Abstract
Objective
To determine the role of toll-like receptor 2 in cardiac dysfunction during polymicrobial sepsis.
Design
Controlled animal study.
Setting
University hospital research laboratory.
Subjects
Male C57BL/6, wild-type, toll-like receptor 2−/−.
Intervention
Polymicrobial peritonitis, a clinically relevant model of sepsis, was generated by cecum ligation and puncture. Wild-type and toll-like receptor 2−/− mice were divided into sham and cecum ligation and puncture groups. The sham animals underwent laparotomy but without cecum ligation and puncture. Twenty-four hours after surgeries, the cardiac function was assessed by serial echocardiography in vivo, a pressure transducer catheter was inserted into the left ventricles of isolated hearts (Langendorff model), and in vitro measurement of Ca2+ transients and sarcomere shortening in adult cardiomyocytes were isolated from the sham and septic animals. In addition, myocardial and serum cytokines, blood white blood cell counts, peritoneal neutrophil recruitment, chemokine receptor expression, and survival rates were examined.
Measurements and Results
Compared to septic wild-type mice, toll-like receptor 2−/− mice had markedly improved cardiac function during sepsis, as demonstrated by in vivo tissue Doppler imaging, better-preserved left ventricle function in isolated heart, and improved sarcomere shortening measured in single cardiomyocytes. There was also a significant survival benefit in toll-like receptor 2−/− mice compared to wild-type mice. These favorable outcomes in toll-like receptor 2−/− mice were associated with attenuated cardiodepressant cytokine levels in the myocardium and serum and enhanced neutrophil migratory function.
Conclusions
These studies suggest that toll-like receptor 2 signaling plays a critical role in mediating cardiomyopathy, deleterious myocardial and systemic inflammation, and high mortality during polymicrobial sepsis.
Keywords: Toll-like receptor 2, sepsis, cardiac dysfunction, inflammation, neutrophils
Sepsis is the systemic inflammatory response syndrome that occurs during infection and has an estimated prevalence of 751,000 cases each year in the United States, >50% of which receive intensive care (1). Age-adjusted severe sepsis mortality rates increase from 30.3 to 49.7 per 100,000 population during the 10 yrs between 1993 and 2003 (2). Undoubtedly, given an aging population with increasing numbers of patients infected with treatment-resistant organisms and patients with compromised immune system, the frequency of severe sepsis is increasing (1, 2).
Whereas normal or elevated cardiac output and decreased systemic vascular resistance are often the early clinical manifestations of adequately resuscitated septic patients (3), left ventricular depression and acute dilation may occur later, which represent a main feature of severe sepsis and contribute to its high mortality (1, 4, 5). Several cellular and molecular mechanisms have been proposed as the possible contributors of septic cardiomyopathy, e.g., suppressive proinflammatory cytokines, mitochondrial dysfunction, apoptosis, and cardiac hibernation (5). But, the signaling mechanisms that lead to these critical events during sepsis remain incompletely understood.
Germline-encoded innate immune receptors such as toll-like receptors (TLRs) represent the first line of host defense against pathogen invasion. They play a pivotal role in host innate immune response and modulate adaptive immunity (6). However, recent studies also have suggested that inappropriate host response via TLR-dependent mechanisms may contribute to the pathogenesis of sepsis (7, 8) and myocardial injury (9). The heart expresses at least two receptors involved in TLR signaling, i.e., TLR2 and TLR4 (10–13). Various studies in different models have suggested that these receptors are partly responsible for cardiac dysfunction in certain conditions. For example, TLR4 is essential for endotoxin-induced left ventricle (LV) dysfunction (12), whereas TLR2 is involved in peptidoglycan-associated lipoprotein-induced (13) and Staphylococcus aurous-induced (11) cardiac dysfunction in cardiomyocytes and isolated heart, respectively. It remains unclear, however, whether TLR2 mediates cardiac dysfunction during polymicrobial sepsis in vivo and the underlying mechanisms during severe polymicrobial sepsis.
Here, we sought to test the hypothesis that TLR2 signaling is essential for cardiac dysfunction in mice with severe polymicrobial sepsis. We found that compared to septic wild-type mice, mice deficient of TLR2 had better-preserved cardiomyocyte function, attenuated systemic inflammation with reduced production of cardiodepressive cytokines in the heart, improved neutrophil migratory function to infectious site, and markedly improved overall survival.
Materials and Methods
Mouse Model of Polymicrobial Sepsis Induced by Cecum Ligation and Puncture
All animal experiments were performed with the approval of the Animal Care Committee of Massachusetts General Hospital; 8- to 12-wk-old male mice were used for the studies. All mice were housed in the same conditions and fed the same bacteria-free diet. TLR2−/− mice were generated by Takeuchi et al (14). Polymicrobial peritonitis was generated by cecum ligation and puncture (CLP) (15, 16). The cecum was ligated 1.0 cm from the tip of cecum, punctured twice with an 18-gauge needle, and squeezed gently to expel a small amount of fecal materials before being returned to the abdominal cavity. The sham-operated mice underwent laparotomy but without CLP. After surgery, prewarmed normal saline (0.1 mL/gram body weight) was administered subcutaneously.
Echocardiography in Mice
Before, 12 hrs, or 24 hrs after laparotomy or CLP, mice were lightly anesthetized with ketamine (0.02 mg/g). Transthoracic echocardiographic images were obtained and interpreted by an echocardiographer blinded to the experimental groups using a 13.0-MHz linear probe (Vivid 7; GE Medical System, Milwaukee, WI). M-mode images were obtained from a parasternal short-axis view at mid-ventricular level with a clear view of papillary muscle. Tissue Doppler imaging was collected at a frame rate of 224–483 frames per second and a depth of 1 cm. Fractional shortening was defined as [(LV internal dimension diastole – LV internal dimension systole)/LV internal dimension diastole] × % (17, 18). Strain rate of the posterior wall was analyzed offline in an EchoPAC workstation (GE Healthcare, Wauwatosa, WI) (19). In brief, a region of interest (axial distance, 0.2 mm; width, 0.6 mm) was manually positioned in the middle of the posterior wall. A strain length of 0.5 mm was used. Peak systolic strain rate was measured. The values of five consecutive cardiac cycles were averaged.
