CD 14 receptors and downregulates TH2 activity. The presence of a dominant TH1 immune response is critical during early postnatal life, when exposure to high levels of antigen first occurs, as this initial response is likely to determine lifelong reactivity to that antigen and TH2 predominance is likely to promote a deleterious allergic sensitization and predisposition to 28,32,33
A polymorphism in the promoter region of the deoxyribonucleic acid (DNA) encoding CD14 is thought to be responsible for the reduced levels of circulating sCD14 found in AD. It is thought that AD patients with reduced sCD14 levels have decreased TH1 reactivity with increased TH2 reactivity. This increase in TH2 reactivity enhances allergic sensitization and the risk for becoming atopic.34 Zdolsek et al32 demonstrated an inverse correlation between CD14 and IgE levels in AD patients, thus supporting the relationship between CD 14 levels and allergic sensitization in people with AD.32 In addition, it has been demonstrated that low levels of CD14 in maternal breast milk correlated with an increased susceptibility for the child to develop AD.32,33
Zdolsek and Rothenbacher individually found that children who were breastfed for six months had the lowest odds ratio of developing AD when the sCD14 levels were high in the mothers’ breast milk.32,33 Given the conclusion of this study, it may be possible to mitigate a CD-14 deficient child’s propensity to develop AD by giving the child breast milk with high levels of CD14; however, this is unconfirmed and further testing is needed. Either way, decreased levels of CD 14 appear to be associated with the development of AD in children.
NODI belongs to a family of NOD receptors present in the cell cytosol and is composed of an N-terminal caspase recruitment domain, a centrally located NOD, and multiple C-terminal leucine-rich repeats; it is expressed constitutively by epithelial cells.35,36 While cell membrane PRRs, such as TLRs and CD14, respond to extracellular PAMPs, cytosolic PRRs like NOD recognize PAMPs that cross the plasma membrane.35,36 A large number of subtypes of NODs are described and genetic variations of some have been associated with the development of some inflammatory diseases. Polymorphisms in the subtype NODI are associated with AD.28,29 Under normal physiological condition, a functional NODI binds to degradation products of bacterial cell wall components of Gram-positive and Gram-negative bacteria.39 After NODI binds to the peptidoglycan of a bacterial cell wall, a signal transduction cascade is initiated, which causes translocation of nuclear factor kappa beta (NF-κβ) to the nucleus. NF-κβ then induces the transcription of genes encoding AMPs, pro-inflammatory cytokines, and chemokines, and is crucial for effective antimicrobial host responses.28,29
The association of AD and NODI polymorphisms and haplotypes has been analyzed in three large cohorts. In these cohorts, 2 of the 11 known NODI single nucleotide polymorphisms were found to be associated exclusively with AD while one of NODI haplotypes showed significant association with AD.28,40 These NODI polymorphisms in AD patients alter the inherent ability of skin to recognize bacterial pathogens and elicit protective immunologic and inflammatory responses and therefore lead to an increased susceptibility for bacterial colonization and infection.
AMPs belong to a group of peptides that possess activity against bacteria, viruses, and fungi. AMPs are expressed in skin and mucosal epithelia and act to prevent pathogen colonization of host tissues by direct microbial killing, chemotaxis, and modification of inflammation responses. These AMPs are cationic in nature and destroy bacteria by literally creating holes in their negatively charged cell walls.41,42 Defensins and catheHcidins are AMPS that have diminished expression/function in AD skin.5,16 Diminished expression of defensins and cathelicidins predisposes the skin of AD patients to microbial colonization and infection.
