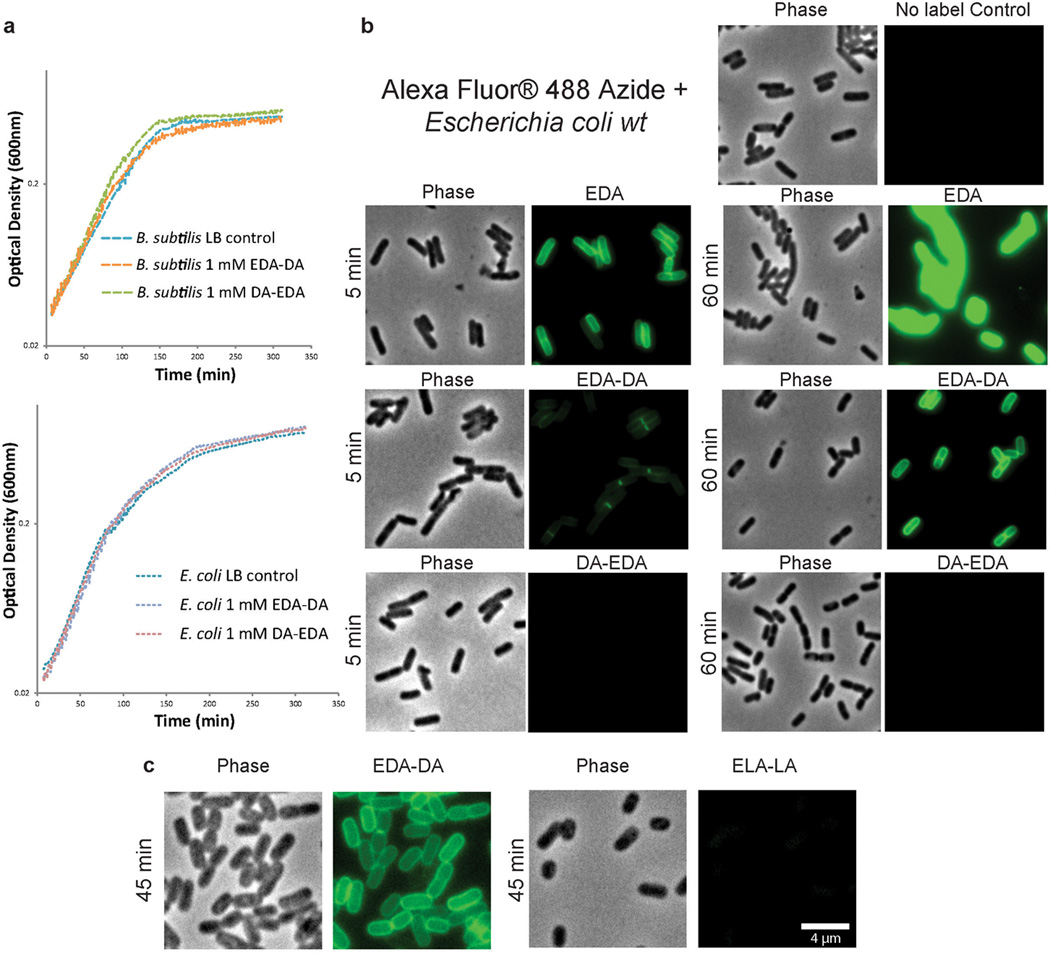
Extended Data Figure 2. D-enantiomer dipeptide probes do not affect growth in rich media, but differentially and specifically label PG of E. coli and B. subtilis.
a, Growth of wild-type E. coli and B. subtilis in the presence of experimental concentrations of EDA-DA or DA-EDA. A representative growth curve from two biological replicates, each with three technical replicates, is shown. b, Phase contrast and epifluorescence microscopy of E. coli grown with 0.5 mM alkyne containing EDA-DA, DA-EDA or as a positive control with EDA at five minutes and 60 minutes. These samples together with unlabeled controls were ‘clicked’ to Alexa Fluor 488 azide and imaged. When the alkyne is on the C-terminus (DA-EDA), the labeling is not apparent. Signal from N-terminally tagged dipeptide (EDA-DA) is significantly higher, but still lower than EDA and the patterns of labeling at the earlier time points are different. This is probably due to periplasmic incorporation of D-amino acids (e.g. EDA) by E. coli L,D-transpeptidases, which result in more efficient peripheral labeling in addition to labeling due to lipid II-dependent PG synthesis. Therefore, in bacteria that have active L,D-transpeptidases, the cytoplasmic PG labeling through dipeptide probes provides a better measure of lipid II-dependent PG synthesis than single D-amino acids. The experiment was conducted twice and images are representative of a minimum of five fields viewed per condition/time point per replicate. c, Comparison of the labeling in E. coli grown with 0.5 mM alkyne containing EDA-DA or the L-enantiomer control Ethynyl-L-alanine-L-alanine (ELA-LA) for 45 min and clicked as above shows that the labeling is D-enantiomer specific. Images are representative of a minimum of four fields viewed per replicate and the experiment was conducted twice.