Measurements of Tail-Cuff Blood Pressure in Awake Mice
Systolic blood pressure was measured with a noninvasive tail-cuff machine (XBP 1000; Kent Scientific, Torrington, CT) in awake wild-type (WT) mice and TLR2−/− mice before and after surgeries as described previously (20).
Langendorff Perfusion of Isolated Heart
Mice were heparinized (1000 IU/kg, intraperitoneal) and euthanized. The hearts were excised and aortae were cannulated and retrograde-perfused at a constant rate (3 mL/min) with modified Krebs-Henseleit buffer. After 10 mins of perfusion, LV systolic pressure, LV end-diastolic pressure, and heart rate were recorded for 30 mins, as described previously (20).
Cardiomyocyte Sarcomere Shortening and Intracellular Calcium
Sarcomere shortening and [Ca2+]i transients were recorded simultaneously on an IonOptix system (Milton, MA) as described previously (13, 21, 22). Adult cardiomyocytes were incubated with membrane permeable fluorescent indicator fura-2 AM (1 μM) (Molecular Probes; Invitrogen, Carlsbad, CA) and probenecid (0.5 mM). Cardiomyocytes were perfused with 1.2 mM Ca2+ Tyrode solution and electrically paced at 1, 2, 4, and 6 Hz via platinum wires. The Ca2+ transients and sarcomere shortening were analyzed based on single-cell-averaged tracing. The final values were derived from 16 to 27 individual cells in each group and calculated for statistical analysis. At least four mice from each mouse group were used to prepare cardiomyocytes for the functional studies.
Polymerase Chain Reaction
TLR2 genotyping was performed by polymerase chain reaction using a primer set specific for TLR2 WT and TLR2−/− as described previously (23).
Flow Cytometry Analysis of Peritoneal Neutrophils
Cells in peritoneal lavage were collected and total cell numbers were manually counted. A fraction of cells (5 × 105) from the peritoneal lavage was labeled with Ly-6C GR-1 (BD Biosciences, San Jose, CA) and CXCR2 monoclonal antibodies (R&D Systems, Minneapolis, MN), respectively. Neutrophils were gated on GR-1 and CXCR2 for neutrophil percentage in the recruited peritoneal cells and the CXCR2 expression density on neutrophils. The total neutrophil number in the peritoneum was calculated by multiplying the total cell number with the percentage of neutrophils.
Multiplex Cytokine Immunoassays
Twenty-four hours after sham or CLP procedures, mouse blood or the heart was collected. Sera were prepared at 4°C and stored at −80°C. The hearts were frozen in liquid nitrogen. Serum or myocardial cytokine concentrations were determined using a fluorescent bead-based multiplex immunoassay (Luminex, Austin, TX). Briefly, antibody for each cytokine covalently immobilized to a set of microspheres by manufacturer (Millipore, Billerica, MA). After overnight incubation, cytokines bound on the surface of microspheres were detected by a cocktail of biotinylated antibodies. After binding of streptavidin–phycoerythrin conjugates, the reporter fluorescent signal was measured with a Luminex 200 reader (Luminex Co.). Final cytokine concentrations were calculated based on a standard cytokine curve obtained in each experiment.
Mortality Study
Mice were observed every 24 hrs for up to 14 days.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism 5 software (La Jolla, CA). The distributions of the continuous variables were expressed as the mean ± se. The p values of cytokine production analysis were applied on the log10 scale and based on a two-way analysis of variance (Fig. 4A). For those cytokine production values below detection limit, imputed values at the detection limit were used in a non-parametric test and the p values were based on Mann-Whitney test (Fig. 4B). Student's t test was used for statistical analysis as shown in Figure 5. All other data were analyzed with two-way analysis of variance with Bonferroni post hoc tests for statistic significance. The survival data were analyzed with a log-rank test. The null hypothesis was rejected for p < .05.
Figure 4.
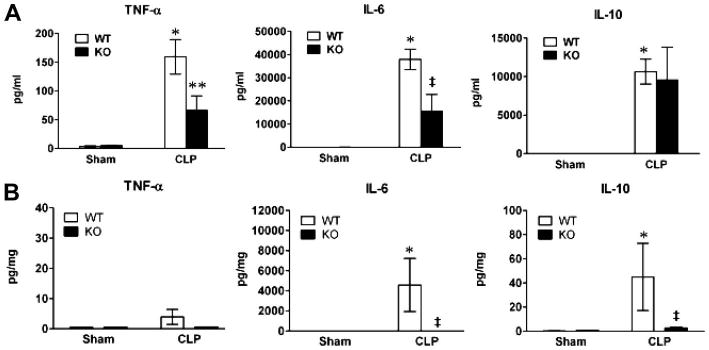
Toll-like receptor 2 (TLR2)−/− mice had attenuated serum and myocardial cytokine levels during polymicrobial sepsis. The two groups of mice, wild-type (WT) and TLR2−/−, underwent sham or cecum ligation and puncture (CLP) procedures. Twenty-four hours later, blood and the hearts were collected. Serum and tissue tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 were measured with a multiplex fluorescent bead-based immunoassay. A, Serum cytokines. Each error bar represents mean ± se of four to seven mice. *p < .001 vs. WT sham; **p < .05 and ‡p < .01 vs. WT CLP; n = 5–7. B, Myocardial cytokines. The hearts were harvested from a separate set of mice. *p < .05 vs. WT sham; ‡p < .05 vs. WT CLP; n = 5. KO, knockout (TLR2−/−).