Defensins are small cysteine-rich peptides with a molecular mass between 3 and 5 kDa. They are classified into two main categories, alpha- and beta-defensins, based on the Hnking pattern of their six cysteine residues. The alpha-defensins are predominantly found in neutrophils and in small intestinal Paneth cells, whereas the beta-defensins have been isolated from both leukocytes and epithelial cells. HBD-1 is constitutively expressed in epidermis and sweat ducts and HBD-2 and HBD-3 are inducible by bacterial infection and cytokines IL-1 and TNF. HBD-3 has been shown to have strong antibacterial activity against S. aureus and to possess other biological activities, including chemokine-like activities, immunomodulating properties, and assistance in coordinating activities of the innate and the adaptive immune systems.42,43
Cathelicidins are found in keratinocytes, intestinal cells, mast cells, sweat, saliva, and the vernix caseosa. Their secretion is induced by trauma, infection, inflammation, and vitamin D. Once secreted, cathelicidins bind to negatively charged bacterial membranes and induce a leakage of bacterial contents, leading to an inflammatory response and cytokine secretion. In keratinocytes, they are stored in lamellar bodies along with lipid precursors, secreted into the granular and spinous layer, and during periods of AMP activation are processed to form smaller peptides with greater antimicrobial, anti-inflammatory, and vasoactive properties, such as LL-37.41,42
Ong et5 al have documented that there are lower levels of LL-37 (a catabolized cathelicidin) and HBD-2 in both acute and chronic lesions of atopic skin compared to psoriasis lesions and normal skin.5,16 It has been suggested that these decreases in AMP are a result of the increased TH2 mediated inflammation and IL-4 expression seen in AD, gene polymorphisms, or a combination of the two.28
Given the abnormalities of the innate immune system identified in AD patients, it is understandable why such patients have difficulty mounting an appropriate defense against some bacterial and viral infections, such as staphylococcal pyodermas and eczema herpeticum. AD patients appear to have impaired recognition of microbial organisms via defects in PPRs, such as TLR2, NODI, and CD14. In addition, the decrease in some AMPs predisposes both normal-appearing and eczematous atopic skin to microbial colonization and infection. However, as demonstrated in Figure 2, there are other factors in AD that also contribute to the increased colonization, infection, and allergic sensitization seen in AD, including structural and functional SC barrier defects, pH alteration, decrease in SC ceramides, and psychological stress.
Conclusion
Defects in both the structural and functional integrity of the SC barrier and cutaneous immune defense system occur as inherent aberrations in atopic dermatitis. These two components of our “defense against the outside world” are complex, multifaceted, and interdependent. In this three-part article series, the authors have described the structural and functional changes of the SC that relate to its barrier functions and aberrant immunologic responses that are associated with atopic skin. They have also reviewed how these abnormalities interconnect and correlate with clinical manifestations of AD. An increased understanding of the multiple pathways, polymorphisms, and dysregulations that are operative in AD have made it clear that the SC is a dynamic constituent of the cutaneous defense system, subject to both innate structural defects, and vulnerabilities induced by impairment and dysregulation of immune detection and response. Development of an appropriate approach for prevention and treatment must take into consideration the multiple pathophysiological variables in AD that involve both the structure and function of the SC barrier and its closely related “partner in crime”, the immune response system.
Footnotes
Disclosure:Dr. Levin is an advisory board participant and consultant for Onset Dermatologies and Galderma Laboratories. Dr. Friedlander is a consultant, advisory board participant, clinical ivestigator, and/or speaker for Galderma, Top MD, Valeant, and Onset Dermatologies. Dr. Del Rosso is consultant, advisory board participant, clinical investigator, and/or speaker for Allergan, Bayer Healthcare, Eisai, Galderma, Medicis (a division of Valeant), Obagi Medical Products, Onset Dermatologies, PharmaDerm, Primus, Tromius, Quinnova, Ranbaxy, Taro Pharmaceuticals, TriaBeauty, Unilever, Valeant, and Warner-Chilcott.