Figure 5.
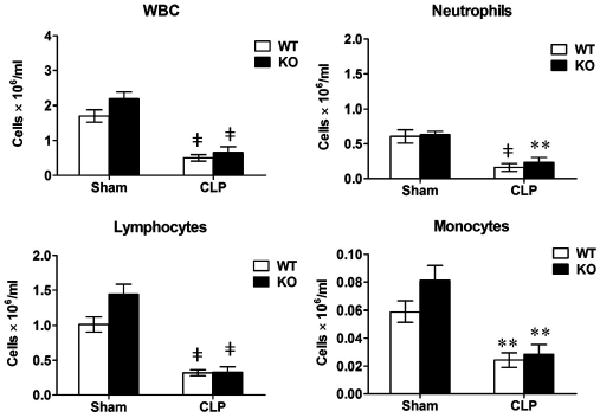
Polymicrobial sepsis leads to severe leucopenia. The two groups of mice, wild-type (WT) and toll-like receptor 2−/−, underwent sham or cecum ligation and puncture (CLP) procedures. Twenty-four hours later, total white blood cells (WBC), neutrophils, lymphocytes, and monocytes were counted. Each error bar represents mean ± se of six to nine mice. **p < .01; ‡p < .001 vs. the sham mice. KO, knockout (toll-like receptor 2−/−).
Results
TLR2−/− Mice Have Improved Cardiac Function Compared to WT Mice During Polymicrobial Sepsis
We performed CLP to induce polymicrobial sepsis in both WT and TLR2−/− mice. As shown in Table 1, 24 hrs after CLP, WT mice had severe hypotension with a 64% decrease in mean blood pressure and hypothermia despite fluid resuscitation after CLP surgery. In comparison, TLR2−/− mice subjected to CLP exhibited improved hemodynamics with 30% decrease in mean blood pressure and near-normal body temperature. There were no changes in hemodynamics or body temperature in mice that underwent sham operation in both animal groups.
Table 1.
General characteristics of wild-type and toll-like receptor 2−/− (knockout) septic mice
| Sham | CLP | |||
|---|---|---|---|---|
| Baseline | 24 hrs | Baseline | 24 hrs | |
| Weight, grams | ||||
| WT | 28.1 ± 2.1 | 26.3 ± 1.8 | 27.5 ± 0.7 | 26.7 ± 0.8 |
| KO | 28.9 ± 0.7 | 27.5 ± 0.8 | 29 ± 0.7 | 27.8 ± 0.7 |
| Mean blood pressure, mm Hg | ||||
| WT | 84 ± 1 | 83 ± 8 | 93 ± 2 | 33 ± 6b |
| KO | 88 ± 5 | 81 ± 6 | 88 ± 4 | 62 ± 7a,c |
| Temperature, °C | ||||
| WT | 37.0 ± 0.6 | 37.1 ± 0.1 | 37.1 ± 0.2 | 33.5 ± 1.2a |
| KO | 37.2 ± 0.5 | 37.5 ± 0.1 | 37.4 ± 0.2 | 35.8 ± 0.7 |
WT, wild-type; KO, knockout mice; CLP, cecum ligation and puncture.
p < 0.05 vs. CLP baseline;
p < .001 vs. CLP baseline and sham 24 hrs;
p < .05 vs. WT CLP 24 hrs. WT: n = 6–10; KO: n = 6–9.
Serial echocardiography performed before and at 12 and 24 hrs after CLP or sham operation indicated that polymicrobial sepsis induced a progressive reduction in LV contractile function. Strain rate, which measures the rate of LV posterior wall deformation, as illustrated in Figure 1, represents a sensitive index of myocardial function and correlates well with LV hemodynamic indexes, such as end-systolic volume elastance and the first derivative of developed LV pressure (19). Strain rate was reduced by 33% at 12 hrs and 43% at 24 hrs after CLP in WT mice (Table 2). In comparison, TLR2−/− mice had a partially preserved strain rate after CLP. It is noteworthy that supplemental normal saline (0.1 mL/g body weight) was administered subcutaneously after either sham or CLP surgery to replace the volume loss and to minimize the preload reduction that is often associated with sepsis (4, 24). Conventional echocardiography measurements confirmed that LV function was better-preserved 24 hrs after CLP in TLR2−/− mice, with smaller LV internal dimensions and higher fractional shortening than WT mice, although neither LV internal dimension nor fractional shortening measurements in WT mice showed any statistically significant changes over the same period (Table 2).
Figure 1.
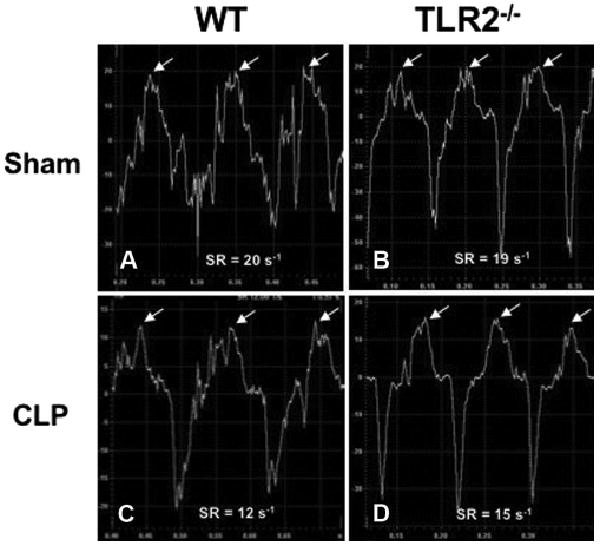
Representative images of strain rate. Wild-type (WT) and toll-like receptor 2 (TLR2)−/− mice were subjected to sham or cecum ligation and puncture (CLP) procedures. Twenty-four hours later, tissue Doppler imaging was performed and strain rate of left ventricle posterior wall was analyzed. Time–strain rate (SR) curve is represented. Peak radial SR is indicated by white arrows.
Table 2.
Serial echocardiographic measurements before and after cecum ligation and puncture
| Sham | CLP | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 hrs | 24 hrs | Baseline | 12 hrs | 24 hrs | |
| Heart rate, bpm | ||||||
| WT | 657 ± 10 | 682 ± 13 | 678 ± 6 | 659 ± 9 | 545 ± 31c | 540 ± 31c |
| KO | 645 ± 11 | 663 ± 10 | 650 ± 17 | 650 ± 5 | 620 ± 21 | 560 ± 26c |
| Strain rate, 1/sec | ||||||
| WT | 20 ± 1 | 21 ± 2 | 20 ± 2 | 21 ± 2 | 14 ± 2c | 12 ± 2c |
| KO | 19 ± 2 | 20 ± 1 | 19 ± 3 | 20 ± 1 | 17 ± 2a | 15 ± 1b,c |
| LV internal dimension diastole, mm | ||||||
| WT | 3.2 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.0 | 3.3 ± 0.1 | 3.2 ± 0.1 |
| KO | 3.1 ± 0.1 | 3.1 ± 0.0 | 3.1 ± 0.0 | 3.2 ± 0.0 | 3.3 ± 0.0 | 3.1 ± 0.1 |
| LV internal dimension systole, mm | ||||||
| WT | 1.6 ± 0 | 1.6 ± 0 | 1.6 ± 0.0 | 1.5 ± 0 | 1.7 ± 0.1 | 1.74 ± 0.1 |
| KO | 1.4 ± 0.1 | 1.5 ± 0 | 1.4 ± 0 | 1.5 ± 0 | 1.6 ± 0 | 1.4 ± 0.1a |
| Fractional shortening, % | ||||||
| WT | 51 ± 2 | 50 ± 1 | 49 ± 1 | 51 ± 1 | 47 ± 2 | 46 ± 3 |
| KO | 53 ± 1 | 52 ± 1 | 53 ± 1 | 53 ± 1 | 52 ± 1 | 53 ± 1a |
WT, wild-type; KO, knockout; CLP, cecum ligation and puncture; LV, left ventricle.
p < 0.05 and
p < .01 vs. WT CLP;
p < .01 vs. Baseline. WT: n = 6–10; KO: n = 6–9.
To confirm the tissue Doppler imaging findings of LV contractile dysfunction after sepsis and the impact of TLR2 deficiency, we isolated the hearts from both sham and septic mice at 24 hrs and perfused the hearts in a Langendorff system with oxygenated buffer at a constant flow. The ex vivo system measures LV contractile function with a constant preload that may not be achievable in an in vivo septic condition. Under these conditions, the hearts derived from WT mice that underwent CLP surgeries had decreased LV function, as demonstrated by 36% and 38% reduction in LV developed pressure and rate pressure product, respectively (p < .01), and by 32% and 40% reduction in the maximum first derivative of developed LV pressure and the minimum first derivative of developed LV pressure, respectively (p < .01). Remarkably, TLR2−/− mice subjected to CLP had preserved LV contractile function with near-normal LV diastolic pressure, rate pressure product, and the first derivative of developed LV pressure (Fig. 2).
Figure 2.
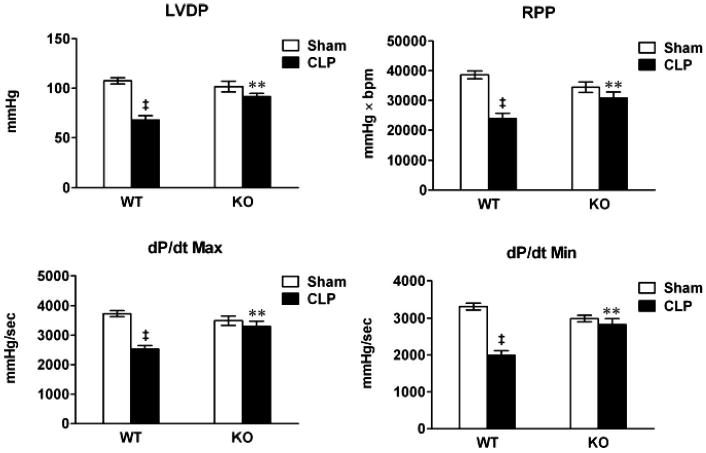
Toll-like receptor 2−/− mice have improved left ventricle function compared to wild-type (WT) after cecum ligation and puncture (CLP) as measured in isolated hearts. WT and toll-like receptor 2−/− mice underwent sham or CLP surgeries. Twenty-four hours later, the hearts were isolated and perfused with cell-free buffer in a Langendorff system. Left ventricle contractile functions were measured as described in Materials and Methods. LVDP, left ventricle developed pressure; RPP, rate pressure product = heart rate × LVDP; dP/dtmax, the maximum first derivative of LVDP; dP/dtmin, the minimum first derivative of LVDP; KO, knockout (toll-like receptor 2−/−). **p < .01 vs. WT CLP; ‡p < .001 vs. WT sham. n = 6–8.
To further confirm the impact of TLR2 deficiency on the intrinsic cardiomyocyte dysfunction after CLP, we isolated adult cardiomyocytes from mice 24 hrs after sham or CLP surgery and examined cardiomyocyte function as measured by sarcomere shortening and [Ca2+]i transients. As shown in Figure 3, sarcomere shortening was reduced by 59% (p < .001) and peak change in [Ca2+]i attenuated by 27% (p < .05) in WT mice subjected to CLP compared to cells from WT mice subjected to sham surgery. By contrast, TLR2−/− cardiomyocytes maintained normal sarcomere shortening and peak change in [Ca2+]i after CLP, although these cells appeared to have somewhat lower values at the baseline in sarcomere shortening and peak Ca2+ transients compared to WT cells. Importantly, compared to the cells isolated from WT mice subjected to CLP, TLR2−/− cardiomyocytes have improved sarcomere shortening after CLP (p < .01).