References
- 1.Fallon PG, Sasaki T, Sandilands A, et al. A homozygous frameshift mutation in the mouse FLG gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41(5):602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9(5):437–446. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28(3):769–779. doi: 10.1002/(SICI)1521-4141(199803)28:03<769::AID-IMMU769>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Lasse S, Lee P, et al. Development of atopic dermatitis-like skin disease from the chronic loss of epidermal caspase-8. Proc Natl Acad Sci USA. 2010;107(51):22249–22254. doi: 10.1073/pnas.1009751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic 16. dermatitis. N Engl J Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 6.Nylander-Lundqvist E, Back O, Egelrad T, et al. IL-1 beta activation in human epidermis. J Immunol. 1996;157:1699–1704. [PubMed] [Google Scholar]
- 7.Elias PM, Ansel JC, Woods LD, et al. Signaling networks in barrier homeostasis. The mystery widens. Arch Dermatol. 1996;132(12):1505–1506. [PubMed] [Google Scholar]
- 8.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10(3):119–126. [PubMed] [Google Scholar]
- 9.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak N, Baurecht H, Schäfer T, et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol. 2008;128:1430–1435. doi: 10.1038/sj.jid.5701190. [DOI] [PubMed] [Google Scholar]
- 11.Hon KL, Ching GK, Ng PC, et al. Exploring CCL18, eczema severity and atopy. Pediatr Allergy Immunol. 2011;22(7):704–707. doi: 10.1111/j.1399-3038.2011.01174.x. [DOI] [PubMed] [Google Scholar]
- 12.de Nadaï, Charbonnier AS, Chenivesse C, et al. Involvement of CCL18 in allergic asthma. J Immunol. 2006;176(10):6286–93. doi: 10.4049/jimmunol.176.10.6286. [DOI] [PubMed] [Google Scholar]
- 13.Hatano Y, Terashi H, Arakawa S, et al. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786–792. doi: 10.1111/j.0022-202X.2005.23651.x. [DOI] [PubMed] [Google Scholar]
- 14.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberg KM, Man MQ, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. :3262–3269. doi: 10.4049/jimmunol.171.6.3262. 2003171. [DOI] [PubMed] [Google Scholar]
- 17.Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung DY, Harbeck R, Bina P, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis: evidence for a new group of allergens. J Clin Invest. 1993;92:1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong HH, Oh J, Deming C, Conlan S, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Research. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. Eur J Cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 22.Chun HJ, Zheng L, Ahmad M, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 23.Grewe M, Bruijnzeel-Koomen CA, Schöpf E, et al. A role for Thl and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 24.Scharschmidt TC, Segre JA. Modeling atopic dermatitis with increasingly complex mouse models. J Invest Dermatol. 2008;128:1061–1064. doi: 10.1038/sj.jid.5701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallo RL, Huttner KM. Antimicrobial peptides: an emerging concept in cutaneous biology. J Invest Dermatol. 1998;111(5):739–743. doi: 10.1046/j.1523-1747.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- 26.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272(5258):50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 27.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 28.Biedermann T. Dissecting the role of infections in atopic dermatitis. Acta Derm Venereol. 2006;86(2):99–109. doi: 10.2340/00015555-0047. [DOI] [PubMed] [Google Scholar]
- 29.Weidinger S, Klopp N, Rummler L, et al. Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J Allergy Clin Immunol. 2005;116(1):177–184. doi: 10.1016/j.jaci.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, et al. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113(3):565–567. doi: 10.1016/j.jaci.2003.12.583. [DOI] [PubMed] [Google Scholar]
- 31.Lai Y, Cogen AL, Radek KA, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130(9):2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zdolsek HA, Jenmalm MC. Reduced levels of soluble CD14 in atopic children. Clin Exp Allergy. 2004;34(4):532–539. doi: 10.1111/j.1365-2222.2004.1921.x. [DOI] [PubMed] [Google Scholar]
- 33.Rothenbacher D, Weyermann M, Beermann C, et al. Breastfeeding, soluble CD 14 concentration in breast milk and risk of atopic dermatitis and asthma in early childhood: birth cohort study. Clin Exp Allergy. 2005;35(8):1014–1021. doi: 10.1111/j.1365-2222.2005.02298.x. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Interleukin-12: a cytokine produced by antigenpresenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 35.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2001;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 36.Athman R, Philpott D. Innate immunity via Toll-like receptors and Nod proteins. Curr Opin Microbiol. 2004;7:25–32. doi: 10.1016/j.mib.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90(5):525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 38.Hauser C, Wuethrich B, Matter L, et al. Staphylococcus aureus skin colonization in atopic dermatitis patients. Dermatohgica. 1985;170(1):35–39. [PubMed] [Google Scholar]
- 39.Inohara N, Chamafflard M, McDonald C, et al. NODLRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 40.Abecasis GR, Cookson WO, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet. 2000;8:545–551. doi: 10.1038/sj.ejhg.5200494. [DOI] [PubMed] [Google Scholar]
- 41.Durr M, Peschel A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defence. Infect Immun. 2002;70(12):6515–6517. doi: 10.1128/IAI.70.12.6515-6517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harder J, Schröder JM. Antimicrobial peptides in human skin. Ckem Immunol Allergy. 2005;86:22–41. doi: 10.1159/000086650. [DOI] [PubMed] [Google Scholar]
- 43.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Ghem. 1997;272(24):15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]