Figure 3.
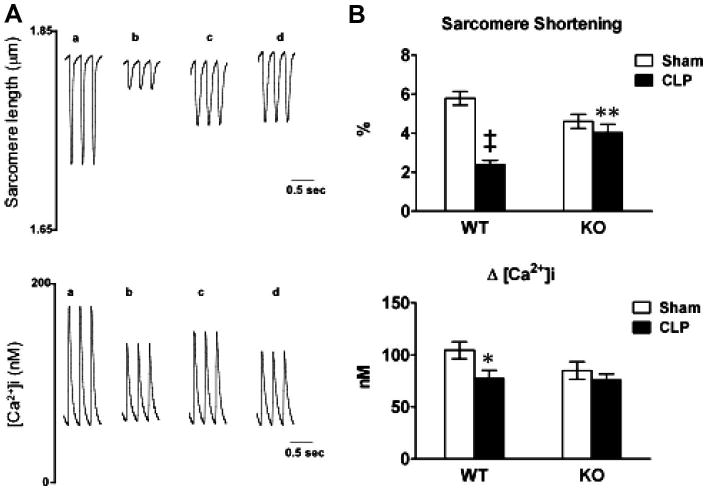
Toll-like receptor 2 (TLR2)−/− mice have improved cardiomyocyte function after polymicrobial sepsis. Wild-type (WT) and TLR2−/− mice underwent sham or cecum ligation and puncture (CLP) procedures. Twenty-four hours later, the hearts were harvested and cardiomyocytes were isolated. A, Representative tracing of sarcomere shortening and Ca2+ transients in cardiomyocytes isolated from WT (a, b) and TLR2−/− (c, d) mice subjected to either sham (a, c) or CLP (b, d) surgeries. B, Accumulated data of sarcomere shortening and Ca2+ transients. Each error bar represents mean ± se. The data in each group were recorded from 16 to 27 single adult cardiomyocytes isolated from more than four mice. * p < .05 vs. WT sham; ** p < .01 vs. WT CLP; ‡p < 0.001 vs. WT sham. KO, knockout (TLR2−/−).
Cardiac and Serum Cytokine Production in Response to Peritoneal Polymicrobial Sepsis Is TLR2-Dependent
Cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α are potent proinflammatory mediators that are known for contributing to septic cardiomyopathy (5, 25), whereas IL-10 is anti-inflammatory (3). To determine the role of TLR2 signaling in cytokine production during polymicrobial sepsis, we subjected WT and TLR2−/− to CLP or sham surgeries. As illustrated in Figure 4, 24 hrs after CLP, WT mice showed a robust cytokine response with elevated serum levels of TNF-α, IL-6 (26.4 vs. 37,942.9 pg/mL), and IL-10 (11.2 vs. 10627.1 pg/mL). In contrast, TLR2−/− mice subjected to CLP had markedly reduced pro-inflammatory cytokines, IL-6, and TNF-α, but the same level of IL-10, compared with septic WT mice. In the myocardium, there was a marked increase in IL-6 (0.9 vs. 4579 pg/mg protein) and IL-10 (0.4 vs. 45 pg/mg protein) production in WT mice after CLP. TLR2-deficient mice had markedly attenuated myocardial cytokine production (Fig. 4B). There was no significant change in myocardial TNF-α (Fig. 4B) or IL-6 (data not shown) after CLP.
TLR2-Deficiency Had No Impact on Leukopenia Induced by Polymicrobial Sepsis
Leucopenia and associated immune paralysis represent a key feature of severe sepsis and may contribute to its high mortality (3, 26). To determine whether TLR2 signaling contributes to leukocyte reduction during severe sepsis, we next examined total white blood cell, neutrophils, lymphocytes, and monocytes in the two groups of mice, WT and TLR2−/−, after CLP. Twenty-four hours after CLP, there was a dramatic reduction, i.e., 71%, 74%, 70%, and 72%, in the numbers of white blood cells, neutrophils, lymphocytes, and monocytes, respectively, in septic WT mice as compared to the sham-operated WT mice. Interestingly, systemic TLR2 deficiency had no significant impact to the degree of leucopenia induced by polymicrobial sepsis when compared to WT (Fig. 5).
Neutrophil Recruitment to Infectious Peritoneum Is Enhanced in TLR2−/− Mice
Neutrophils play an important role in innate immune response to invading pathogens, such as bacterial clearance and systemic cytokine responses. To assess the impact of TLR2 signaling on neutrophil function, we next examined neutrophil recruitment into the peritoneal space after CLP. Twenty-four hours after CLP, a large number of neutrophils [(10 ± 2) × 106] were recruited into the peritoneal space of WT mice as compared to sham-operated controls [(2 ± 1) × 106] (p < .05) (Fig. 6A). There were significantly more neutrophils (28 ± 4 × 106) recruited to the peritoneum in TLR2−/− mice compared to WT mice after CLP (p = .001). Approximately 85% of peritoneal cells recruited into the peritoneum were neutrophils in both strains of septic mice compared to approximately 30% in the sham controls (Fig. 6B). Furthermore, the peritoneal neutrophils harvested from the CLP mice, either WT or TLR2−/−, had marked reduction in the expression of the chemokine receptor CXCR2 compared to the sham mice. But no difference in CXCR2 expression was observed between WT and TLR2−/− peritoneal neutrophils after CLP (Fig. 6C, D).
Figure 6.
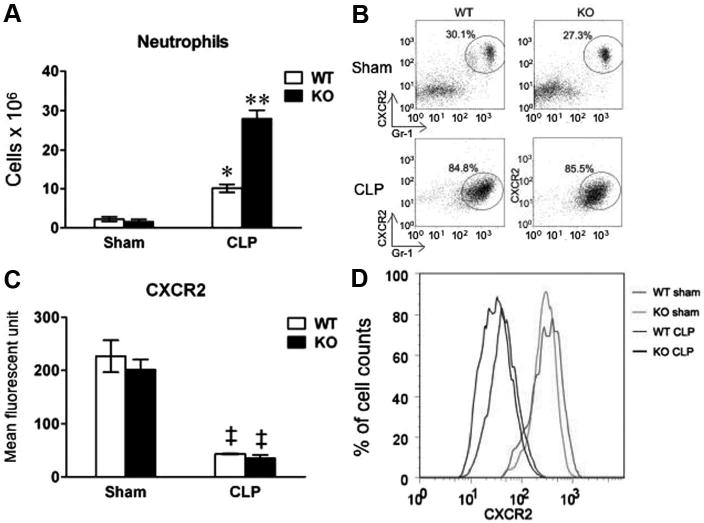
Peritoneal neutrophil recruitment and chemokine expression after cecum ligation and puncture (CLP). Mice underwent sham or CLP procedures. Twenty-four hours later, peritoneal cells were harvested from lavage fluid and manually counted. The same numbers of cells (5 × 105) from each mouse were loaded for fluorescence-activated cell-sorting (FACS) analysis. A, Total peritoneal neutrophils in the peritoneum. Neutrophil numbers were calculated based on the total cells numbers manually counted multiplied by the percentage of neutrophils as measured by FACS. Each error bar represents mean ± se of four mice. *p < .05 vs. the sham mice; **p = .001 vs. wild-type (WT) CLP. B, A representative example of FACS plots of peritoneal neutrophils from WT and toll-like receptor 2 (TLR2)−/− mice. The cells were gated on CXCR2 (chemokine receptor) and GR-1 (neutrophil marker). The cells within the circle represent neutrophils. The percentage of neutrophils is shown. C, CXCR2 expression on neutrophils recruited to the peritoneum in WT and TLR2−/− mice. Each error bar represents mean ± se of four mice. ‡p < .001 vs. the sham mice. D, A representative example of FACS of neutrophil CXCR2 expression in WT sham (red), TLR2−/− sham (green), WT CLP (blue), and TLR2−/− CLP mice (black). KO, knockout (TLR2−/−).
High Mortality Induced by Polymicrobial Sepsis Is Dependent on TLR2 Expression
The mouse model of polymicrobial sepsis led to a high mortality rate (64% at 48 hrs after CLP 91% at 96 hrs and 2 wks) in WT mice. In contrast, TLR2−/− mice showed marked reduction in the mortality rate after CLP (22% at 48 hrs, 33% at 96 hrs, and 56% at 2 wks) (p < .05) (Fig. 7).
Figure 7.
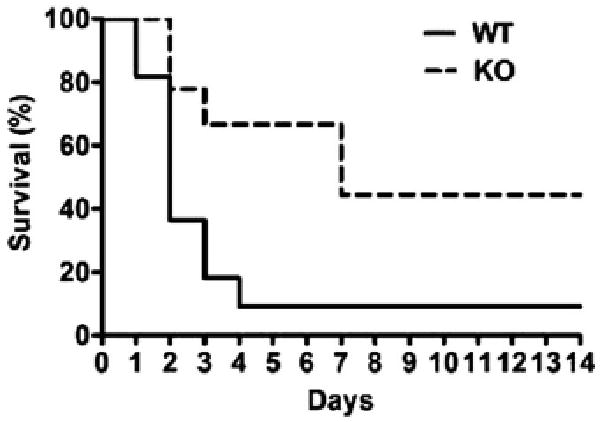
Impact of toll-like receptor 2 (TLR2) deficiency on the mouse mortality after cecum ligation and puncture. Survival rate of wild-type (WT) and TLR2−/− mice were recorded up to 14 days after cecum ligation and puncture. WT, n = 11; TLR2−/−, n = 9. p < .05. KO, knockout (TLR2−/−).
Discussion
The present study demonstrated a critical role of TLR2 in cardiac dysfunction during severe sepsis induced by polymicrobial infection. Wild-type mice had severe sepsis within 24 hrs after infection with clinical manifestations typical for septic shock, such as hypothermia, severe hypotension, markedly elevated serum and myocardial cytokine level, severely impaired cardiac contractile function, and high mortality. We found that TLR2-deficient mice had markedly improved cardiac function and survival during sepsis. These favorable outcomes in TLR2−/− mice were associated with significant reduction in the myocardial and serum levels of the cytokines and enhanced neutrophil recruitment into infectious peritoneal cavity.
The CLP model used in the present study represents a clinically relevant model of sepsis (15, 16) and has been validated in several laboratories (16, 27). Hotchkiss et al (28) have demonstrated that both Gram-positive and Gram-negative organisms are present in blood cultures in CLP mice but not in sham-operated mice. These include Proteus mirabilis, Streptococcus faecium, Enterobacter cloacae, Escherichia coli, Bacillus species, and Enterobacter faecium (28). TLR2 signaling has been implicated in recognition of Gram-positive bacterial wall components such as S. aurous peptidoglycan, whereas TLR4 recognizes lipopolysaccharide, a component of Gram-negative bacteria such as E. coli (14). However, we have previously shown that peptidoglycan-associated lipoprotein, a naturally occurring TLR2 agonist and a ubiquitous Gram-negative bacterial outer membrane protein that is shed by Gram-negative bacteria (e.g., E. coli) into the circulation of septic animals (29), causes inhibitory effect on cardiomyocyte function in vitro (13). Infusion of the Gram-positive bacteria S. aurous to isolated heart also induces LV dysfunction (11). Whereas these studies suggest a role of TLR2 in modulating cardiac function, the experimental conditions of those studies may not reflect the complex nature that often accompanies severe sepsis in vivo, such as profound immune activation/suppression and significant hemodynamic disturbance. Furthermore, clinical septic shock and septic cardiomyopathy often occur after polymicrobial infection, such as open-wound trauma and bowel perforation, whereas single purified bacteria or bacterial component were used and administered in vitro in these previous studies.
Severe sepsis is often featured clinically by a dramatic decrease in systemic vascular resistance as manifested as refractory hypotension. Decreased preload as well as afterload could affect overall cardiac contractile function. Host neurohormonal response and administration of vasoactive reagents can mask cardiac dysfunction during severe sepsis. In this study, we have taken several approaches aiming to accurately measure cardiac dysfunction during polymicrobial sepsis. First, all mice were given additional fluid after surgery to minimize hypovolemia often associated with sepsis. Second, we used strain rate by tissue Doppler imaging to noninvasively measure LV contractile function. As noted, strain rate represents the rate of myocardial deformation and can reliably measure LV function (19, 30, 31). It is also more sensitive and less dependent on loading condition than ejection fractions (31, 32). Whereas the conventional echocardiographic measurements such as LV internal dimension and fractional shortening are often used in routine clinical practice, these parameters are limited by both preload and afterload dependencies. The advantage of strain rate over the conventional parameters was evident in the present study. Polymicrobial sepsis-induced cardiomyopathy was demonstrated by a progressive decrease in strain rate within 12–24 hrs after CLP, whereas LV internal dimension and fractional shortening did not change significantly within the same period. Third, we isolated the hearts from sham and septic mice and measured LV contractile function in a Langendorff system. The ex vivo system allows us to measure LV function at a constant preload that is often impossible under in vivo septic conditions and, in the meantime, avoid some of systemic neurohormonal factors that may impact on myocardial function in vivo. Again, the ex vivo studies clearly indicate that polymicrobial sepsis induces a significant reduction in LV contractile function, whereas the hearts from TLR2−/− mice maintained normal LV function. Finally, we tested sarcomere shortening and Ca2+ transients in cardiomyocytes isolated from septic WT and TLR2−/− mice. Consistent with in vivo and ex vivo studies, cardiomyocytes isolated from septic TLR2−/− exhibited significant improvement in sarcomere shortening compared to WT cardiomyocytes. Whereas the downstream intracellular signaling that leads to cardiomyocyte dysfunction remains to be investigated, this series of studies clearly indicate that TLR2 activation is a critical signaling pathway leading to cardiac dysfunction during polymicrobial sepsis.
Cytokines, such as TNF-α, IL-6, and IL-1β, have been implicated as cardiode-pressants responsible for septic cardiomyopathy (5, 25, 33), and their plasma levels correlate with patient survival during sepsis (34). The blunted proinflammatory cytokine production in TLR2−/− mice after polymicrobial infection is likely to be one of the mechanisms that contribute to the better-preserved cardiac function and improved survival in TLR2−/− mice after CLP. Our study extended the work of Peck-Palmer et al (35) and Alves-Filho, et al (7), who examined the role of TLR2/4-MyD88 signaling in lymphocyte apoptosis and serum cytokine production during sepsis. We demonstrated the specific role of TLR2 signaling in the cardiodepressant cytokine production in the heart during sepsis. These findings suggest an important role for TLR2 signaling by cardiomyocytes as a critical mediator in cardiac dysfunction and systemic inflammation during polymicrobial sepsis. It is possible that TLR2-dependent signaling in cardiomyocytes is directly responsible for the CLP-induced cardiomyocyte dysfunction as supported by our previous in vitro work (13). However, we cannot exclude the possibility that inflammatory cytokines produced from other sources depress cardiomyocyte function during sepsis. Further studies using tissue-specific TLR2-deficient mice are needed to determine the cell type responsible for the myocardial dysfunction observed in our study.
Neutrophils have been regarded as double-edged swords in sepsis (3). They provide the first line of defense against invading pathogens by phagocytosing, killing, and digesting bacteria and fungi. Transmigration of neutrophils from microcirculation to inflammation site requires a chemoattractant gradient. The ability of neutrophils to effectively migrate to infectious site represents a vital part of host defense and may impact on the outcome of sepsis (36, 37). However, neutrophils also contribute to the immunopathogenesis of sepsis by releasing a variety of inflammatory cytokines, lipid mediators, and oxygen radicals on stimulated by bacterial components (38). In the present study, we demonstrate that polymicrobial infection induces peritoneal neutrophil infiltration and systemic TLR2 deficiency further enhances neutrophils migration into the peritoneal space after CLP, a finding consistent to that reported by Alves-Filho et al (7). In that study, the investigators propose that bacterial sepsis impairs neutrophil migratory function by down-regulation of its CXCR2, a chemokine receptor critical for neutrophil chemotaxis function. They demonstrate that direct neutrophil TLR2 activation is essential for the CXCR2 down-regulation and the impairment of neutrophil chemotaxis. Furthermore, we found no difference in CXCR2 expression by peritoneal neutrophils between WT and TLR2−/− mice. This is likely attributable to the fact that the peritoneal neutrophils have been exposed to and activated by a variety of chemokines and cytokines, such as CXCL2 and TNF-α. These cytokines can down-regulate CXCR2 expression in neutrophils (39–42). Thus, it seems reasonable to speculate that in addition to TLR2 signaling, other signaling pathways activated by cytokines/chemokines during polymicrobial sepsis may also play an important role in modulating neutrophil CXCR2 expression and function.
The present study demonstrates that systemic TLR2 deficiency confers a significant survival benefit in TLR2−/− mice after severe sepsis. Whereas the exact mechanisms leading to the survival benefit in TLR2−/− mice need to be further investigated, improved LV contractile function and cardiovascular hemodynamics, enhanced neutrophil migratory function, and attenuated systemic inflammation all likely contribute to the improved survival of these septic TLR2−/− mice.
In summary, our data suggest that TLR2 signaling is essential for the development of cardiac dysfunction and contributes to the high mortality during polymicrobial intra-abdominal sepsis. This study indicates that specific targeting of TLR2 signaling pathway may confer a novel therapeutic benefit in the clinical management of severe sepsis and septic cardiomyopathy.
Acknowledgments
The authors would like to thank Dr. Judith Hellman of University of California at San Francisco and Dr. Ulrich Schmidt of Massachusetts General Hospital for the helpful discussions on the project. We appreciate Dr. Hui Zheng of MGH Biostatistics Center for his advice on statistical analysis.
This work was supported by National Institutes of Health grant GM-080906 and American Heart Association grant 0755890T.
Footnotes
The authors have not disclosed any conflicts of interest.
Contributor Information
Lin Zou, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Department of Ultrasound Medicine, Central South University, China.
Yan Feng, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Yu-Jung Chen, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Rui Si, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Shiqian Shen, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Qichang Zhou, Department of Ultrasound Medicine, Central South University, China.
Fumito Ichinose, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Marielle Scherrer-Crosbie, Cardiac Ultrasound Laboratory, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Wei Chao, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 5.Flierl MA, Rittirsch D, Huber-Lang MS, et al. Molecular events in the cardiomyopathy of sepsis. Mol Med. 2008;14:327–336. doi: 10.2119/2007-00130.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 7.Alves-Filho JC, Freitas A, Souto FO, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A. 2009;106:4018–4023. doi: 10.1073/pnas.0900196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plitas G, Burt BM, Nguyen HM, et al. Toll-mlike receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1–H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantz S, Kobzik L, Kim YD, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knuefermann P, Sakata Y, Baker JS, et al. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation. 2004;110:3693–3698. doi: 10.1161/01.CIR.0000143081.13042.04. [DOI] [PubMed] [Google Scholar]
- 12.Nemoto S, Vallejo JG, Knuefermann P, et al. Escherichia coli LPS-induced LV dysfunction: Role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol. 2002;282:H2316–H2323. doi: 10.1152/ajpheart.00763.2001. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Bagchi A, Zhao H, et al. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med. 2007;35:886–892. doi: 10.1097/01.CCM.0000256723.37586.A2. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 15.Chaudry IH, Wichterman KA, Baue AE. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]
- 16.Baker CC, Chaudry IH, Gaines HO, et al. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 17.Feigenbaum H, Popp RL, Wolfe SB, et al. Ultrasound measurements of the left ventricle. A correlative study with angiocardiography. Arch Intern Med. 1972;129:461–467. [PubMed] [Google Scholar]
- 18.Hataishi R, Rodrigues AC, Neilan TG, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 19.Sebag IA, Handschumacher MD, Ichinose F, et al. Quantitative assessment of regional myocardial function in mice by tissue Doppler imaging: Comparison with hemodynamics and sonomicrometry. Circulation. 2005;111:2611–2616. doi: 10.1161/CIRCULATIONAHA.104.474411. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Zhao H, Xu X, et al. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol. 2008;295:H1311–H1318. doi: 10.1152/ajpheart.00119.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Zhao H, Graveline AR, et al. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;291:H1900–H1909. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose F, Buys ES, Neilan TG, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res. 2007;100:130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 23.von Meyenburg C, Hrupka BH, Arsenijevic D, et al. Role for CD14, TLR2, and TLR4 in bacterial product-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R298–R305. doi: 10.1152/ajpregu.00659.2003. [DOI] [PubMed] [Google Scholar]
- 24.Parrillo JE. Septic shock–vasopressin, norepinephrine, and urgency. N Engl J Med. 2008;358:954–956. doi: 10.1056/NEJMe0800245. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes CJ, Jr, Akamine N, Knobel E. Myocardial depression in sepsis. Shock. 2008;30(Suppl 1):14–17. doi: 10.1097/SHK.0b013e3181818617. [DOI] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Coopersmith CM, McDunn JE, et al. The sepsis seesaw: Tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remick DG, Newcomb DE, Bolgos GL, et al. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Swanson PE, Cobb JP, et al. Apoptosis in lymphoid and parenchymal cells during sepsis: Findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Liang MD, Bagchi A, Warren HS, et al. Bacterial peptidoglycan-associated lipoprotein: A naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J Infect Dis. 2005;191:939–948. doi: 10.1086/427815. [DOI] [PubMed] [Google Scholar]
- 30.Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–1164. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg NL, Firstenberg MS, Castro PL, et al. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 32.Gorcsan J, III, Strum DP, Mandarino WA, et al. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation. 1997;95:2423–2433. doi: 10.1161/01.cir.95.10.2423. [DOI] [PubMed] [Google Scholar]
- 33.Goldfarb RD, Glock D, Kumar A, et al. A porcine model of peritonitis and bacteremia simulates human septic shock. Shock. 1996;6:442–451. doi: 10.1097/00024382-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 35.Peck-Palmer OM, Unsinger J, Chang KC, et al. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J Leukoc Biol. 2008;83:1009–1018. doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- 36.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 37.Alves-Filho JC, de Freitas A, Spiller F, et al. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 39.Allen SJ, Crown SE, Handel TM. Chemokine: Receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 40.Neel NF, Schutyser E, Sai J, et al. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16:637–658. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khandaker MH, Mitchell G, Xu L, et al. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood. 1999;93:2173–2185. [PubMed] [Google Scholar]
- 42.Asagoe K, Yamamoto K, Takahashi A, et al. Down-regulation of CXCR2 expression on human polymorphonuclear leukocytes by TNF-alpha. J Immunol. 1998;160:4518–4525. [PubMed] [Google Scholar]


